Abstract
Although astronauts’ cardiovascular function is normal while they are in space, many have altered haemodynamic responses to standing after they return to Earth, including inordinate tachycardia, orthostatic hypotension, and uncommonly, syncope. Simulated microgravity impairs vagal baroreceptor–cardiac reflex function and causes orthostatic hypotension. Actual microgravity, however, has been shown to either increase, or not change vagal baroreflex gain. In this study, we tested the null hypothesis that spaceflight does not impair human baroreflex mechanisms. We studied 11 American and two German astronauts before, during (flight days 2–8), and after two, 9- and 10-day space shuttle missions, with graded neck pressure and suction, to elicit sigmoid, vagally mediated carotid baroreflex R–R interval responses. Baseline systolic pressures tended to be higher in space than on Earth (P= 0.015, repeated measures analysis of variance), and baseline R–R intervals tended to be lower (P= 0.049). Baroreceptor–cardiac reflex relations were displaced downward on the R–R interval axis in space. The average range of R–R interval responses to neck pressure changes declined from preflight levels by 37% on flight day 8 (P= 0.051), maximum R–R intervals declined by 14% (P= 0.003), and vagal baroreflex gain by 9% (P= 0.009). These measures returned to preflight levels by 7–10 days after astronauts returned to Earth. This study documents significant increases of arterial pressure and impairment of vagal baroreflex function in space. These results and results published earlier indicate that microgravity exposure augments sympathetic, and diminishes vagal cardiovascular influences.
Introduction
Prior to the first space mission, scientists predicted that exposure to microgravity would lead to failure of critical organ systems, including particularly, the cardiovascular system. Although the first human in space, the Soviet cosmonaut Uri Gagarin, returned to Earth from an 89 min orbital mission with no apparent disability, the ninth man in space, the American astronaut Wally Schirra, returned after 9 h 13 min in space and reported dizziness upon standing up. Orthostatic symptoms occur commonly after space missions (Fritsch et al. 1992). Indeed, fully 9 of 14 astronauts (including seven of the astronauts we studied) could not stand motionless for 10 min without experiencing symptoms of dizziness or presyncope, and two additional astronauts could stand, but experienced dizziness by the end of 10 min of standing (Buckey et al. 1996).
Since experimental baroreceptor denervation causes profound orthostatic hypotension (Persson et al. 1988), many studies have sought a contribution of baroreflex impairment to the orthostatic hypotension seen after exposure to microgravity or its terrestrial analogues. In one study (Convertino et al. 1990), we showed that weightlessness, as simulated by prolonged head-down bed rest (Kakurin et al. 1976), provokes post-bed rest orthostatic intolerance, in proportion to the reduction of vagally mediated carotid baroreceptor–cardiac reflex gain that also occurs. Subsequently, we showed that actual microgravity reduces baroreflex gain in astronauts studied before and after space missions (Fritsch et al. 1992; Fritsch-Yelle et al. 1994). On landing day, however, astronauts may be excited, fatigued and sleep-deprived; they may have been given pharmacological agents; they may have been given salt and fluid prior to landing (which may induce vomiting); and they may have lost body mass (presumably, fluid) acutely during the return to Earth (Drummer et al. 2000).
Thus, although data obtained before and after space missions support conclusions drawn from studies of volunteers before and after simulated weightlessness, they leave open the question, Are human baroreflexes impaired during space missions, under more controlled conditions than those that obtain on landing day? A recent study (Di Rienzo et al. 2008) documented supranormal vagal baroreflex gain early, and normal gain late during space missions. Another (Beckers et al. 2009) reported no significant change of vagal baroreflex function in space. We conducted our study to test the null hypothesis that spaceflight does not impair human baroreflex mechanisms. Our study differs from others conducted on astronauts in space, in that we report data from 13 astronauts – a larger number of subjects than studied heretofore.
Methods
Subjects and missions
We studied 11 male and two female astronauts during two space shuttle missions: the 9-day Spacelab Life Sciences-1 mission (SLS-1), and the 10-day second German Spacelab mission (D-2) – missions STS-40 and STS-55 of the space shuttle Columbia. The Institutional Review Boards of the Hunter Holmes McGuire Department of Veterans Affairs Medical Center, the Medical College of Virginia at Virginia Commonwealth University, the German Aerospace Research Establishment, and the Human Research Policy and Procedures Committee, National Aeronautics and Space Administration (NASA) Johnson Space Center all approved the research protocol, which conformed with the provisions of the Declaration of Helsinki. All astronauts gave their written informed consent prior to participation. All were healthy and had passed the NASA class III physical examination (Pool et al. 1994). At the time of the missions, the astronauts’ average age was 40 (range: 31–47) years.
Preflight measurements were made on all subjects (n= 13) 45 or 10 days prior to the launch of the mission. Inflight measurements were made on flight days 2 (n= 12), 4 (n= 11), 6 (n= 10), and 8 (n= 13). Postflight measurements were made on landing day (n= 12), the day after landing (n= 13), and 4 (n= 13), 7–10 (n= 11), and 30 (n= 9) days after landing.
Baroreflex test
We studied astronauts in the supine position before and after the space missions, and the astronauts studied themselves in the upright position during the space missions. We recorded the electrocardiogram, respiration (nasal thermistor, or abdominal bellows connected to a strain-gauge pressure transducer), and neck chamber pressure (strain-gauge pressure transducer). Sphygmomanometric arterial pressures were measured on Earth by physicians and physiologists, and in space, by crew members who were fully trained to perform sphygmomanometry (as well as other aspects of the research). Three crew members of the SLS-1 mission were physicians. Blood pressure was measured three times, before, during and after baroreflex testing, and results were averaged. Baseline R–R intervals were measured during held expiration immediately before each neck pressure sequence (see below), and averaged.
