Abstract
Two of the most successful and widely used tests developed by Arthur Benton and colleagues are the Facial Recognition Test (FRT) and Judgment of Line Orientation test (JLO), which probe visuoperceptual and visuospatial functions typically associated with right hemisphere structures, especially parietal, occipitoparietal, and occipitotemporal structures. Taking advantage of a large database of focal lesion patients (the Iowa Neurological Patient Registry), we used a new lesion-deficit mapping technique to investigate the neuroanatomical correlates of FRT and JLO performance. For the FRT, there were 201 patients with relevant data; of these, 38 were impaired on the FRT, and failure was most strongly associated with lesions in the right posterior-inferior parietal and right ventral occipitotemporal (fusiform gyrus) areas. For the JLO, there were 181 patients with relevant data; of these, 23 were impaired on the JLO, and failure was most strongly associated with lesions in the right posterior parietal region. These findings put new empirical teeth in the localizing value of the FRT and JLO tests, and extend and sharpen previous work which had pointed to right posterior structures as being important for FRT and JLO performance.
INTRODUCTION
At the 20th Annual Meeting of the International Neuropsychological Society in February of 1992, Arthur Benton delivered the Herbert Birch Memorial Lecture, in which he reprised the highlights of developments in clinical neuropsychology from 1960 to 1990. In this presentation, Benton credited Henry Hécaen and Oliver Zangwill, circa 1960, with altering thinking at the time regarding hemispheric laterality, and specifically, for transforming the long-held doctrine of left-hemisphere dominance to an account of hemispheric asymmetry. The idea that we speak with the left hemisphere was firmly established. The work by Hécaen and Zangwill showed conclusively that right hemisphere damage led to defects in visuospatial and visuoconstructional abilities (for summaries, see Hécaen & Albert, 1978; Zangwill, 1960). Thus, rather than considering the left hemisphere as the dominant, major seat of important cognitive functions, and the right hemisphere as a minor, less important player (or “relatively retarded,” as Sperry colorfully put it in his 1981 Nobel Laureate address published in 1982), the hemispheres came to be seen as having different types of specialization, each with its own particular importance depending on the task at hand.
Benton, himself, contributed in no small measure to this shift of conceptualization. Some of his most influential and enduring contributions on this topic come from his development, standardization, and adaptation for clinical use, of several clever techniques for measuring visuoperceptual and visuospatial abilities. In particular, he and his colleagues developed the Facial Recognition Test (FRT) and the Judgment of Line Orientation Test (JLO), and both measures have been widely adopted in clinical neuropsychological practice and today are used in neuropsychological laboratories the world over.
What is known about the neuroanatomical correlates of the FRT and the JLO tests? Although Benton did considerable preliminary work in linking these tests to the right hemisphere, and to right posterior (especially parietal, occipitoparietal, and occipitotemporal regions) sectors in particular, the actual empirical work that has investigated the neuroanatomical correlates of FRT and JLO is surprisingly meager, and much of what is available was performed prior to the availability of modern neuroimaging technology. The current study was designed to provide an update on the neuroanatomical correlates of FRT and JLO, using contemporary lesion-deficit analysis methods and taking advantage of a large database at the University of Iowa. Before going into the current study, however, a brief review of previous work on the neuroanatomical basis of FRT and JLO performance is provided.
Facial Recognition Test
One of the earliest reports relevant to this topic was published by De Renzi and Spinnler (1966). Their article, curiously, is actually centered mainly on the topic of prosopagnosia; however, the task they utilized was in fact more of a perceptual matching task than a true face “identification” task. Participants were required to study an unfamiliar face for 15 seconds, and then the study face was removed and participants were asked to choose from an array of 20 different faces (including the study face) the one they had just studied (only 4 trials were administered). Patients with right hemisphere damage had poorer performance than did patients with left hemisphere damage and neurological comparison patients (patients with no neurological disease above the thoracic spinal level). The authors called this “face recognition,” but they acknowledged that their findings do not really speak to the central issue of prosopagnosia per se. In this vein, it is important to underscore that the designation of prosopagnosia is properly restricted to a disorder of face identity recognition, i.e., knowing who a particular person is from their face, not simply discriminating one unfamiliar face from another (De Renzi and Spinnler in fact allude to this in their notation that prosopagnosic patients have impaired recognition of “even those faces that have a name, for instance those of persons they know well”).
