Abstract
A sensitive, selective, accurate and robust LC-MS/MS method was developed and validated for the quantitative determination of glucocorticoids in rabbit ocular tissues. Samples were processed by a simple liquid- liquid extraction procedure. Chromatographic separation was performed on Phenomenex reversed phase C18 gemini column (50 × 4.6 mm i.d.,) with an isocratic mobile phase composed of 30% of acetonitrile in water containing 0.1% of formic acid, at a flow rate 0.2 mL/min. Dexamethasone (DEX), prednisolone (PD) and hydrocortisone (HD) were detected with proton adducts at m/z 393.20→355.30, 361.30→147.20 and 363.20→121.0 in multiple reaction monitoring (MRM) positive mode respectively. Finally, 50µL of 0.1% novel DEX mixed micellar formulation was topically administered to a rabbit eye and concentrations were measured. The method was validated over a linear concentration range of 2.7–617.6 ng/mL. Lower limit of quantitation (LLOQ) of DEX and PD was measured in the concentration range of 2.7 and 11.0 ng/mL respectively. The resulting method demonstrated intra and inter-day precision within 13.3 % and 11.1 % and accuracy within 19.3 % and 12.5 % for DEX and PD, respectively. Both analytes were found to be stable throughout freeze–thaw cycles and during bench top and postoperative stability studies (r2 > 0.999). DEX concentrations in various ocular tissue samples i.e., aqueous humor, cornea, iris ciliary body, sclera and retina choroid were found to be 344.0, 1050.07, 529.6, 103.9 and 48.5 ng/mg protein respectively. Absorption of DEX after topical administration from a novel aqueous mixed micellar formulation achieved therapeutic concentration levels in posterior segment of the rabbit eye.
Keywords: Glucorticosteroids, LC-MS/MS, rabbit ocular matrix, validation, dexamethasone, topical administration
1. Introduction
Glucocorticoids (GC) belong to a class of steroid hormones high binding affinities with GC receptor [1]. Such binding trigger analogous effects, which are either mediated slowly via nuclear receptors or rapidly via non-genomically, mediated membrane-associated receptors [2]. GC receptors are found inside the cells of most vertebrate tissues [3]. Cortisol or hydrocortisone (HD) is a naturally occurring GC in humans, which regulates vital metabolic, immunologic, cardiovascular and homeostatic functions. GC exhibit anti-inflammatory properties and inhibit inflammatory response in all stages [4, 5]. GC derivatives are synthetic compounds with pharmacologic properties similar to cortisol and include dexamethasone (DEX), prednisolone (PD), triamcinolone and betamethasone [6, 7]. These compounds are widely recommended in replacement therapy for glucocorticoid deficient patients. Owing to the potential anti-angiogenic, anti-edematous, anti-apoptotic and anti-proliferative effects GC based drugs gained wide use in the treatment of both anterior and posterior ocular segment diseases such as allergic conjunctivitis, herpes zoster keratitis, corneal injury, age-related macular degeneration, proliferative vitreoretinopathy and diabetic macular edema [8].
Drug delivery to retina, choroid and vitreous is virtually important for controlling posterior segment disorders. An ideal drug delivery system should be capable of delivering therapeutic drug concentrations to this target tissues with minimal or no side effect [9]. Currently corticosteroids are administered through local (eye-drop suspensions, ointments, implants and intravitreal injections) and systemic routes (oral and parenteral). Though intravitreal injection directly delivers the drug to vitreous bathing neural retina, it is associated with inherent potential side effects such as retinal detachment, increased intraocular pressure, hemorrhage, endophthalmitis, cataract which limit long term therapy [10]. Topical administration of drugs in the form of eye drops is considered to be the most patient compliant treatment. However, only a small fraction of eye dose reach the posterior segment after topical administration [3].
Quantification of steroids following topical administration in various ocular tissues especially retina requires a rapid, sensitive and robust bioanalytical method. Analysis of GC molecules is somewhat distribute, because of its lipophilic nature. Moreover, these lipophilic compounds are extensively distributed in tissue space resulting in very low biological matrix concentrations. Various analytical methods have been reported in literature to estimate GC concentrations in biological samples. These include high performance liquid chromatography (HPLC) [11, 12] gas chromatography [13, 14], radioimmunoassay (RIA) [15] and capillary separations coupled with various detectors [16, 17]. HPLC technique utilizing ultra-violet (UV) detection suffers from disadvantages such longer retention time, tedious extraction procedure and inadequate limit of quantitation (LOQ) [18]. Though fluorescence and gas chromatography are highly sensitive they require derivatization steps which are time consuming [12]. RIA usually suffers from cross-reactivity and hence resulting in lack of adequate selectivity for GC [15, 20].
Recently, LC-MS/MS has been applied extensively for the quantitative estimation of drugs in various biological matrices such as plasma, serum and urine due to its sensitivity, selectivity and reproducibility. To the best of our knowledge, no validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method has been reported in literature for the quantitative estimation of corticosteroids in ocular matrices and vitreous humor samples.
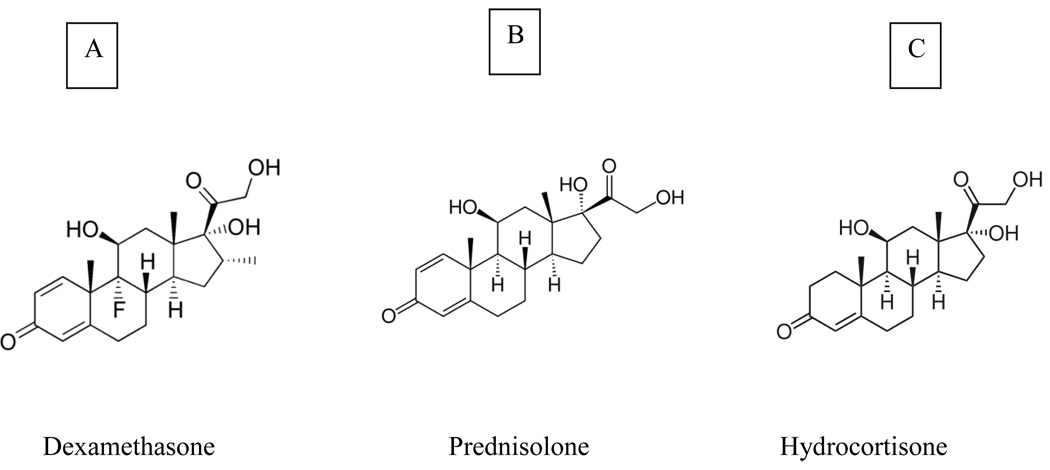
In particular, tandem triple-quadrupole mass spectrometry in the multiple reaction monitoring mode provides uncompromised sensitivity and selectivity [21]. The aim of the present study is to develop and validate a selective, rapid, rugged and reproducible LC-MS/MS assay for the quantitative determination of corticosteroids in ocular tissue matrices. This method is successfully validated and applied to the quantitative determination of DEX in rabbit ocular tissues following topical administration in a novel mixed micellar eye drop formulation. DEX, PD and HD structures are shown in Fig 1.