We described the method for studying vagal baroreflex function earlier (Eckberg & Fritsch, 1993). Each astronaut wore a custom-made, tightly fitting synthetic rubber chamber that sealed against the mandible, upper chest and posterior neck. A stepping motor-controlled bellows varied pressure within the chamber systematically. After about 5 s of held expiration at a normal end-expiratory volume, neck pressure increased to about 40 mmHg. Then, after about 5 s at this level, pressure was lowered by successive 15 mmHg, R-wave triggered decrements, to about –65 mmHg. Thus, carotid artery dimensions were reduced (Kober & Arndt, 1970), and then increased progressively, by negative pressure steps superimposed on the naturally occurring arterial pulse; the nominal range of pressure changes was 105 mmHg. Each neck pressure sequence was applied seven times, and responses were averaged.
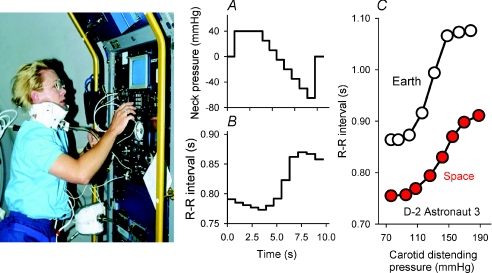
Figure 1 shows astronaut Rhea Seddon studying her own baroreflexes during the SLS-1 mission, and data from a second astronaut recorded before and during flight day 8 of the D-2 mission. In this and other figures, data recorded in space are shown in red. Figure 1A shows a stylized neck pressure sequence, and Fig. 1B shows R–R intervals recorded during an actual neck pressure sequence and telemetered from space. Since abrupt R–R interval fluctuations reflect changes of vagal-cardiac nerve activity linearly (Katona et al. 1970), the neck pressure–R–R interval relation (Fig. 1C) may characterize the entire classical (Koch, 1931) vagal-cardiac baroreflex relation, and define threshold, linear and saturation ranges. In this astronaut, the maximum slope was 5.38 before the mission, and 2.63 ms mmHg−1 on flight day 8 (red). An earlier Earth-based study (Eckberg et al. 1992) conducted with similar equipment in 26 healthy subjects studied twice 10 weeks apart showed that both the stimulus and the vagal response to neck pressure changes are highly reproducible.
Figure 1. Baroreflex testing.
The left panel shows astronaut Rhea Seddon performing baroreflex testing on herself during the SLS-1 mission. The custom-fabricated neck chamber was sealed around the mandible, posterior neck and chest with synthetic rubber. A, a stylized neck pressure sequence; B, R–R interval responses to the stimulus profile, telemetered from space; C, baroreceptor stimulus–R–R interval response relations for a different astronaut, studied preflight and on flight day 8 during the D-2 mission. In this and other figures, data from space are shown in red.
Carotid distending pressure was taken as systolic pressure less neck chamber pressure. We measured maximum and minimum R–R intervals, the R–R interval range (maximum minus minimum R–R intervals), and the maximum slope (derived from linear regression of the three consecutive data pairs that yielded the greatest slope). Typically, this sequence overlaps the baseline arterial pressure, and thus reflects responses to carotid distending pressure changes above and below baseline levels.
Statistics
Statistical analyses were performed with SigmaStat 3.1 (Systat Software, Point Richmond, CA, USA). We used one-way repeated measures analysis of variance with individual (not group) data for data sets that were distributed normally, and Holm–Sidak pairwise multiple comparison analysis to identify a group or groups that differed from others. We used Friedman's repeated measures analysis of variance on ranks for data sets that were not distributed normally and Tukey's pairwise multiple comparison test to identify a group or groups that differed from others.
Due to the similarity of responses 10 and 30 days after the mission, and the limited number of subjects studied at 30 days (n= 9), we excluded 30 day data from the analysis. Since many of the measured parameters appeared to change in a linear fashion across experimental days, we analysed individual (not group) data with linear regression from preflight through flight day 8, and from flight day 8 through 10 days after return. Nearly all results were normally distributed, and are reported as mean values ± 95% confidence intervals.
Results
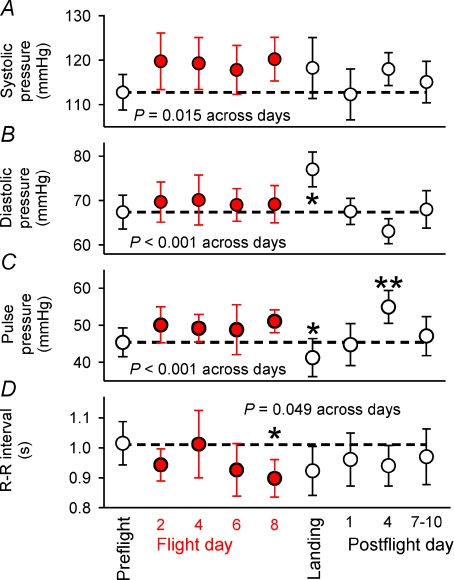
Figure 2 depicts mean baseline arterial pressures and R–R intervals and their 95% confidence intervals for all subjects for all sessions. All four sets of data were statistically significant across experiment days: systolic, diastolic and pulse pressures tended to be higher than preflight levels (P= 0.015, 0.001, and 0.001), and R–R intervals tended to be lower (P= 0.049). Mean diastolic pressure (Fig. 2B) was significantly higher on landing day (*) than on all other days. Pulse pressure (Fig. 2C) on landing day was significantly lower than on flight day 8 (*), and pulse pressure on postflight day 4 was significantly higher than preflight, landing day and postflight day 1 (**). Mean R–R intervals (Fig. 2D) were significantly lower on flight day 8 than preflight. The falling R–R interval trend over the inflight sessions was broken on flight day 4; the punctate return of mean R–R intervals to the preflight level on this day was mirrored by baroreflex-mediated R–R interval changes (see below).