Warrington and James (1967) replicated and extended the findings from De Renzi and Spinnler, employing both a task of immediate recognition of unfamiliar faces and a task requiring identification of famous faces. Patients with right hemisphere damage were inferior to patients with left hemisphere damage and comparison patients (patients with peripheral nerve lesions) on both tasks. Interestingly, though, the patients with right hemisphere damage did not show a strong pattern of failing both tasks: of 21 patients who failed one or the other task, only 5 failed both, and Warrington and James suggested that the two tests “are measures of different functions.”
The first report that employed the FRT as we know it today was published in 1968 by Benton and Van Allen. This study explicitly removed any memory component, and focused on a procedure that purely tapped perceptual discrimination and matching. Specifically, participants were given a target unfamiliar face, and asked to match it to (1) identical front-view photographs, (2) three-quarter view photographs, and (3) front-view photographs under different lighting. This task was administered to patients with left or right hemisphere disease and healthy comparison participants, and it was found that patients with right hemisphere damage performed significantly lower than the other two groups (the left hemisphere group was slightly below the healthy group, but not significantly so). Moreover, “failure” on the test (performance below a cutoff score) was much more common in the right hemisphere group. The investigators did not find sex effects in the healthy comparison group (no gender information was provided for the lesion participants). There was no obvious association of performance on the FRT with specific subregions within hemisphere. Benton and Van Allen noted that the relationship of their findings to the symptom of facial agnosia “remains problematic,” and they specifically noted that their task called for “discrimination of unfamiliar faces” (as opposed to recognition of facial identity). Nonetheless, the test was labeled as the Facial Recognition Test, and the name has stuck, even though it is misleading in the sense that it implies some kind of recognition process in the task, which is not the case. (We have often used the label of “Facial Discrimination Test” to refer to the FRT, to be clear about its processing requirements, but the fact remains that the test is published as the Facial Recognition Test, so we are reluctant to depart entirely from the standard name. Hence, in the current study we will use the standard “Facial Recognition Test” designation, while cautioning that the FRT is not a test of face recognition per se.)
The same year as Benton and Van Allen’s paper on the FRT, De Renzi and colleagues (De Renzi, Faglioni, & Spinnler, 1968) published another study that focused on perceptual matching and brief memory (immediate, 30 second delay) for unfamiliar faces. Confirming and extending their previous results, the authors found that right hemisphere damaged patients, especially those with left visual field defects (implying retro-Rolandic lesions), performed more poorly on the various tasks than left hemisphere damaged patients. This included one test that called for matching a profile face (~three-quarters) with front-views, a task that is very similar to part II of the Benton and Van Allen FRT. The authors argued that the visual perceptual capacities needed for discrimination and immediate memory for unfamiliar faces appeared to be strongly related to right hemisphere structures, especially retro-Rolandic ones.
The full version of the Benton and Van Allen FRT comprised 22 items calling for a total of 54 responses. Benton and colleagues noted that the test could require 20 minutes or more to administer to brain-damaged patients, and in an effort to shorten the test for more practical clinical usage, Levin et al. (Levin, Hamsher, & Benton, 1975) published a short form of the FRT that had 13 items requiring 27 responses. The short form correlated highly with the full test in both healthy participants (r = .88) and patients with brain disease (r = .92). Levin et al. cautioned that scores in the borderline range on the short form (between 36 and 41, prorated) should probably prompt full examination with the long form in order to get the most reliable estimate of patient performance, but over time, the short form of the FRT found its way into widespread usage, and this is the form that is typically used in our Program. (We have on occasion probed the extent to which administration of the additional items in the long form changes the final score of a patient, and it rarely does by more than a point or two. Thus, we have used primarily the short form, as published by Levin et al. (1975).)
In a later study from Benton’s group, the emphasis on the FRT being associated with right hemisphere functioning was qualified somewhat (Hamsher, Levin, & Benton, 1979). Specifically, the authors reported that FRT performance was not only affected by right hemisphere lesions (especially posterior ones), but also by left hemisphere lesions that produced significant language comprehension defects. Hamsher et al., however, explained at some length that the poor performance of this latter group was not attributable to a basic inability to understand the test instructions, and the authors cited several factors that supported the claim that these patients did comprehend the basic demands of the task. Hamsher et al. went on to argue for the idea that facial discrimination was a “bihemispheric” process, and not exclusively under the purview of the right hemisphere.