Fig 1.
Corticosteroid chemical structures [A] DEX, [B] PD and [C] HD with parent molecular weights: 392.464, 360.444, and 362.465 Da respectively
2. Experimental
2.1.Materials
DEX, PD and HD were purchased from Sigma Chemical Co (St. Louis, MO). HPLC grade methanol, acetonitrile, diethyl ether, dichloromethane, isopropyl alcohol and analytical grade formic acid, perchloric acid and potassium chloride were procured from Fisher Scientific (New Brunswick, NJ). Ultrapure water from MilliQ-system (Millipore, Molshecin France) was used through the study. All chemicals were of HPLC grade and used as received without further purification.
2.2.Preparation of stock and standard solutions
DEX, PD and HD stock solutions were prepared at 1 mg/ml in methanol. Calibration curve dilutions (30.88, 24.70, 19.79, 14.83, 9.63, 5.30, 1.59, 0.55 and 0.14µg/mL) and quality control (QC) dilutions (24.70, 14.83, 5.30, 1.59, 0.55 and 0.14 µg/mL) consisting of DEX and PD were prepared in 20% v/v methanol. Stock dilution of internal standard (HD) at 10µg/mL was prepared in 20% v/v methanol in water. All the solutions were stored at −80 °C until further use.
2.3.Liquid-Chromatographic Operating conditions
Chromatographic analysis was carried out on API 2000 triple quadruple linear ion Qtrap mass spectrometer. High performance liquid chromatographic system consists of Agilent 1100 LC Quaternary pump, Agilent 1100 well plate, autosampler (Agilent technologies, Wilmington, DE) with a reversed phase gemini C18 column (50 × 4.6 mm i.d, 5µm, Phenomenex, Torrance, CA). Isocratic mobile phase composed of 30% of acetonitrile in water containing 0.1% of formic acid, was at a flown rate of 0.2 mL/min.
2.4.Mass spectrometer operating conditions
MDS Sciex API 2000 Triple Quadrupole linier QTrap mass spectrometry (Applied Biosystems/MDS Sciex, Faster City, CA) system interfaced by turbo ion spray (TIS) with positive ion source in MRM mode was applied for detection. Ultra high pure nitrogen served as collisionally activated dissociation (CAD) at 4 psi and curtain gas at 20 psi. Nebulizer and turbo gas were optimized at 40 and 50 psi respectively. The TIS temperature was maintained at 200°C, with source voltage and dwell time optimized at 5200V and 400 milliseconds respectively. Mass dependent parameters were tuned and optimized for DEX, PD and HD. Parent and daughter ions obtained by direct infusion mode (10µL/min) was injected with built-in Harvard infusion syringe pump and were optimized.
2.5.Preparation of reference standard solution
Reference standard solution (600ng/mL) was prepared from stock solutions of DEX and PD by adding 25 µL of 10µg/mL IS stock dilution. System performance test was conducted by injecting six replicate injections of reference standard solution every day and validation parameters were checked. Percentage of coefficient of variation (%CV) was calculated before each run and found to be less than 4.0 %.
2.6. Preparation of calibration curve(CC) and quality control(QC) standards
Validation parameters were carried out according to FDA May 2001 guidelines [22]. LLOQ was considered as 2.70 ng/mL for DEX and 11.00 ng/mL for PD. ULOQ was considered to be 617.60 ng/mL for both DEX and PD. Working calibration curve dilutions were spiked at 0.2 % v/v in a homogenized rabbit ocular tissue matrix and ocular fluids to obtain various (617.60, 494.00, 395.20, 296.60, 192.60, 106.00, 31.80, 11.00 and 2.70 ng/mL for DEX) spiked CC standards. High QC (494.00 ng/mL), middle QC (296.60 ng/mL), other middle QC (106.00 ng/mL), low QC (11.00 ng/mL) and LLOQ QC (11.00 ng/mL for PD and 2.70 ng/mL for DEX) standards were also prepared in a similar manner.
2.7. Procedure for ocular matrix and fluid sample preparation and extraction
Homogenized rabbit ocular matrices and fluid samples were thawed at room temperature and vortexed. Using calibrated pipettes, 100 µL of samples were aliquoted into a 1.5 mL poly propylene microcentrifuge tubes (PPMCT), followed by addition of 25 µL of 10.0 µg/mL freshly prepared IS solution to all samples, except for blank. These solutions were vortexed for 30 seconds, to which 50 µL of 20 % (v/v) perchloric acid was added, and again vortexed for 30 seconds to precipitate proteins. The samples were extracted by the addition of 1000 µL of methyl tertiary butyl ether (MTBE) followed by vortexing for approximately 2 min. After centrifugation at 12000 rpm at 4°C for 25 min, 850 µL of organic layer was transferred to a pre-labeled fresh 1.5 mL of poly propylene microcentrifuge tube and evaporated in the Speed Vac® at 35°C for 60 min. The residue was reconstituted with 100 µL of mobile phase, vortexed for 30 seconds and transferred into a pre-labeled HPLC autosampler vial with silanized inserts. A 25 µL of the resulting solution was injected into LC-MS/MS.