Figure 2. Mean (± 95% confidence limits) baseline arterial pressures and R–R intervals associated with each baroreflex test.
Dashed lines indicate preflight averages. Results from repeated measures analysis of variance are given. The significance of pairwise differences was determined with the Holm–Sidak test. In panel B: *diastolic pressure was significantly higher on landing day than on all other experimental days. In panel C: *pulse pressure was significantly lower on flight day 8 than preflight, and **significantly higher on postflight day 4 than preflight, landing day, and postflight day 1. In panel D: *R–R intervals were significantly lower on flight day 8 than preflight.
Baroreflex response relations
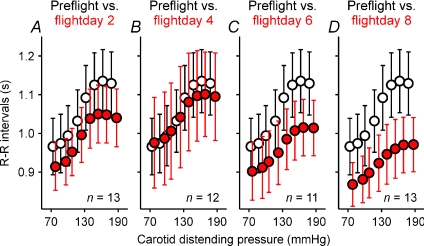
Figure 3 shows mean pre- and inflight (red) baroreflex response relations and R–R interval and neck pressure 95% confidence intervals. In general, baroreflex relations tended to be displaced downwards as the space mission wore on, and baroreflex ranges and slopes tended to diminish. On the earliest day in space (flight day 2, Fig. 3A, red) the baroreflex relation was displaced downward. However, on the fourth day in space (Fig. 3B, red), R–R intervals at the lowest carotid distending pressures were slightly greater than those recorded preflight. By flight day 8 (Fig. 3D, red), however, the 95% confidence intervals of R–R intervals at the highest carotid distending pressures did not overlap those recorded preflight.
Figure 3. Mean ± 95% confidence intervals for preflight and inflight vagal baroreflex relations.
Confidence limits for carotid distending pressures are obscured by the symbols. Open circles, preflight values; red circles inflight values.
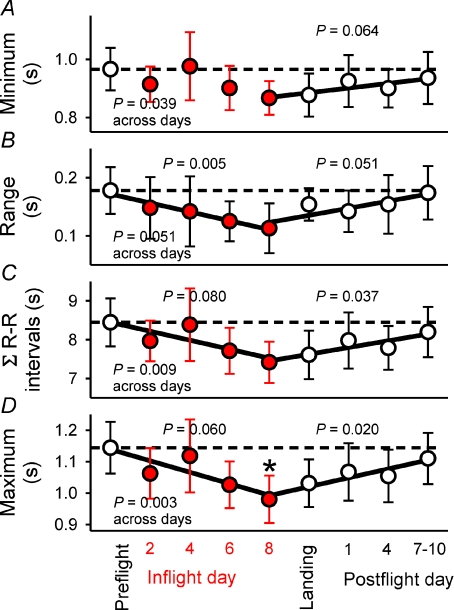
Figure 4 summarizes several R–R interval responses to neck pressure sequences. A prominent feature of these four panels is that the data inscribe V-shapes: measurements tended to decline progressively from preflight levels as the mission wore on, and to increase progressively after astronauts returned to Earth. Levels of significance were derived from parametric or non-parametric repeated measures analysis of variance across study days, and from linear regression for measurements from preflight to flight day 8, and from flight day 8 until the last postflight session. All measurement changes but one (R–R interval range, Fig. 4B, P= 0.051) were statistically significant across study days. The range of mean R–R interval changes (Fig. 4B) fell significantly (P= 0.005) from 0.178 preflight, to 0.113 s by flight day 8, a 37% reduction, and the maximum mean R–R intervals (Fig. 4D) fell significantly (P= 0.003) from 1.11 preflight to 0.98 s by flight day 8, a 13% reduction.
Figure 4. Mean ± 95% confidence intervals for R–R interval changes provoked by baroreflex stimuli.
Regressions for data from preflight to flight day 8 and from flight day 8 until postflight days 7–10 are shown with corresponding levels of significance. C, the integrals for all R–R interval responses to stimulus sequences. *Maximum R–R intervals during baroreflex testing were significantly lower on flight day 8 than preflight.
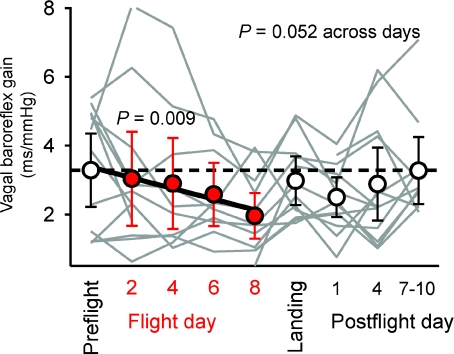
Figure 5 shows individual (grey), and mean vagal baroreflex gains (see Methods) and their 95% confidence intervals for all subjects for all sessions. As expected (Eckberg & Kuusela, 2005), individual vagal baroreflex gains varied widely between individuals and within the same individual across study days. As with R–R interval measurements (Fig. 4), the pattern of vagal baroreflex gain changes tended to be V-shaped. However, changes across experimental days were not significant (P= 0.052). The regression of vagal baroreflex gain from preflight levels to flight day 8 was highly significant (Fig. 5, red, P= 0.009).