In the latest version of the manual for Benton’s tests (Benton, Sivan, Hamsher, Varney, & Spreen, 1994), the studies cited in support of the neural correlates of the FRT are mainly those reviewed above. It is noted that the Hamsher et al. (1979) study seems to provide the one discrepant finding (with left hemisphere patients with comprehension defects having impaired FRT performance); otherwise, the FRT has been consistently linked to right hemisphere functioning, and especially posterior sectors of the right hemisphere (the authors note that this discrepant result “remains to be resolved”). And as noted below, a study by Mehta and colleagues (Mehta, Newcombe, & Damasio, 1987) that utilized the FRT found that patients with right hemisphere damage had the poorest performance, compared to patients with left hemisphere damage and healthy comparison participants. Lezak and colleagues (Lezak, Howieson, & Loring, 2004) also emphasize the association between FRT and the right parietal region. As far as sex effects are concerned, in the Benton et al. manual it is noted that men and women tend to perform similarly on the FRT, and there is no “correction” for sex (there are corrections for education and age). This conclusion was also supported by a large-scale study of normal individuals by Schretlen and colleagues (Schretlen, Pearlson, Anthony, and Yates, 2001), which found that FRT performances were very comparable for men and women.
Wasserstein, Zappulla, Rosen, and Gerstman (1984) reported FRT performance in 8 patients with right hemisphere lesions, and 4 of the patients had the “expected” poor performance while 4 were entirely normal. Only one of the patients, though, had a lesion that significantly involved the parietal region, and most of the patients had brain tumors (6/8), making lesion-deficit conclusions from this study questionable (cf. Anderson, Damasio, & Tranel, 1990). Another study from this group was reported more recently (Wasserstein, Barr, Zappulla, & Rock, 2004), but again, “most” of the patients had brain tumors, and inferences about lesion-deficit relationships are problematic. The focus of the Wasserstein et al. 2004 study was on how the perceptual demands of facial discrimination may relate to other perceptual capacities such as “closure” (e.g., as in the Mooney closure stimuli), but one finding reported by the authors was that a large percentage of right hemisphere lesion patients (80%) performed below the median of the left hemisphere patient group on the Benton FRT. (Again, we would use caution in interpreting the lesion-deficit results from both of the Wasserstein et al. studies, given the nature of the patient material and the small sample sizes.)
Püschel and Zaidel (1994) used the Benton/Van Allen face stimuli and a tachistoscopic presentation format, and found that there was a left hemifield (right hemisphere) advantage only for the “shadowed” (different lighting) stimuli, and not for the front-view or three-quarters view items. The authors suggested that this result could help explain the puzzling finding (e.g., as in Hamsher et al., 1979) whereby left hemisphere patients sometimes fail the FRT—that is, only one portion of the FRT (the “different lighting” condition) is truly a “pure” right hemisphere task, whereas the other portions are bihemispheric. The authors acknowledge, however, that there are substantial differences between unlimited free viewing (as in the standard administration of the FRT) versus tachistoscopic viewing, and this makes it difficult to draw direct comparisons to the FRT as used in clinical practice.
Trahan (1997) reported a study of stroke patients studied fairly acutely (within 6 weeks of onset), and confirmed that failure on the FRT was strongly associated with right hemisphere lesions. Not surprisingly, when patients had left visual neglect the FRT failure was even more prominent. FRT failure in left hemisphere patients was much less frequent (albeit not zero), and Trahan notes that this result is generally compatible with Hamsher et al.’s (1979) previous finding. The study did not address the issue of intrahemispheric localization, and Trahan noted that most of the lesions were middle cerebral artery strokes that involved both anterior and posterior sectors of the hemisphere.
In summary, there is consistent evidence that FRT performance, which is properly described as “visuoperceptual discrimination of unfamiliar faces” (and not facial “recognition”), is related to right hemisphere processing. Within the hemisphere, there is some indication that the posterior sector is more critical, but there is actually not much empirical evidence for this. There is an inkling that the left hemisphere makes a non-negligible contribution, and for the simpler aspects of the test (front-view and three-quarter view matching), may perhaps even be of comparable competence as the right hemisphere. We did not find any functional imaging studies using FRT stimuli.
