Abstract
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset neurodegenerative disorder characterized by selective loss of motor neurons (MNs). Twenty percent of familial ALS cases are associated with mutations in Cu2+/Zn2+ superoxide dismutase (SOD1). To specifically understand the cellular mechanisms underlying mutant SOD1 toxicity we have established an in vitro model of ALS using rat primary MN cultures transfected with an adenoviral vector encoding a mutant SOD1, G93A-SOD1. Transfected cells undergo axonal degeneration and alterations in biochemical responses characteristic of cell death such as activation of caspase-3. Vascular endothelial growth factor (VEGF) is an angiogenic and neuroprotective growth factor that can increase axonal outgrowth, block neuronal apoptosis and promote neurogenesis. Decreased VEGF gene expression in mice results in a phenotype similar to that seen in patients with ALS, thus linking loss of VEGF to the pathogenesis of MN degeneration. Decreased neurotrophic signals prior to and during disease progression may increase MN susceptibility to mutant SOD1-induced toxicity. In this study we demonstrate a decrease in VEGF and VEGFR2 levels in the spinal cord of G93A-SOD1 ALS mice. Furthermore, in isolated MN cultures, VEGF alleviates the effects of G93A-SOD1 toxicity and neuroprotection involves phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling. Overall, these studies validate the usefulness of VEGF as a potential therapeutic factor for the treatment of ALS and give valuable insight into the responsible signaling pathways and mechanisms involved.
Keywords: Amyotrophic lateral sclerosis, Neuroprotection, Mechanism, Phosphatidylinositol 3-kinase/protein kinase B, Vascular endothelial growth factor
INTRODUCTION
ALS is an adult-onset neurodegenerative disorder characterized by progressive degeneration of upper and lower motor neurons (MNs) resulting in weakness, paralysis and subsequent death. It has an incidence of approximately 3–5 cases per 100,000 in the United States and consists of both sporadic and familial forms which are clinically and pathologically indistinguishable. Familial ALS accounts for approximately 10% of all cases, one fifth of which are associated with mutations in the Cu2+/Zn2+ superoxide dismutase (SOD1) gene, with G93A-SOD1 being the best characterized mutation (Rosen et al., 1993). Mutations in SOD1 result in a toxic gain of unknown function (Valentine et al., 2005).
Vascular endothelial growth factor (VEGF), first characterized as an angiogenic factor, is known to have neurotrophic effects in the nervous system. VEGF can increase axonal outgrowth (Sondell et al., 2000; Rosenstein et al., 2003), block neuronal apoptosis (Matsuzaki et al., 2001; Li et al., 2003; Sun et al., 2003) and promote neurogenesis (Jin et al., 2002; Zhu et al., 2003). Within the nervous system there are 5 different isoforms of VEGF (also called VEGF-A). The most commonly studied isoform of VEGF is VEGF165, however the full biological array of VEGF isoforms may offer increased efficacy (Carmeliet, 2003; Whitlock et al., 2004; Amano et al., 2005). VEGF receptor-2 (VEGFR2) stimulation leads to the activation of multiple signaling pathways including the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and p44/42 mitogen activated protein kinase (p44/42 MAPK) pathways (Matsuzaki et al., 2001).
VEGF was first linked to ALS pathogenesis in 2001 by Carmeliet and colleagues (Oosthuyse et al., 2001). In the study, mice lacking the hypoxia response element in the VEGF promoter developed adult-onset muscle weakness and neurodegeneration identical to that of ALS. Further studies by the same group later identified 2 VEGF haplotypes associated with increased risk for developing ALS when VEGF alleles from 600 individuals with ALS and 1,000 controls were sequenced (Lambrechts et al., 2003). Since that time, numerous studies have validated the link between VEGF and ALS and reports have begun to examine VEGF as a potential therapy for ALS. In spinal cord cultures, VEGF treatment protects against glutamate-induced MN death (Tolosa et al., 2008). Retrograde delivery of VEGF165 to MNs by gene therapy increases survival of G93A-SOD1 transgenic mice (Azzouz et al., 2004), and the G93A-SOD1 transgenic rat model has also shown evidence of increased survival in response to treatment with intracerebroventricular delivery of VEGF165 (Storkebaum et al., 2005).
While in vitro and in vivo studies have examined the neuroprotective potential of VEGF, the mechanisms of VEGF neuroprotection against mutant SOD1 toxicity have not been established. This study utilizes primary MN cultures transfected with G93A-SOD1 to evaluate the effects of VEGF on mutant SOD1 toxicity. G93A-SOD1 transfection of primary MNs results in biochemical and morphological defects representative of MN degeneration in ALS. We demonstrate that VEGF attenuates G93A-SOD1-induced caspase-3 cleavage and DNA fragmentation, which is mediated by activation of PI3K/Akt signaling via VEGFR2. This study proposes that VEGF activation of PI3K/Akt signaling constitutes a conserved mechanism that can intervene against cellular insults in ALS and ameliorate disease progression.
METHODS
Animal care
All animals used for these studies were housed in a pathogen-free environment, and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines. Animal care standards described in the National Institute of Health Guide for Care and Use of Laboratory Animals are adhered to in order to ensure limited discomfort and distress.
Isolation of mouse spinal cords
End-stage G93A-SOD1 transgenic mice and age-matched controls were euthanized at 120 d of age. Whole spinal cords were collected and fresh tissue was frozen for RNA extraction or mounted in Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Sakura, Tokyo, Japan) prior to sectioning. Embedded spinal cords were mounted and 18 µm sections were cut using a Leica CM 1850 cryostat onto glass slides.
Primary motor neuron culture
All culture reagents were obtained from Sigma (St. Louis, MO) unless otherwise noted. The primary MN treatment media includes Neurobasal Medium (Gibco BRL, Invitrogen, Carlsbad, CA) with 2.5 mg/ml albumin, 2.5 µg/ml catalase, 2.5 µg/ml SOD, 0.01 mg/ml transferrin, 15 µg/ml galactose, 6.3 ng/ml progesterone, 16 µg/ml putrescine, 4 ng/ml selenium, 3 ng/ml β-estradiol, 4 ng/ml hydrocortisone and 1X penicillin/streptomycin/neomycin (Gibco BRL). For initial plating, 1X B-27 additives (Gibco BRL) and 2 µM l-glutamine are also included. Growth media contains all the components of the culture media plus 1X B-27 additives.
