Abstract
β-peptides possess several features that are desirable in peptidomimetics; they are easily synthesized, fold into stable secondary structures in physiologic buffers, and resist proteolysis. They can also bind to a diverse array of proteins to inhibit their interactions with α–helical ligands. β–peptides are not usually cell permeable, however, and this feature limits their utility as research tools and potential therapeutics. Appending an Arg8 sequence to a β–peptide improves uptake but adds considerable mass. We reported that embedding a small cationic patch within a PPII, α– or β–peptide helix improves uptake without the addition of significant mass. In another mass-neutral strategy, Verdine, Walensky, and others have reported that insertion of a hydrocarbon bridge between the i and i+4 positions of an α–helix also increases cell uptake. Here we describe a series of β–peptides containing diether and hydrocarbon bridges and compare them on the basis of cell uptake and localization, affinities for hDM2, and 14-helix structure. Our results highlight the relative merits of cationic patch and hydrophobic bridge strategies for improving β–peptide uptake and identify a surprising correlation between uptake efficiency and hDM2 affinity.
β-peptides1-4 possess several features that are desirable in peptidomimetics;5,6 they are easily synthesized, fold into helices1-3,7 in physiologic buffers,8 and resist proteolysis.9 They also bind in vitro to proteins such as hDM2,10-14 hDMX,10 gp41,15,16 and others,17-19 and inhibit their interactions with α-helical ligands. β-peptides are not usually cell permeable, however, and this feature limits their utility as research tools and potential therapeutics. Appending an Arg8 sequence to a β-peptide can improve uptake20,21 but adds considerable mass. We reported that embedding a small cationic patch within a PPII,22 α-23 or β-peptide11 helix improves uptake without the addition of significant mass.24,25 Similarly, Verdine, Walensky, and others26-33 reported that insertion of a hydrocarbon bridge (a “staple”) between the i and i+4 positions of an α-helix34 increases uptake.26,29,32,34-38 Here we describe a variety of β-peptides containing diether- and hydrocarbon bridges and compare them on the basis of cell uptake and localization, affinity for hDM2, and 14-helix structure. Our results highlight the relative merits of cationic patch and hydrophobic bridge strategies for improving β-peptide uptake and identify an unprecedented correlation between uptake efficiency and hDM2 affinity in vitro.
Our studies began with an analysis of available x-ray39,40 and NMR structures13,41 of β-peptide 14-helices to identify those position pairs that would best tolerate an ether42,43 or hydrocarbon34 bridge. This analysis, supported by recent work of Perlmutter42 and Seebach44 suggested that a 21-atom bridge could be accommodated between most i and i+3 positions of a 14-helix. To test this prediction, we synthesized an analog of β-peptide 27 containing (O-allyl)-β3-L-Ser at positions 3 and 6 (2(3-6), Figure 1), and subjected it to on-resin ring-closing metathesis using bis(tricyclohexylphosphine)benzylidene ruthenium (IV) dichloride34 to generate 2(3-6)s.45 The circular dichroism (CD) spectra of 2, 2(3-6) and 2(3-6)s were identical (Figure S1), indicating that this 21-atom diether bridge is accommodated between positions 3 and 6. Introduction of the diether bridge did not significantly increase or decrease the extent of 14-helix structure as judged by CD.
Figure 1.
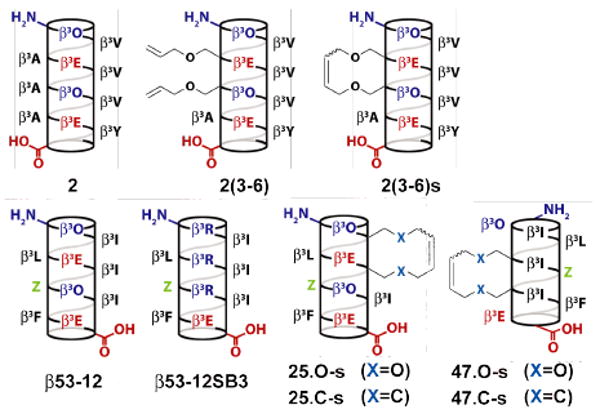
Helical net representation of β-peptides studied herein. β3-homoamino acids are identified by the single-letter code used for the corresponding α–amino acid. Orn represents ornithine. Z represents 3-(S)-3-amino-4-(2-trifluoromethylphenyl)-butyric acid.
