Abstract
Acute or short-term exposure to high doses of methylmercury (MeHg) causes a well-characterized syndrome that includes sensory and motor deficits. The environmental threat from MeHg, however, comes from chronic, low-level exposure, the consequences of which are poorly understood. Selenium (Se), an essential nutrient, both increases deposition of mercury (Hg) in neurons and mitigates some of MeHg's neurotoxicity in the short term, but it is unclear whether this deposition produces long-term adverse consequences. To investigate these issues, adult Long Evans rats were fed a diet containing 0.06 or 0.6 ppm of Se as sodium selenite. After 100 days on these diets, the subjects began consuming 0.0, 0.5, 5.0, or 15 ppm of Hg as methylmercuric chloride in their drinking water for 16 months. Somatosensory sensitivity, grip strength, hind-limb cross (clasping reflex), flexion, and voluntary wheel-running in overnight sessions were among the measures examined. MeHg caused a dose- and time-dependent impairment in all measures, No effects appeared in rats consuming 0 or 0.5 ppm of Hg. Somatosensory function, grip strength, and flexion were among the earliest signs of exposure. Selenium significantly delayed or blunted MeHgs effects. Selenium also increased running in unexposed animals as they aged, a novel finding that may have important clinical implications. Nerve pathology studies revealed axonal atrophy or mild degeneration in peripheral nerve fibers, which is consistent with abnormal sensorimotor function in chronic MeHg neurotoxicity. Lidocaine challenge reproduced the somatosensory deficits but not hind-limb cross or flexion. Together, these results quantify the neurotoxicity of long-term MeHg exposure, support the safety and efficacy of Se in ameliorating MeHg's neurotoxicity, and demonstrate the potential benefits of Se during aging.
Keywords: Methylmercury, selenium, aging, adult-onset, peripheral nerves, sensory-motor, wheel-running
1. Introduction
Methylmercury (MeHg) is well known as a developmental neurotoxicant [28,29,40,52], but the consequences of long-term, chronic exposure have received much less attention, especially as it relates to the process of aging. The tragic episodes at Minamata and Niigata, Japan [15] and in Iraq [2,3] exemplify the effects of high exposures in adults and, in Minamata victims, the aging nervous system. Individuals exposed as adults displayed signs of parethesia, ataxia, auditory and visual dysfunction, dysarthria and, in some cases, [1,14] weakness. As the Minamata population aged, it became evident that many of these effects were highly persistent [33,51]. Adult victims began to show age-related deficits in activities of daily living at approximately 50 years of age [23] that were as severe as those observed in much older, unexposed individuals from nearby villages.
While incidents of acute and high level MeHg-exposure are striking, they rarely occur. Chronic exposure to low levels of MeHg is more common and represents an important but poorly understood environmental threat to human health. Such exposures do not result in the overt signs seen at high doses but careful neuropsychological testing provides evidence of subclinical sensory deficits, impaired manual dexterity, and perhaps motor weakness and fatigue [5,12,24].
Adult-onset MeHg neurotoxicity is notable for the latent period between exposure and the appearance of neurological signs, a period that can span weeks to months [2,55], or even years [23]. Sensorimotor and related neurological deficits, including paralysis, hind-limb crossing, and unsteady gait, can be induced in adult rodents [11,41,46,47]. In animal models, as in humans, the effects show a latent period, and can be highly persistent after both high [41] and more moderate [11] exposure levels.
Postmortem examination of victims of high level, acute MeHg exposure showed varying degrees of neuronal loss, especially in sensory regions of the cortex, cerebellar granular cells, and the primary motor cortex [9] as well as peripheral nerves [13], a pattern also observed in rodent models [41]. These clinical observations and animal studies suggest that peripheral nerves are especially vulnerable. Short term exposure to 5 mg/kg/day, of MeHg, s.c., for 25 days, produced no detectable histopathology in cerebellum or visual cortex, a reversible decrease of local cerebral glucose utilization, the sparing of motor fibers, but permanent axonal degeneration in myelinated sensory peripheral nerve fibers [46,47]. Motor nerves are not invulnerable, however, as muscle atrophy was observed in 21% of Minamata victims [53], with short-term, high dose exposure correlated with extensive motor fiber damage [49]. Together, these findings indicate a particular vulnerability of peripheral sensory systems, and perhaps motor systems, to adult-onset exposures.
Methylmercury (MeHg) exposure occurs primarily through the consumption of fish, also a source of nutrients like selenium (Se), a micronutrient that is required for the activity of many antioxidant enzymes that perform vital functions in brain and endocrine tissues [6,7]. Se forms a covalent bond with mercury (Hg) with an affinity much higher than that of sulfur [37,50]. Oral Se administration substantially elevates the deposition of mercury in dorsal root ganglia [42,43] and increases brain Hg content [31]. Somewhat paradoxically, Se also protects against some neurological consequences of Hg exposure [37,42,43]. This protection could arise because the Hg-Se bond sequesters Hg, thus making it biologically inactive. It has also been suggested that decreases in bioavailable Se due to the tight Hg-Se bond may contribute to the neurological signs observed following MeHg exposure. Theoretically, then, Se supplementation could replace Se that was inactivated due to Hg binding [37]. While this might imply that high levels of Se would be protective, the long-term effects of co-administration of MeHg and Se are unknown and the elevated Hg deposition associated with co-exposures raises a concern.
To address these issues, the present study uses a rat model of combined oral exposures to MeHg and Se over a period of over 16 months, a regimen that models human exposures. A 2 (diet) X 4 (MeHg) full factorial design permitted the detection of not only the long-term effects of MeHg-Se co-exposure but also the long-term benefits of dietary Se on natural aging. Methylmercury levels were selected to span a broad range of exposures and Se levels used were on the low and high end of nutritional recommendations for laboratory rats [10,39]. Behavioral measures of sensory-motor damage were combined with nerve pathology, skin biopsies to examine the status of somatosensory end organs, and pharmacological deadening of peripheral fibers using lidocaine, to gain a comprehensive picture of the interplay among aging, MeHg exposure, and dietary Se.
2. Methods
2.1 Subjects
One hundred thirty two female Long-Evans rats, approximately 60 days of age, were purchased from Harlan Laboratories (Indianapolis, IN). Subjects were housed in polycarbonate shoebox-cages (2 animals per cage) with open wire tops and hardwood chips (Harlan Teklad Sani Chips) bedding. A Flexan© diagonal barrier separated cage mates, who were always in the same experimental group. This separation ensured that all animals received their correct allotment of food and water. The colony was maintained on a 12-hour light-dark cycle (lights on at 6:00 am) in a temperature and humidity controlled, AAALAC accredited facility. Each subject was implanted subcutaneously with an electronic chip (BioMedic Data Systems Inc, Seaford, DE) containing a unique identification number. Animals were quarantined before entering the colony. Seven days after entering the colony, subjects were assigned to diet groups and fed ad libitum until body weight reached approximately 240 – 250 g at which time food was restricted to maintain this weight. When subjects reached approximately 12 months of age, their body weights were increased to 250 – 260gms. Weight was maintained by recording the weight of the animals twice per week and adjusting their feed accordingly. The animals were maintained at this level in order to ensure their health and not subject them to conditions of obesity by allowing unrestrictive feeding. Two Se diets and four MeHg exposures produced a 2 (diet) X 4 (MeHg) full factorial design. There were 16–17 animals in each of the eight experimental groups. Animals were approximately 20.5 months old at the end of the study.
2.2 Diet and MeHg exposure
Diets (Research Diets, New Brunswick, NJ) were based on the AIN 93 semi purified formulation, and were adjusted for Se content to conform to the concentrations reported below. Seven days after entering the colony, subjects were randomly assigned to one of two isocaloric diet groups. The "Low" and "High" Se diets contained 0.06 and 0.6 ppm (nominally), respectively. These were the same casein-based semi-purified diets used in an earlier study [28] in which Se concentration ranged from 0.05 to 0.1 ppm in the Low Se diet, and from 0.6 to 0.9 ppm in the High Se diet. Se intake was estimated to be 2.4 µg/kg/day and 24.0 µg/kg/day (0.03 and 0.3 µmols/kg/day) for the Low and High diets respectively (Table 1). Hg in the purified diet is below the level of detection of 50 ppb.
Table 1.