Primary MNs were isolated according to our previously published protocol (Vincent et al., 2004). Briefly, spinal cords from E15 Sprague-Dawley rat embryos were collected, perineural membranes were removed, and the tissue was chopped into 2–3 mm pieces. Cells were dissociated by incubating the tissue in 0.5% trypsin/EDTA for 10 min at 37°C followed by trituration with a serum-coated glass pipette for 1 min. The resulting cell suspension was layered on a 5.4% Optiprep solution in Leibowitz’s L-15 media (Gibco BRL) and centrifuged for 15 min at 2,000 × g. The MN were collected from the top layer, washed in fresh L-15 media and resuspended in culture media. MN density was adjusted to between 2 × 104 and 2 × 106 MN/ml and 50 µl of the suspension was applied to poly-l-lysine-coated glass coverslips in a 24-well plate or 1 ml of the suspension was applied to poly-l-lysine-coated 6-well plates. After 24 h, the MNs on coverslips were fed with 250 µl growth media and the MNs in 6-well plates were fed with 2 ml growth media.
MN treatment
All MNs spent 3 d in culture prior to treatment. Three adenoviruses, Ad5CMV humanSOD1-G93Amutant (AdmSOD), AdCMV humanSOD1 wildtype (AdwtSOD1) and control Ad5CMVeGFP (AdGFP) were purchased from the Gene Transfer Vector Core at the University of Iowa (Iowa City, IA). Viral transfection of primary MNs was performed as previously described (Vincent et al., 2004). Briefly, virus (0–1000 plaque-forming units (pfu) per MN) was added after 2 d in culture and MNs were incubated for 24 h prior to harvesting cell lysates or fixing for immunostaining or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) labeling. For all VEGF experiments, MN were changed to treatment media 4 h prior to addition of VEGF165 (Sigma). LY294002, U0126 and SU1498 inhibitors (Calbiochem, La Jolla, CA) were added 1 h prior to VEGF at a concentration of 20µM.
Immunostaining
Immunostaining was carried out by standard protocols as previously described (Kim et al., 1997). Briefly, for spinal cords, sections were permeabilized with 0.1% Triton/PBS and then blocked in 5% normal goat serum/0.1% Triton/PBS. VEGFR1, VEGFR2 and VEGFR3 primary antibodies (Cell Signaling, Danvers, MA) were incubated overnight. Sections were then incubated in Alexafluor-594-conjugated secondary antibody (Molecular Probes, Invitrogen, Carlsbad, CA) followed by mounting in ProLong Gold (Molecular Probes, Invitrogen). Images were collected using an Olympus BX-51 microscope. For cultured primary MNs, cells were grown and treated on glass coverslips followed by fixation in 4% PFA for 5 min. Immunostaining was performed as described above for spinal cord sections. Primary antibodies used include phospho-VEGFR2-Y951 (Cell Signaling), phospho-VEGFR2-Y1175 (Cell Signaling), VEGFR2 (Cell Signaling) and ChAT (Calbiochem).
RNA isolation and PCR
Total RNA was isolated from G93A-SOD1 transgenic and age-matched control mouse spinal cords using the Qiagen (Valencia, CA) RNeasy kit according to the manufacturer’s instructions. cDNA was obtained by reverse transcription using random primers followed by PCR using Promega (Madison, WI) PCR MasterMix and the appropriate primer pairs for VEGF family ligands, VEGFRs and actin. Forward and reverse primers were obtained from Integrated DNA Technologies, Inc. (Coralville, IA) for the following sequences: VEGF-A-forward 5’-ATGCGGATCAAACCTCACCAAAGC-3’; VEGF-A-rev 5’-TTCGTTTAACTCAAGCTGCCTCGC-3’; VEGF-B-forward 5’-AATGCAGATCCTCATGATCC-3’; VEGF-B-rev 5’-TCTGGCTTCACAGCACTCTC-3’; VEGF-C-forward 5’-AACGTGTCAAGAAATCAGCC-3’; VEGF-C-rev 5’-AGTCCTCTCCCGCAGTAATCC-3’; PlGF-forward 5’-ACAGAAGTGGAAGTGGTG-3’; PlGF-rev 5’-GGCTAATAAATAGAGGGTAGG-3’; VEGFR1-forward 5’-GAAGCGGTTCACCTGGACTGAGACC-3’; VEGFR1-rev 5’-GGCTTTGCTGGGGGGATTTCTCTAA-3’; VEGFR2-forward 5’-TGGGAAACCTCCTGCAAGCAAATG-3’; VEGFR2-rev 5’-TTCTGATGCAAGGACCATCCCACT-3’; VEGFR3-forward 5’-CAGACAGACAGCGGGATGGTGC-3’; VEGFR3-rev 5’-AGGCTGTAGTGGGGGTGGGACA-3’; actin-forward 5’-TGAGAGGGAAATCGTGCGTGACAT-3’; actin-rev 5’-ACTCCTGCTTGCTGATCCACATCT-3’. Results of at least 4 independent experiments were analyzed by densitometry using Quantity One 1-D Analysis software (BioRad Laboratories, Inc.) and normalized to actin.
Western blotting
Western blotting was performed as previously described (Vincent et al., 2004). MN lysates were prepared by scraping cells in RIPA buffer (20 mM Tris, pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1 mM Na deoxycholate, 1% Triton X-100, 0.1 trypsin units/µl aprotinin, 10 mg/ml leupeptin and 50 mg/ml PMSF. Equal amounts of protein were loaded in each lane of a polyacrylamide gel, with gel percentages (either 7.5% or 12.5%) dependent on the size of the protein of interest. Nitrocellulose membranes were incubated with primary antibody overnight at 4°C, and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. Primary antibodies used are from Cell Signaling unless otherwise indicated: phospho-p44/42 (Thr202/Tyr204) MAPK; phospho-Akt (Ser473); cleaved caspase-3 (Asp175); GAPDH (Chemicon, Temecula, CA); Anti-human SOD1 (Medical & Biological Laboratories Co., Nagoya, Japan); GFP. Antibody binding was developed with LumiGLO Reagent and Peroxide (Cell Signaling) and exposed to Kodak BioMax XAR film (Sigma). Each experimental paradigm was tested on three separate occasions using different cultures or tissue samples. Membranes were reprobed for GAPDH to confirm equal protein loading and densitometry results were normalized to GAPDH.