Figure 5. Mean ± 95% confidence intervals and individual (grey) vagal baroreflex gains for all subjects for all experimental sessions.
Regression of baroreflex gains from preflight to flight day 8 was highly significant (P= 0.009). Individual data illustrate the great variability of vagal baroreflex gain among healthy subjects.
Discussion
Most astronauts experience symptoms of lightheadedness when they stand on Earth immediately after space missions (Buckey et al. 1996); such abnormal orthostatic responses may reflect space-induced vagal baroreflex derangement (Convertino et al. 1990). We studied 13 astronauts before, during, and after two 8-day space sojourns, and tested the null hypothesis that spaceflight does not impair human baroreflex mechanisms. Our results reject this hypothesis and provide two unique insights into human autonomic adaptations to space. Exposure to microgravity (1) systematically reduces vagal baroreflex gain, R–R interval responses to changes of baroreceptor input, and baseline R–R intervals, and (2) raises baseline arterial pressures. These, and results published previously, provide a coherent, internally consistent view of human autonomic responses to space travel: microgravity exposure augments sympathetic, and diminishes vagal influences.
Numerous approaches have been devised to simulate the effects of microgravity on Earth; however, it is likely that no approach faithfully replicates the physiological challenges of microgravity. Consider sympathetic responses to one popular microgravity analogue, head-down bed rest (Kakurin et al. 1976). Head-down bed rest does not alter sympathetic activity, as reflected by plasma noradrenaline levels (Convertino et al. 1990) or directly recorded muscle sympathetic nerve activity (Pawelczyk et al. 2001). Spaceflight, however, augments sympathetic activity, as reflected by plasma (Norsk et al. 1995; Ertl et al. 2002) and platelet (Christensen et al. 2005) noradrenaline concentrations, and whole-body noradrenaline spillover and muscle sympathetic nerve activity (Ertl et al. 2002). Therefore, in this discussion we focus on results from human studies conducted in space.
Baseline haemodynamic measurements in space
It is surprising that after nearly a half century of manned spaceflight, there is disagreement over how space travel affects simple resting haemodynamic measurements. As others have discussed (Baevsky et al. 2007; Di Rienzo et al. 2008; Verheyden et al. 2009), much of this disagreement arises from the experimental conditions that obtain on Earth and in space. On Earth, the supine position may most closely replicate conditions in space: autonomic mechanisms are materially altered when supine subjects sit or stand (Pickering et al. 1971; Burke et al. 1977; Norsk et al. 2006). Therefore, differences between data from space and data from Earth, recorded with subjects sitting (Verbanck et al. 1997; Norsk et al. 2006), semi-supine (Baevsky et al. 2007), or standing (Shykoff et al. 1996; Migeotte et al. 2003; Beckers et al. 2009) are difficult to interpret.
Four groups compared R–R intervals and arterial pressures recorded in space with those recorded on Earth, with subjects unambiguously in the supine position. Shykoff et al. (1996) and Migeotte et al. (2003) and their coworkers studied groups of six and four astronauts, and found no significant difference between R–R intervals measured on Earth and in space. Cox et al. (2002), who studied the same four astronauts as Migeotte, reported insignificant reductions of baseline R–R intervals and increases of arterial pressures in space.
Verheyden et al. (2009) studied 11 astronauts, and reported that baseline heart rates and blood pressures are comparable in space and on Earth. We are not certain why this conclusion, drawn from a relatively large cohort of astronauts, differs from our own. One possibility is that the astronauts Verheyden studied were in better physical condition in space than they were on Earth. In space, astronauts exercised for up to 2 h, three out of four days, but on Earth, they did not follow any set exercise programme. Since exercise training lowers resting heart rate and blood pressure (Kingwell et al. 1992), responses to training may have prevented the heart rate and blood pressure increases that we observed in space.
Arbeille et al. (2001) studied Russian astronauts and reported that their mean R–R intervals declined by 11% by the end of the first week in space. We confirm this result, and also document an average R–R interval reduction of 11%. In our study, mean R–R intervals, with the exception of flight day 4, were consistently below preflight levels (Fig. 2D, P= 0.049). Shortened R–R intervals in space may reflect diminished vagal-cardiac nerve activity; when arterial pressure is varied pharmacologically, R–R intervals provide a more faithful estimate of changing levels of vagal-cardiac nerve activity than respiratory-frequency R–R interval fluctuations (Eckberg et al. 1988). We extend Arbeille's study by showing that arterial pressures are increased in space (Fig. 2).
Our baseline measurements and those of Arbeille et al. (2001) speak to concerns expressed by others regarding ‘paradoxical’ results obtained in space. Verheyden et al. (2009) and Norsk et al. (2006) and their coauthors could not reconcile findings of increased sympathetic nerve activity and vasopressor hormone levels in space with their conclusions that the ‘circulation [is] chronically dilated’ or ‘relaxed’ in space. Changes of arterial pressure, heart rate, and cardiac output in space are not paradoxical when they are compared with measurements made in the supine position on Earth. The combination of increased blood pressure (Fig. 2A), increased sympathetic activity, however measured (Kvetňanskýet al. 1991; Norsk et al. 1995; Ertl et al. 2002), increased systemic (Norsk et al. 2006) and calf (Watenpaugh et al. 2001) vascular resistance, increased levels of vasopressor hormones (Norsk et al. 1995), reduced cardiac outputs (Arbeille et al. 2001), and reduced R–R intervals (Fig. 2D) and vagal baroreflex responsiveness (Figs 3–5) are internally consistent. Space travel augments sympathetic and reduces vagal cardiovascular influences.