2.8.Method Validation tests
2.8.1. Selectivity
Selectivity was performed by analyzing the blank ocular matrix samples (samples prepared according to described in extraction procedure section 1.7) from six different rabbit sources to test for matrix interference with retention times of DEX, PD and IS HD were evaluated with LLOQ concentration. Six samples were injected into LC-MS/MS to determine selectivity for blank. Peak areas of blank interferences should not be more than 20 % of mean peak area of LLOQ of DEX and PD and should not be more than 5 % of mean peak area of HD samples.
2.8.2. Sensitivity and LLOQ evaluation
Sensitivity was achieved by analyzing spiked and processed LLOQ to sample from selected/screened blank matrix. Six LLOQ samples were injected into LC-MS/MS to determine LLOQ sensitivity for DEX and PD.
2.8.3. Precision and accuracy
Method validation was performed according to most recent recommendation outlined by the FDA in May 2001 [22]. Three precision and accuracy batches containing calibration curve standards and six replicates of QC standard samples at four concentration levels were performed in rabbit ocular tissue matrices as well as in normal phosphate buffer samples were extracted and analyzed in three different runs along with one standard zero (blank without IS) and one standard blank (blank with IS) (one used to check the interference contribution at the retention time of analytes).
2.8.4. Within–run and between-run variability
In the case of within-run evaluation a minimum of six QC at each concentration levels were analyzed with one calibration curve standards in one single precision and accuracy batch. For the between-run at least eighteen QC at each concentration level were analyzed in at least three different precision and accuracy batches in three different days.
2.8.5. Ruggedness Batch
One set of calibration curve along with six replicates of QC standard samples at four concentration levels were retrieved from −80 °C freezer, processed and analyzed. The batches were processed according extraction method described in section 1.6) by another analyst or by a different HPLC column (without altering the chemistry and brand of the column).
2.8.6. Bench-top stability
The spiked calibration curve and QC standards samples were stored at −80°C. Low and high QC (n=6) were retrieved from −80°C freezer and these kept at room temperature on bench top for six hrs. The stability samples were processed and extracted along with the freshly spiked calibration curve standards.
2.8.7. Freeze-thaw stability
Low and high QC samples (n=6) were retrieved from −80°C freezer after three freeze-thaw cycles according to the FDA guide lines. The stability samples were processed and extracted along with the freshly spiked calibration curve standards.
2.8.8. Extraction recovery
Extraction recovery of DEX and PD was determined by analysis of six replicates at low and high QC standards samples concentration for DEX and PD and at one concentration for the IS HD. Absolute extraction recovery was determined by comparing peak areas of the analytes obtained from the extracted QC ocular matrix samples to unextracted standards (post spiked). A relative extraction ratios was determined by comparing peak area ratios of the analytes to internal standard obtained from the extracted QC (ocular matrix samples versus unextracted standards).
2.9.Quantitative determination DEX from rabbit ocular tissues
2.9.1. Animals studies
New Zealand albino adult male rabbits, weighed between 2.0 to 2.5 kg, were obtained from Myrtle’s Rabbittry (Thompson Station, TN) and housed in accordance with US Department of Agricultural and Association for Assessment and Accreditation of Laboratory Animal Care international guidelines. All protocols were reviewed and approved by Animal Care and Use Committee of University of Missouri Kansas City. Studies performed were in accordance with the Association for Research in Vision and Ophthalmology Regulations and Standards (ARVORS) guidelines. Rabbits were anesthetized with subcutaneous ketamine (30 mg/kg). Ten minutes after anesthesia, the rabbit’s head was held to keep the left eye horizontal and 50µL of 0.1% dexamethasone a novel mixed micellar formulation (Vitamin E TPGS - 4.5% and Octoxynol-40 – 2.0%) eye drops were instilled using a micropipette at the center of lower cul-de-sac. During the instillation, the lower eyelid was pulled slightly away from the globe and was returned to normal position immediately after instillation. After 60 minutes, rabbits were euthanized using 1 mL of intravenous injection of Fatal- Plus® (Vertech Pharmaceuticals Corporation, Dearborn, Mich) (39% sodium pentobarbital) into the marginal ear vein. Following euthanasia, the eye ball was enucleated immediately (on an average within 150 seconds) and transferred to a beaker containing ice-cold phosphate buffer (pH 7.4). Repetitive washings were carried out in cold phosphate buffer to remove any adsorbed drug on to the surface. Aqueous humor was withdrawn by limbal paracentesis and then vitreous humor was aspirated using a 1 mL tuberculin syringe after making a tiny incision at sclera-limbus junction. The enucleated eyeball was cut open and the following tissues were dissected: cornea, iris-ciliary body (ICB), lens, retina-choroid (RC) and sclera were all collected into pre-weighed vials immediately after euthanasia. After dissection, the tissues were dried with Kim wipes® and weighed. Protein content in the aqueous and vitreous humor was measured by the method of Bradford (Bio-Rad protein estimation kit, Hercules, CA). All tissue samples were stored at −80°C before further analysis.
2.9.2. Preparation of samples
Tissues were homogenized in 500 µL chilled (4°C) phosphate buffer (pH 7.4) for about 4 min with a tissue homogenizer (Tissue Tearor, Model 985-370; Dremel Multipro, Racine, WI) in an ice bath, with the exception of sclera, which required 1.5 ml. One hundred micro liters of aqueous humor (AH) and vitreous humor (VH) were collected for analysis without further processing. Subsequently 100 µL of the tissue homogenates (cornea, iris-ciliary body, lens, retina-choroid and sclera), aqueous humor and vitreous humor were collected for further sample processing.
2.9.3. Dexamethasone ocular tissue sample extraction
Dexamethasone was extracted from ocular tissue homogenates by a simple liquid-liquid extraction as described previously. All tissue samples were analyzed with freshly prepared calibration curve and quality control standards. Calibration curve (2.70– 617.60 ng/mL) and QC standards were prepared by spiking appropriate abounds in seven different blank ocular matrices (control tissues) with varying concentrations of dexamethasone with PD as an IS. All standards and samples were analyzed with LC-MS/MS.