Judgment of Line Orientation
An early study by Warrington and Rabin (1970) reported that patients with right parietal lesions had impaired performance on a variety of visual perceptual matching tasks, including a task that required matching of the slopes of line segments. Picking up on the line slope task idea, and using a tachistoscopic stimulus presentation, Benton, Hannay, and Varney (1975) found that patients with right hemisphere lesions performed worse than patients with left hemisphere lesions and healthy comparison participants (the latter two groups did not differ significantly) on judgments of line orientation. Benton et al. commented that poor performance was associated especially with frontal-parietal, frontal-temporal, and parietal damage (but not with strictly prefrontal damage), although no actual neuroanatomical analysis was performed. Right-hemisphere damaged men (9/16 defective) and women (4/6 defective) appeared to have similar failure rates.
In a follow-up study, Benton and colleagues (Benton, Varney, & Hamsher, 1978) adapted the line orientation stimuli to be more suitable for clinical application, and not dependent on a tachistoscope to administer. The full line segments that had been used in the tachistoscope version proved too easy in free viewing conditions, so Benton et al. created a version of the test in which the full line segments were used as practice items, and for the test items, only shortened, partial line segments were used (making the task more difficult). Benton et al. (1978) replicated the basic finding that patients with right hemisphere damage were inferior to patients with left hemisphere damage and healthy comparison participants on judgments of line orientation; again, left hemisphere and normal groups were not significantly different. This study also replicated the within-hemisphere finding of the previous study, namely, that more posterior right hemisphere lesions (parietal, parietal-occipital, parietal-temporal) were associated with poor performance, whereas right prefrontal lesions were not. The sex of the brain-damaged participants was not reported, but the authors did note that 2 points were added to the scores of women to “correct” their scores to be comparable to those of men (a correction for education was also incorporated).
Some years later, Hannay and colleagues (Hannay, Falgout, Leli, Katholl, Halsey, & Wills, 1987) used a focal cerebral blood flow measurement (133Xe inhalation) to show that performance on a judgment of line orientation task produced blood flow changes in the right temporal-occipital region. Hannay et al. used a two-alternative forced choice matching format, and their stimuli actually had some important differences from the line orientation task that Benton and colleagues (1978) had been using. Specifically, 80% of the stimuli called for a shape (or configuration) discrimination, rather than a spatial judgment per se, and this would help explain why the blood flow changes in the Hannay et al. study were mainly temporal-occipital, rather than temporal-parietal as might have been expected if the stimuli had been more strictly spatial in nature. In any event, the Hannay et al. results were broadly consistent with the lesion evidence from Benton and colleagues (1978). Also, a similar result was reported by Gur and colleagues (Gur, Gur, Obrist, Skolnick, & Reivich, 1987), who found that performance of a judgment of line orientation task differentially increased cerebral blood flow in the right hemisphere (as contrasted with blood flow changes during performance of a verbal analogies task).
Discrepant results were reported by Mehta et al. (Mehta, Newcombe, & Damasio, 1987), who found that in a sample of men with penetrating missile brain wounds, participants with left hemisphere injuries performed significantly worse on the JLO test than healthy comparison participants. Participants with right hemisphere damage had lower JLO scores than the comparison group, but actually performed better (although not statistically so) than the left hemisphere group. (As noted earlier, the Benton FRT was also employed in this study, and on that measure the RH group performed significantly below the comparison group. The LH group had a lower mean score than the comparison group, but this difference was not statistically significant.) Mehta and Newcombe (1991) reported similar results in a subsequent study that included mostly (75%) the same patients as who had been reported in the 1987 study, again finding that the LH group was significantly inferior to a healthy comparison group on JLO performance, whereas the RH group was not significantly below the comparison group (although the RH mean score was lower than that of the comparison group). Both studies make the point that aphasic handicaps in the LH group cannot explain their spatial processing deficits, although neither study provides much information about language performances in the LH patients, and the 1987 study does not comment on comprehension per se. The two Mehta studies seem to raise a question about whether there might be a significant left hemisphere contribution to the processing requirements for JLO, although it has to be said that these results appear at odds with the prevailing lore in the field, not to mention nearly all other empirical reports of JLO findings.