TUNEL
MNs were grown and treated on glass coverslips followed by fixation in 4% PFA for 5 min. TUNEL was used to detect DNA fragmentation as an indication of apoptotic death. MN plating densities were equal between treatment conditions and were adjusted to 50 MN/mm2. TUNEL analysis included blinded counting of at least 10 representative fields per condition in at least 3 independent experiments per our published protocol for an average total of approximately 300 MNs per condition (Russell et al., 2002; Sullivan et al., 2007). Samples were labeled with digoxygenin-dUTP and then stained with horseradish peroxidase-conjugated anti-digoxygenin antibody using the ApopTag Plus In Situ Apoptosis Peroxidase Detection Kit (Chemicon). Alternatively, for detection of fluorescent TUNEL signal, MNs were grown and treated in 96-well culture plates. After fixation, TUNEL processing was carried out as described above with a Fluorecein-labeled conjugate using the ApopTag Plus In Situ Apoptosis Fluorescein Detection Kit (Chemicon). Fluorescent signal was detected and recorded using a Fluoroskan Ascent FC mircoplate reader (Labsystems, Helsinki, Finland) (Vincent et al., 2007).
Statistical analysis
Results are representative of at least 3 independent experiments. Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparison test to compare individual data points using Prism software version 3.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
VEGF and VEGFRs in the spinal cord
To confirm the potential of VEGF to affect MNs in ALS, MNs from G93A-SOD1 and wildtype mice were first analyzed for the expression of VEGFRs. Spinal cord tissue collected from endstage G93A-SOD1 transgenic mice at 120 d of age (Fig. 1B,D,F) and age-matched control mice (Fig. 1A,C,E) were immunostained for VEGFR1, VEGFR2 and VEGFR3. VEGFR expression is evident in ventral horn MNs in control mice and is also evident in G93A-SOD1 transgenic mice, despite the decreased number of MNs present. To quantify the relative levels of VEGF family ligands and VEGFR expression, total RNA was isolated from spinal cords and analyzed by PCR (Fig. 1G). Equal levels of total RNA from spinal cords of transgenic and control mice was confirmed by PCR for actin (data not shown). There is approximately a 50% reduction in the levels of both VEGF-A and VEGFR2 in G93A-SOD1 transgenic mouse spinal cords compared to control spinal cords following quantification by densitometry. PCR results also confirm that expression of other VEGF family ligands and VEGFRs did not demonstrate a compensatory upregulation to the decreased levels of VEGF-A and VEGFR2. These data indicate a decrease in VEGF and VEGFR2 levels in G93A-SOD1 transgenic mice and confirm the expression of VEGFRs on ventral horn MNs in ALS mice.
Figure 1. VEGFR expression in G93A-SOD1 and nontransgenic mouse spinal cords.
Immunostaining for VEGFR1 (A–B), VEGFR2 (C–D) and VEGFR3 (E–F) in nontransgenic (A, C, E) and G93A-SOD1 (B, D, F) mouse spinal cord sections. Arrows point to VEGFR expression in MNs in the ventral horn. (G) PCR on RNA isolated from G93A-SOD1 and nontransgenic control mouse spinal cords for VEGF family ligands and VEGFR RNA. Densitometry followed by one-way ANOVA and Tukey’s multiple comparison test demonstrates significant decreased expression of VEGF and VEGFR2 RNA in G93A-SOD1 spinal cords compared to nontransgenic control spinal cords (* P < 0.01). Results are normalized to actin. Scale bar 50 µm.
VEGF signaling
Immunostaining of MNs isolated from E15 rat spinal cord confirms expression of VEGFR2 in cultured primary MNs, as indicated by ChAT co-staining to identify MNs (Fig. 2A–C). Exogenous addition of VEGF promotes phosphorylation of VEGFR2 at Y951 and Y1175 (Fig. 2D–G). These data demonstrate that VEGFR2 is expressed and capable of VEGF-induced phosphorylation and activation in our model system.
Figure 2. VEGFR expression and activation by VEGF in cultured primary MNs.
Immunostaining of primary MNs for VEGFR2 (A) and the MN marker ChAT (B) demonstrates expression of VEGFR2 in MNs (C). Treatment with VEGF (250 ng/ml, 30 min) promotes activation of VEGFR2 as indicated by phosphorylation of Y951 (E) and Y1175 (G) throughout the MN cell body and axon (ChAT positive; arrows) upon VEGF stimulation. Phosphorylation is not seen in the absence of VEGF treatment (D, F). Scale bar 50 µm.
VEGF activates multiple signaling pathways in MNs. To determine which pathways are activated by VEGF in primary MNs, we treated cultures with VEGF (0–500 ng/ml) for 30 min. Dose-dependent phosphorylation of Akt and p44/42 MAPK was observed that reached maximal activation at a concentration of 250 ng/ml VEGF, while no alterations in JNK or p38 MAPK signaling were detected (data not shown). The temporal activation of PI3K/Akt and p44/42 MAPK signaling was also examined. Addition of VEGF (250 ng/ml) resulted in sustained phosphorylation of Akt after 5 min which persisted for at least 60 min, while p44/42 MAPK phosphorylation peaked at 15 min (Fig.3A). Activation of both PI3K/Akt and p44/42 MAPK signaling are prevented by inhibition of VEGFR2 using SU1498 (20µM; Fig. 3B). The pathway-specific inhibitors LY294002 and U0126 prevent VEGF-induced phosphorylation of Akt and p44/42 MAPK, respectively (Fig. 3B).
Figure 3. VEGF signaling in cultured primary MNs.
