Abstract
The skeletal muscle exercise pressor reflex (EPR) induces increases in heart rate (HR) and mean arterial pressure (MAP) during physical activity. This reflex is activated during contraction by stimulation of afferent fibres responsive to mechanical distortion and/or the metabolic by-products of skeletal muscle work. The molecular mechanisms responsible for activating these afferent neurons have yet to be identified. It has been reported that activation of the transient receptor potential vanilloid 1 (TRPv1) receptor within skeletal muscle (localized to unmyelinated afferent fibres) elicits increases in MAP and HR similar to those generated by the EPR. Thus, we hypothesized that stimulation of the TRPv1 receptor during muscle contraction contributes to the activation of the EPR. The EPR was activated by electrically induced static muscle contraction of the hindlimb in decerebrate Sprague–Dawley rats (n= 61) before and after the administration of the TRPv1 receptor antagonists, capsazepine (Capz; 100 μg/100 μl), iodoresinaferatoxin (IRTX; 1 μg/100 μl), or Ruthenium Red (RR; 100 μg/100 μl). Static muscle contraction alone induced increases in both HR (8 ± 2 bpm) and MAP (21 ± 3 mmHg). The HR and MAP responses to contraction were significantly lower (P < 0.05) after the administration of Capz (2 ± 1 bpm; 7 ± 1 mmHg, respectively), IRTX (3 ± 2 bpm; 5 ± 3 mmHg, respectively) and RR (0 ± 1, bpm; 5 ± 2 mmHg, respectively). These data suggest that the TRPv1 receptor contributes importantly to activation of the EPR during skeletal muscle contraction in the rat.
Introduction
The skeletal muscle exercise pressor reflex (EPR) contributes importantly to blood pressure and heart rate (HR) control during exercise primarily by increasing sympathetic nerve activity (Kaufman & Forster, 1996). In 1972, the EPR was characterized in the cat and it was determined that activation of group III and group IV small diameter afferent neurons by muscle contraction initiate this reflex (McCloskey & Mitchell, 1972). Group III and group IV afferent neurons comprise the small diameter afferent neurons that innervate skeletal muscle (Kaufman et al. 1983). Most group III fibres are activated by stimulation of receptors responsive to the mechanical distortion of their receptive fields and are characterized as stretch sensitive (Kaufman et al. 1984). The majority of group IV fibres are activated by the stimulation of chemically sensitive receptors responsive to the metabolic by-products of muscular work (Kaufman et al. 1984). Despite this knowledge, the receptors activating the group III and IV fibres that are important to EPR function are largely unknown and remain a source of controversy.
The transient receptor potential vanilloid 1 (TRPv1) receptor, previously termed the capsaicin or vanilloid receptor 1 (VR1), is localized on the group IV afferent neuron and has been demonstrated to mark these afferent fibres (Michael & Priestley, 1999). Stimulation of this receptor with exogenous agonists such as capsaicin produces increases in blood pressure and HR that are similar to those elicited during exercise (Kaufman et al. 1982; Li et al. 2004; Smith et al. 2005b). Given its anatomical location and demonstrated ability to alter cardiovascular haemodynamics, the TRPv1 receptor serves as a good candidate for mediating EPR activity. While stimulation of group IV afferent neurons is known to contribute to EPR activity (Kaufman et al. 1983; Stebbins & Longhurst, 1989), it remains unclear whether the TRPv1 receptors localized to these neurons are involved in mediating EPR function. Recent studies in cats indicate that the TRPv1 receptor is not related to the EPR (Kindig et al. 2005). In contrast, studies in humans, albeit through indirect evidence, suggest that activation of this receptor may be necessary to elicit a normal cardiovascular response to exercise (Dawson et al. 2004).
Using a rat model of exercise, our laboratory has previously demonstrated that the blood pressure and HR responses to activation of the EPR are similar to humans under both physiological (Smith et al. 2001) and pathological conditions (Smith et al. 2003, 2005a,b). Additionally, we have demonstrated that intra-arterial hindlimb injection of capsaicin (a specific exogenous activator of the TRPv1 receptor) produces significant increases in both mean arterial pressure (MAP) and HR in normal healthy rats (Smith et al. 2005b). This capsaicin-induced increase in HR and MAP was successfully blocked with the competitive antagonist for the TRPv1 receptor, capsazepine (Smith et al. 2005b). These studies indicate that selective activation of the TRPv1 receptor can evoke a rise in both MAP and HR in the rat similar to that evoked by the EPR in both rats and humans. Therefore, we hypothesized that stimulation of the TRPv1 receptor during muscle contraction contributes to the activation of the EPR. To test this hypothesis, we used three different antagonists of the TRPv1 receptor and determined the effect of each on the cardiovascular response to static muscle contraction (concurrent stimulation of group III and IV fibres) (Mitchell et al. 1983), passive muscle stretch (primary stimulation of group III fibres) (Stebbins et al. 1988; Hayes & Kaufman, 2001), and intra-arterial injection of capsaicin in the hindlimb (primary stimulation of group IV fibres) (Kaufman et al. 1982) in the rat model described. The results of these studies indicate that stimulation of the TRPv1 receptor in skeletal muscle activates the EPR in the rat.
Methods
Ethical approval
Experiments were performed in 61 male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) greater than 13 weeks of age (weight range 350–450 g). The described procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. All studies were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and comply with the policies of The Journal of Physiology (Drummond, 2009).