Dietary Se and Hg concentrations and estimated intakes.
| Hg concentration in water (ppm) |
Se concentration in diet (ppm) |
Estimated Hg intake (µg/kg/day) |
Estimated Se intake (µg/kg/day) |
Estimated Hg intake (µmols/kg/day) |
Estimated Se intake (µmols/kg/day) |
Estimated consumed Hg:Se Molar Ratio |
|---|---|---|---|---|---|---|
| 0 | 0.06 | ~ | 2.4 | ~ | 0.03 | ~ |
| 0.5 | 0.06 | 40 | 2.4 | 0.19 | 0.03 | 6.3 |
| 5 | 0.06 | 400 | 2.4 | 1.9 | 0.03 | 63 |
| 15 | 0.06 | 1200 | 2.4 | 5.6 | 0.03 | 187 |
| 0 | 0.6 | ~ | 24 | ~ | 0.30 | ~ |
| 0.5 | 0.6 | 40 | 24 | 0.19 | 0.30 | 0.63 |
| 5 | 0.6 | 400 | 24 | 1.9 | 0.30 | 6.3 |
| 15 | 0.6 | 1200 | 24 | 5.6 | 0.30 | 18.7 |
MeHg exposure to 0, 0.5, 5, or 15 ppm of Hg as methylmercuric chloride in drinking water began 108 days after arrival in the colony and 100 days after the onset of diet exposure. The first three concentrations (0, 0.5, 5.0 ppm of Hg as MeHg) have previously been reported as concentrations that produce approximately 0, 40, and 400 µg/kg/day of Hg intake [32], and the 15 ppm of Hg group likely consumed approximately 1200 µg/kg/day based on the consumption of the other groups. Subjects from each diet group were randomly assigned to one of the four MeHg groups. Mercury to Se (Hg:Se) ratios, for subjects that were exposed to MeHg in their drinking water, ranged 300-fold, from 0.63 to 187 (Table 1).
2.3 Overnight running
Eight running wheels, 35.5 cm in diameter and 14 cm wide with wire mesh floors, were used for testing. A 21.6×11.4×11.4 cm chamber containing a water bowl was always accessible. Subjects were divided into groups of eight, counterbalanced across experimental groups, and randomly assigned to a running wheel approximately 2–3 hours after receiving their daily food ration. All wheels performed similarly. Sessions began between 3:00 and 4.00 p.m. and continued for 16.5 hours, 12 of which were during the dark cycle. The pre-MeHg exposure baseline test commenced 56 days before MeHg administration began. The second test sessions commenced 28 days after MeHg administration began, and then occurred approximately every two months for 16 months. A total of six tests were conducted. At each time point, 132 rats were tested over the course of 17 days.
2.4 Forelimb Grip Strength
A 7.7 cm deep by 11.4 cm grid (0.33 cm diameter wire, forming 1.9 cm squares) was attached to a force gauge (Imada, Northbrook, IL) that was clamped to a table. The grid projected down at approximately 45° when attached. A rat was held by the tail and torso, allowed to grip the top bar of the grid with its forelimbs, and was then pulled away in a rapid horizontal motion. The tester was blind to treatment. The mean peak grip of three trials was used for analysis. The same experimenter performed all trials for all test sessions. The first baseline session was conducted 28 days before MeHg administration began. Thereafter, testing was conducted approximately every 10 weeks for 16 months for a total of seven sessions.
2.5 Tail-pressure sensitivity
The same Imada digital force gauge was assembled with a 90° chisel point attachment. The rat was wrapped gently in a towel to occlude its vision and restrict motion, and placed on a flat surface with its tail exposed. While holding the subject gently but firmly, the chisel point was applied vertically to the subjects’ tail approximately 5 cm from the tip, with pressure increasing over a period of approximately 1 second. The force gauge was removed when the subject flinched, flicked its tail, or squeaked. If the rat did not react then a force of 2 kg (the gauge's maximum) was recorded. The mean of three readings was used for analysis. The tester was blind to treatment. Testing followed the same timeline as the forelimb grip test for a total of 7 sessions.
2.6 Flexion and hindlimb cross (HLC)
Observations were conducted periodically after exposure began and then weekly starting 56 days after MeHg administration. The tester, blind to treatment held the subject perpendicular to the floor by the base of its tail for approximately 3 sec. Two categories were used for flexion: “normal” if the hindlimb digits were splayed with toes pointed away from the body, and “flexion” if all five hindlimb digits of either or both feet curved inward, as in the form of a clenched fist. Hind limb cross was scored as: “normal” if both hind limbs were splayed outward, “partial cross” if the hindlimbs were adducted toward the midline without crossing it or if only one limb crossed the midline, and “full cross” if both hindlimbs crossed the midline. Flexion and HLC were assessed at the same time.
2.7 Lidocaine challenge
In a separate group of six rats, peripheral fibers in the hind legs and tail were temporarily numbed to determine which signs of MeHg toxicity could be reproduced by reduced activity of peripheral fibers. A 0.2 ml volume of a 1% (10 mg/ml) lidocaine hydrochloride solution was injected between the greater trochanter and the ischial tuberosity, with the bevel of the 25 gauge needle turned toward the femoral head [27]. Once the needle encountered the body of the ischium, the lidocaine was injected over a period of 3 seconds. Injections were bilateral. Then, to numb the tail, a 0.1 ml volume of the 1% lidocaine solution was injected at the base of the rat’s tail at the 2 and 10 o’clock positions. Efficacy of the injection was verified by the absence of a toe- or tail-pinch reflex. An additional six animals received a saline (vehicle) injection at the same site.
Tests for each rat were performed prior to injections and again 10 minutes after the last injection. Hindlimb cross, flexion, and tail-pressure sensitivity were tested as described above. Proprioceptive performance was assessed based on several tests. In one, the rat was wrapped gently in a towel and the dorsal surface of a back paw was positioned on a table surface. Rats without sciatic nerve block will reposition their feet immediately so that the plantar surface touches the table [27]. All adjustments occurred within one sec, although the rat was always given ten sec to respond. Nociception was evaluated with skin (lateral metatarsus) and deep bone (fifth toe) pinch, using forceps tips.
2.8 Pathology
Skin biopsies were taken from the left paw using a dermal biopsy punch that was 2 mm in diameter (Miltex Instrument Inc., York, PA) when subjects were approximately 380 days old, following 9.5 and 6.8 months of exposure to Se and MeHg, respectively. Subjects were anesthetized with a ketamine/xylazine solution (75 mg/kg ketamine and 5 mg/kg xylazine). The skin surface was sterilized with chlorhexidine. The dermal punch was pushed perpendicular to the surface of the skin and rotated in a clockwise direction. The wound was sealed with tissue adhesive. Samples were fixed in 4% paraformaldehyde over night and sent to one of the authors (JL), who was blind to treatments. Skin biopsies were embedded into optimal cutting temperature (OCT) medium after the fixation and sectioned into 40µm thickness vertically in a cryostat. Sections were then stained with antibodies against PGP9.5 (protein gene product – 9.5 a protein specifically expressed on axonal membranes), to label epidermal nerve fibers as described before [25]. Quantification of epidermal nerve fiber density was made under fluorescence microscopy [17,22]. Sample sizes for the Low Se diet were 8, and 10, from the 0, and 5 ppm MeHg groups, respectively. For the High Se diet, sample sizes were 9, 7, and 5 from the 0, 5, and 15 ppm MeHg groups, respectively. There were no samples from the Low Se/15 ppm Hg group due to high mortality from MeHg toxicity.
At the end of the study, six rats on the Low Se diet were perfused transcardially with 4% paraformaldehyde. Cases selected included two controls with no neurological signs, two exposed with no neurological signs one exposed with hindlimb cross but no flexion and one exposed with both hindlimb cross and flexion. All exposed cases were in the Low Se/5 ppm Hg group. The exposed animals showed elevation of pressure thresholds in the tail sensitivity test. Dorsal/ventral roots and sciatic nerves were dissected and fixed in 4% paraformaldehyde + 3.5% glutaraldehyde fixative over night. Nerves were then embedded into Epon and sectioned into 1µm thickness. Sections were stained with Toluidine blue and examined under light microscopy [56].
2.9 Criteria for euthanasia
All animals were physically examined at least twice weekly, and usually more frequently. An animal was euthanized if there was rapid weight loss, failure to eat, inability to reach food or water, or failure to explore or locomote when placed on an open surface.
2.10 Data analysis
Stratified Kaplan-Meier estimates followed by a log-rank test were conducted for the dichotomous measures of mortality, flexion, and HLC. The independent variables were age (in weeks) for mortality and exposure duration for flexion and HLC. For flexion and HLC, a subject was right censored if it died before the sign was first detected. Right censoring is required in survival analysis to indicate the subject did not complete the duration of the experiment long enough to experience the measured variable i.e., they did not exhibit flexion or HLC before they died.