In order to evaluate the relative uptake of bridged β-peptides in the context of a functional molecule of diverse sequence, we synthesized a series of variants of β53-12,10 an inhibitor of p53-hDM2 complexation (Figure 1). These variants contained either (O-allyl)-β3-L-Ser (to generate a diether bridge) or (S)-3-aminooct-7-enoic acid (to generate a hydrocarbon bridge) at i and i+3 positions 2 and 5 (25.O-s and 25.C-s, respectively) or 4 and 7 (47.O-s and 47.C-s, respectively). According to the CD spectra (Figure 2), all bridged β-peptides assumed a 14-helical structure and were modestly more helical than unbridged analogs (Figure S2).
Figure 2.
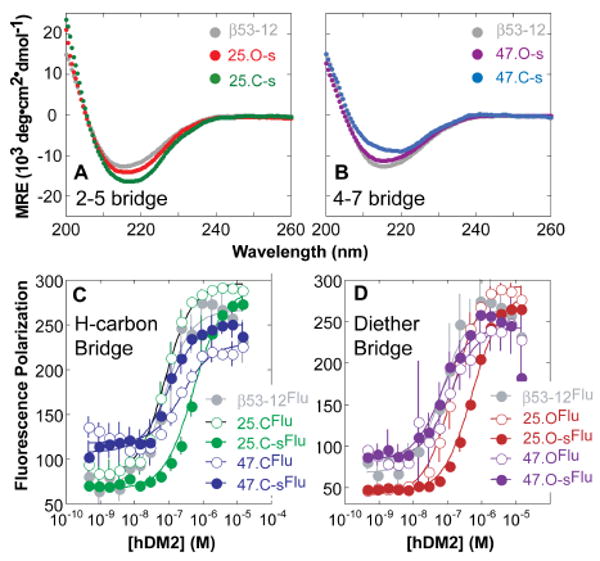
CD analysis of β-peptides containing hydrocarbon or diether bridges between residues (A) 2 and 5 or (B) 4 and 7. Fluorescence polarization (FP) analysis of hDM2 binding by β-peptides containing (C) hydrocarbon or (D) diether bridges.
As a prelude to evaluating cell uptake and localization, we employed a direct fluorescence polarization assay to compare hydrocarbon and diether bridged β-peptides on the basis of affinity for hDM21-188 (Figure 2B). β-peptides containing a diether or hydrocarbon bridge between positions 4 and 7 bound hDM21-188 2-fold better (Kd = 53.9 ± 22.7 and 94.1 ± 18.4 nM, respectively) than the corresponding unbridged analogs (Kd = 114 ± 28 and 253 ± 75 nM, respectively), in line with analogous comparisons in an α–peptide context.35 By contrast, β-peptides containing a diether or hydrocarbon bridge between positions 2 and 5 bound hDM21-188 between 4 and 8-fold worse (Kd = 548 ± 58 and 546 ± 96 nM, respectively) than unbridged analogs (Kd = 139 ± 13 and 68.1 ± 7.8 nM, respectively). In silico analysis suggests that the lower hDM21-188 affinity of β-peptides 25.C-s and 25.O-s results from steric hindrance between the hydrocarbon bridge and the hDM2 surface that is absent in the complex with peptides 47.C-s and 47.O-s (Figure 3, compare A and B).
Figure 3.
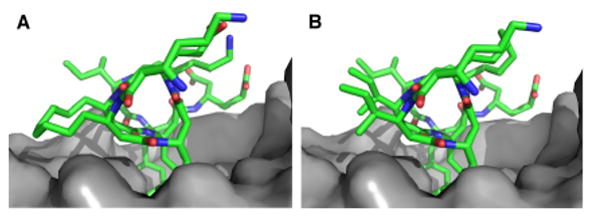
Computational model of hDM2 (grey) in complex with (A) 25.C-s or (B) 47.C-s.45
We next set out to monitor the mammalian cell uptake and sub-cellular localization of diether- and hydrocarbon bridged β-peptides based on β53-12. Uptake was monitored using flow cytometry, whereas sub-cellular localization was assessed using confocal microscopy (Figure 4). β-peptides containing diether or hydrocarbon bridges between positions 4 and 7 were taken up significantly more efficiently (MCF = 8.21 ± 0.45 and 8.63 ± 0.77, respectively) than unbridged analogs (MCF = 3.23 ± 0.31 and 2.63 ± 0.32, respectively), irrespective of bridge structure. By contrast, β-peptides containing diether or hydrocarbon bridges between positions 2 and 5 were taken up poorly, irrespective of bridge structure, and behaved much like the unbridged analogs. In all cases, as judged by flow cytometry, the greatest uptake was observed with β-peptide β53-12SB3, which contains a cationic patch on one 14-helix face but no bridge of any kind (Figure 4AB).
Figure 4.