Physiological preparations
General surgical procedures
Rats were initially anaesthetized with isoflurane gas (2–4% in 100% oxygen) and subsequently intubated for mechanical ventilation as previously described (Smith et al. 2001). As necessary, isoflurane levels were increased when any of the following were present: (i) withdrawal reflex to pinching of the hindpaw; (ii) presence of a corneal reflex and/or (iii) spontaneous increases in HR. Fluid-filled catheters (PE-50, polyethylene tubing) were placed within the right jugular vein as well as both carotid arteries. A haemodynamic stabilizing solution (2 ml 1 m NaHCO3, 10 ml 5% dextrose, 38 ml Ringer solution) was continuously infused via the jugular vein (3–5 ml h−1 kg−1) (Quintin et al. 1989). Blood pressure was recorded by connecting one of the carotid arterial catheters to a pressure transducer (model DTX plus-DT, NN12, Ohmeda/GE Healthcare). MAP was obtained by integrating the arterial pressure signal with a time constant of 1–4 s. HR was measured from the blood pressure pulse wave by using a biotachometer (Gould Instruments/LDS Test and Measurement LLC, Middleton, WI, USA). After initial instrumentation, animals were decerebrated and rendered insentient. The brain was sectioned rostral to the superior colliculus and the forebrain was aspirated. Subsequently, small pieces of oxidized regenerated cellulose (Ethicon, Somerville, NJ, USA) were placed on the exposed surfaces of the brain and the cranial cavity was packed with cotton balls. Dexamethasone (0.2 mg) was given intramuscularly to minimize oedema (Tian & Duffin, 1996). Post-decerebration, isoflurane anaesthesia was discontinued and a minimum recovery period of one hour was employed before beginning any experimental procedure. Animals were placed in a stereotaxic head unit (David Kopf Instruments, Tujunga, CA, USA) and customized spinal frame throughout the remainder of experimentation.
Surgical procedures for muscle contraction and stretch
A laminectomy exposing the lower lumbar portions of the spinal cord (L2 to L6) was performed. Bipolar platinum stimulating electrodes were placed around the cut peripheral ends of the L4 and L5 ventral rootlets. Exposed neural tissue was covered in a pool of mineral oil. The pelvis was stabilized with steel posts within the customized spinal frame. The right hindlimb was fixed in one position using a clamp around the tibial bone. The gastrocnemius and soleus muscles of the right hindlimb were isolated and the calcaneal bone cut. The Achilles’ tendon was connected to a force transducer (FT-10, Grass Technologies/Astro-Med Inc., West Warwick, RI, USA) allowing the measurement of muscle tension.
Surgical procedures for hindlimb intra-arterial injections
The circulation of the right hindlimb was isolated to allow injection of chemicals into the arterial supply of the muscle. A catheter (PE-10, polyethylene tubing) was placed in the left common iliac artery. The tip of the catheter was advanced to the bifurcation of the abdominal aorta. This surgical procedure allowed chemicals to be directly injected into the circulation of the right hindlimb via the right common iliac artery. A reversible vascular occluder was placed around the common iliac vein emptying the right hindlimb to limit drug delivery to the limb.
Experimental protocols
General protocols
The EPR and its individual mechanically and metabolically sensitive afferent components were preferentially activated via static hindlimb muscle contraction, passive hindlimb muscle stretch and hindlimb intra-arterial chemical injection, respectively. These procedures were performed before and after the administration of the TRPv1 antagonists capsazepine (Capz; 100 μg/100 μl), iodoresinaferatoxin (IRTX; 1 μg/100 μl) and/or Ruthenium Red (RR; 100 μg/100 μl).
Hindlimb muscle contraction
Electrically induced static contraction of the right gastrocnemius and soleus muscles was used to activate both the mechanically and metabolically sensitive components of the EPR concurrently (i.e. group III and IV afferent fibres, respectively). With the use of constant current stimulation (3 times motor threshold (the minimum current required to produce a muscle twitch), 0.1 ms pulse duration, 40 Hz), 30 s contractions were produced by excitation of the L4 and L5 ventral roots.
Capz experiments
Baseline resting tension was set to 100 g and the muscle was contracted using the stimulus parameters described. Subsequently, either saline (a control measure) or Capz was injected into the hindlimb arterial supply (0.1 ml volume). Upon substance injection, the circulation of the hindlimb was occluded for 2 min to trap the injectate in the leg following which a contraction was performed. This chronological sequence was chosen as it has been previously reported to be effective when using Capz to antagonize TRPv1 receptors (Smith et al. 2005b). This injection/occlusion/contraction procedure was repeated an additional three times with a minimum of 15 min between contractions.
IRTX and RR experiments
Baseline resting tension was set to 100 g and the muscle was contracted. Subsequently, saline, IRTX or RR was injected into the hindlimb arterial supply (0.1 ml volume). Upon substance injection, the circulation of the hindlimb was occluded for 10 min. This was followed by a 5 min period in which the hindlimb was freely perfused. At the conclusion of the 5 min period, the muscle was contracted. After a 15 min recovery period, the muscle was again contracted in the absence of saline, IRTX or RR. The chronological order for chemical injection, hindlimb occlusion and free perfusion was chosen as it has previously been reported to be effective when using IRTX to antagonize TRPv1 receptors (Kindig et al. 2005). In addition, the sequence of contractions in IRTX and RR experiments was designed to mimic those previously used in cats to facilitate comparisons between studies (Kindig et al. 2005).