3. Results and Discussion
3.1. Liquid-Chromatographic Operating conditions
The goal of this work was to develop and validate a simple, rapid and sensitive assay method for quantitative determination of glucocorticoids by extraction of ocular tissues. Chromatographic conditions, especially the composition and acidic nature of the mobile phase, were optimized to achieve best resolution. It also increased the signal to mix ratio and to minimized run times. Total run time of the method was 7.0 min at 200 µL/min flow rate and injection volume was 25 µL. Under these conditions the retention times of the chromatographic peaks were 4.27, 3.99 and 4.07 minutes for DEX, PD and HD respectively.
3.2. Mass spectrometer operation condition
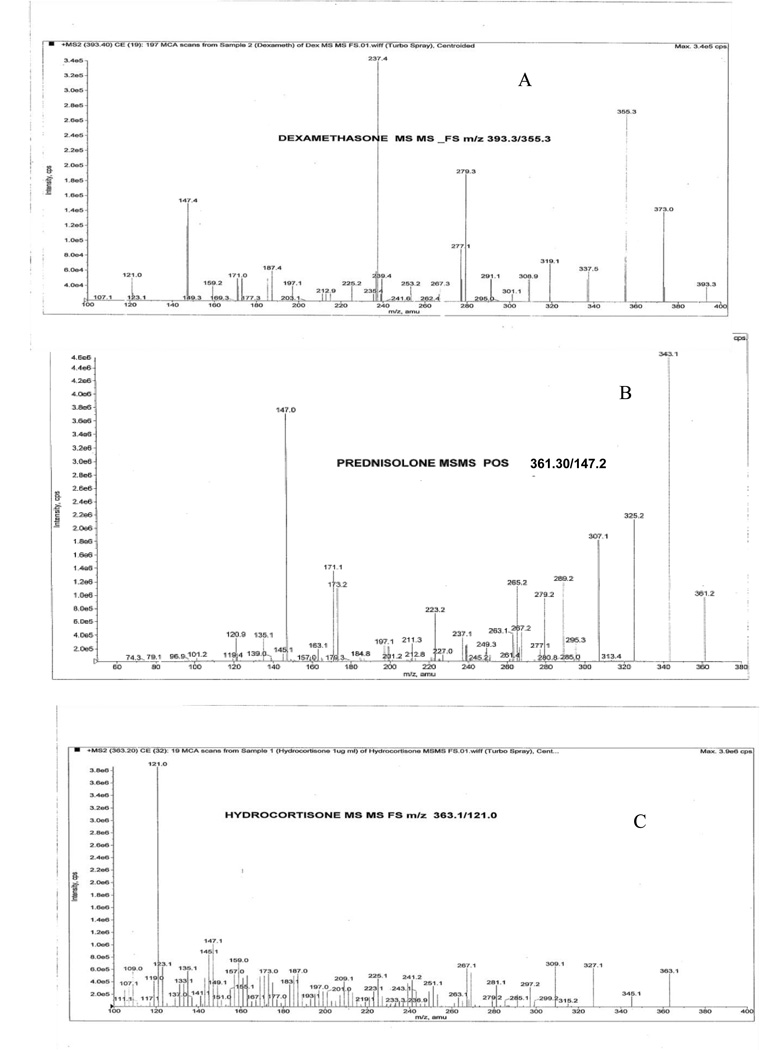
In order to optimize electrospray ionization condition for DEX, PD and HD, full scan mass spectra were acquired in the positive ion mode. During a direct infusion experiment, the mass spectra for DEX, PD and the internal standard HD revealed peaks at mass- to- charge ratio (m/z) 393.20, 361.30 and 363.20 respectively as protonated molecular ions [M+H]+. The most stable abundant fragment ion observed in each product MS/MS spectrum were at m/z 355.30 for DEX, 147.20 for PD and 121.0 for IS. These spectra were achieved by optimizing the collision energies at 18.0 V, 30.0 V and 18.0 V respectively as shown in Fig 2. Quantitative determination was performed in MRM scan positive ion mode with the following mass transitions: 393.20→355.30 for DEX, 361.30→147.20 for PD and 363.20→121.0 for HD as illustrated in Fig 2.
Fig 2.
MS/MS mass spectrum [A] for DEX m/z 393.3→355.3, [B] for PD m/z 361.3→147.2 and [C] for HD 363.1→121.0
3.3. Selectivity
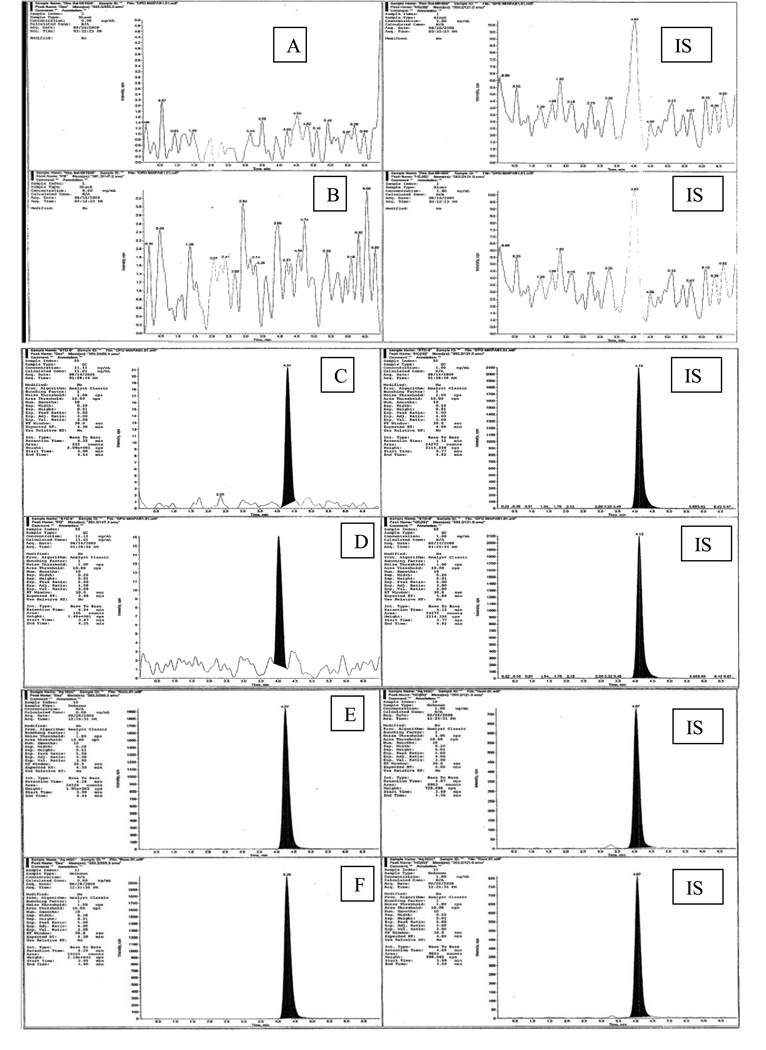
A typical chromatogram from blank (free of analytes and IS) and ocular matrix spiked with DEX and PD at LLOQ and ULOQ concentration levels along with IS are shown in Fig 3. No interfering peaks from blank samples were observed.