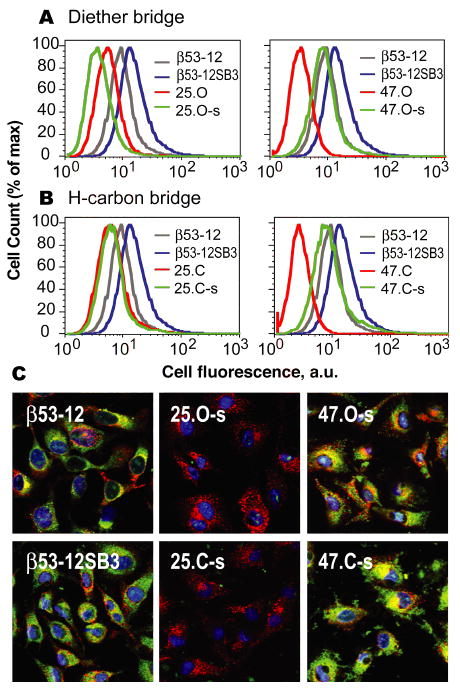
HeLa cell uptake and localization of Flu-labeled β-peptides. (A,B) HeLa cells were incubated with 2 μM β–peptide for 4 h, treated with 0.25% trypsin for 10 min, washed with cold DMEM and PBS, and analyzed using flow cytometry. (C) Confocal microscopy of HeLa cells treated with 20 μM of the indicated β-peptide (green), 5 mg•mL-1 Alexa Fluor 647-transferrin (red) and 150 nM Hoescht 33342 (blue).
The localization of bridged β-peptides upon cell uptake was explored in more detail using confocal microscopy. HeLa cells were treated with fluorescently labeled β-peptide (green) as well as Alexa Fluor® 647 labeled transferrin and Hoescht 33342 to visualize recycling endosomes46,47 (red) and nuclei (blue). β-peptides containing a diether or hydrocarbon bridge between positions 4 and 7 are distributed widely among Tf+ and Tf- endosomes, as well as nuclear and cytosolic compartments, whereas those containing the analogous bridge between positions 2 and 5 are not (Figure 3). Indeed, β-peptides containing a diether or hydrocarbon bridge between positions 2 and 5 are taken up more poorly than the unbridged analog (Figure S4). These results highlight an intriguing correlation between hDM2 affinity and cell uptake; it is possible that the structural features that lower hDM2 affinity (Figure S3) also lower uptake efficiency. Indeed, it appears that for these β-peptides, an increase in 14-helix secondary structure does not necessarily confer increased cell uptake.26
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM 74756), the National Foundation for Cancer Research, and a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (J.M.). A.D.B. is grateful to Bristol-Myers Squibb for a graduate research fellowship.
Footnotes
Supporting Information Available: β-peptide synthesis, binding and cell uptake assays, and confocal microscopy images. This material is available free of charge on the Internet at http://pubs.acs.org.
References
- 1.Cheng RP, Gellman SH, DeGrado WF. Chem Rev. 2001;101:3219–3232. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
- 2.DeGrado WF, Schneider JP, Hamuro Y. J Pept Res. 1999;54:206–217. doi: 10.1034/j.1399-3011.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 3.Gellman SH. Acc Chem Res. 1998;31:173–180. [Google Scholar]
- 4.Seebach D, Overhand M, Kunhle FNM, Martinoni B, Oberer L, Hommel U, Widmer H. Helv Chim Acta. 1996;79:913–941. [Google Scholar]
- 5.Bautista AD, Craig CJ, Harker EA, Schepartz A. Curr Opin Chem Biol. 2007;11:685–592. doi: 10.1016/j.cbpa.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kritzer JA, Stephens OM, Guarracino DA, Reznik SK, Schepartz A. Bioorg Med Chem. 2004;13:11–16. doi: 10.1016/j.bmc.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kritzer JA, Tirado-Rives J, Hart SA, Lear JD, Jorgensen WL, Schepartz A. J Am Chem Soc. 2005;127:167–178. doi: 10.1021/ja0459375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart SA, Bahadoor ABF, Matthews EE, Qiu XJ, Schepartz A. J Am Chem Soc. 2003;125:4022–4023. doi: 10.1021/ja029868a. [DOI] [PubMed] [Google Scholar]
- 9.Frackenpohl J, Arvidsson PI, Schreiber JV, Seebach D. ChemBioChem. 2001;2:445–455. doi: 10.1002/1439-7633(20010601)2:6<445::aid-cbic445>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Harker EA, Daniels DS, Guarracino DA, Schepartz A. Bioorg Med Chem. 2009;17:2038–2046. doi: 10.1016/j.bmc.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harker EA, Schepartz A. ChemBioChem. 2009;10:990–993. doi: 10.1002/cbic.200900049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J Am Chem Soc. 2004;126:9468–9469. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]
- 13.Kritzer JA, Luedtke NW, Harker EA, Schepartz A. J Am Chem Soc. 2005;127:14584–14585. doi: 10.1021/ja055050o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray JK, Gellman SH. Pept Sci. 2007;88:657–686. doi: 10.1002/bip.20741. [DOI] [PubMed] [Google Scholar]