Western blotting of primary MNs treated with VEGF. (A) Time course of Akt and p44/42 MAPK phosphorylation in response to VEGF (250 ng/ml) treatment. (B) Pretreatment (1 h) with inhibitors to the PI3K/Akt pathway (LY294002; 20µM), p44/42 MAPK pathway (U0126; 20µM), and VEGFR2 (SU1948; 20µM) prevent activation of the respective signaling pathways in response to VEGF treatment.
G93A-SOD1 effects on primary MN
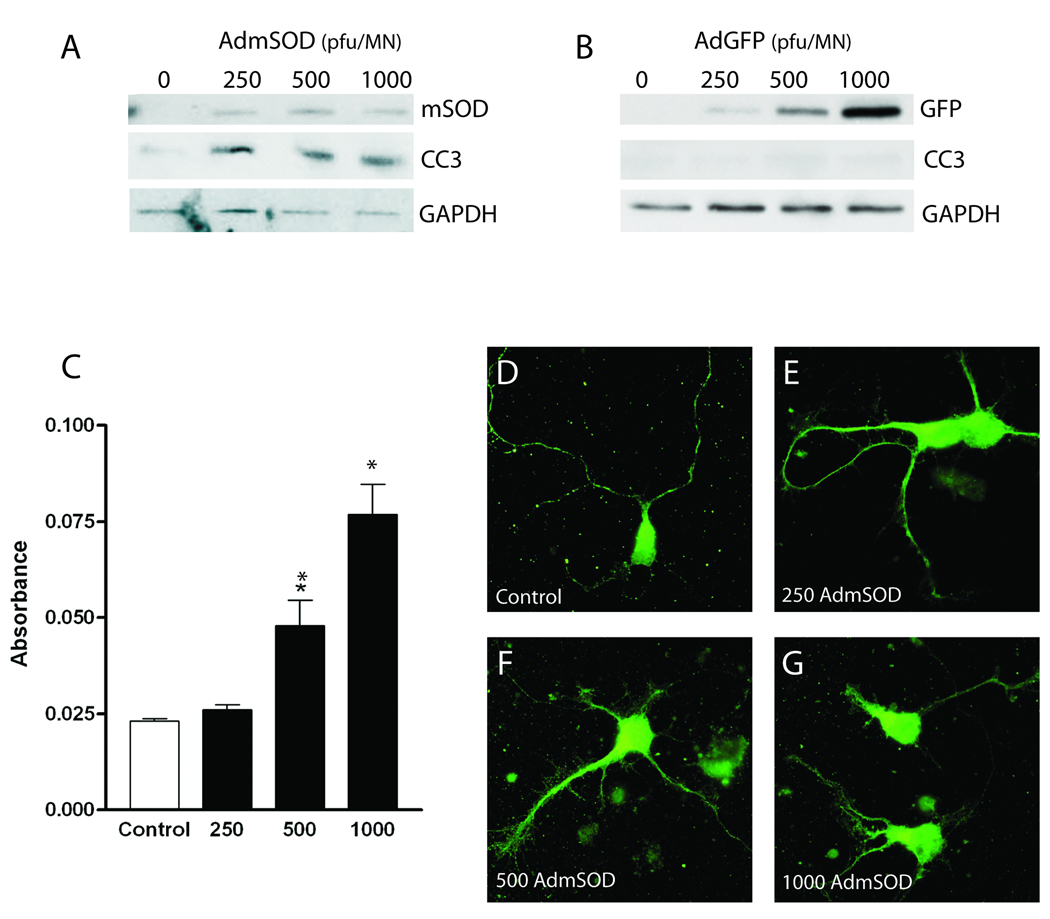
Expression of G93A-SOD1 in primary MNs results in biochemical and morphological characteristics resembling ALS. Transfection with AdmSOD (0–1000 pfu/MN) promotes expression of the mutant protein and a corresponding cleavage of caspase-3 (Fig. 4A), indicative of apoptotic cell death, while transfection with AdGFP does not promote caspase-3 cleavage, eliminating any toxic effects due to viral titer alone (Fig. 4B). Activation of apoptotic death by G93A-SOD1 in primary MNs is further confirmed using fluorescent TUNEL labeling, which results in a dose-dependent increase in DNA fragmentation (Fig 4C). Furthermore, comparable overexpression of wtSOD1 followed by TUNEL analysis indicates that there is no apparent toxicity associated with overexpression of SOD1 itself (Fig. 5A).
Figure 4. Effects of G93A-SOD1 on cultured primary MNs.
Caspase-3 cleavage in response to adenoviral transfection of cultured primary MNs with (A) AdmSOD (0–1000 pfu/MN) or (B) AdGFP (0–1000 pfu/MN). Western blotting demonstrates dose-dependent caspase-3 cleavage in response to G93A-SOD1 expression in cultured primary MNs, while expression of comparable amounts of GFP do not, ruling out toxic effects of viral titer alone. (C) Fluorescent TUNEL staining of AdmSOD-transfected primary MNs. Results are averaged from three independent experiments and analyzed for statistical significance using one-way ANOVA followed by Tukey’s multiple comparison test (* P < 0.01). (D–G) Immunostaining of MNs transfected with AdmSOD for the MN marker ChAT demonstrate dose-dependent effects of G93A-SOD1 on MN morphology.
Figure 5. VEGF neuroprotection against G93A-SOD1 in cultured primary MNs.
(A) TUNEL labeling demonstrates increased DNA damage associated with 500 and 1000 pfu/MN AdmSOD transfection, which is significantly attenuated in the presence of VEGF (250 ng/ml). Transfection with equal concentrations of AdwtSOD1 does not result in increased DNA damage. MN densities are equivalent between treatments groups and results are averaged over at least 10 fields of 3 independent experiments and analyzed for statistical significance using one-way ANOVA followed by Tukey’s multiple comparison test (*P < 0.05). (B) Western blotting demonstrates decreased levels of G93A-SOD1-induced caspase-3 cleavage with VEGF treatment. Pretreatment (4 h pre) is the most efficacious, with decreased efficacy associated with VEGF cotreatment or posttreatment (18 h post).