Fig 3.
Chromatograms in MRM mode : (A) blank of DEX, (B) blank of PD, (IS ) blank of HD, (C) LLOQ of DEX, (D) LLOQ of PD, (E) ULOQ of DEX, (F) ULOQ of PD and ( IS) of HD
3.4. Sensitivity and LLOQ evaluation
Sensitivity and lower limit of quantitation (LLOQ) for DEX and PD were optimized at 2.70 and 11.00 ng/mL respectively. The mean within and between day precision and accuracy for DEX at 2.70ng/mL and for PD at 11.00 ng/mL are summarized in table 1A.
Table 1.
Precision and accuracy, plasma stabilities of DEX and PD in rabbit ocular matrix
| Table 1 (A and B) : Within batch assay precision and accuracy for DEX and PD CCS (n=2), QC (n=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DEX | PD | ||||||||
| Name | Nominal conc.(ng/mL) |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
| CCS-1 | 617.60 | 624.20 | 115.27 | 18.5 | 101.1 | 610.29 | 10.34 | 1.7 | 98.8 |
| CCS-2 | 494.08 | 499.55 | 29.63 | 5.9 | 101.1 | 490.01 | 5.76 | 1.2 | 99.2 |
| CCS-3 | 395.26 | 428.24 | 7.95 | 1.9 | 108.3 | 404.84 | 13.55 | 3.3 | 102.4 |
| CCS-4 | 296.45 | 303.74 | 28.16 | 9.3 | 102.5 | 295.88 | 0.81 | 0.3 | 99.8 |
| CCS-5 | 192.69 | 176.82 | 6.11 | 3.5 | 91.8 | 185.94 | 9.55 | 5.1 | 96.5 |
| CCS-6 | 105.98 | 99.59 | 2.06 | 2.1 | 94.0 | 103.91 | 2.93 | 2.8 | 98.0 |
| CCS-7 | 31.79 | 35.78 | 0.76 | 2.1 | 112.5 | 34.17 | 3.36 | 9.8 | 107.5 |
| CCS-8 | 11.13 | 11.38 | 1.04 | 9.1 | 102.2 | 10.88 | 0.35 | 3.2 | 97.8 |
| CCS-9 | 2.70 | 3.14 | 0.32 | 10.2 | 116.3 | ||||
| High QC | 494.08 | 530.12 | 10.28 | 1.9 | 107.3 | 515.11 | 15.66 | 3.0 | 104.3 |
| Middle QC | 296.45 | 301.20 | 6.79 | 2.3 | 101.6 | 273.99 | 5.26 | 1.9 | 92.4 |
| Middle1 QC | 105.98 | 109.06 | 3.34 | 3.1 | 102.9 | 98.91 | 0.86 | 0.9 | 93.3 |
| LOQ | 11.13 | 10.59 | 0.54 | 5.1 | 95.1 | 11.01 | 1.17 | 10.6 | 101.1 |
| LLOQ | 2.70 | 3.20 | 0.42 | 13.3 | 118.5 | ||||
| Table 1 (C) : Between batch assay precision and accuracy for DEX and PD CCS (n=2), QC (n=18) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DEX | PD | ||||||||
| Name | Nominal conc.(ng/mL) |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
| CCS-1 | 617.60 | 596.65 | 45.70 | 7.7 | 96.6 | 572.67 | 11.79 | 2.1 | 92.7 |
| CCS-2 | 494.08 | 450.59 | 30.75 | 6.8 | 91.2 | 432.29 | 4.86 | 1.1 | 87.5 |
| CCS-3 | 395.26 | 422.25 | 11.05 | 2.6 | 106.8 | 427.34 | 3.85 | 0.9 | 108.1 |
| CCS-4 | 296.45 | 313.61 | 33.98 | 10.8 | 105.8 | 281.96 | 10.78 | 3.8 | 95.1 |
| CCS-5 | 192.69 | 206.98 | 8.45 | 4.1 | 107.4 | 210.01 | 4.16 | 2.0 | 109.0 |
| CCS-6 | 105.98 | 103.38 | 14.35 | 13.9 | 97.5 | 115.21 | 2.39 | 2.1 | 108.7 |
| CCS-7 | 31.79 | 31.58 | 5.85 | 18.5 | 99.3 | 35.76 | 0.06 | 0.2 | 112.5 |
| CCS-8 | 11.13 | 11.15 | 0.79 | 7.1 | 100.2 | 11.50 | 1.28 | 11.1 | 103.3 |
| CCS-9 | 2.70 | 3.06 | 0.35 | 11.3 | 113.2 | ||||
| High QC | 494.08 | 530.12 | 10.27 | 1.9 | 107.3 | 441.82 | 32.41 | 7.3 | 98.3 |
| Middle QC | 296.45 | 301.19 | 6.79 | 2.3 | 101.5 | 284.82 | 25.76 | 9.0 | 96.1 |
| Middle1 QC | 105.98 | 109.06 | 3.15 | 3.1 | 102.9 | 103.98 | 5.79 | 5.6 | 98.1 |
| LOQ | 11.13 | 10.59 | 0.54 | 5.1 | 96.3 | 11.63 | 1.24 | 10.7 | 104.5 |
| LLOQ | 2.70 | 3.22 | 0.11 | 3.5 | 119.3 | ||||
| Table 1 (D and E ) : Matrix stability assay precision and accuracy for DEX and PD CCS (n=2), bench top QC ( n=6), freeze and thaw QC (n=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DEX | PD | ||||||||
| Name | Nominal conc.(ng/mL) |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
Mean conc.(ng/mL) |
SD (±) |
% CV |
% Mean accuracy |
| CCS-1 | 617.60 | 616.77 | 6.55 | 1.1 | 99.9 | 596.87 | 46.02 | 7.7 | 96.6 |
| CCS-2 | 494.08 | 560.58 | 132.81 | 23.7 | 113.5 | 439.72 | 15.37 | 3.5 | 89.0 |
| CCS-3 | 395.26 | 441.82 | 51.09 | 11.6 | 111.8 | 419.24 | 15.30 | 3.6 | 106.1 |
| CCS-4 | 296.45 | 334.28 | 23.21 | 6.9 | 112.8 | 298.85 | 13.10 | 4.4 | 100.8 |