Running, grip strength, and tail-pressure sensitivity were normalized for each animal by dividing the value seen at each age by the baseline value taken before exposure began. Normalized values were analyzed with repeated measures ANOVAs, with time of testing as the repeated measure and experimental group as a between-subjects factor. Because of the high early mortality rate in the 15.0 ppm MeHg group, data were analyzed twice. First, a 4(MeHg)×2(Se)×T (= 3 or 4 sessions) factorial design was used for running, grip strength, and tail-pressure sensitivity. Data were then re-analyzed without the 15.0 ppm MeHg group, but with all testing sessions (6 running wheel, 7 forelimb grip strength and 7 tail-pressure sensitivity) included. A log transform was used for forelimb grip and tail-pressure sensitivity to stabilize variability across groups. The following effects were tested 1) a main effect of exposure duration evaluating age-related differences, 2) a MeHg X time interaction, to evaluate the effects of MeHg over time, 3) a Se X time interaction to evaluate the effects of dietary Se over time, and 4) a MeHg X Se X time interaction, to evaluate protection by dietary Se of MeHg’s chronic neurotoxicity. Only the results of repeated measures analyses (main effects of duration and interactions of duration with exposure) are emphasized and p values > 0.1 are not reported. Significant individual comparisons against pre-exposure sessions are summarized in Table 2 in the results rather than as symbols on the figures.
Table 2.
| Running wheels | |||||||
|---|---|---|---|---|---|---|---|
| Se Diet |
Hg concentration |
6(30) weeks |
17(41) weeks |
27(51) weeks |
38(62) weeks |
49(74) weeks |
|
| Low | 0.0 ppm | ~ | ~ | ~ | ~ | ~ | |
| Low | 0.5 ppm | ~ | ~ | ~ | ~ | p = .005 | |
| Low | 5.0 ppm | ~ | ~ | ~ | ~ | ~ | |
| Low | 15.0 ppm | p = .048 | p <.001 | n/a | n/a | n/a | |
| High | 0.0 ppm | ~ | p = .05 | p = .024 | p = .008 | p < .001 | |
| High | 0.5 ppm | ~ | ~ | ~ | p = .003 | p = .004 | |
| High | 5.0 ppm | ~ | ~ | ~ | ~ | ~ | |
| High | 15.0 ppm | ~ | p < .001 | p < .001 | n/a | n/a | |
| Tail Sensitivity | |||||||
| Se Diet |
MeHg concentration |
7(31) weeks |
11(35) weeks |
17(41) weeks |
28(52) weeks |
40(64) weeks |
51(76) weeks |
| Low | 0.0 ppm | ~ | ~ | p = .014 | ~ | p < .001 | p < .001 |
| Low | 0.5 ppm | ~ | ~ | ~ | p = .003 | p < .001 | p = .001 |
| Low | 5.0 ppm | p = .017 | ~ | ~ | p = .004 | p < .001 | p = .001 |
| Low | 15.0 ppm | ~ | p < .001 | n/a | n/a | n/a | n/a |
| High | 0.0 ppm | ~ | ~ | ~ | p = .009 | p < .001 | p < .001 |
| High | 0.5 ppm | p = .045 | ~ | ~ | ~ | p < .001 | p = .001 |
| High | 5.0 ppm | ~ | ~ | p = .003 | p = .032 | p < .001 | p < .001 |
| High | 15.0 ppm | ~ | p = .007 | p < .001 | p < .001 | n/a | n/a |
| Forelimb Grip Strength | |||||||
| Se Diet |
MeHg concentration |
9(33) weeks |
16(40) weeks |
23(47) weeks |
31(55) weeks |
41(65) weeks |
50(75) weeks |
| Low | 0.0 ppm | ~ | ~ | ~ | ~ | p = .002 | ~ |
| Low | 0.5 ppm | ~ | ~ | p = .003 | ~ | p = .008 | p = .001 |
| Low | 5.0 ppm | ~ | p < .001 | p < .001 | p < .001 | p < .001 | p = .001 |
| Low | 15.0 ppm | ~ | p = .01 | ~ | ~ | ~ | ~ |
| High | 0.0 ppm | ~ | ~ | p = .002 | p = .012 | p < .001 | p = .005 |
| High | 0.5 ppm | ~ | ~ | p < .001 | ~ | p = .005 | p = .022 |
| High | 5.0 ppm | ~ | ~ | ~ | ~ | ~ | p = .017 |
| High | 15.0 ppm | p = .05 | p < .001 | p < .001 | p < .001 | n/a | n/a |
P values represent the results of post hoc, paired t-test’s comparing a post-exposure session against the pre-exposure baseline.
“Weeks” indicates duration of exposure. Numbers in parenthesis represent the age of the animal.
n/a indicates that animals were dead during this period.
The independence of these measures was determined by examining their correlations among controls of approximately the same age. A correlation of zero would imply that two measures are unrelated to each other. The extent to which performance decrements tended to cluster was evaluated by examining the correlations among these measures in animals exposed to 5 ppm of Hg. The 15 ppm Hg exposed groups were not included in these correlational analyses because the onset of signs occurred so rapidly in this group.
3. Results
3.1 Mortality
Of the original 132 subjects 84 survived to the end of the experiment. Twelve died of various causes unrelated to MeHg exposure (hairballs, sedative overdose during a procedure, or no determinable cause) distributed evenly across exposure groups as follows. For those on the Low Se diet there were 2, 0, 4, and 0 deaths in the 0, 0.5, 5, and 15 ppm MeHg groups, respectively. For those on the High Se diet there were 2, 1, 3, and 0 such deaths in the 0, 0.5, 5, and 15 ppm MeHg groups, respectively.
Thirty six subjects were euthanized because of advanced MeHg neurotoxicity, including all 34 of the 15.0 ppm MeHg animals and 2 from the Low Se/ 5.0 ppm MeHg group.
The 15.0 ppm MeHg subjects had lower survival rates than the other MeHg exposure groups (log rank test χ2(3, 129) = 195, p < .001) and all deaths were due to MeHg-related euthanasia. Of this group, subjects maintained on the High Se diet survived longer than subjects maintained on the Low Se diet (χ2(1, 34) = 19.5, p < .001). Further analysis indicated that the High Se/5.0 ppm MeHg group survived significantly longer than the Low Se/5 ppm Hg group (χ2(1, 33) = 3.91 p = .048). Mean survival times for the Low Se/15 ppm Hg, High Se/15 ppm Hg and Low Se/5 ppm Hg exposure groups were 36.6, 52.0, and 89.1 weeks respectively.
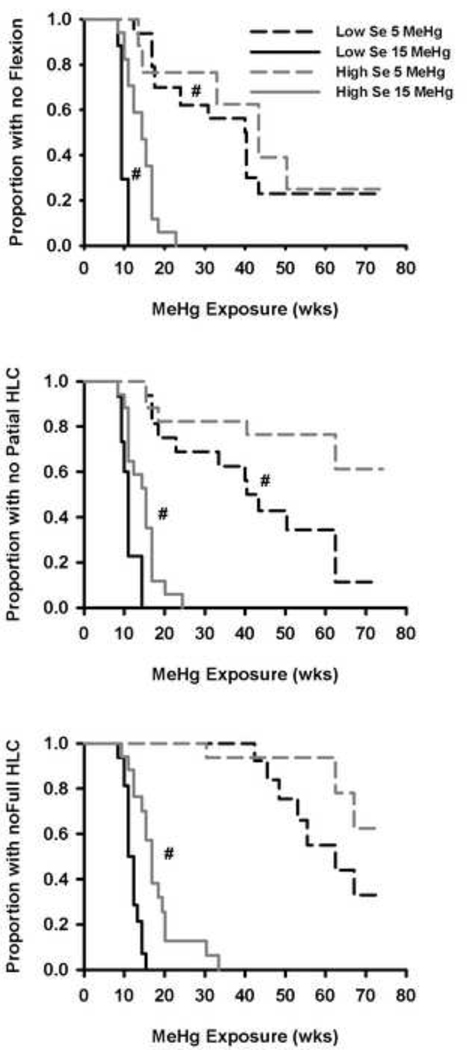
3.2 Flexion and HLC: Effects of MeHg and amelioration by Se
Figure 1 displays the Kaplan – Meier survival curves showing the onset of flexion (top), partial HLC (middle) and full HLC (bottom), for the 5.0 and 15.0 ppm MeHg groups, separated by diet. None of these signs were seen in the other groups except for one case of flexion in the Low Se/0.5 ppm MeHg group. The Low Se/ 5.0 ppm MeHg group had a shorter latency to flexion than the High Se/5.0 ppm MeHg group (median of 40 vs. 58 weeks, χ2(1, 28) = 4.84, p =.028). In the 15.0 ppm MeHg groups, the Low Se group had a shorter latency to flexion than the High Se group (median of 9.5 and 14 weeks, respectively; χ2(1, 32) = 19.7, p < .001). The Low Se/5.0 ppm MeHg group showed signs of partial HLC earlier than the High Se/5.0 ppm MeHg group (χ2(1, 32) = 5.18, p = .023; Figure 1, center), but not for full HLC (χ2(1, 32) = 2.50, p = .113; see Figure 1, bottom). For the 15 ppm MeHg group, the Low Se subjects always showed MeHg effects significantly earlier than the High Se subjects (partial HLC, χ2(1, 32) = 12.1, p = .001; full HLC, χ2(1, 32) = 15.4, p < .001). Flexion preceded partial HLC which preceded full HLC by 1.4 to 13 weeks, depending on the condition.