Axonal degeneration is associated with ALS pathogenesis (Fischer et al., 2004; Fischer and Glass, 2007); therefore we next examined MN morphology. Expression of G93A-SOD1 in primary MNs promotes dose-dependent axonal degeneration represented by decreased axon length and aberrant branching (Fig. 4D–G), while transfection with AdGFP does not promote axonal degeneration, again eliminating any toxic effects due to viral titer alone (data not shown). These data validate AdmSOD transfection of primary MNs as a suitable model system for examining neuroprotection in ALS.
Mechanisms of VEGF neuroprotection
To examine VEGF neuroprotection in ALS, we treated primary MNs with 500 or 1000 pfu/MN AdmSOD in the absence or presence of VEGF and measured DNA damage using the TUNEL assay (Fig. 5A). VEGF significantly decreased the level of DNA damage associated with G93A-SOD1 expression. To determine the temporal efficacy of VEGF in AdmSOD-treated MN, VEGF was administered to MN cultures at the time of AdmSOD transfection or added 4 hr pre-transfection or 18 hr post-transfection and caspase-3 activation was examined by Western blotting (Fig. 5B). All VEGF treatments decrease caspase-3 cleavage associated with G93A-SOD1, with pre-treatment conferring the greatest efficacy. These data confirm that VEGF is neuroprotective against G93A-SOD1 caspase-3 cleavage and DNA damage in primary MNs.
To determine what signaling pathways activated by VEGF are important in neuroprotection, we utilized pathway-specific inhibitors for PI3K/Akt and p44/42 MAPK signaling pathways and assessed the ability of VEGF to attenuate G93A-SOD1 DNA damage using the TUNEL assay (Fig. 6). Inhibition of the VEGFR2 with SU1498 completely prevented the neuroprotective effects of VEGF. LY294002, which prevents signaling via the PI3K/Akt pathway, also completely prevented VEGF neuroprotection, while inhibition of p44/42 MAPK signaling using U0126 did not impact the neuroprotective potential of VEGF. These data indicate that VEGF neuroprotection against G93A-SOD1 is mediated by activation of the PI3K/Akt pathway via VEGFR2 activation.
Figure 6. Identification of VEGF signaling pathways involved in neuroprotection against G93A-SOD1.
TUNEL labeling to identify signaling pathways responsible for VEGF neuroprotection against G93A-SOD1-induced DNA fragmentation. Treatment with AdmSOD (1000 ng/ml) results in DNA damage and is significantly attenuated by VEGF treatment (250 ng/ml). Inhibition of VEGFR2 signaling with SU1498 (20µM; 1 h pretreatment) prevents VEGF neuroprotection. Inhibition of PI3K/Akt signaling with LY294002 (20µM; 1 h pretreatment) also prevents VEGF neuroprotection, while inhibition of p44/42 MAPK signaling with U0126 (20µM; 1 h pretreatment) has no effect on VEGF neuroprotection. Results are averaged over at least 10 fields of 3 independent experiments and analyzed for statistical significance using one-way ANOVA followed by Tukey’s multiple comparison test (*P < 0.01 compared to mSOD + VEGF).
DISCUSSION
To date there are no effective treatments for ALS and the mechanisms of disease onset and progression have not been determined. In addition to the toxic gain-of-function mechanism associated with SOD1 mutations, genetic mutations have also been identified in Alsin, dynactin, angiogenin, senataxin and vesicle-associated membrane protein/synaptobrevin-associated membrane protein B (Pasinelli and Brown, 2006; Gonzalez de Aguilar et al., 2007; Valdmanis and Rouleau, 2008). Additional proposed mechanisms of ALS include autoimmune and inflammatory responses (Appel et al., 1991; Appel et al., 1994), neurofilament aggregation (Lin et al., 2004; Ge et al., 2005), mitochondrial impairment (Dupuis et al., 2004; Bacman et al., 2006; Vande Velde et al., 2008), oxidative stress (Yim et al., 1996; Liu et al., 2007), glutamate toxicity (Rothstein et al., 1990; Coyle and Puttfarcken, 1993) and altered neurotrophism (Ekestern, 2004). The combination of multiple insults and attenuated protective responses, which are all reflected in gene profiling data from ALS patients (Wang et al., 2006), compounds the neurodegenerative phenotype of the MNs. The development of therapies that can target multiple facets of the disease will play a crucial role in understanding and treating ALS. The current study investigates the therapeutic potential of a neurotrophic factor, VEGF, using the ALS model system created by expressing G93A-SOD1 in primary MNs. Expression of G93A-SOD1 in cultured primary MNs induces biochemical and morphological characteristics of cell death resembling human ALS (Fig. 4). Overexpression of wildtype SOD1, on the other hand, does not promote DNA damage (Fig. 5A). In agreement with this, the literature strongly supports that only expression of mutant SOD1promotes disease (Gurney et al., 1994; Reaume et al., 1996), confirming that the observed toxicity is due to expression of mutant SOD1 and not overexpression of SOD1 itself.
Utilizing cultured E15 rat primary MNs enables us to examine the effects of VEGF directly on G93A-SOD1-expressing MNs. While some studies have examined MNs isolated from transgenic rodent models of ALS expressing various forms and levels of mutant SOD1 (Raoul et al., 2002; Spalloni et al., 2004; Raoul et al., 2006; Lobsiger et al., 2007), utilizing rat primary MNs allows for control over the expression levels and toxicity of G93A-SOD1 (Howland et al., 2002; Alexander et al., 2004; Goos et al., 2007). This system results in the morphological and biochemical alterations associated with ALS MN degeneration including axonal degeneration, activation of caspase-3 and DNA damage indicative of cell death (Fig. 4; (Martin, 1999; Li et al., 2000; Fischer et al., 2004; Fischer and Glass, 2007)). The observed severity of alterations follow a dose-dependant association with mutant SOD1 expression levels in cultures from both transgenic animal models expressing different copy numbers of the mutant SOD1 protein and wildtype animals transfected with the mutant SOD1 protein (Howland et al., 2002; Alexander et al., 2004). Taken together, while other models exist to study neuroprotection in ALS, primary rat MNs provide an ideal controllable system for the mechanistic studies undertaken here.