Hindlimb passive muscle stretch
Passively stretching hindlimb skeletal muscle does not increase muscle metabolism. Therefore, this technique is commonly used to preferentially stimulate stretch-sensitive afferent fibres in muscle (i.e. predominantly group III fibres) (Stebbins et al. 1988; Kim et al. 2007). Using a calibrated 9.5 mm rack and pinion system (Harvard apparatus), the gastrocnemius and soleus muscles were passively stretched at muscle tensions approximately equivalent to those achieved during maximal static contractions.
Capz experiments
Prior to the administration of Capz, the hindlimb muscle was stretched as a control. After a 15 min recovery period, Capz was injected into the hindlimb arterial supply (0.1 ml volume) and the circulation of the hindlimb was occluded for 2 min. Following this period, the muscle was again stretched. This injection/occlusion/stretch procedure was repeated an additional three times with a minimum of 15 min between each stretch.
IRTX and RR experiments
During a pre-treatment period, the gastrocnemius and soleus muscles were stretched. Subsequently, either IRTX or RR was injected into the hindlimb arterial supply (0.1 ml volume) and the circulation of the hindlimb occluded for 10 min. This was followed by a 5 min period in which the hindlimb was freely perfused after which the muscles were again stretched. This injection/occlusion/stretch procedure was repeated an additional three times with a minimum of 15 min between each stretch.
Hindlimb intra-arterial chemical injection
Capsaicin is a known agonist of the TRPv1 receptor which is predominantly localized to chemically sensitive, group IV afferent neurons (Jansco et al. 1977; Michael & Priestley, 1999). In order to establish the effectiveness of the TRPv1 antagonists used in this study, capsaicin (0.10 μg/100 μl) was injected into the arterial supply of the right hindlimb before and after the administration of IRTX and RR. The effectiveness of the dose of Capz used in this study on capsaicin-induced increases in MAP and HR has been established in a previous report from our laboratory (Smith et al. 2005b) and, therefore, was not repeated in this investigation. During a pre-treatment period, capsaicin (0.1 ml volume) was injected into the arterial supply of the hindlimb and trapped for 2 min by pulling the reversible occluder around the right common iliac vein. Following this, either IRTX or RR was injected into the hindlimb arterial supply (0.1 ml volume) and the circulation of the hindlimb occluded for 10 min. The hindlimb was then freely perfused for 5 min. After this 5 min period, capsaicin was again injected into the hindlimb arterial supply and trapped for 2 min. After a sufficient recovery period (minimum of 15 min), the capsaicin injection procedure was repeated in the absence of IRTX or RR. To prevent muscle contractions or twitches as a result of injection of capsaicin, brief neuromuscular blockade (2 min; 1 mg ml−1 vecuronium bromide, Organon/Merck) was induced before each capsaicin delivery.
At the conclusion of all experiments, insentient decerebrated animals were killed by intravenous injection of saturated potassium chloride (4 m, 2 ml kg−1). Use of this procedure adheres to the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association.
Data acquisition
Baseline values for all variables (e.g. HR, MAP, tension) were determined by evaluating 30 s of recorded data before a given contraction, stretch or capsaicin injection. The peak response for each variable was defined as the greatest change from baseline elicited by the experimental manoeuvre. All cardiovascular and force data were acquired, recorded and analysed using data acquisition software (Spike 2, v. 3) for the CED micro 1401 system (Cambridge Electronic Design, Cambridge, UK).
Statistical analyses
Statistical analyses were performed using analysis of variance (ANOVA). A one-way repeated measures ANOVA was utilized in protocols in which one factor was considered. A two-way ANOVA was utilized when two factors were statistically examined. When ANOVA was found to be significant, a Student–Newman–Keuls multiple comparison test was utilized to identify differences between specific group means (SigmaStat, Systat Software Inc., San Jose, CA, USA). The significance level was set at P < 0.05. Results are presented as means ±s.e.m.
Results
The mean body weight of the 61 male Sprague–Dawley rats used in the investigation was 406 ± 7 g. Baseline MAP and HR were 113 ± 3 mmHg and 408 ± 8 bpm, respectively, in these decerebrate animals.
Capsazepine reduces the cardiovascular response to static muscle contraction but has no effect on the response to passive muscle stretch
As stated previously, the dose of Capz used in this investigation (100 μg/100 μl) has been shown to effectively attenuate the cardiovascular response to the intra-arterial hindlimb administration of the TRPv1 agonist capsaicin (Smith et al. 2005b). Therefore, this dose of Capz was used to assess the contribution of TRPv1 receptor activation to the HR and MAP responses to muscle contraction and stretch.