| CCS-5 | 192.69 | 174.53 | 24.59 | 14.1 | 90.6 | 201.63 | 16.02 | 7.9 | 104.6 |
| CCS-6 | 105.98 | 108.21 | 1.29 | 1.2 | 102.1 | 114.79 | 1.80 | 1.6 | 108.3 |
| CCS-7 | 31.79 | 33.94 | 5.78 | 17.0 | 106.7 | 32.44 | 4.63 | 14.3 | 102.0 |
| CCS-8 | 11.13 | 11.52 | 0.27 | 2.3 | 103.5 | 10.44 | 0.93 | 8.9 | 93.8 |
| CCS-9 | 2.70 | 3.15 | 0.64 | 20.2 | 116. 7 | ||||
| Table 1 (D) : Bench top stability samples precision and accuracy for DEX and PD QC (n=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| High QC | 494.08 | 457.63 | 32.96 | 7.2 | 92.6 | 462.17 | 25.15 | 5.4 | 93.5 |
| Middle QC | 31.79 | 29.14 | 1.62 | 5.5 | 91.7 | 33.46 | 2.91 | 8.7 | 105.3 |
| Table 1 (E) : Freeze and thaw stability samples precision and accuracy for DEX and PD QC (n=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| High QC | 494.08 | 499.33 | 44.59 | 8.9 | 101.1 | 433.61 | 36.36 | 8.4 | 87.8 |
| Middle QC | 31.79 | 32.19 | 2.67 | 8.1 | 101.2 | 32.19 | 3.53 | 11.0 | 101.3 |
CCS : Calibration curve standard, QC : Quality control standard, % CV : Coefficient of variation SD: Standard deviation
3.5. Precision and accuracy
Precision and accuracy batches were determined by analysis of QC samples. Within the batch assay, precision and accuracy are evaluated by multiple analyses (n=6) of the five QC samples (2.70, 11.00, 106.00, 296.60 and 494.00 ng/mL) during a validation run and presented in Table 1(A and B). Percent CV for DEX (10.2 – 13.3 %) and PD (3.2–10.6 %) was observed at LLOQ of 2.70 ng/mL and 11.00 ng/mL respectively. Linearity of DEX and PD was obtained over the concentration range of 2.70 −617.60 and 11.00–617.60 ng/mL respectively in a rabbit ocular tissue matrix, serum and isotonic phosphate buffer solution (pH=7.4). Linear regression, “no weighting” mean linear correlation coefficient (r) were calculated to be 0.9990 and 0.9989 for PD and DEX respectively. Regression equation (y) = 0.000543x + 0.0019 for DEX where as slope (m) is 0.000543, intercept(c) is 0.0019 and regression equation (y) = 0.000251x + 0.0000706 for PD where as slope (m) is 0.000251, intercept(c) is 0.0000706. Regression equation (y) = m x + c, where y- axis is the area ratio of analyte area and IS area, x –axis is the concentration ratio of analyte concentration and IS concentration, m is the slope of the linear regression line and c is the intercept point of the linear regression line and the y- axis. Between day assay precision and accuracy evaluated by multiple analyses of the QC samples during each validation run are presented in table 1 A, B and C. The precision was within 11.3 % for DEX and 11.1 % for PD at LLOQ.
3.6. Stability of analytes in ocular matrix
Stability of stored QC ocular matrix samples was determined with freshly spiked calibration curve standards. Freeze-thaw stability studies were carried out with low and high QC in replicates of 6 for both DEX and PD. In each freeze-thaw cycle samples were frozen for at least 24 hrs before thawing at room temperature. For bench top stability studies the QC samples (n=6) were retrieved from −80 °C storage and kept at room temperature for 6 hrs before processing. Stability assessments of DEX and PD under various anticipated conditions were revealed. Preclinical samples are received, stored and processed as presented in table 1E with low (31.80 ng/mL) and high QC (494.00 ng/mL) concentrations for both analytes. Both DEX and PD appears to be stable through multiple freezing and thawing cycles. DEX appears to be less degradation than PD through multiple freezing and thawing cycles. Bench top stability studies are stable for 6 hrs at room temperature. Results from these studies are summarized in table 1D. No significant degradation of DEX and PD were observed in any of the sample preparation.