Figure 1.
Kaplan – Meier survival curves showing the proportion of animals not showing flexion (top), partial HLC (center) or full HLC (bottom). # indicates there was a significant difference between Se groups for a common MeHg exposure (α = 0.05).
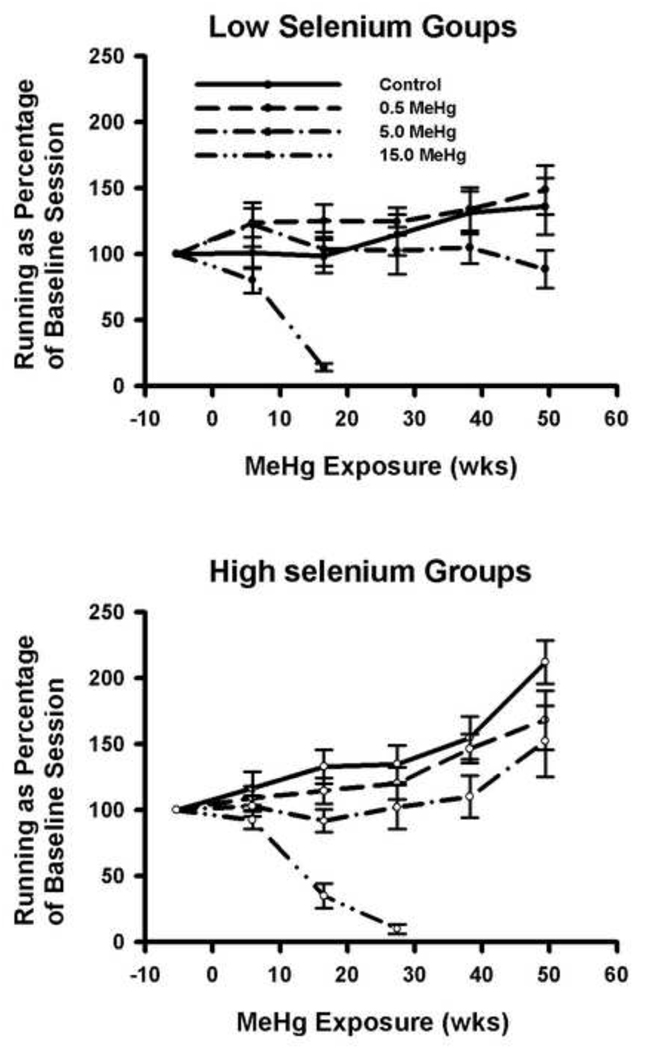
3.3 Se increases running in aging animals; MeHg decreases it
Figure 2 displays the distance run at different time points throughout the study, normalized against pre-baseline levels. The average distance run during baseline was 1.93 km, and this was similar for all exposure groups. At the end of the experiment, subjects in the High Se group more than doubled the distance run to approximately 4 km, while in the Low Se/ 0 MeHg group, only a 30% increase was seen (Figure 2; p < 0.05 for difference between the 0 MeHg groups, other p values in Table 2). The Se X exposure-duration interaction was significant (F(5,405) = 4.2, p = 0.001).
Figure 2.
Distance run as a percentage of the pre-exposure baseline. Error bars = SEM. Average distance run during the pre-exposure baseline was 1.93 km in a 14.5 hour session.
Repeated measures analysis of all groups over the first three sessions (all animals) and all sessions (excluding the 15.0 ppm MeHg group from analysis) revealed a main effect of exposure duration (F(2, 228) = 17.0, p < 0.001 for sessions 1–3; F(5, 405) = 19.2, p <.001 for all sessions), and an interaction between exposure duration and MeHg (F(6, 228) = 16.7, p < .001 for sessions 1–3; F(10, 405) = 3.25, p < 0.001 for all sessions). These data suggest that increases in running observed in control animals were prevented by MeHg exposure for both diet groups.
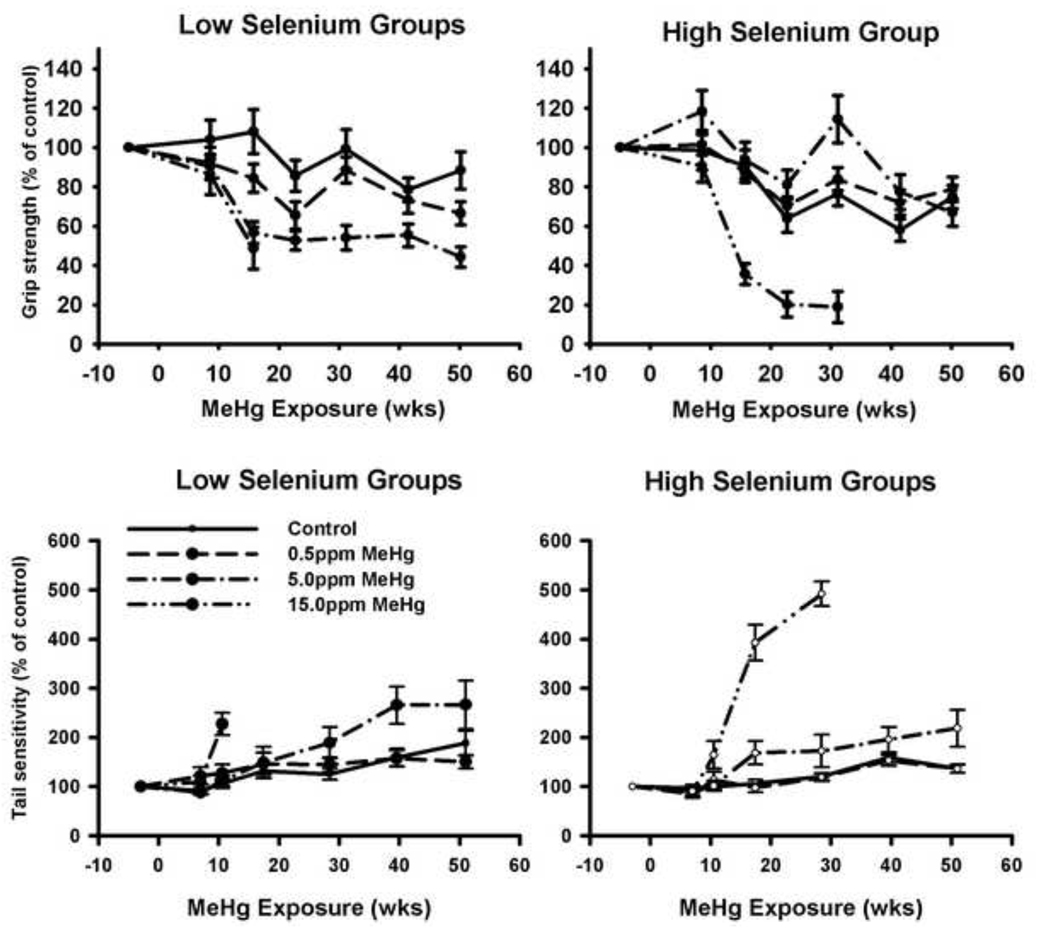
3.4 Forelimb grip was diminished by MeHg, with amelioration by Se
Average forelimb grip strength during baseline sessions was 377 g, and did not differ across exposure groups. For controls, forelimb grip strength declined by approximately 20% with age (individual p values in Table 2). Figure 3 (top) shows forelimb grip strength as a percent of the baseline session. Data from the first three sessions (all animals) and from all sessions (excluding the 15.0 ppm MeHg group) were analyzed separately due to attrition in the high MeHg group. Data were log-transformed prior to statistical analyses. Repeated measures ANOVA revealed a main effect of exposure duration (F(2, 226) = 61.1, p < 0.001 for sessions 1–3; F(6, 492) = 34.4, p <. 001 for all sessions) and an interaction between exposure duration and MeHg (F(6, 226) = 15.8, p < .001 for sessions 1–3; F(12, 492) = 4.12, p = 0.001 for all sessions). No interaction between exposure duration and Se was observed for sessions 1–3 (F(2, 226) = .292, p = .747 for session 1–3) but a marginal one occurred for all sessions (F(6, 492) = 2.051, p = .058).
Figure 3.
Forelimb grip strength (top) and tail pressure sensitivity (bottom) as a percentage of the baseline session before MeHg exposure began. Error bars = SEM. Average grip strength was 377 gms during the pre-exposure baseline. Average sensitivity was 450.7 gm. during the pre-exposure baseline.