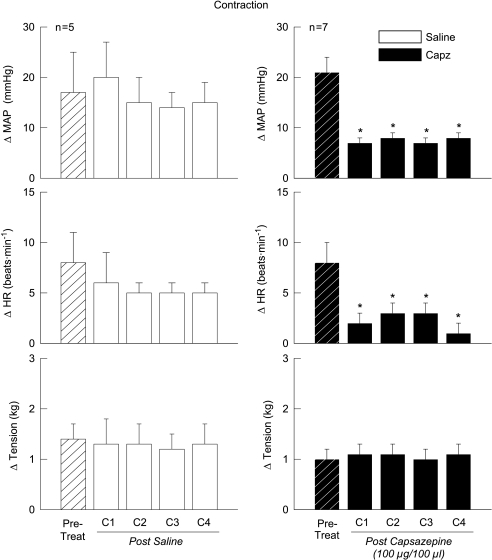
Compared to baseline haemodynamics, activation of the EPR via static muscle contraction induced increases in MAP and HR (Fig. 1). The contraction-induced elevations in MAP and HR were not altered by the intra-arterial administration of saline within the hindlimb and were maintained over a series of four consecutive contractions of equal tension development (Fig. 1, left panel). In contrast, injecting Capz (a competitive, selective TRPv1 antagonist) into the hindlimb arterial supply reduced the contraction-evoked increase in MAP and HR by 14 ± 1 mmHg and 6 ± 1 bpm, respectively, over four tension-equivalent contractions (Fig. 1, right panel).
Figure 1. Capsazepine (Capz) attenuates the cardiovascular response to static muscle contraction.
The intra-arterial administration of the TRPv1 receptor antagonist Capz into the hindlimb significantly reduced the changes in MAP and HR in response to activation of the exercise pressor reflex. The administration of saline had no effect on the cardiovascular responses to contraction. Pre-Treat, contractions prior to chemical injection; C1–C4, contractions 1–4 after saline or Capz injection. *Significance from pre-treatment trial (P < 0.05).
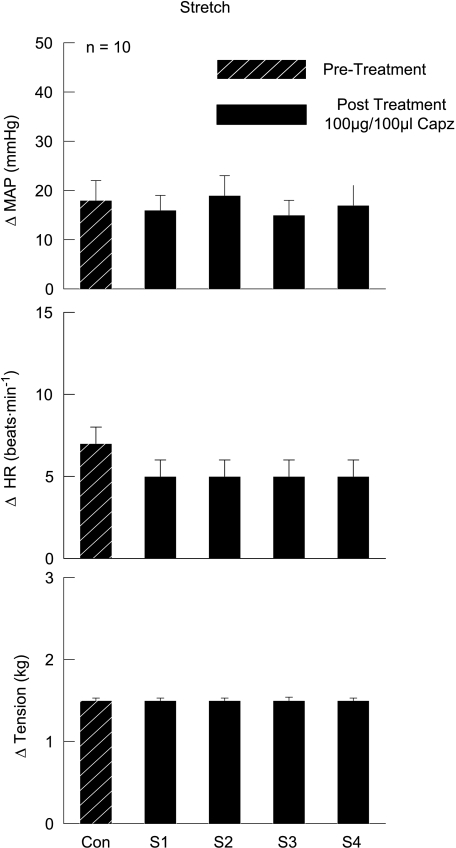
Passively stretching the gastrocnemius and soleus muscles of the hindlimb produced increases, above baseline, in MAP (18 ± 1 mmHg) and HR (7 ± 1 bpm) (Fig. 2). During consecutive stretches of equal tension development, the intra-arterial injection of Capz within the circulation of the hindlimb did not alter these haemodynamic responses. Given that Capz was found to attenuate the cardiovascular response to muscle contraction in this investigation, the inability of Capz to alter the response to stretch is not due to the dose administered.
Figure 2. Effects of capsazepine (Capz) on the cardiovascular response to passive muscle stretch.
The TRPv1 antagonist Capz had no effect on the MAP and HR responses to passive muscle stretch. Con, control stretch prior to Capz injection; S1–S4, stretches 1–4 after the intra-arterial hindlimb administration of Capz.
IRTX effectively attenuates the pressor response to capsaicin injection and muscle contraction but not stretch
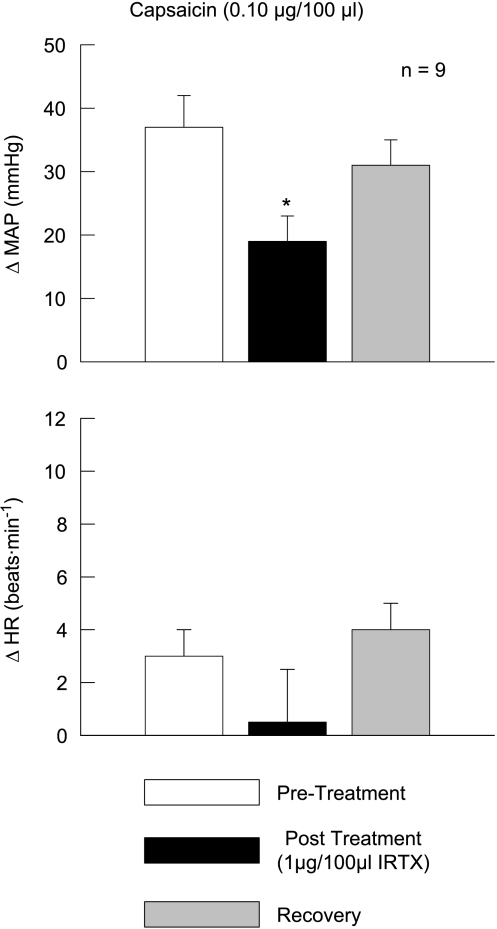
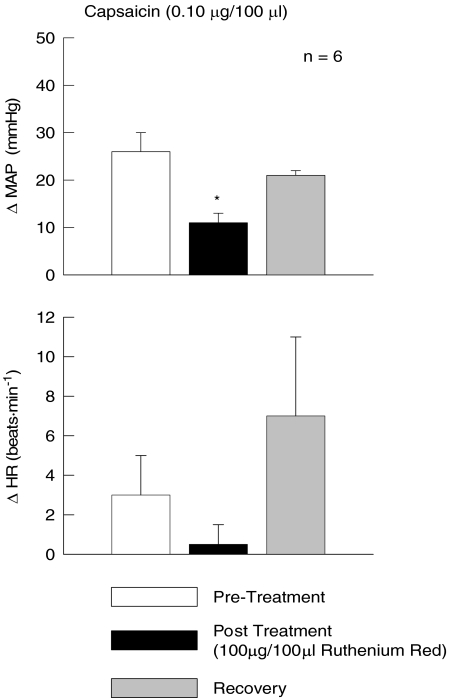
To establish that the dose of IRTX (1 μg/100 μl) utilized in this study was efficacious, IRTX was injected into the arterial supply of the hindlimb and then challenged by administration of the TRPv1 agonist capsaicin (Fig. 3). Capsaicin (0.10 μg/100 μl) evoked large elevations in MAP (37 ± 5 mmHg) that were reduced by approximately 50% in the presence of this dose of IRTX.