Recently, the role of mutant SOD1 in ALS pathogenesis has been examined in both MNs and glia. In agreement with our findings in MN cultures, studies indicate that expression of G93A-SOD1 exclusively in MNs is sufficient to promote protein aggregation and onset of MN degeneration (Jaarsma et al., 2008). Expression of G93A-SOD1 in glia, however, appears to play a more important role in disease progression (Clement et al., 2003; Di Giorgio et al., 2007; Yamanaka et al., 2008). These focused studies are in agreement with the current understanding of the role of G93A-SOD1 in MNs and enable us to examine the contribution and mechanisms of VEGF neuroprotection at the level of the MN.
Loss of VEGF was first associated with ALS pathogenesis in 2001 when the deletion of the hypoxia response element in the VEGF promoter in transgenic mice resulted in a phenotype essentially identical to that of human ALS (Oosthuyse et al., 2001). Within the G93A-SOD1 mouse spinal cord we observe a decrease in VEGF and VEGFR2 levels concurrent with loss of large MNs (Fig. 1). This decrease in VEGF and VEGFR2 expression is not associated with a compensatory upregulation of other VEGF family members (Fig. 1G). Reductions in VEGF and VEGFR2 are also evident in ALS patients (Brockington et al., 2006) and the role of VEGF in ALS is further supported by evidence that mutant SOD1 destabilizes VEGF mRNA (Lu et al., 2007). Reduced VEGFR2 levels can potentially result in decreased responsiveness to VEGF neurotrophism, or reduced VEGF may increase MN susceptibility to additional cellular insults promoting disease progression.
In order for VEGF treatment to be efficacious in ALS, remaining MNs in the spinal cord must be capable of responding to VEGF. Immunostaining for VEGFRs in G93A-SOD1 mouse spinal cords demonstrates that the remaining MNs express all 3 VEGFRs (Fig. 1B,D,F), and expression of VEGFR2 is also demonstrated in cultured primary MNs (Fig. 2A–C). Furthermore, cultured primary MNs respond to VEGF treatment, as evidenced by phosphorylation and activation of VEGFR2 (Fig. 2D–G). These data confirm that MNs in the spinal cord and in our culture system are capable of responding to exogenous VEGF application.
The current studies demonstrate that VEGF can protect MNs from G93A-SOD1-induced damage. Addition of VEGF significantly prevents G93A-SOD1-induced DNA fragmentation (Fig. 5A) and caspase-3 cleavage (Fig. 5B). While VEGF provides lower levels of neuroprotection when administered concurrent with or following G93A-SOD1 transfection compared to pretreatment, the ability to decrease caspase-3 cleavage in this in vitro system that represents an endstage disease phenotype implies that VEGF treatment efficacy, when translated into the human MN environment, could be effective throughout the disease course. These findings are congruent with previous reports supporting the neuroprotective effects of VEGF in ALS. Retrograde delivery of VEGF to MNs by gene therapy increases survival of G93A-SOD1 transgenic mice (Azzouz et al., 2004). Systemic delivery of VEGF also decreases astrogliosis and increases neuromuscular junctions in these mice (Zheng et al., 2007). Double transgenic mice expressing G93A-SOD1 and increased VEGF levels demonstrate delayed MN loss, delayed motor impairment, and increased survival (Wang et al., 2007). G93A-SOD1 transgenic rats also show evidence of increased survival in response to treatment with intracerebroventricular delivery of VEGF (Storkebaum et al., 2005). G93A-SOD1 expression in a zebrafish model of ALS also demonstrates attenuation of G93A-SOD1-induced neuronal defects with increased VEGF levels, and exacerbated defects with decreased VEGF concentrations (Lemmens et al., 2007).
Our findings demonstrate that VEGF is neuroprotective against G93A-SOD1 toxicity (Figure 5). This data suggests that the decreased toxicity and increased MN survival in response to VEGF treatment, which is supported in additional reports (Li et al., 2003; Zheng et al., 2004; Dewil et al., 2007; Zheng et al., 2007), may indicate a role for VEGF neuroprotection in ALS. In fact, VEGF is protective against glutamate toxicity in spinal cord cultures, one of the main non-neuronal mechanisms of MN damage in ALS (Tovar et al., 2007; Tolosa et al., 2008). These data, along with the finding of reduced VEGF levels in transgenic ALS mouse spinal cords (Figure 1G), suggest that the loss of VEGF neurotrophism may be a contributing factor to MN survival in ALS. Restoration of VEGF levels, therefore, is supported as a viable approach to treating MN degeneration in ALS.
VEGF activates many signaling pathways in MNs including PI3K/Akt and p44/42 MAPK (Fig. 3). We have previously demonstrated that PI3K/Akt and p44/42 MAPK signaling are involved in the neuroprotective effects of insulin-like growth factor-I (IGF-I) against glutamate toxicity (Vincent et al., 2004). We are able to address the contribution of these signaling pathways to VEGF neuroprotection in our model system using the pathway-specific inhibitors LY294002 and U0126 to prevent PI3K/Akt and p44/42 MAPK signaling, respectively (Vlahos et al., 1994; Favata et al., 1998). Work in our laboratory and others (Kim et al., 1998; Leinninger et al., 2004; Vincent et al., 2004; Vincent et al., 2007) have extensively examined and optimized these inhibitors for dosing regimens and their selective effectiveness in neuronal cultures, and at the concentrations used for these studies, inhibitor concentrations are sufficient to prevent activation of the respective pathways (Fig. 3B) with minimal toxicity. Unlike results from studies examining IGF-I neuroprotection (Vincent et al., 2004), our results demonstrate that VEGF-induced PI3K/Akt signaling is neuroprotective against G93A-SOD1 toxicity, with no apparent involvement of p44/42 MAPK signaling (Fig. 6). The role of PI3K/Akt in ALS has been previously examined in various systems with conflicting results. Familial and sporadic ALS patients have decreased levels of phosphorylated Akt in MNs, as also evidenced in G93A-SOD1 mice prior to symptom onset (Dewil et al., 2007). Examination of phosphorylated Akt levels in G93A-SOD1 mouse spinal cords in an alternate study, however, demonstrated no change in phosphorylated Akt (Peviani et al., 2007). Despite these discrepancies, constitutive activation of Akt in G37R-SOD1-expressing N2a cells provides neuroprotection (Dewil et al., 2007). Furthermore, neuroprotective effects of intracerebroventricular VEGF treatment in G93A-SOD1 rats results in increased phospho-Akt levels (Dewil et al., 2007). Therefore, VEGF specifically mediates its protective effects via PI3K/Akt signaling, which may constitute a conserved mechanism for VEGF neuroprotection in ALS.