A three-way interaction was found among exposure duration, MeHg, and Se (F(6, 226) = 3.1, p = .006 for session 1–3; F(12, 492) = 3.51, p < 0.001 for all sessions) indicating that MeHg-related declines in grip strength were greater in the animals on the Low-Se diet than in those on the High-Se diet (p values in Table 2). Post-hoc t-tests also revealed a significant effect of diet on grip strength at 23 wks (F(1, 31) = 4.31, p = .046), 31 wks (F(1, 31) = 6.68, p = .015), and 41.5 wks (F(1, 31) = 7.67, p = .009).
3.5 Tail-pressure sensitivity was diminished by MeHg, ameliorated by Se
The average force required to elicit a response in the tail-pressure sensitivity test during baseline (pre-exposure) sessions was 450 g. No significant differences were detected between groups but the threshold for unexposed animals increased by about 50% by the end of the experiment (p values in Table 2). Figure 3 (bottom) shows tail-pressure sensitivity tests as a percent of the baseline session. Repeated measures analysis of the first 4 sessions, which included the 15 ppm MeHg subjects, revealed a main effect of exposure duration (F(3, 312) = 74.8, p < 0.001), a significant interaction between exposure duration and MeHg (F(9, 312) = 14.3, p < .001), and a significant three-way interaction among exposure duration, MeHg and Se (F(9, 312) = 2.35, p = 0.014).
Repeated measures analysis of all the sessions (omitting data from the 15.0 ppm MeHg group due to attrition) revealed a main effect of exposure duration (F(6, 462) = 75.5, p < .001), a significant interaction between session and MeHg (F(12, 462) = 4.03, p <.001), and a significant three-way interaction among exposure duration, MeHg exposure levels and Se diet (F(12, 462) = 2.11, p = 0.016). Mercury accelerated the age-related decrease in tail sensitivity, especially in the Low Se group.
3.6 Independence, and clustering of performance measures
For unexposed animals, correlations across performance tests from both diet groups were indistinguishable from zero (r range 0.015 to 0.25), suggesting that our measures were independent of one another. For exposed animals, many correlations were significantly different from zero (Table 3), indicating that performance deficits clustered together.
Table 3.
The Pearson correlation coefficients (r) between variables at comparable times of testing.
| Exposure Week | Correlations | |||||
|---|---|---|---|---|---|---|
| Variables | Run | Grip | Low Se, 5 ppm | N | High Se, 5 ppm. | N |
| Run and Grip | 6(30) | 9(33) | 0.51* | 16 | ||
| 17(41) | 16(40) | 0.63** | 16 | 0.63** | 17 | |
| 27(51) | 31(55) | 0.72** | 16 | |||
| 38(62) | 41(65) | 0.85*** | 15 | 0.56* | 15 | |
| 49(73) | 50(74) | 0.73** | 11 | |||
| Run and Tail | Run | Tail | ||||
| 17(41) | 16(40) | −0.64** | 16 | |||
| 27(51) | 27(51) | −0.52* | 16 | −0.53* | 14 | |
| 38(62) | 40(64) | −0.65** | 15 | −0.56* | 14 | |
| 49(73) | 51(75) | −0.74** | 11 | |||
| Grip and Tail. | Grip | Tail | ||||
| 17(41) | 17(41) | −0.49* | 16 | |||
| 31(55) | 28(52) | −0.78*** | 16 | −0.81*** | 14 | |
| 41(65) | 41(65) | −0.72** | 15 | −0.61* | 14 | |
| 51(75) | 50(74) | −0.81** | 11 | |||
indicates significant to α = .05
indicates significant to α = .01
indicates significant α < .001.
Numbers in parentheses indicate the age of the animals in weeks at the time of testing. N indicates the animals in the group at the time of comparison.
3.7 Lidocaine challenge
Of the animals receiving lidocaine, none showed full hind-limb cross or flexion and only two out of six showed partial hind-limb cross. The effectiveness of the lidocaine challenge was verified in the effects of somatosensory and pain testing. No animal responded to metatarsus, fifth toe, or tail pinch, and none showed postural adjustment. Further, no subject showed a tail-pressure reflex even at 2 kg of pressure.
Of the six animals receiving vehicle injections, none showed hind-limb cross, flexion, or ataxia. All six responded to metatarsus, tail, and fifth toe pinch and showed immediate postural adjustment. The average pressure to produce a tail-pressure reflex in the six vehicle-exposed animals was 481 g.
3.8 Peripheral pathology demonstrates axonal degeneration or atrophy in sensory myelinated nerve fibers and motor nerve fibers
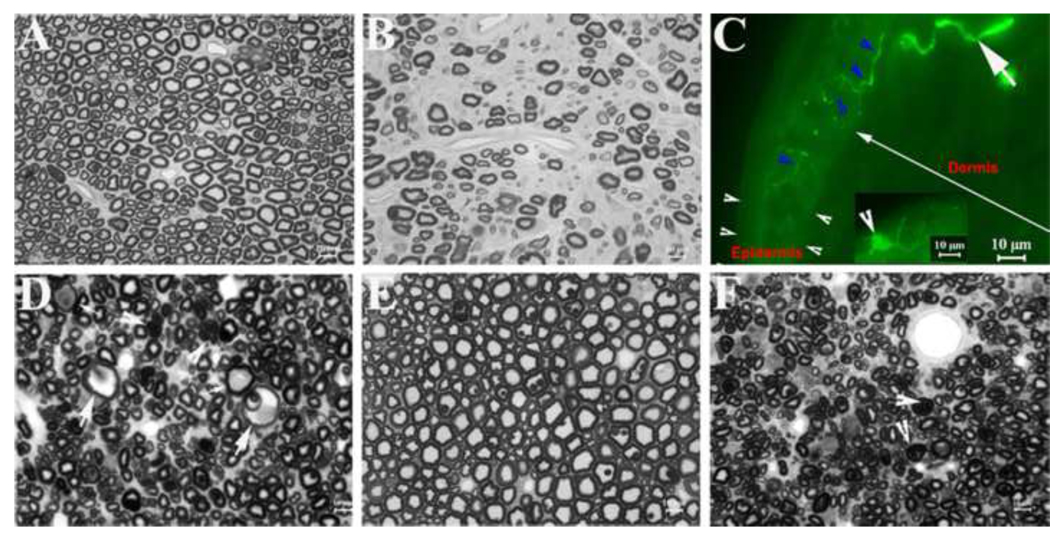
Table 4 summarizes the pathology observed in sciatic nerves and in dorsal and ventral roots from two controls and four animals exposed to 5 ppm MeHg. Nerve samples from subjects exposed to 15 ppm MeHg were not available. A loss of myelinated nerve fibers was conspicuous in all available sciatic nerves of the 5 ppm MeHg group rats, but fibers were normal in an age- and diet-matched control rat (Figures 4A and B). This pathological change in 5 ppm MeHg sciatic nerves appeared diffuse, and there was a large amount of collagen deposition between nerve fibers.
Table 4.
Neurolological signs and dorsal and ventral root fiber pathology.
| Rat ID # |
Chronic MeHg Exposure |
Chronic Se Exposure |
Hindlimb Cross |
Flexion | Ventral Roots |
Dorsal Roots |
|---|---|---|---|---|---|---|
| 1109 | 0 ppm Hg | Low Se | NO | NO | OK | OK |
| 1110 | 0 ppm Hg | Low Se | NO | NO | OK | OK |
| 1313 | 5.0 ppm Hg | Low Se | NO | NO | OK | Axonal degeneration |
| 1316 | 5.0 ppm Hg | Low Se | NO | NO | OK | Axonal degeneration |
| 1314 | 5.0 ppm Hg | Low Se | YES | NO | OK | Swollen six fold |
| 1312 | 5.0 ppm Hg | Low Se | YES | YES | DAMAGE |
Figure 4.
Nerve pathology and skin biopsy: A. This is a semithin section of sciatic nerve from a control rat. Myelinated nerve fibers with different diameters are present and free of any pathology. B. A semithin section of sciatic nerve from a 5 ppm MeHg treated rat shows conspicuous loss of myelinated nerve fibers with a large amount of collagen deposition between nerve fibers. C. A skin biopsy from the paw of a rat exposed to 5 ppm MeHg was sectioned into 40µm thickness and stained with antibodies against PGP9.5. Nerve fibers (solid arrowheads; green color) were revealed in the epidermis (between white arrowheads) and dermis (long white arrow). Inset: A dendritic cell was detected in the epidermis (arrowhead in inset). Based on its morphological feature [22], it is likely a Langerhans cell. D. A semithin section of dorsal root from a 5 ppm rat shows many atrophic axons. Some axons are swollen (large arrows) or occupied by accumulated materials (small arrows) or obliterated by ‘collapsed myelin’ (arrowheads). All these findings are consistent with axonal degeneration. E. A semithin section of a 5 ppm ventral root appears normal and has numerous myelinated nerve fibers. F. A semithin section of ventral root from rat #1312 shows diffuse atrophic axons. Myelin in some nerve fibers is collapsed (arrowheads), which makes axonal space invisible under light microscopy.