Figure 3. Iodoresinaferatoxin (IRTX) effectively blocks the pressor response to capsaicin.
The MAP response to the intra-arterial hindlimb injection of the TRPv1 agonist capsaicin was reduced by the presence of IRTX. Pre-Treatment, capsaicin injection prior to IRTX treatment; Post Treatment, capsaicin injection after IRTX treatment; Recovery, capsaicin injection after recovery from IRTX treatment. *Significance from pre-treatment and recovery trials (P < 0.05).
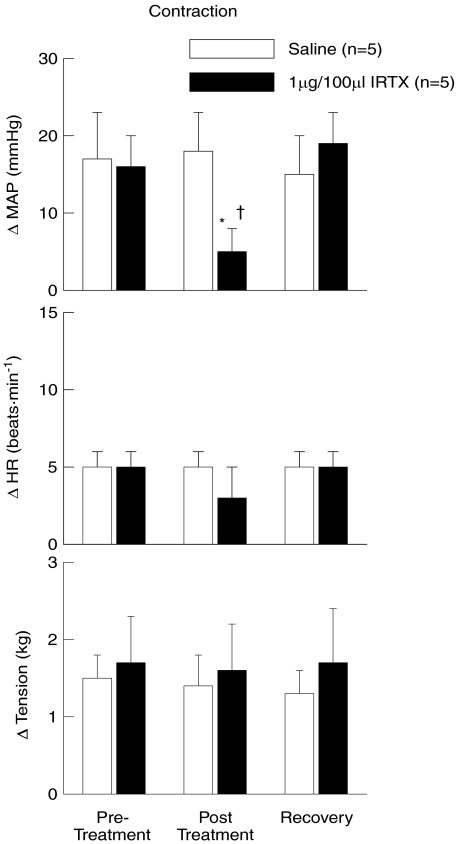
The effect of introducing the selective TRPv1 antagonist IRTX into the arterial supply of the hindlimb on the cardiovascular response to static muscle contraction is presented in Fig. 4. Prior to IRTX injection, the MAP and HR responses to contraction were 16 ± 4 mmHg and 5 ± 1 bpm, respectively. After IRTX injection, the pressor response was significantly reduced to 5 ± 3 mmHg while the HR response was not significantly affected (3 ± 2 bpm). After allowing a 15 min recovery period from IRTX administration, the response to contraction was fully restored (MAP, 19 ± 4; HR, 5 ± 1). The introduction of saline into the intra-arterial supply of the hindlimb had no effect on the cardiovascular response to muscle contraction.
Figure 4. Iodoresinaferatoxin (IRTX) reduces the pressor response to static muscle contraction.
The intra-arterial administration of the TRPv1 receptor antagonist IRTX into the hindlimb significantly reduced the changes in MAP in response to activation of the exercise pressor reflex. The change in HR was relatively unaffected by IRTX injection. The administration of saline had no effect on the cardiovascular responses to contraction. Pre-Treatment, contractions prior to chemical injection; Post Treatment, contractions after saline or IRTX injection; Recovery, contractions after recovery from chemical injection. *Significance from pre-treatment and recovery trials within IRTX treated group (P < 0.05). †Significance from all saline trials (P < 0.05).