3.7. Extraction recoveries
Recovery of corticosteroids from ocular matrix by protein precipitation followed by liquid-liquid extraction procedure resulted in clean samples with high extraction efficiency. We have investigated various sample extraction methods including protein precipitation and liquid-liquid extraction with different organic solvents (acetonitrile, methanol, isopropyl alcohol, perchloric acid, ethyl acetate, methyl (t)-butyl ether, dichloromethane, hexane and diethyl ether). Perchloric acid and isopropyl alcohol efficiently precipitated proteins from the serum and vitreous fluid. Addition of saturated potassium chloride solution further improved accuracy but reduces recovery of analytes. The above sample preparation and extraction method produced no significance endogenous chromatographic peak interference from rabbit vitreous and plasma. The extraction recoveries of the two analytes were: DEX 86.9 % at LQC and 91.6 % at HQC, PD 86.4 % at LQC and 79.1 at HQC. Extraction recovery (%) expressed as the ratio of the mean peak area of the analytes spiked into ocular matrix before extraction to the mean peak area of the analytes spiked into ocular matrix after extraction multiplied by 100. Post spiked samples are treated as 100 % recovery samples. These samples are bypass to a various matrices constituent interferences during the extraction process except ion suppression. Usually ion suppression is occurred after extraction. Ocular matrices are clean and less protein and other constituents than human/animal serum/plasma matrices. Therefore high recovery of analytes is obtained from ocular matrices.
3.8. Quantitative determination DEX from rabbit ocular tissues
Various concentrations (30.88, 24.70, 19.79, 14.83, 9.63, 5.30, 1.59, 0.55 and 0.14µg/mL) were prepared freshly after drug added solution ( 0.2 % v/v) to seven different ocular tissue matrices in order to obtain various (617.60, 494.00, 395.20, 296.60, 192.60, 106.00, 31.80, 11.00 and 2.70 ng/mL) calibration curve standards. High QC (494.00 ng/mL), middle QC (296.60 ng/mL), middleO QC (106.00 ng/mL), low QC (11.00 ng/mL) and LLOQ QC (11.00 ng/mL for PD and 2.70 ng/mL for DEX) standards were also prepared in a similar manner. The sample volume injected was 25 µl and run time was 7 min. MRM positive ion mode was utilized for quantitative estimation. Lower limit of quantification was found to be 2.7ng/ml. Reproducibility of back-calculated DEX and PD concentrations in calibrators was within the Food and Drug Administration (FDA) bioanalytical guide line limits [17]. The precision and accuracy of the quantitative results were within validation limits (mean RSD 6.5 %, mean accuracy 9.0 %); the peak areas were also reproducible (RSD 4.67%). Quantitative determination of DEX in different ocular tissues after topical administration is shown in Table 2. Sixty minutes after topical application to a rabbit eye, DEX concentration in the anterior (cornea, aqueous humor and lens) and posterior segments (sclera, vitreous humor, and retina choroid and iris ciliary bodies) was determined. DEX concentrations of 1415.01, 1459.60, 652.73 and 675.58 ng/gm (four times dilution factor was applied to those cornea samples, values are 353.75 × 4, 364.90 × 4, 163.18 × 4 and 168.90 × 4 ng/gm respectively) of tissue and 464.80, 401.05, 314.94 and 195.13 ng/mg protein were quantified in the cornea and aqueous humor respectively. For the posterior segment, of DEX concentrations in the sclera, retina choroid and iris ciliary bodies were observed to be 180.20, 77.49 and 54.06 ng/gm of tissue, and 27.36, 80.15, 50.29 and 36.27 ng/gm of tissue, and 922.20, 630.27 ng/gm (two times dilution factor is applied values are 461.10 × 2 and 315.14 × 2 ng/gm), 265.98 and 299.76 ng/gm of tissue respectively. DEX concentration in the lens and vitreous humor were below LLOQ (LLOQ = 2.7 ng/mL). Mean concentration and standard deviations of DEX in various ocular tissue matrices are summarized in Table 2. A novel 0.1% mixed micellar formulation of DEX showed high concentration levels in back of the eye (retina-choroid = 49 ng/gm of tissue).
Table 2.
Dexamethasone drug levels in ocular tissues from 0.1% mixed micellar formulation
| Average back-calculated calibration curve standards for DEX (n=7) | |||||
|---|---|---|---|---|---|
| Name | Nominal conc.(ng/mL) |
Mean conc.(ng/mL) |
SD (±) |
% CV | % Mean accuracy |
| CCS-1 | 617.60 | 593.15 | 9.69 | 1.6 | 96.0 |
| CCS-2 | 494.08 | 460.95 | 20.38 | 4.4 | 93.3 |
| CCS-3 | 395.26 | 402.59 | 25.26 | 6.3 | 101.9 |
| CCS-4 | 296.45 | 285.68 | 5.64 | 2.0 | 96.3 |
| CCS-5 | 192.69 | 181.27 | 7.85 | 3.3 | 94.1 |
| CCS-6 | 105.98 | 103.51 | 5.22 | 5.0 | 97.7 |
| CCS-7 | 31.79 | 34.41 | 5.09 | 14.8 | 108.2 |
| CCS-8 | 11.13 | 11.07 | 0.65 | 5.9 | 100.6 |
| CCS-9 | 2.70 | 2.71 | 0.30 | 11.2 | 100.4 |
| DEX drug levels in ocular tissues from 0.1% mixed micellar formulation after topical administration | ||
|---|---|---|
| Name of rabbit ocular tissue |
Mean amount of DEX conc. ng/ gm tissue or gm protein |
SD(±) |
| Retina Choroid | 49 | 23.11 |
| Aqueous Humor | 344 | 116.69 |
| Iris Ciliary Muscle | 530* | 309.08 |
| Sclera | 104 | 67.09 |
| Cornea | 1051* | 446.84 |
| Lens | BLOQ | |
| Vitreous Humor | BLOQ | |
BLOQ : Below limit of quantification; LOQ : Limit of quantification ( 2.70 ng/mL)
Note: Applied dilution factors for cornea (four times) and iris ciliary muscle (two times) samples
4. Conclusion
A sensitive and selective LC-MS/MS method for the determination of DEX and PD in rabbit ocular matrix was developed and validated. Total run time for the method was 7 minutes. Ion suppression was not a significant occurrence for both analytes and internal standard. Protein precipitation followed by liquid- liquid extraction was a useful method development process to avoid matrix ionization effects. Quantitative measurements of samples resulted in consistent values with high accuracy. The peak area response of DEX and PD remained unchanged upon repeated injections which show the robustness of this analytical method. This method was used successfully for the quantitative determination of DEX for the posterior and anterior segments of the eye tissues and PD. A novel 0.1% mixed micellar formulation successfully delivered DEX to the posterior segment of the eye especially for retina choroid. This method may also be applicable as ocular microdialysis analysis technique to deliverse pharmacokinetic profiles of glucocorticoids in anterior and posterior segments of the eye.