Vagal baroreflex mechanisms in space
Two studies (Iellamo et al. 2006; Beckers et al. 2009) evaluated the vagal baroreflexes of nine astronauts in space, and in the supine position on Earth, and showed no significant change of baroreflex gain in space. We report a different result: in 13 astronauts, vagal-cardiac reflex relations shifted progressively downward (Fig. 3), and R–R interval responses to baroreceptor stimulation (Fig. 4) and baroreflex gains (Fig. 5) fell during 8 days in space. These changes paralleled reductions of baseline R–R intervals (Fig. 2). The study of Di Rienzo et al. (2008) of vagal baroreflexes in space is not comparable to ours, in that these authors made their observations on Earth with subjects in the sitting position. However, one result from their study is relevant to our own observations: in space: baroreflex gains were significantly greater early than late during the mission. We confirmed this observation and documented a highly significant (Fig. 5, P= 0.009) downward trend of baroreflex gains as the duration of microgravity exposure lengthened.
Our observations in humans are complemented by studies of Shimizu and his colleagues (Yamasaki et al. 2004a,b; Waki et al. 2005) in animals. They showed that in developing rats, the proportion of unmyelinated aortic depressor nerve fibres and their numbers are diminished by spaceflight. Moreover, aortic nerve activity triggered by arterial pressure elevations, and baroreflex-mediated heart rate responses are less in animals flown in space than in animals on Earth, and they have higher baseline heart rates. Importantly, 30 days after landing, differences between baroreflex responses of animals flown in space and animals studied on Earth were no longer significant.
Potential physiological bases for autonomic changes in microgravity
Blood volume
Red cell mass, and plasma and blood volumes decline within the first day after astronauts enter microgravity, and stay at subnormal levels as long as astronauts remain in space (Alfrey et al. 1996). Hypovolaemia in space is sufficient to explain several important haemodynamic responses to microgravity, including reductions of left ventricular diastolic volume (9%), stroke volume (17%), and cardiac output (8%, Arbeille et al. 2001). Left ventricular stroke volume reductions reduce carotid artery pulsations (Hastings et al. 2007), and increase muscle sympathetic nerve activity (Levine et al. 2002; Convertino & Cooke, 2002; Charkoudian et al. 2005). Cardiac output reductions appear to increase muscle sympathetic nerve activity (Charkoudian et al. 2005). Increased sympathetic activity opposes vagal heart rate inhibition (Taylor et al. 2001) and vagal baroreflex responses (Eckberg et al. 1976).
Vestibular
Most astronauts experience motion sickness in space (Davis et al. 1988), and display altered vestibular function following space missions (Young et al. 1993; Merfeld, 1996; Clément et al. 2007). In an elegant series of experiments conducted on rats flown in space, Ross (1993; 1994; 2000); reported significantly increased numbers of ribbon synapses and sphere-like ribbons in hair cells of utricular maculae. These changes correlated with functional disability when rats returned to Earth. In humans, vestibular stimulation increases muscle sympathetic nerve activity (Ray, 2000), and contributes to autonomic responses to upright posture (Sauder et al. 2008). Conceivably, upregulation of vestibular receptors in space helps to maintain normal sympathetic baroreflex responses to upright posture following spaceflight (Levine et al. 2002).
Muscle
Antigravity muscles atrophy in space (Riley et al. 1996). Muscle atrophy is likely to increase central command (greater effort must be expended to perform the same work with weakened muscles), and may also alter mechano- and metaboreceptor afferent responses to exercise (Tipton, 2003). However, if muscle atrophy influenced our results, the effect was likely to have been small: Fu et al. (2002) showed that arterial pressure, heart rate, and muscle sympathetic nerve responses to handgrip followed by forearm ischaemia are normal in space. Although we are aware of no studies of human forearm muscle mass in space, it is likely that forearm muscles respond to reduced levels of exertion during lifting in space, by atrophying. Physical detraining, including that caused by bed rest, itself may provoke parallel reductions of vagal baroreflex responses and baseline R–R intervals (Hughson et al. 1994).
Limitations
The challenges attending attempts to perform meaningful, scientifically valid research on humans in space are daunting. Arguably, the largest of these is the often very small number of subjects who can be studied (this issue was encapsulated by Pawelczyk (2006) in his Perspectives article, ‘Big concepts small N’). Although we studied baroreflex function in more astronauts (n= 13) than other authors, we cannot exclude the possibility that some of our negative results reflect β-statistical errors. Similarly, we are unable to draw meaningful conclusions regarding possible sex differences in responses to microgravity (Harm et al. 2001); we studied only two women, and during some experimental sessions, only one. A second limitation is that vagal baroreflex gain fluctuates hugely in healthy humans, from moment-to-moment, and from day-to-day (Eckberg & Kuusela, 2005; Westerhof et al. 2006). This problem can be mitigated if the number of subjects is large enough; indeed, we (Eckberg et al. 1992) showed that with an n of 26, responses to the vagal baroreflex provocation we used are highly reproducible.