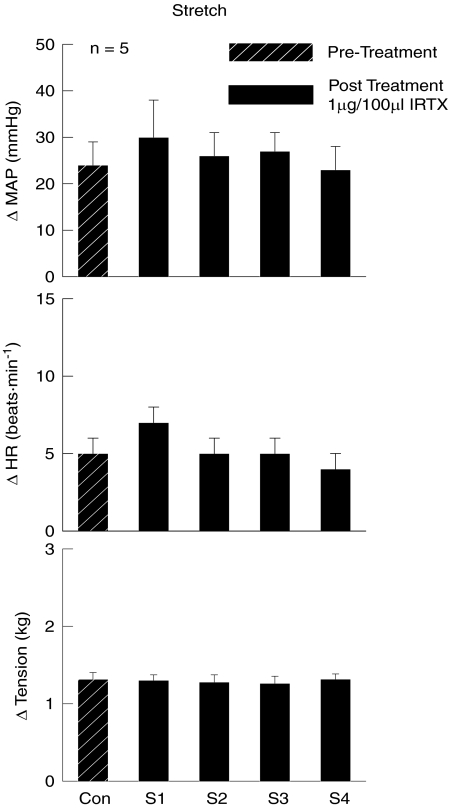
Similar to Capz, intra-arterial hindlimb administration of IRTX did not alter the pressor or tachycardic responses to passive muscle stretch (Fig. 5). The initial increases in MAP (24 ± 5 mmHg) and HR (5 ± 1 bpm) evoked by stretch prior to IRTX injection were not significantly altered during four consecutive stretches of equal tension development after IRTX administration.
Figure 5. Effects of iodoresinaferatoxin (IRTX) on the MAP and HR responses to passive muscle stretch.
The TRPv1 antagonist IRTX had no effect on the cardiovascular responses to passive muscle stretch. Con, control stretch prior to IRTX injection; S1–S4, stretches 1–4 after the intra-arterial hindlimb administration of IRTX.
The effects of Ruthenium Red on the cardiovascular response to capsaicin injection, muscle contraction and stretch are similar to those of Capz and IRTX
As in IRTX experiments, the pressor response to the intra-arterial hindlimb injection of capsaicin was significantly attenuated by the administration of RR (Fig. 6). The latter finding confirms that the dose of RR (100 μg/100 μl) used in this study was sufficient to antagonize the effect of capsaicin at the TRPv1 receptor.
Figure 6. Ruthenium Red effectively blocks the MAP response to capsaicin.
The pressor response to the intra-arterial hindlimb injection of the TRPv1 agonist capsaicin was reduced by the presence of Ruthenium Red. Pre-Treatment, capsaicin injection prior to Ruthenium Red treatment; Post Treatment, capsaicin injection after Ruthenium Red treatment; Recovery, capsaicin injection after recovery from Ruthenium Red treatment. *Significance from pre-treatment and recovery trials (P < 0.05).
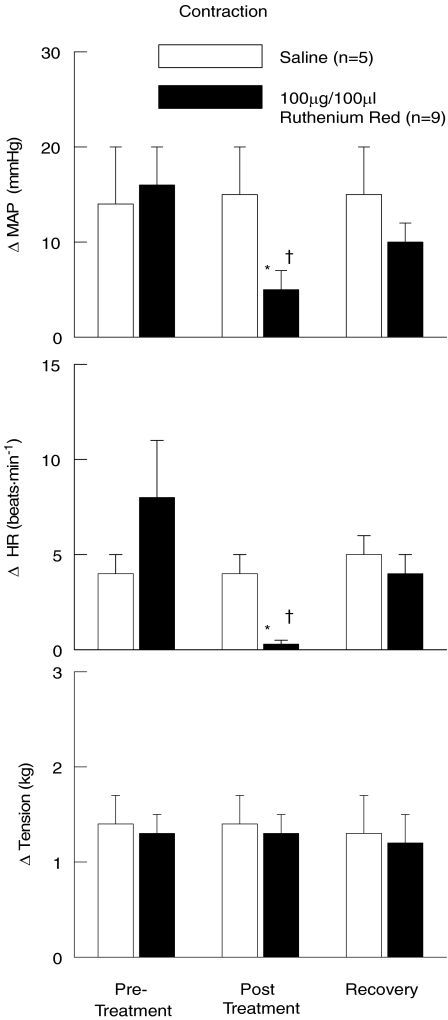
In this series of experiments, the cardiovascular response to static muscle contraction was assessed before and after the intra-arterial hindlimb administration of RR (Fig. 7). In all trials, static muscle contraction elicited increases in MAP and HR as compared to baseline. These increases in cardiovascular haemodynamics were significantly attenuated by injecting RR into the arterial supply of the hindlimb prior to contraction. After allowing sufficient time for RR to be removed from the hindlimb circulation, the MAP and HR responses to contraction were not statistically different from pre-treatment responses. In control experiments, the intra-arterial hindlimb injection of saline had no effect on the cardiovascular response to muscle contraction.
Figure 7. Ruthenium Red attenuates the pressor and tachycardic responses to static muscle contraction.
The intra-arterial administration of the TRPv1 receptor antagonist Ruthenium Red into the hindlimb significantly reduced the changes in MAP and HR in response to activation of the exercise pressor reflex. The administration of saline had no effect on the cardiovascular responses to contraction. Pre-Treatment, contractions prior to chemical injection; Post Treatment, contractions after saline or Ruthenium Red injection; Recovery, contractions after recovery from chemical injection. *Significance from pre-treatment and recovery trials within Ruthenium Red treated group (P < 0.05). †Significance from all saline trials (P < 0.05).