Acknowledgements
This research was supported by the National Institutes of Health grants R01 EY 09171-12 and R01 EY 10659-10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cato AC, Wade E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. BioEssays. 1996;18(5):371–378. doi: 10.1002/bies.950180507. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H. [Molecular mechanism of glucocorticoid action] Ryumachi. 2002;42(1):13–22. [PubMed] [Google Scholar]
- 3.Vazzana M, Vizzini A, Salerno G, Di Bella ML, Celi M, Parrinello N. Expression of a glucocorticoid receptor (DlGR1) in several tissues of the teleost fish Dicentrarchus labrax. Tissue & Cell. 2008;40(2):89–94. doi: 10.1016/j.tice.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. (Lond) 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandt J, Luhder F, McPherson KG, de Graaf KL, Tischner D, Wiehr S, Herrmann T, Weissert R, Gold R, Reichardt HM. Enhanced glucocorticoid receptor signaling in T cells impacts thymocyte apoptosis and adaptive immune responses. Am. J. Pathology. 2007;170(3):1041–1053. doi: 10.2353/ajpath.2007.060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr. Opin Ophthalmol. 2004;15(3):211–220. doi: 10.1097/01.icu.0000120711.35941.76. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J, Ingber DE. Angiostatic steroids. Method of discovery and mechanism of action. Ann. Surg. 1987;206(3):374–383. doi: 10.1097/00000658-198709000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv. Ophthalmol. 2008;53(2):139–149. doi: 10.1016/j.survophthal.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert. Opin. Drug Deliv. 2007;4(4):371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 10.Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm. Res. 2009;26(5):1197–1216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibata N, Hayakawa T, Takada K, Hoshino N, Minouchi T, Yamaji A. Simultaneous determination of glucocorticoids in plasma or urine by high-performance liquid chromatography with precolumn fluorimetric derivatization by 9-anthroyl nitrile. J. Chromatogr B. Biomed. Sci. Appl. 1998;706(2):191–199. doi: 10.1016/s0378-4347(97)00557-4. [DOI] [PubMed] [Google Scholar]
- 12.Glowka FK, Karazniewicz M, Lipnicka E. RP-HPLC method with fluorescence detection for determination of small quantities of triamcinolone in plasma in presence of endogenous steroids after derivatization with 9-anthroyl nitrile; pharmacokinetic studies. J. ChromatogrB. Anal. Technol. Biomed. Life Sci. 2006;839(1–2):54–61. doi: 10.1016/j.jchromb.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Shibasaki H, Nakayama H, Furuta T, Kasuya Y, Tsuchiya M, Soejima A, Yamada A, Nagasawa T. Simultaneous determination of prednisolone, prednisone, cortisol, and cortisone in plasma by GC-MS: estimating unbound prednisolone concentration in patients with nephrotic syndrome during oral prednisolone therapy. J. Chromatogr B. Anal. Technol. Biomed. Life Sci. 2008;870(2):164–169. doi: 10.1016/j.jchromb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.N'Gankam V, Uehlinger D, Dick B, Frey BM, Frey FJ. Increased cortisol metabolites and reduced activity of 11beta-hydroxysteroid dehydrogenase in patients on hemodialysis. Kidney Int. 2002;61(5):1859–1866. doi: 10.1046/j.1523-1755.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Tunn S, Pappert G, Willnow P, Krieg M. Multicentre evaluation of an enzyme-immunoassay for cortisol determination. J. Clin. Chem. Clin. Biochem. 1990;28(12):929–935. doi: 10.1515/cclm.1990.28.12.929. [DOI] [PubMed] [Google Scholar]
- 16.Noe S, Bohler J, Keller E, Frahm AW. Determination of prednisolone in serum: method development using solid-phase extraction and micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 1998;18(3):471–476. doi: 10.1016/s0731-7085(98)00035-1. [DOI] [PubMed] [Google Scholar]
- 17.Lemus Gallego JM, Perez Arroyo J. Determination of prednisolone and the most important associated compounds in ocular and cutaneous pharmaceutical preparations by micellar electrokinetic capillary chromatography. J. Chromatogr B. 2003;784(1):39–47. doi: 10.1016/s1570-0232(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 18.Frerichs VA, Tornatore KM. Determination of the glucocorticoids prednisone, prednisolone, dexamethasone, and cortisol in human serum using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr B. 2004;802(2):329–338. doi: 10.1016/j.jchromb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Rivero-Marabe JJ, Maynar-Marino JI, Garcia-de-Tiedra MP, Galan-Martin AM, Caballero-Loscos MJ, Maynar-Marino M. Determination of natural corticosteroids in urine samples from sportsmen. J. Chromatogr B. Biomed. Sci. Appl. 2001;761(1):77–84. doi: 10.1016/s0378-4347(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 20.Ionita IA, Fast DM, Akhlaghi F. Development of a sensitive and selective method for the quantitative analysis of cortisol, cortisone, prednisolone and prednisone in human plasma. J. ChromatogrB. Anal. Technol. Biomed. Life Sci. 2009;877(8–9):765–772. doi: 10.1016/j.jchromb.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Ismaiel OA, Halquist MS, Elmamly MY, Shalaby A, Karnes HT. Monitoring phospholipids for assessment of matrix effects in a liquid chromatography-tandem mass spectrometry method for hydrocodone and pseudoephedrine in human plasma. J. Chromatogr B. 2007;859(1):84–93. doi: 10.1016/j.jchromb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, Food and Drug Administration (FDA) [last accessed May 2001];Center for Drug Evaluation and Research (CDER), Guidance for Industry, Bioanalytical Method Validation.