In an earlier study, rats exposed chronically to MeHg demonstrated that MeHg-induced neurodegeneration preferentially in large DRG neurons while small neurons were relatively spared [44]. To evaluate this pathological feature in our model, we performed immunohistochemistry on skin biopsies (Figure 4C) with antibodies against PGP9.5 to label epidermal nerve fibers [22]. These sensory fibers (c-fibers) represent axonal terminals extended mainly from the small DRG-neurons. The average density was 4.1 (SD=1.1) fibers/mm. No effect of MeHg (F(1,29) = 0.38, p = 0.54), or Se (F(1,29) = 0.81, p = 0.38), and no MeHg X Se interaction (F(1,29) = 0.06, p = 0.82), was noted on fiber density in the epidermis in an analysis that included both diets and animals exposed to 0 or 5.0 ppm of MeHg. Including the additional five animals exposed to 15 ppm MeHg did not change the outcome of the analysis substantively. There was no significant difference in fiber density between asymptomatic animals and those showing flexion or hind-limb cross. These results are consistent with the notion that small DRG neurons are resistant to MeHg toxicity even after 6.8 months of exposure to MeHg.
Axonal degeneration was observed in all available dorsal roots collected from animals exposed to 5 ppm MeHg and maintained on the Low Se diet. Some axons were atrophic with collapsed myelin or were severely swollen with accumulation of intra-axonal materials (Figure 4D). No changes of de- or re-myelination, such as denuded axons or onion bulbs, were observed. These findings are consistent with elevated thresholds observed on the tail-pressure tests in these exposed rats.
Ventral roots from four 5 ppm MeHg group rats were also examined. One was normal (Figure 4E), while two showed some atrophic axons or an occasional degenerated nerve fiber. One (#1312) had diffuse axonal atrophy with a few degenerated fibers (Figure 4F). This rat was the only animal examined that showed the flexion response.
We noticed that there are PGP9.5-positive dendritic cells in the epidermis (inset in Figure 4C). Based on previous publications, these cells are likely Langerhans cells [26]. These cells were also manually counted. The average density was 2.5 cells/mm. No effect of MeHg (F(1,29) = 2.8, p = 0.11), Se (F(1,29) = 0.25, p = 0.624) and no MeHg X Se interaction (F(1,29) = 0.39, p = 0.54) was noted in an analysis that included both diets and animals exposed to 0, or 5.0 ppm of MeHg. Including the animals exposed to 15 ppm MeHg did not change the results substantively.
4. Discussion
The present study revealed a dose and time-dependent neurotoxicity of chronic exposure to MeHg using tests of somatosensory function, peripheral motor systems, and running in overnight sessions. The doses used ranged from one that was without effect in any animal to a dose that was high enough to produce deficits in every exposed animal within 35 weeks. The two groups that were not exposed to MeHg also provided novel data on the roles of dietary selenium and aging on these measures. Functional deficits were consistent with the pathological findings in peripheral sensory and motor nerves. The duration of the study was 16 months, so very long-term effects of both MeHg and dietary Se exposure could be identified. In addition, MeHg was consumed orally via drinking water throughout the study period. This stands in contrast to exposure regimens that employ routes of administration such as injection and gastric intubation, which deliver MeHg in a pulsatile temporal pattern with high transient exposures. Thus, the present study is relevant to a stable pattern of human dietary MeHg consumption.
4.1 Selectivity of the neurological tests
Correlations among the different neurological tests were examined to determine the degree to which these tests provided independent measures of function. For unexposed animals, the inter-test correlations among wheel-running, grip strength, and tail-pressure sensitivity were indistinguishable from zero, suggesting that these measures were indeed independent of one another. For exposed animals, however, the inter-test correlations were much higher, ranging from 0.51 to 0.85; performance on one test accounted for 25% to 70% of the variability on another test. For impaired animals, the correlations between grip strength and tail-pressure sensitivity were the strongest. Since these tests were independent of one another in controls, this could mean that degradation of sensory and motor systems occurred in parallel. Alternatively, it could imply that impairment in one functional domain contributed to poor performance in the others in exposed animals. For example, subtle somatosensory deficits may have caused an early release from the grip strength apparatus.
4.2 MeHg exposure produced a cascade of neurobehavioral signs
Both sensory and motor effects of chronic MeHg exposure have been reported in clinical populations [12,16,24] and the laboratory model described here reproduced these and documented a cascade of signs in the animals exposed to 5 ppm of MeHg. The first signs to appear were flexion in the peripheral digits, weaker forelimb grip, and reduced somatosensory function in the tail. These signs tended to co-occur even though they reflect different underlying pathology (detailed below). Hind-limb cross and changes in running occurred later in exposure. In contrast to the slow progression of signs in the 5 ppm MeHg group, the 15 ppm MeHg dosage was so toxic that all signs tended to appear nearly simultaneously.
Diminished somatosensory function surely reflects pathology in peripheral sensory fibers, which showed the most severe damage at the end of the study. The flexion response likely reflects damage to motor pathways. Of the animals on which we had pathology results from the motor fiber-containing ventral root, the only one that showed flexion was also the one with the most advanced neuropathology.
Distance run increased through the course of the study, which ended when animals were approximately 20 to 21 months of age This outcome was unexpected in light of previous reports that, in rats, voluntary running decreases with age after about six to seven months old [18–20]. The reasons for the discrepancy cannot be identified with certainty, but the intermittency of the opportunity to run and degree of food restriction may be important. In the present study, running was examined only every two months whereas in the other cited studies there was continuous access to the running wheel. Thus, animals in the present study might be running-deprived” and, given the importance of running for rats, this could result in a progressively higher level of running with each opportunity to do so [36,45]. In addition, food restriction tends to increase running [35], and the animals in the present study were more food restricted than those in the above cited studies. If animals had been allowed to age further, a decrease in running would likely have occurred as noted in an earlier study in which the animals were allowed to run until they were 2 ½ years of age [11].
MeHg prevented the age-related increase in running in overnight sessions. It is noteworthy that running increased even as animals were experiencing diminished grip strength and somatosensory deficits. This suggests that some compensation for sensorimotor deficits occurred that enabled them to run. This is consistent with observations that running is a highly valued activity for laboratory housed rats [4].
The diminished running (relative to unexposed controls) observed among MeHg-exposed animals might be due to reduced endurance or elevated fatigue, but other possibilities can be noted. For example, it could be due to reduced bursts of running early in the session when the animal is first placed into the wheel rather than larger fatigue-induced decreases late in the session. Alternatively, it could also reflect diminished control by the photoperiod in exposed rats because a portion of the session occurred during the light period and another portion occurred during the dark period. Unfortunately, due to the nature of the equipment used, a time-course for running is not available, prohibiting us from distinguishing among these and other possibilities.
4.3 Neuropathology
Previous studies have shown mild functional abnormalities and normal morphological findings in the CNS of chronically MeHg-exposed rats that show f axonal degeneration in peripheral sensory nerves [46,47]. Skin biopsies enabled the evaluation of sensory nerve axon terminals in the present study. As these are the most remote part of the neuron from the cell body, they ought to be especially sensitive to insults against sensory neurons. Surprisingly, our results showed no difference between the MeHg group and controls. The presence of normal epidermal nerve fiber density in our study, even after nearly 7 months of exposure and detectable loss of sensory function, reveals remarkable resistance of these axons and their related small DRG-neurons to MeHg toxicity. This finding is consistent with observations in a previous study [44]. At the present, the biological substrates for this resistance are unclear, but it is reasonable to speculate that some molecular property intrinsic to small DRG neurons might play a role.
Langerhans cells were found in the epidermis of skin biopsies in the present study. A previous study suggests an increase of these cells during acute sensory nerve damages [26] and likely reflects a reactive change of these cells to dermal nerve injuries. However, this increase was not found in our rats exposed to MeHg. This discrepancy is not necessarily surprising since our skin biopsies were taken 7 months after the exposure. The reactive change of Langerhans cells may have faded away after the acute phase of MeHg toxicity.
Significant axonal degeneration was noted in the dorsal roots of the 5 ppm MeHg exposed animals, and the sciatic nerves in these subjects showed a loss of myelinated nerve fibers. These findings are consistent with diminished response to tail-pressure observed in these exposed rats. Overall, axonal degeneration in dorsal roots, reflecting proximal damage, was milder than that in the sciatic nerves, reflecting more distal damage, suggesting a length-dependent susceptibility to axonal degeneration.