Clinical implications
Autonomic responses to microgravity may have important functional consequences for astronauts after they return to Earth. Convertino et al. (1990) reported strong correlations among vagal-cardiac baroreflex impairment, heart rate increases in the upright position, and the occurrence of presyncope in subjects after prolonged head-down bed rest. Fritsch et al. (1992) found a strong correlation between subjects’ operating positions on their sigmoid carotid baroreflex relations and the occurrence of presyncope after spaceflight. Our results may also have implications for patients. Heart disease impairs vagal baroreflex responses (Eckberg et al. 1971; Sopher et al. 1990), and impaired baroreflex responsiveness constitutes a major risk factor for death after myocardial infarction (La Rovere et al. 1998), including particularly, dysrhythmic sudden death (Billman et al. 1982).
In conclusion, we evaluated vagal baroreflex responses of astronauts before, during and after two space missions, and tested the null hypothesis that spaceflight does not impair human baroreflex mechanisms. We show that vagal baroreflex gain and baroreflex-triggered R–R interval responses steadily decline from preflight levels during exposure to microgravity, and return to preflight levels by 10 days after astronauts return to Earth. Baseline R–R interval reductions and arterial pressure increases paralleled degradation of vagal baroreflex responses. Our results are consistent with other studies of human responses to microgravity, and point to reduced vagal, and enhanced sympathetic influences in space.
Acknowledgments
We thank the dedicated American and German crew members who served as subjects for this research. This work was supported by contracts from the National Aeronautics and Space Administration.
Author contributions
D.L.E. conceived the research, invented the neck chamber and the neck pressure stimulus algorithm, analysed the data and wrote the first and final drafts of the manuscript; J.R.H. performed some of the studies, performed some statistical analyses, and contributed importantly to the writing of the manuscript; L.A.B. supported all aspects of the research and collated the data for analysis; T.E.B. and J.A.T. conducted some of the studies and contributed to manuscript revisions; and R.G. designed, built and programmed the equipment used in space and on Earth.
References
- Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol. 1996;81:98–104. doi: 10.1152/jappl.1996.81.1.98. [DOI] [PubMed] [Google Scholar]
- Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol. 2001;86:157–168. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- Baevsky RM, Baranov VM, Funtova II, Diedrich A, Pashenko AV, Chernikova AG, Drescher J, Jordan J, Tank J. Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J Appl Physiol. 2007;103:156–161. doi: 10.1152/japplphysiol.00137.2007. [DOI] [PubMed] [Google Scholar]
- Beckers F, Verheyden B, Liu J, Aubert AE. Cardiovascular autonomic control after short-duration spaceflights. Acta Astronaut. 2009;65:804–812. [Google Scholar]
- Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Burke D, Sundlöf G, Wallin BG. Postural effects on muscle nerve sympathetic activity in man. J Physiol. 1977;272:399–414. doi: 10.1113/jphysiol.1977.sp012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen NJ, Heer M, Ivanova K, Norsk P. Sympathetic nervous activity decreases during head-down bed rest but not during microgravity. J Appl Physiol. 2005;99:1552–1557. doi: 10.1152/japplphysiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- Clément G, Denise P, Reschke MF, Wood SJ. Human ocular counter-rolling and roll tilt perception during off-vertical axis rotation after spaceflight. J Vestib Res. 2007;17:209–215. [PubMed] [Google Scholar]
- Convertino VA, Cooke WH. Relationship between stroke volume and sympathetic nerve activity: new insights about autonomic mechanisms of syncope. J Gravit Physiol. 2002;9:P-63–P-66. [PubMed] [Google Scholar]
- Convertino VA, Doerr DF, Eckberg DL, Fritsch JM, Vernikos-Danellis J. Head-down bed rest impairs vagal baroreflex responses and provokes orthostatic hypotension. J Appl Physiol. 1990;68:1458–1464. doi: 10.1152/jappl.1990.68.4.1458. [DOI] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KUO, Kuusela TA, Levine BD, Cooke WH, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC, Jr, Baisch FJ, Blomqvist CG, Robertson D, Eckberg DL. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva's manoeuvre. J Physiol. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JR, Vanderploeg JM, Santy PA, Jennings RT, Stewart DF. Space motion sickness during 24 flights of the space shuttle. Aviat Space Environ Med. 1988;59:1185–1189. [PubMed] [Google Scholar]
- Di Rienzo M, Castiglioni P, Ielamo F, Volterrani M, Pagani M, Mancia G, Karemaker JM, Parati G. Dynamic adaptation of cardiac baroreflex sensitivity to prolonged exposure to microgravity: data from a 16-day spaceflight. J Appl Physiol. 2008;105:1569–1575. doi: 10.1152/japplphysiol.90625.2008. [DOI] [PubMed] [Google Scholar]