VEGF efficacy has also been previously examined in models of sporadic ALS. Familial ALS and sporadic ALS are clinically and pathologically indistinguishable and it is suggested that both forms of the disease potentially result from a common mechanism. Reports on sporadic ALS demonstrate an involvement of non-neuronal glutamate toxicity in MN degeneration and disease progression (Rothstein et al., 1990; Coyle and Puttfarcken, 1993; Clement et al., 2003; Boillee et al., 2006; Di Giorgio et al., 2007; Yamanaka et al., 2008). In fact, VEGF decreases MN loss by over 75% in a rat model of excitotoxic MN degeneration (Tovar et al., 2007). Activation of PI3K/Akt signaling by IGF-I is also associated with neuroprotection against glutamate toxicity (Vincent et al., 2004). Tolosa and colleagues demonstrated direct neuroprotection by VEGF via PI3K/Akt signaling against excitotoxic stress (Tolosa et al., 2008).
Taken together, the current studies strengthen the role of VEGF as a therapy for ALS. We have established that VEGF-mediated PI3K/Akt signaling is an important mechanism for protection against G93A-SOD1 in MNs. Induction of PI3K/Akt signaling by VEGF may constitute a conserved neuroprotective mechanism for MNs in response to a number of cellular insults in ALS pathogenesis including mutant SOD1 and glutamate toxicity. These data support the idea that G93A-SOD1 promotes destabilization of VEGF mRNA and decreased protein levels. The reduced protein level propagates susceptibility onto the MNs to additional cellular insults, contributing to disease onset. MNs remaining in the ALS spinal cord, however, are capable of responding to VEGF stimulation, and thus can respond to therapeutic exogenous VEGF administration via activation of PI3K/Akt signaling resulting in neuroprotection (Fig. 7).
Figure 7. Model of G93A-SOD1 and VEGF effects on MNs.
Expression of G93A-SOD1 in MNs results in decreased endogenous production of VEGF and the activation of caspase-3 ultimately resulting in cell death. Addition of exogenous VEGF activates VEGFR2 and subsequent PI3K/Akt and p44/42 MAPK signaling. Activation of PI3K/Akt signaling prevents G93A-SOD1-induced caspase-3 activation and cell death.
Determining the most efficacious delivery mechanism for VEGF is important for achieving neuroprotection in ALS, and gene-based therapies offer much potential for effective delivery of therapeutics to MNs (Federici and Boulis, 2006). Achieving the maximal impact of VEGF neurotrophism is also important to consider. The current studies investigate the efficacy of VEGF165, one of the five VEGF splice variants normally expressed in the nervous system. Induction of the full biological array of VEGF isoforms, however, may promote an additive effect on neuroprotection (Whitlock et al., 2004; Amano et al., 2005) and increased potency and longevity of VEGF neuroprotection may be observed. Studies are currently underway to establish a more efficacious delivery mechanism using gene-based treatment strategies to increase PI3K/Akt activation in ALS using the full biological array of VEGF splice variants.
ACKNOWLEDGEMENTS
The authors would like to thank the ALS Association, the A. Alfred Taubman Medical Research Institute, and the Program for Neurology Research and Discovery for supporting our research in ALS. SAS is supported by NIH T32 NS007222-26. The authors would also like to thank Ms. Judith Boldt for excellent secretarial support during the preparation of this manuscript.
REFERENCES
- Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD. Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res. 2004;130:7–15. doi: 10.1016/j.molbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Amano H, Hackett NR, Kaner RJ, Whitlock P, Rosengart TK, Crystal RG. Alteration of splicing signals in a genomic/cDNA hybrid VEGF gene to modify the ratio of expressed VEGF isoforms enhances safety of angiogenic gene therapy. Mol Ther. 2005;12:716–724. doi: 10.1016/j.ymthe.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Appel SH, Engelhardt JI, Garcia J, Stefani E. Autoimmunity and ALS: a comparison of animal models of immune-mediated motor neuron destruction and human ALS. Adv Neurol. 1991;56:405–412. [PubMed] [Google Scholar]
- Appel SH, Smith RG, Engelhardt JI, Stefani E. Evidence for autoimmunity in amyotrophic lateral sclerosis. J Neurol Sci. 1994;124 (Suppl):14–19. doi: 10.1016/0022-510x(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Bacman SR, Bradley WG, Moraes CT. Mitochondrial involvement in amyotrophic lateral sclerosis: trigger or target? Mol Neurobiol. 2006;33:113–131. doi: 10.1385/MN:33:2:113. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Brockington A, Wharton SB, Fernando M, Gelsthorpe CH, Baxter L, Ince PG, Lewis CE, Shaw PJ. Expression of vascular endothelial growth factor and its receptors in the central nervous system in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65:26–36. doi: 10.1097/01.jnen.0000196134.51217.74. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dewil M, Lambrechts D, Sciot R, Shaw PJ, Ince PG, Robberecht W, Van den Bosch L. Vascular endothelial growth factor counteracts the loss of phospho-Akt preceding motor neurone degeneration in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2007;33:499–509. doi: 10.1111/j.1365-2990.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis. 2004;1:245–254. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- Ekestern E. Neurotrophic factors and amyotrophic lateral sclerosis. Neurodegener Dis. 2004;1:88–100. doi: 10.1159/000080049. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Federici T, Boulis NM. Gene-based treatment of motor neuron diseases. Muscle Nerve. 2006;33:302–323. doi: 10.1002/mus.20439. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Glass JD. Axonal degeneration in motor neuron disease. Neurodegener Dis. 2007;4:431–442. doi: 10.1159/000107704. [DOI] [PubMed] [Google Scholar]
- Ge WW, Wen W, Strong W, Leystra-Lantz C, Strong MJ. Mutant copper-zinc superoxide dismutase binds to and destabilizes human low molecular weight neurofilament mRNA. J Biol Chem. 2005;280:118–124. doi: 10.1074/jbc.M405065200. [DOI] [PubMed] [Google Scholar]