In contrast to the sensitivity of the dorsal roots, the ventral roots' pathology was less severe. It has been reported that motor nerves are spared in cases of MeHg toxicity [46,47], raising questions about how muscle atrophy [53] and or other motor effects could occur. Our results suggest one explanation for the motor deficits caused by chronic MeHg exposure: Perhaps pathological changes in motor fibers also represent a length-dependent process, in which disruption first appears distally in the sciatic nerve or at the neuromuscular junction [8], and, only later, following continued exposure, in the more proximal ventral roots. Furthermore, atrophic motor axons may be dysfunctional prior to degeneration.
Taken together, morphological evaluation confirms previous reports of preferential damage to large sensory neurons or axons and provides evidence of peripheral motor nerve abnormalities that have not been described before. If motor nerve pathology takes a long time to develop, then this could explain why pathological changes in motor nerves were absent or unreported in previous studies [46,47]. The longest exposure duration from which pathology has been described is 90 days, which is much shorter than the duration of 16 months in the present study.
Performance of the neurobehavioral tests described here requires participation of sensorimotor pathways as well as CNS-neurons, so these tests alone cannot distinguish abnormalities in the CNS from those in peripheral nerves. Some tendencies can be noted, however, Grip reflects forelimb strength, but could also be influenced by sensory loss because gripping the grid used in this task requires proprioception. It should be noted, however, that grip strength testing was conducted only on animals that could grip the metal grid at the beginning of the task, so sufficient sensory function had to be in place for the test to be initiated (i.e., if there was sensory loss then it was only partial). The observation that most behavioral tasks involve multiple components of nervous system function does not affect our conclusions about motor or sensory dysfunction by the chronic exposure of MeHg or about protection by Se (see below).
It has been suggested that the hind limb cross (HLC) characteristic of chronic MeHg exposure is due to pathology in the dorsal root ganglion [54] and, in fact, there was axonal degeneration and atrophic axons appearing in dorsal roots, while sciatic nerves showed a loss of myelinated nerve fibers. To determine whether loss of sensory nerves could result in HLC, sciatic nerve function was eliminated reversibly by lidocaine in normal animals. A lidocaine dose that eliminated a pinch response on the hind paws, eliminated sensitivity of the tail to applied pressure up to 2 kg, and produced a gait disturbance, did not produce the flexion response or HLC seen in the MeHg-exposed animals, suggesting that its cause lies elsewhere.
4.4 Se ameliorates some neurobehavioral signs of chronic MeHg exposure
The present study provided compelling evidence that long-term dietary Se ameliorated MeHg-induced deficits. Evidence for this beneficial effect is shown in every functional measurement as well as in overall survival. These results should alleviate concerns about the possibility that the long-term co-administration of Se and MeHg exacerbates neurological deficits by increasing deposition of Hg in neurons [44]. This finding has direct public health implications, assuming model relevance, but does not imply that dietary Se can reverse or otherwise be used to treat patients exposed chronically to MeHg.
The pattern of Se-Hg interaction effects depended upon the daily Hg dose and the dependent variable examined. The most striking effects of Se lay in its substantial delay of the onset of flexion and hind-limb cross. Dietary Se in the high-normal range delayed the appearance of these signs by one to four months, as compared with Se in the low-normal range. While the effect was less dramatic, Se also moderated the effects of MeHg on grip strength, generally delaying MeHg-induced weakening of the forelimbs.
As noted above, Se produced an overall increase in running in overnight sessions. MeHg exposure attenuated this increase in a dose-related fashion. The evidence for this lies in the presence of statistical interactions of duration with both Se and MeHg. However, the absence of a significant MeHg X Se X duration interaction suggests that Se failed to confer protection from MeHg-induced decreases in running. Within each nutrition group, the pattern of MeHg effects was similar.
While it is clear that selenium is protective against MeHg neurotoxicity, quantifying the relative protection by specific Se intake presents a challenge. An interesting approach has been to relate neurotoxicity to the ratio of Hg to Se in the blood or brain [37], the diet, or to a “Health Benefit Value,” an index that incorporates the ratio and absolute contents of Hg and Se in the diet [21,37]. In those studies, the appearance of growth retardation and hindlimb cross in post-weaning rats on high MeHg diets (50–60 µmoles/kg or 10–12 ppm in feed} depended on the concentration of Se in the diet and in the brain. The Health Benefit Value and Hg:Se ratios in brain or blood accounted for MeHg’s neurotoxicity better than tissue content did, but the range of tissue concentrations examined was narrow since only one MeHg dose resulted in neurotoxicity.
A stronger test of hypotheses regarding Hg:Se ratios would be to examine several neurobehavioral endpoints, over a range of MeHg exposures, and durations long enough that signs associated with lower levels have time to appear, as done in the present study. Here, the concentration of MeHg in the diet was the most important determinant of neurotoxicity on all endpoints examined, but dietary selenium influenced the toxicity at a particular exposure level. Thus, the order of toxicity here was Low Se/15 ppm MeHg > High Se/15 ppm MeHg > Low Se/5 ppm MeHg > High Se/5 ppm MeHg. For the 0 and 0.5 ppm MeHg groups only Se effects were detected in the present study. As noted in Table 1, the order of toxicity predicted by strict dietary molar ratios would be different. Not noted in Table 1 is the prediction based on Health Benefit Values. That sequence would be would be Low Se/15 ppm MeHg > Low Se/5 ppm MeHg > High Se/15 ppm MeHg > 0.5 ppm MeHg (both diets) > High Se/5 ppm MeHg > Low Se/0.5 ppm MeHg > High Se/0.5 ppm MeHg. Brain ratios are also likely to be important. As reported in Newland et al.,[31] using a similar exposure regimen (without the 15 ppm MeHg group) drinking water concentration of MeHg was the major determinant of brain Hg, while dietary Se played a secondary role, similar to that noted in the present study of neurotoxicity. Taken together, the present report suggests that dietary selenium modulates the neurotoxicity associated with adult-onset MeHg exposure neurotoxicity on the endpoints studied here, but the most important influence on MeHg neurotoxicity is the dose of MeHg, however, the exact mechanisms by which selenium confers protection cannot be ascertained from these data. This conclusion is restricted to chronic, adult-onset exposure. With developmental exposure, dietary Se had little influence over MeHgs neurotoxicity [29].
4.5 Aging and Se
With normal aging, somatosensory thresholds became elevated and grip strength declined by about 20% over the course of the study (see fig. 3), a magnitude similar to that seen in high-rate operant behavior [30]. In contrast, running distance only increased in the unexposed animals. Interestingly, high dietary Se potentiated this age-related increase in running in all but the 15 ppm MeHg exposure groups. Unexposed animals on the High Se diet ran twice as far at the end of the study as at the beginning of the study and 60% further than animals on the Low Se diet. Even MeHg-exposed animals on the High Se diet showed age-related increases in running and did so as grip strength and somatosensory function declined.
The Se-induced increase in running in aging animals was unexpected and is novel. Its mechanism is unknown, but could be related to Se's antioxidant effects. Physical activity promotes the release of reactive oxygen species and thiol groups and dietary supplementation with Vitamin E and Se [38] or dietary Se [48] attenuates this release and enhances exercise.
4.6 Concluding remarks
There appears to be a threshold above which MeHg produces overt neurological signs and below which they do not appear, even with prolonged exposure. This would be expected based on earlier observations of dose-related accumulation of Hg in the nervous system with chronic exposure [11,31]. This is not to say that there are no effects of the lowest exposure level of 0.5 ppm MeHg, only that none were detected with the tests used here.
Se, a nutrient found in many fish, has been hypothesized to confer protection against, even prevent, MeHg's neurotoxicity. In a previous study, it was reported that dietary docosahexaenoic acid, another fish-borne nutrient, did not protect against the neurotoxicity of chronic MeHg [11], although it may have benefits of its own [34]. Here, we report strong evidence that dietary Se delays the appearance of selected neurological signs of long-term, adult-onset MeHg exposure. In addition, benefits of long-term consumption of Se were seen in unexposed animals, especially as they approached old age, suggesting that this nutrient may confer some protection against aging as well.