- Drummer C, Hesse C, Baisch F, Norsk P, Elmann-Larsen B, Gerzer R, Heer M. Water and sodium balances and their relation to body mass changes in microgravity. Eur J Clin Invest. 2000;30:1066–1075. doi: 10.1046/j.1365-2362.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Abboud FM, Mark AL. Modulation of carotid baroreflex responsiveness in man: effects of posture and propranolol. J Appl Physiol. 1976;41:383–387. doi: 10.1152/jappl.1976.41.3.383. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Convertino VA, Fritsch JM, Doerr DF. Reproducibility of human vagal carotid baroreceptor-cardiac reflex responses. Am J Physiol Regul Integr Comp Physiol. 1992;263:R215–R220. doi: 10.1152/ajpregu.1992.263.1.R215. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Fritsch JM. How should human baroreflexes be tested? News Physiol Sci. 1993;8:7–12. doi: 10.1152/physiologyonline.1993.8.1.7. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kuusela TA. Human vagal baroreflex sensitivity fluctuates widely and rhythmically at very low frequencies. J Physiol. 2005;567:1011–1019. doi: 10.1113/jphysiol.2005.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand. 1988;133:221–231. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC, Jr, Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ, Robertson D. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch JM, Charles JB, Bennett BS, Jones MM, Eckberg DL. Short-duration spaceflight impairs human carotid baroreceptor-cardiac reflex responses. J Appl Physiol. 1992;73:664–671. doi: 10.1152/jappl.1992.73.2.664. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol. 1994;77:1776–1783. doi: 10.1152/jappl.1994.77.4.1776. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD, Pawelczyk JA, Ertl A, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D, Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm DL, Jennings RT, Meck JV, Powell MR, Putcha L, Sams CP, Schneider SM, Shackelford LC, Smith SM, Whitson PA. Invited Review: Gender issues related to spaceflight: a NASA perspective. J Appl Physiol. 2001;91:2374–2383. doi: 10.1152/jappl.2001.91.5.2374. [DOI] [PubMed] [Google Scholar]
- Hastings J, Shibata S, Shook R, Okazaki K, Conner C, Palmer MD, Fu Q, Levine BD. Muscle sympathetic nerve activity is dependent on stroke volume via carotid artery distortion during orthostatic stress. FASEB J. 2007;21:750. Abstract. [Google Scholar]
- Hughson RL, Maillet A, Gharib C, Fortrat JO, Yamamoto Y, Pavy-Letraon A, Rivière D, Güell A. Reduced spontaneous baroreflex response slope during lower body negative pressure after 28 days of head-down bed rest. J Appl Physiol. 1994;77:69–77. doi: 10.1152/jappl.1994.77.1.69. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Di Rienzo M, Lucini D, Legramante JM, Pizzinelli P, Castiglioni P, Pigozzi P, Pagani M, Parati G. Muscle metaboreflex contribution to cardiovascular regulation during dynamic exercise in microgravity insights: from mission STS-107 of the space shutle Columbia. J Physiol. 2006;572:829–838. doi: 10.1113/jphysiol.2005.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakurin LI, Lobachik VI, Mikhailov VM, Senkevich YA. Antiorthostatic hypokinesia as a method of weightlessness simulation. Aviat Space Environ Med. 1976;47:1083–1086. [PubMed] [Google Scholar]
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Dart AM, Jennings GL, Korner PI. Exercise training reduces the sympathetic component of the blood pressure-heart rate baroreflex in man. Clin Sci. 1992;82:357–362. doi: 10.1042/cs0820357. [DOI] [PubMed] [Google Scholar]
- Kober G, Arndt JO. Die Druck-Durchmesser-Beziehung der A. carotis communis des wachen Menschen. Pflugers Arch. 1970;314:27–39. doi: 10.1007/BF00587044. [DOI] [PubMed] [Google Scholar]
- Koch E. Die reflektorische Selbststeuerung des Kreislaufes. In: Kisch B, editor. Ergebnisse der Kreislaufforschung. Dresden: Steinkopff; 1931. [Google Scholar]
- Kvetňanský R, Noskov VB, Blazicek P, Gharib C, Popova IA, Gauquelin G, Macho L, Guell A, Grigoriev AI. Activity of the sympathoadrenal system in cosmonauts during 25-day space flight on station Mir. Acta Astronaut. 1991;23:109–116. doi: 10.1016/0094-5765(91)90106-f. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Robertson D, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamnic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM. Effect of spaceflight on ability to sense and control roll tilt: human neurovestibular studies on SLS-2. J Appl Physiol. 1996;81:50–57. doi: 10.1152/jappl.1996.81.1.50. [DOI] [PubMed] [Google Scholar]
- Migeotte P-F, Prisk GK, Paiva M. Microgravity alters respiratory sinus arrhythmia and short-term heart rate variability in humans. Am J Physiol Heart Circ Physiol. 2003;284:H1995–H2006. doi: 10.1152/ajpheart.00409.2002. [DOI] [PubMed] [Google Scholar]
- Norsk P, Damgaard M, Petersen L, Gybel M, Pump B, Gabrielsen A, Christensen NJ. Vasorelaxation in space. Hypertension. 2006;47:69–73. doi: 10.1161/01.HYP.0000194332.98674.57. [DOI] [PubMed] [Google Scholar]
- Norsk P, Drummer C, Röcker L, Strollo F, Christensen NJ, Warberg J, Bie P, Stadeager C, Johansen LB, Heer M, Gunga H-C, Gerzer R. Renal and endocrine responses in humans to isotonic saline infusion during microgravity. J Appl Physiol. 1995;78:2253–2259. doi: 10.1152/jappl.1995.78.6.2253. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA. Big concepts, small N. J Physiol. 2006;572:607–608. doi: 10.1113/jphysiol.2006.108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk JA, Zuckerman JH, Blomqvist CG, Levine BD. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H2230–H2239. doi: 10.1152/ajpheart.2001.280.5.H2230. [DOI] [PubMed] [Google Scholar]