In the manual for Benton’s tests (Benton et al., 1994), the authors assert that right posterior lesions are the most consistent correlate of failure on JLO, and they mention that the relationship between poor JLO performance and right hemisphere disease has been “fully confirmed.” Findings from Levick (1982), Trahan (1991), and Hamsher, Capruso, and Benton (1992) are adduced in support of this claim (we note, however, that the Levick study is a Master’s thesis and the Trahan study is an abstract). Hamsher et al. (1992) tackled directly the contrary Mehta et al. (1987) study, and reported on JLO performances in groups of patients with focal LH damage or RH damage. The standard finding of poorer JLO performance in the RH patients was noted, and failure rate on JLO was also much higher in the RH group. Hamsher et al. suggested that the discrepant Mehta et al. results might be explained by the “atypical nature of their case material,” and by the fact that Mehta et al. did not age-correct the JLO scores of their participants. In the latest version of Lezak’s tome on neuropsychological assessment (Lezak et al., 2004), the association between JLO performance and right posterior brain regions continues to be emphasized.
Trahan (1998) reported JLO performances in patients with right or left hemisphere lesions caused by stroke, tested within 6 weeks of onset (this appears to be mostly the same sample reported for the Trahan (1997) paper on FRT performance, as described earlier). The study confirmed earlier work in finding that JLO failure was substantially higher in patients with right hemisphere damage, and not surprisingly, JLO performance was even worse when the patients had left visual neglect. JLO failure in the left hemisphere group was not common, but it did occur in about one-fourth (26%) of the sample. As in the FRT study (Trahan, 1997; see above), the author was not able to comment on within-hemisphere localization, as many of the patients had lesions that involved both anterior and posterior sectors.
One modern functional imaging study using JLO stimuli has been reported (Kesler, Haberecht, Menon, Warsofsky, Dyer-Friedman, Neely, & Reiss, 2004). The investigators used “easy” and “hard” JLO-type items. The easy ones had only 5 line foils, whereas the hard items used 11 line foils (as in the standard array in the clinical version of JLO). The authors found that performance on the difficult JLO items activated not only visuospatial areas such as the parietal and occipital regions, but also dorsolateral prefrontal structures, suggesting an “executive function” aspect to task performance. Activations tended to be bilateral, but more extensive in the right hemisphere.
To summarize, the empirical work available in regard to the neuroanatomical correlates of JLO performance consistently points to a significant role of the right hemisphere, especially posterior sectors including parietal and parietal-occipital or parietal-temporal regions. Most of this work, though, was performed more than two decades ago, and there are no large-scale studies that have revisited this issue using modern lesion-deficit analysis techniques and capitalizing on advances in neuroimaging technology.
The Current Study
In the current investigation, we took advantage of a large database at the University of Iowa, to explore anew the neuroanatomical correlates of FRT and JLO. The database comprises a Neurological Patient Registry maintained in the Division of Behavioral Neurology and Cognitive Neuroscience in the Department of Neurology at the University of Iowa. We capitalized on this resource to test the following hypotheses: (1) Impaired performance on the Benton Facial Recognition Test will be associated with lesions in right parietal and right occipitotemporal regions; and (2) Impaired performance on the Benton Judgment of Line Orientation Test will be associated with lesions in the right parietal and right occipitoparietal regions.
METHODS
Participants
The participants were neurological patients with focal brain damage selected from the Iowa Neurological Patient Registry in the Division of Behavioral Neurology and Cognitive Neuroscience at the University of Iowa. Participants were included if they had (1) an FRT or JLO performance from the chronic epoch (defined as 3 months or more post lesion onset), and (2) a single, focal lesion in one hemisphere (or, in bilateral cases, two focal lesions, one in each hemisphere; see below). We also required that the participants be right-handed, which we defined as +70 to +100 on the Geschwind-Oldfield Handedness Questionnaire (this requirement was implemented to avoid ambiguities associated with hemispheric laterality in non-right-handers). For the FRT, we had 201 patients who met these entry criteria, and for JLO we had 181 patients who met the criteria. For the 201 FRT patients, there were 91 with unilateral left hemisphere lesions, 77 with unilateral right hemisphere lesions, and 33 with bilateral lesions. For the 181 JLO patients, there were 75 with unilateral left hemisphere lesions, 75 with unilateral right hemisphere lesions, and 31 with bilateral lesions.