Acknowledgments
Supported by NIEHS grants ES 10835 (MCN) and ES 06639 (JL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Amin-zaki L, Majeed MA, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. Br Med J. 1978;1:613–616. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 3.Bakir F, Rustam H, Tikriti S, Al-Damluji SF, Shihristani H. Clinical and epidemiological aspects of methylmercury poisoning. Postgrad Med J. 1980;56:1–10. doi: 10.1136/pgmj.56.651.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Beuter A, de Geoffroy A, Edwards R. Analysis of rapid alternating movements in Cree subjects exposed to methylmercury and in subjects with neurological deficits. Environ Res. 1999;80:64–79. doi: 10.1006/enrs.1998.3885. [DOI] [PubMed] [Google Scholar]
- 6.Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5:75–79. doi: 10.1046/j.1523-5408.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Burk RF, Laevander OA. Selenium. In: Shils ME, Olsen JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. Baltimore, MD: Williams and Williams; 1999. pp. 265–276. [Google Scholar]
- 8.Candura SM, D'Agostino G, Castoldi AF, Messori E, Liuzzi M, Manzo L, Tonini M. Effects of mercuric chloride and methyl mercury on cholinergic neuromuscular transmission in the guinea-pig ileum. Pharmacol Toxicol. 1997;80:218–224. doi: 10.1111/j.1600-0773.1997.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 9.Castoldi AF, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Rev Environ Health. 2003;18:19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Council NR. Nutrient Requirements of Laboratory Animals, Editoin Edition. Washington, DC: Academy Press; 1995. [Google Scholar]
- 11.Day JJ, Reed MN, Newland MC. Neuromotor deficits and mercury concentrations in rats exposed to methyl mercury and fish oil. Neurotoxicol Teratol. 2005;27:629–641. doi: 10.1016/j.ntt.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Dolbec J, Mergler D, Sousa Passos CJ, Sousa de Morais S, Lebel J. Methylmercury exposure affects motor performance of a riverine population of the Tapajos river, Brazilian Amazon. Int Arch Occup Environ Health. 2000;73:195–203. doi: 10.1007/s004200050027. [DOI] [PubMed] [Google Scholar]
- 13.Eto K, Tokunaga H, Nagashima K, Takeuchi T. An autopsy case of minamata disease (methylmercury poisoning)--pathological viewpoints of peripheral nerves. Toxicol Pathol. 2002;30:714–722. doi: 10.1080/01926230290166805. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda Y, Ushijima K, Kitano T, Sakamoto M, Futatsuka M. An analysis of subjective complaints in a population living in a methylmercury-polluted area. Environ Res. 1999;81:100–107. doi: 10.1006/enrs.1999.3970. [DOI] [PubMed] [Google Scholar]
- 15.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 16.Harada M. Neurotoxicity of methylmercury: Minamata and the Amazon. In: Yasui M, Strong JJ, Ota K, Verity MA, editors. Mineral and Metal Neurotoxicology. Boca Raton, FL: CRC Press; 1997. pp. 177–188. [Google Scholar]
- 17.Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO. Exercise and food restriction in rats. J Nutr. 1992;122:774–777. doi: 10.1093/jn/122.suppl_3.774. [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70:1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- 20.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north pacific near Hawaii. Biol Trace Elem Res. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360–371. doi: 10.1002/(sici)1097-4598(199903)22:3<360::aid-mus9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Kinjo Y, Higashi H, Nakano A, Sakamoto M, Sakai R. Profile of subjective complaints and activities of daily living among current patients with Minamata disease after 3 decades. Environ Res. 1993;63:241–251. doi: 10.1006/enrs.1993.1144. [DOI] [PubMed] [Google Scholar]
- 24.Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Bai Y, Ghandour K, Qin P, Grandis M, Trostinskaia A, Ianakova E, Wu X, Schenone A, Vallat JM, et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain. 2005;128:1168–1177. doi: 10.1093/brain/awh483. [DOI] [PubMed] [Google Scholar]
- 26.Lin YW, Tseng TJ, Lin WM, Hsieh ST. Cutaneous nerve terminal degeneration in painful mononeuropathy. Exp Neurol. 2001;170:290–296. doi: 10.1006/exnr.2001.7704. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Popitz-Bergez F, Birknes J, Strichartz GR. The critical role of concentration for lidocaine block of peripheral nerve in vivo: studies of function and drug uptake in the rat. Anesthesiology. 2003;99:1189–1197. doi: 10.1097/00000542-200311000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Newland MC, Donlin WD, Paletz EM, Banna KM. Developmental behavioral toxicity of methylmercury. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton, FL: CRC Press; 2006. pp. 101–146. [PubMed] [Google Scholar]
- 29.Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition: adult effects of fetal exposure in experimental models. Neurotoxicology. 2008;29:783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicol Teratol. 2000;22:819–828. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- 31.Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and blood mercury and selenium after chronic and developmental exposure to methylmercury. Neurotoxicology. 2006;27:710–720. doi: 10.1016/j.neuro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50:106–116. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya T, Imamura K, Kuwahata M, Kindaichi M, Susa M, Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicol Teratol. 2005;27:643–653. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n-3 polyunsaturated fatty acids. Neurotoxicology. 2007;28:707–719. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Premack D, Schaeffer RW. Distributional properties of operant-level locomotion in the rat. J Exp Anal Behav. 1962;5:89–95. doi: 10.1901/jeab.1962.5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Premack D, Schaeffer RW, Hundt A. Reinforcement of Drinking by Running: Effect of Fixed Ratio and Reinforcement Time. J Exp Anal Behav. 1964;7:91–96. doi: 10.1901/jeab.1964.7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralston NV, Ralston CR, Blackwell JL, 3rd, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–811. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Reddy KV, Kumar TC, Prasad M, Reddanna P. Pulmonary lipid peroxidation and antioxidant defenses during exhaustive physical exercise: the role of vitamin E and selenium. Nutrition. 1998;14:448–451. doi: 10.1016/s0899-9007(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 39.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 40.Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology. 1996;17:139–154. [PubMed] [Google Scholar]
- 41.Sakamoto M, Wakabayashi K, Kakita A, Hitoshi T, Adachi T, Nakano A. Widespread neuronal degeneration in rats following oral administration of methylmercury during the postnatal developing phase: a model of fetal-type minamata disease. Brain Res. 1998;784:351–354. doi: 10.1016/s0006-8993(97)01400-5. [DOI] [PubMed] [Google Scholar]
- 42.Schionning JD. Experimental neurotoxicity of mercury. Autometallographic and stereologic studies on rat dorsal root ganglion and spinal cord. APMIS Suppl. 2000;99:1–32. [PubMed] [Google Scholar]
- 43.Schionning JD, Eide R, Ernst E, Danscher G, Moller-Madsen B. The effect of selenium on the localization of autometallographic mercury in dorsal root ganglia of rats. Histochem J. 1997;29:183–191. doi: 10.1023/a:1026493607861. [DOI] [PubMed] [Google Scholar]
- 44.Schionning JD, Larsen JO, Tandrup T, Braendgaard H. Selective degeneration of dorsal root ganglia and dorsal nerve roots in methyl mercury-intoxicated rats: a stereological study. Acta Neuropathol. 1998;96:191–201. doi: 10.1007/s004010050881. [DOI] [PubMed] [Google Scholar]
- 45.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 46.Shigematsu J, Yasuda T, Goto Y, Tanaka K, Tobimatsu S. Chronic effects of methylmercury on the cerebral function in rats. J Neurol Sci. 2000;182:69–75. doi: 10.1016/s0022-510x(00)00454-8. [DOI] [PubMed] [Google Scholar]
- 47.Shigematsu J, Yasuda T, Goto Y, Tanaka K, Tobimatsu S, Kato M. Recovery of brain dysfunction after methylmercury exposure in rats. J Neurol Sci. 2000;182:61–68. doi: 10.1016/s0022-510x(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 48.Soares JC, Folmer V, Rocha JB. Influence of dietary selenium supplementation and exercise on thiol-containing enzymes in mice. Nutrition. 2003;19:627–632. doi: 10.1016/s0899-9007(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 49.Su M, Wakabayashi K, Kakita A, Ikuta F, Takahashi H. Selective involvement of large motor neurons in the spinal cord of rats treated with methylmercury. J Neurol Sci. 1998;156:12–17. doi: 10.1016/s0022-510x(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 50.Sugiura Y, Tamai Y, Tanaka H. Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg Chem. 1978;9:167–180. doi: 10.1016/s0006-3061(00)80288-4. [DOI] [PubMed] [Google Scholar]
- 51.Takaoka S, Fujino T, Sekikawa T, Miyaoka T. Psychophysical sensory examination in individuals with a history of methylmercury exposure. Environ Res. 2004;95:126–132. doi: 10.1016/j.envres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect. 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uchino M, Okajima T, Eto K, Kumamoto T, Mishima I, Ando M. Neurologic features of chronic Minamata disease (organic mercury poisoning) certified at autopsy. Intern Med. 1995;34:744–747. doi: 10.2169/internalmedicine.34.744. [DOI] [PubMed] [Google Scholar]
- 54.Wakabayashi K, Kakita A, Sakamoto M, Su M, Iwanaga K, Ikuta F. Variability of brain lesions in rats administered methylmercury at various postnatal development phases. Brain Res. 1995;705:267–272. doi: 10.1016/0006-8993(95)01208-7. [DOI] [PubMed] [Google Scholar]
- 55.Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110 Suppl 5:851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Chow CY, Sahenk Z, Shy ME, Meisler MH, Li J. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain. 2008;131:1990–2001. doi: 10.1093/brain/awn114. [DOI] [PMC free article] [PubMed] [Google Scholar]