- Persson P, Ehmke H, Kirchheim H, Seller H. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflugers Arch. 1988;411:160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Gribbin B, Petersen ES, Cunningham DJC, Sleight P. Comparison of the effects of exercise and posture on the baroreflex in man. Cardiovasc Res. 1971;5:582–586. doi: 10.1093/cvr/5.4.582. [DOI] [PubMed] [Google Scholar]
- Pool SL, Nicogossian AE, Moseley EC, Uri JJ, Pepper LJ. Medical evaluations for astronaut selection and longitudinal studies. In: Nicogossian A, editor. Space Physiology and Medicine. Philadelphia, PA: Lea & Febiger; 1994. pp. 375–393. [Google Scholar]
- Ray CA. Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2000;279:H2399–H2404. doi: 10.1152/ajpheart.2000.279.5.H2399. [DOI] [PubMed] [Google Scholar]
- Riley DA, Ellis S, Slocum GR, Sedlak FR, Bain JLW, Krippendorf BB, Lehman CT, Macias MY, Thompson JL, Vijayan K, De Bruin JA. In-flight and postflight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. J Appl Physiol. 1996;81:133–144. doi: 10.1152/jappl.1996.81.1.133. [DOI] [PubMed] [Google Scholar]
- Ross MD. Morphological changes in rat vestibular system following weightlessness. J Vestib Res. 1993;3:241–251. [PubMed] [Google Scholar]
- Ross MD. A spaceflight study of synaptic plasticity in adult rat vestibular maculas. Acta Otolarygol. 1994;516(Suppl):3–14. [PubMed] [Google Scholar]
- Ross MD. Changes in ribbon synapses and rough endoplasmic reticulum of rat utricular macular hair cells in weightlessness. Acta Orolaryngol. 2000;120:490–499. doi: 10.1080/000164800750045983. [DOI] [PubMed] [Google Scholar]
- Sauder CL, Leonard TO, Ray CA. Greater sensitivity of the vestibulosympathetic reflex in the upright posture in humans. J Appl Physiol. 2008;105:65–69. doi: 10.1152/japplphysiol.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykoff BE, Farhi LE, Olszowka AJ, Pendergast DR, Rokitka MA, Eisenhardt CG, Morin RA. Cardiovascular response to submaximal exercise in sustained microgravity. J Appl Physiol. 1996;81:26–32. doi: 10.1152/jappl.1996.81.1.26. [DOI] [PubMed] [Google Scholar]
- Sopher SM, Smith ML, Eckberg DL, Fritsch JM, Dibner-Dunlap ME. Autonomic pathophysiology in heart failure: carotid baroreceptor-cardiac reflexes. Am J Physiol Heart Circ Physiol. 1990;259:H689–H696. doi: 10.1152/ajpheart.1990.259.3.H689. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2804–H2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Tipton CM. The autonomic nervous system. In: Tipton CM, editor. Exercise Physiology: People and Ideas. New York: Oxford University Press; 2003. pp. 188–254. [Google Scholar]
- Verbanck S, Larsson H, Linnarsson D, Prisk GK, West JB, Paiva M. Pulmonary tissue volume, cardiac output, and diffusing capacity in sustained microgravity. J Appl Physiol. 1997;83:810–816. doi: 10.1152/jappl.1997.83.3.810. [DOI] [PubMed] [Google Scholar]
- Verheyden B, Liu J, Beckers F, Aubert AE. Adaptation of heart rate and blood pressure to short and long duration space missions. Resp Physiol Neurobiol. 2009;1695:513–516. doi: 10.1016/j.resp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Waki H, Katahira K, Yamasaki M, Nagayama T, Katsuda S, Wago H, Okouchi T, O-Ishi H, Miyake M, Miyamoto Y, Shimizu T. Effects of spaceflight on postnatal development of arterial baroreceptor reflex in rats. Acta Physiol Scand. 2005;184:17–26. doi: 10.1111/j.1365-201X.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Buckey JC, Lane LD, Gaffney FA, Levine BD, Moore WE, Wright SJ, Blomqvist CG. Effects of spaceflight on human calf hemodynamics. J Appl Physiol. 2001;90:1552–1558. doi: 10.1152/jappl.2001.90.4.1552. [DOI] [PubMed] [Google Scholar]
- Westerhof BE, Gisolf J, Karemaker JM, Wesseling KH, Secher NH, van Lieshout JJ. Time course analysis of baroreflex sensitivity during postural stress. Am J Physiol Heart Circ Physiol. 2006;291:H2864–H2874. doi: 10.1152/ajpheart.01024.2005. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Shimizu T, Katahira K, Waki H, Nagayama T, O-Ishi H, Katsuda S, Miyake M, Miyamoto Y, Wago H, Okouchi T, Matsumoto S. Spaceflight alters the fibre composition of the aortic nerve in the developing rat. Neuroscience. 2004a;128:819–829. doi: 10.1016/j.neuroscience.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Shimizu T, Miyake M, Miyamoto Y, Katsuda S, O-Ishi H, Nagayama T, Waki H, Katahira K, Wago H, Okouch T, Nagaoka S, Mukai C. Effects of space flight on the histological characteristics of the aortic depressor nerve in the adult rat: electron microscopic analysis. Biol Sci Space. 2004b;18:45–51. doi: 10.2187/bss.18.45. [DOI] [PubMed] [Google Scholar]
- Young LR, Oman CM, Merfeld D, Watt D, Roy S, DeLuca C, Balkwill D, Christie J, Groleau N, Jackson DK, Law G, Modestino S, Mayer W. Spatial orientation and posture during and following weightlessness: human experiments on Spacelab Life Sciences 1. J Vestib Res. 1993;3:231–239. [PubMed] [Google Scholar]