- Gonzalez de Aguilar JL, Echaniz-Laguna A, Fergani A, Rene F, Meininger V, Loeffler JP, Dupuis L. Amyotrophic lateral sclerosis: all roads lead to Rome. J Neurochem. 2007;101:1153–1160. doi: 10.1111/j.1471-4159.2006.04408.x. [DOI] [PubMed] [Google Scholar]
- Goos M, Zech WD, Jaiswal MK, Balakrishnan S, Ebert S, Mitchell T, Carri MT, Keller BU, Nau R. Expression of a Cu,Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of neuroblastoma cells to infectious injury. BMC Infect Dis. 2007;7:131. doi: 10.1186/1471-2334-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Cheng HL, Margolis B, Feldman EL. Insulin receptor substrate 2 and Shc play different roles in insulin-like growth factor I signaling. J Biol Chem. 1998;273:34543–34550. doi: 10.1074/jbc.273.51.34543. [DOI] [PubMed] [Google Scholar]
- Kim B, Leventhal PS, Saltiel AR, Feldman EL. Insulin-like growth factor-I-mediated neurite outgrowth in vitro requires MAP kinase activation. Journal of Biological Chemistry. 1997;272:21268–21273. doi: 10.1074/jbc.272.34.21268. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, Katayama S, Awata T, Leigh N, Lang-Lazdunski L, Dewerchin M, Shaw C, Moons L, Vlietinck R, Morrison KE, Robberecht W, Van Broeckhoven C, Collen D, Andersen PM, Carmeliet P. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. Faseb J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Van Hoecke A, Hersmus N, Geelen V, D'Hollander I, Thijs V, Van Den Bosch L, Carmeliet P, Robberecht W. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum Mol Genet. 2007;16(19):2359–2365. doi: 10.1093/hmg/ddm193. [DOI] [PubMed] [Google Scholar]
- Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–164. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Research Molecular Brain Research. 2003;111:155–164. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Lin H, Zhai J, Canete-Soler R, Schlaepfer WW. 3' untranslated region in a light neurofilament (NF-L) mRNA triggers aggregation of NF-L and mutant superoxide dismutase 1 proteins in neuronal cells. J Neurosci. 2004;24:2716–2726. doi: 10.1523/JNEUROSCI.5689-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Bao F, Wen J, Liu J. Mutation of superoxide dismutase elevates reactive species: comparison of nitration and oxidation of proteins in different brain regions of transgenic mice with amyotrophic lateral sclerosis. Neuroscience. 2007;146:255–264. doi: 10.1016/j.neuroscience.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Boillee S, Cleveland DW. Toxicity from different SOD1 mutants dysregulates the complement system and the neuronal regenerative response in ALS motor neurons. Proc Natl Acad Sci U S A. 2007;104:7319–7326. doi: 10.1073/pnas.0702230104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zheng L, Viera L, Suswam E, Li Y, Li X, Estevez AG, King PH. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J Neurosci. 2007;27:7929–7938. doi: 10.1523/JNEUROSCI.1877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol. 1999;58:459–471. doi: 10.1097/00005072-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Peviani M, Cheroni C, Troglio F, Quarto M, Pelicci G, Bendotti C. Lack of changes in the PI3K/AKT survival pathway in the spinal cord motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 2007;34:592–602. doi: 10.1016/j.mcn.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci U S A. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Haperin JJ, Herzfeldt B, Van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericack-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown JRH. Mutations in Cu/Zn superoxide dismutase are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- Spalloni A, Albo F, Ferrari F, Mercuri N, Bernardi G, Zona C, Longone P. Cu/Zn-superoxide dismutase (GLY93-->ALA) mutation alters AMPA receptor subunit expression and function and potentiates kainate-mediated toxicity in motor neurons in culture. Neurobiol Dis. 2004;15:340–350. doi: 10.1016/j.nbd.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- Tovar YRLB, Zepeda A, Tapia R. Vascular endothelial growth factor prevents paralysis and motoneuron death in a rat model of excitotoxic spinal cord neurodegeneration. J Neuropathol Exp Neurol. 2007;66:913–922. doi: 10.1097/nen.0b013e3181567c16. [DOI] [PubMed] [Google Scholar]
- Valdmanis PN, Rouleau GA. Genetics of familial amyotrophic lateral sclerosis. Neurology. 2008;70:144–152. doi: 10.1212/01.wnl.0000296811.19811.db. [DOI] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci U S A. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, Imperiale MJ, Boulis NM. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Medicine. 2004;6:79–86. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, Feldman EL. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148:548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wang XS, Simmons Z, Liu W, Boyer PJ, Connor JR. Differential expression of genes in amyotrophic lateral sclerosis revealed by profiling the post mortem cortex. Amyotroph Lateral Scler. 2006;7:201–210. doi: 10.1080/17482960600947689. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock PR, Hackett NR, Leopold PL, Rosengart TK, Crystal RG. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004;9:67–75. doi: 10.1016/j.ymthe.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim MB, Kang JH, Yim HS, Kwak HS, Chock PB, Stadtman ER. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu,Zn-superoxide dismutase mutant: An enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc Natl Acad Sci U S A. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Nennesmo I, Fadeel B, Henter JI. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2004;56:564–567. doi: 10.1002/ana.20223. [DOI] [PubMed] [Google Scholar]
- Zheng C, Skold MK, Li J, Nennesmo I, Fadeel B, Henter JI. VEGF reduces astrogliosis and preserves neuromuscular junctions in ALS transgenic mice. Biochem Biophys Res Commun. 2007;363:989–993. doi: 10.1016/j.bbrc.2007.09.088. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003;17:186–193. doi: 10.1096/fj.02-0515com. [DOI] [PubMed] [Google Scholar]