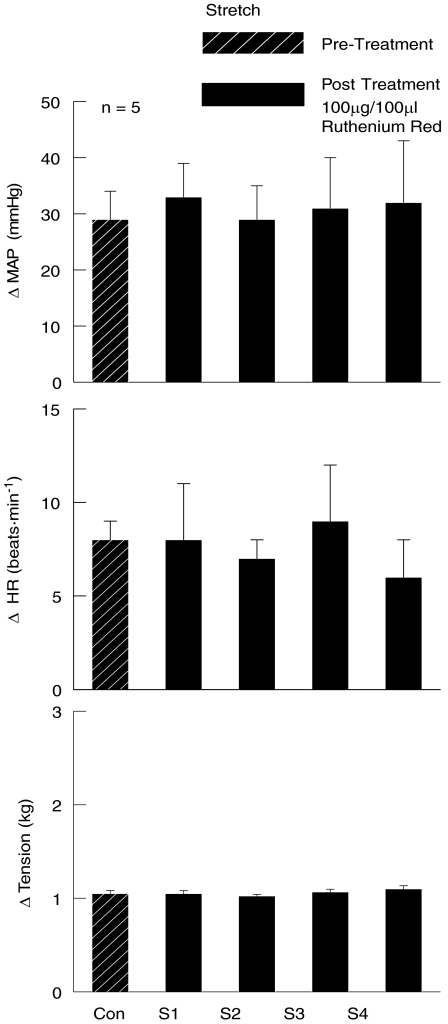
The effect of RR on the cardiovascular response to passive muscle stretch was determined in a separate set of experiments. In these studies, the increases in MAP and HR induced by passively stretching the gastrocnemius and soleus muscles of the hindlimb were unaffected by the introduction of RR into the arterial supply of the hindlimb (Fig. 8).
Figure 8. Effects of Ruthenium Red on the cardiovascular responses to passive muscle stretch.
The TRPv1 antagonist Ruthenium Red had no effect on the MAP and HR responses to passive muscle stretch. Con, control stretch prior to Ruthenium Red injection; S1–S4, stretches 1–4 after the intra-arterial hindlimb administration of Ruthenium Red.
Discussion
While it is well established that the activation of group III and group IV afferent neurons by muscle contraction stimulates the EPR (McCloskey & Mitchell, 1972; Kaufman & Hayes, 2002), the exact receptors that mediate these responses are not well characterized. A study in humans suggests that the TRPv1 receptor plays a role in mediating the EPR (Dawson et al. 2004). However, a recent investigation in cats does not support this concept. Rather, data in cats indicate that the TRPv1 receptor does not contribute to the activation of the EPR (Kindig et al. 2005). We have previously developed a decerebrate rat model for the study of the EPR in rats that evokes cardiovascular responses to exercise similar to those produced in humans (Smith et al. 2001). In the current investigation, we used this model to determine whether the TRPv1 receptor plays a role in mediating the EPR as previously suggested in humans.
In the current studies, we used three different antagonists to explore the role of the TRPv1 receptor in mediating the EPR in the rat. Two of these are competitive antagonists (Capz and IRTX) while RR is a non-competitive antagonist. Further, each of these compounds acts in unique ways to inhibit TRPv1 activity as reviewed by Messeguer et al. (2006). Specifically, the effects of these TRPv1 antagonists on the MAP and HR responses to static muscle contraction, passive stretch and intra-arterial capsaicin injection were investigated. As has been previously shown in this rat model (Smith et al. 2001), static muscle contraction (induced by electrical stimulation of L4 and L5 ventral roots) resulted in significant increases in MAP and HR. Importantly, the MAP responses to activation of the EPR by static muscle contraction were attenuated by all three antagonists. Static muscle contraction activates both group III and group IV afferent neurons (Kaufman et al. 1984). Likewise, the pressor responses evoked by intra-arterial capsaicin injection were significantly attenuated by IRTX and RR in a manner similar to that previously demonstrated with Capz (Smith et al. 2001). Given that capsaicin sensitivity is predominately limited to group IV afferent neurons (Jansco et al. 1977) associated with the chemically sensitive component of the EPR, these findings not only suggest that the doses of antagonist used in the study were effective but also support the concept that TRPv1 receptors located on group IV afferent neurons mediate the cardiovascular response to muscle metabolic activity. In contrast, we observed that none of the TRPv1 antagonists were effective in reducing the MAP and HR responses to passive stretch; a manoeuvre which has been shown to predominately activate group III afferent neurons (Hayes et al. 2005). Collectively, these findings are important for two reasons: (1) the data indicate that stimulation of the TRPv1 receptor contributes to the activation of the EPR in the rat and (2) the evidence suggests that the TRPv1 receptor mediates the activation of chemically sensitive group IV afferent neurons during muscle contraction (i.e. the metabolic component of the EPR). The latter conclusion is based on the finding that the MAP response to muscle contraction was inhibited by the presence of TRPv1 antagonists whereas the pressor response to stretch was unaffected by pharmacological blockade.