- 15.Bautista AD, Stephens OM, Wang L, Domaoal RA, Anderson KS, Schepartz A. Bioorg Med Chem Lett. 2009;19:3736–3738. doi: 10.1016/j.bmcl.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens OM, Kim S, Welch BD, Hodsdon ME, Kay MS, Schepartz A. J Am Chem Soc. 2005;127:13126–13127. doi: 10.1021/ja053444+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English EP, Chumanov RS, Gellman SH, Compton T. J Biol Chem. 2006;281:2661–2667. doi: 10.1074/jbc.M508485200. [DOI] [PubMed] [Google Scholar]
- 18.Lee EF, Sadowsky JD, Smith BJ, Czabotar PE, Peterson-Kaufman KJ, Colman PM, Gellman SH, Fairlie WD. Angew Chem Int Ed. 2009;48:4318–4322. doi: 10.1002/anie.200805761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadowsky JD, Fairlie WD, Hadley EB, Lee HS, Umezawa N, Nikolovska-Coleska Z, Wang S, Huang DCS, Tomita Y, Gellman SH. J Am Chem Soc. 2007;129:139–154. doi: 10.1021/ja0662523. [DOI] [PubMed] [Google Scholar]
- 20.Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SMV, Newham P, Lindsay MA. Br J Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tung CH, Weissleder R. Adv Drug Deliv Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 22.Daniels DS, Schepartz A. J Am Chem Soc. 2007;129:14578–14579. doi: 10.1021/ja0772445. [DOI] [PubMed] [Google Scholar]
- 23.Smith BA, Daniels DS, Coplin AE, Jordan GE, McGregor LM, Schepartz AJ. Am Chem Soc. 2008;130:2948–2949. doi: 10.1021/ja800074v. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence MS, Phillips KJ, Liu DR. J Am Chem Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Proc Natl Acad Sci U S A. 2009;106:6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YW, Verdine GL. Bioorg Med Chem Lett. 2009;19:2533–2536. doi: 10.1016/j.bmcl.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Kutchukian PS, Yang JS, Verdine GL, Shakhnovich EI. J Am Chem Soc. 2009;131:4622–4627. doi: 10.1021/ja805037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden MM, Vera CIR, Song W, Lin Q. Chem Commun. 2009:5588–5590. doi: 10.1039/b912094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moellering RE, Cornejo M, Davis TN, Bianco CD, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelan J. Drug Discov Today. 2004;9:907–907. doi: 10.1016/S1359-6446(04)03268-4. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Zhang H, Debnath AK, Cowburn D. J Biol Chem. 2008;283:16274–16278. doi: 10.1074/jbc.C800048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danial NN, et al. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henchey LK, Jochim AL, Arora PS. Curr Opin Chem Biol. 2008;12:692–697. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]
- 35.Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J Am Chem Soc. 2007;129:2456–2457. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Zhao Q, Bhattacharya S, Waheed AA, Tong X, Hong A, Heck S, Curreli F, Goger M, Cowburn D, Freed EO, Debnath AK. J Mol Biol. 2008;378:565–580. doi: 10.1016/j.jmb.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniels DS, Petersson EJ, Qiu JX, Schepartz A. J Am Chem Soc. 2007;129:1532–1533. doi: 10.1021/ja068678n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman JL, Petersson EJ, Daniels DS, Qiu JX, Schepartz A. J Am Chem Soc. 2007;129:14746–14751. doi: 10.1021/ja0754002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kritzer JA, Hodsdon ME, Schepartz A. J Am Chem Soc. 2005;127:4118–4119. doi: 10.1021/ja042933r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergman YE, Del Borgo MP, Gopalan RD, Jalal S, Unabia SE, Ciampini M, Clayton DJ, Fletcher JM, Mulder RJ, Wilce JA, Aguilar MI, Perlmutter P. Org Lett. 2009;11:4438–4440. doi: 10.1021/ol901803d. [DOI] [PubMed] [Google Scholar]
- 43.Blackwell HE, Grubbs RH. Angew Chem Int Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Ebert MO, Gardiner J, Ballet S, Abell AD, Seebach D. Helv Chim Acta. 2009:2643–2658. [Google Scholar]
- 45.See Supporting Information for details.
- 46.Ghosh R, Gelman D, Maxfield F. J Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins C, Gibson A, Shipman M, Strickland D, Trowbridge I. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