Data from this investigation indicate that a species difference exists with regard to the factors that mediate the EPR. In the cat, Kaufman and colleagues demonstrated that IRTX effectively blocked the pressor response to the intra-arterial administration of capsaicin within the hindlimb (Kindig et al. 2005). However, it was also reported that IRTX did not alter the blood pressure response to static muscle contraction (Kindig et al. 2005). In contrast, Dawson et al. (2004) presented indirect evidence consistent with the concept that the TRPv1 receptor plays a role in mediating the blood pressure response to static muscle contraction in humans. In support of this concept, data from the current study indicate that the TRPv1 receptor is a mediator of the EPR in rats given that three different TRPv1 receptor antagonists were able to attenuate the cardiovascular response to muscle contraction. Moreover, the evidence suggests that the TRPv1 receptor activates the EPR via chemically sensitive group IV afferent fibres in which the receptor is predominantly expressed (Michael & Priestley, 1999). In contrast, the pressor and tachycardic responses to passive muscle stretch (which primarily excite mechanically sensitive group III fibres) were not affected by the TRPv1 receptor antagonists used in this study. Taken together, these studies indicate that the factors that stimulate the EPR in cats may be distinct from those in rats and humans. This could be due to the fact that unmyelinated fibres in the cat demonstrate distinct response properties. For example, it has been demonstrated that nearly 30% of C-fibres (cutaneous fibres that are unmyelinated) in the cat do not encode noxious stimulation (Bessou et al. 1971). Instead, they encode highly discrete innocuous somatosensation in areas such as facial whiskers (Bessou et al. 1971).
Generally, HR responses to muscle contraction and intra-arterial capsaicin injection were reduced after the administration of TRPv1 antagonists. These responses, however, were more variable than those reported for blood pressure. This is most likely due to the relatively small changes in HR evoked by muscle contraction and capsaicin injection. It has been well established in humans and rats that the EPR has a much greater effect on MAP than HR (Mitchell et al. 1989; Kaufman & Forster, 1996; Smith et al. 2005a). As a result, it is not surprising that the reductions in HR induced by the TRPv1 receptor antagonists used in this study did not reach statistical significance under every condition.
It has also been observed in this study that the reduction in EPR activity mediated by the TRPv1 receptor antagonists is partial. While each antagonist significantly reduced the pressor response to static muscle contraction, none of the antagonists completely abolished the response. This finding suggests that other factors are important in activating the EPR during physical activity. It is likely that activation of Group IV afferent neurons is also mediated by non-TRPv1 receptors (Pan et al. 1993; Chen et al. 1995; Lewis et al. 1995; Hanna & Kaufman, 2003; Light et al. 2008; Williams et al. 2008). In addition, receptors localized to group III mechanically sensitive afferent neurons known to be activated by static muscle contraction and passive muscle stretch are requisite for the full activation of the EPR (Hayes & Kaufman, 2001; Middlekauff et al. 2001; Smith et al. 2005a).
In summary, we have evaluated the effect of three different antagonists on three different forms of muscle stimulation to establish a role for the TRPv1 receptor in the activation of the EPR in rats. These data clearly indicate that the TRPv1 receptor activates the EPR during muscle contraction via the chemically sensitive component of the reflex and not by stimulating mechanically sensitive afferent fibres. The data further suggest that the receptor mechanisms inducing EPR activation in rats are similar to those in humans, where the TRPv1 receptor has also been implicated in mediating this process. While the TRPv1 receptor is an important mediator of the EPR via its metabolic component, it is likely that other receptors and/or ion channels localized to both chemically and mechanically sensitive afferent fibres contribute to the activation of this reflex as well.
Acknowledgments
The authors thank Margaret Robledo, Martha Romero and Julius Lamar, Jr. for their outstanding expert technical assistance. This research was supported by grants from the National Institutes of Health (HL-070242 to M.G.G. and HL-088422 to S.A.S.), the American Heart Association (0640007N to M.G.G.), and the Lawson & Rogers Lacy Research Fund in Cardiovascular Diseases (to J.H.M.).
Glossary
Abbreviations
- Capz
capsazepine
- EPR
exercise pressor reflex
- HR
heart rate
- IRTX
iodoresinaferatoxin
- MAP
mean arterial pressure
- RR
Ruthenium Red
- TRPv1
transient potential vanilloid 1
Author contributions
The experiments described in this article were performed in laboratories at UT Southwestern Medical Center at Dallas, TX, USA. All authors contributed to the conception and design of the experiments as well as the collection, analysis and interpretation of data. S.A.S., J.H.M. and M.G.G. drafted the article and revised it critically for important intellectual content with input from all authors. All authors approved the final version submitted.
References
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibres. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Dawson AN, Walser B, Jafarzadeh M, Stebbins CL. Topical analgesics and blood pressure during static contraction in humans. Med Sci Sports Exerc. 2004;36:632–638. doi: 10.1249/01.mss.0000121949.43010.f4. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Jansco G, Kiraly E, Jansco-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology: section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. pp. 381–447. [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibres with endings in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fibre mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol. 2007;292:H866–H873. doi: 10.1152/ajpheart.00771.2006. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol. 2005;288:H1867–H1873. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPv1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its down regulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, et al. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanisms of action. J Appl Physiol. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- Quintin L, Gillon JY, Saunier CF, Ghignone M. Continuous volume infusion improves circulatory stability in anaesthetized rats. J Neurosci Methods. 1989;30:77–83. doi: 10.1016/0165-0270(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mammen PPA, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005a;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005b;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effects of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Longhurst JC. Potentiation of the exercise pressor reflex by muscle ischemia. J Appl Physiol. 1989;66:1046–1053. doi: 10.1152/jappl.1989.66.3.1046. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Williams MA, Smith SA, O’Brien DE, Mitchell JH, Garry MG. The group IV afferent neuron expresses multiple receptor alterations in cardiomyopathic rats: evidence at the cannabinoid CB1 receptor. J Physiol. 2008;586:835–845. doi: 10.1113/jphysiol.2007.140392. [DOI] [PMC free article] [PubMed] [Google Scholar]