All patients had provided informed consent in accordance with the Human Subjects Committee of the University of Iowa and federal guidelines. In connection with their enrollment in the Patient Registry, the patients have been extensively characterized neuropsychologically and neuroanatomically, using standard protocols of the Benton Neuropsychology Laboratory (Tranel, 2007) and the Human Neuroimaging and Neuroanatomy Laboratory (Frank et al., 1997). All data, including the neuropsychological data and the neuroimaging data, were collected in the chronic phrase of recovery, 3 or more months post lesion onset.1
Demographic characteristics of the samples are provided in Table 1, where the groups are broken down according to whether they performed in the impaired or unimpaired range on the FRT or JLO test, respectively (see below for classification). It can be seen that for both tests, the impaired and unimpaired subgroups are very comparable in terms of age, gender ratio, and education. Also, the chronicity data show that all of the subgroups were on average some two to three years post-lesion onset when they received the FRT or JLO tests. For both tests, the impaired and unimpaired subgroups did not differ significantly on the chronicity variable (see Table 1), and the fact that the patients were well into the chronic epoch at the time of FRT or JLO testing helps discount any possibility that recovery factors could account for differences between the impaired and unimpaired groups.
Table 1.
Demographic characteristics of the samples.
| Test/Subgroup | N | Age | Gender | Education | Chronicity | |
|---|---|---|---|---|---|---|
| FRT | Impaired | 38 | 54.2 (13.2) | 11W; 27M | 12.8 (2.4) | 3.2 (4.7) |
| Unimpaired | 163 | 50.8 (15.5) | 72W; 91M | 12.9 (2.4) | 2.4 (3.3) | |
| JLO | Impaired | 23 | 51.7 (13.7) | 13W; 10M | 12.4 (2.1) | 2.3 (3.6) |
| Unimpaired | 158 | 51.1 (15.9) | 62W; 96M | 13.1 (2.5) | 3.0 (3.9) | |
N = number of participants; W = women; M = men. The data for Age, Education, and Chronicity are given as means (SDs in parentheses), in years. Chronicity refers to the time since lesion onset that the patients received the FRT and JLO tests. Subgroup differences in Age, Education, and Chronicity for the two tests (FRT, JLO) were not significant per t-tests (all p’s > .20).
Neuropsychological Data Quantification
The FRT and JLO tests were administered according to the standard instructions described in the manual (Benton et al., 1994). The scoring of both tests followed the recommendations in the manual, and age-, education-, and sex-related corrections were added to the raw scores as appropriate. Specifically, for FRT, scores were corrected for age and education according to the manual (Table 4–6, p. 45), and for JLO, scores were corrected for age and sex according to the manual (Table 5–2, p. 58). Then, using the corrected scores, we applied the cutoffs from the manual to classify patients as “impaired” or “unimpaired.” Specifically, for FRT this meant that scores of 41 or above (corresponding to the 16th %ile or above) were classified as unimpaired, and scores of 40 or less were classified as impaired. (Scores of 39 and 40 are labeled as “borderline” in the Benton Manual, and we put these together with scores below 39 to form one “impaired” group.) For JLO, scores of 21 or above (corresponding to the 22nd %ile or above) were classified as unimpaired, and scores of 20 or less were classified as impaired. (Scores of 19 and 20 are labeled as “borderline” in the Benton Manual, and we put these together with scores below 19 to form one “impaired” group.) For both tests, the rationale for including borderline scores with impaired scores was that we wanted to err on the side of being broadly inclusive for the “impaired” groups, which enhances the power of the lesion-deficit analyses and thereby facilitates the possibility of identifying reliable neuroanatomical correlates for each test. Also, the test score distributions for the FRT and JLO tests are such that the percentiles we adopted for cutoff scores (16th and 22nd for the FRT and JLO tests, respectively) are fairly comparable (see pp. 45 and 59 in the Benton et al., 2004, manual).
Neuroanatomical Data Quantification
The neuroanatomical analysis was based on magnetic resonance (MR) data obtained in a 1.5 Tesla General Electric Sigma scanner with a 3D SPGR sequence yielding 1.5 mm contiguous T1 weighted coronal cuts, or, in a few subjects in whom an MR could not be obtained, on computerized axial tomography (CT) data. Lesion mapping on a reference brain was performed according to MAP-3 lesion analysis methods, using the Brainvox programs (Frank et al., 1997). This method entails a transfer of the lesion brain to a common space in a template brain (see Damasio et al., 2004). In order to facilitate reliable lesion transfer, all major sulci of the lesion brain were color-coded in both the lesion brain and the template brain. Then, the template brain was resliced to match the orientation and thickness of the slices in the native image space of the lesioned brain. After this, the lesion on each native space slice was transferred manually to the corresponding slice in the template brain, respecting identifiable anatomical landmarks. In all instances, this process was performed and reviewed by a neuroanatomical expert. Each lesion was then entered into group lesion overlap analysis in the template brain space (see below).
Data Analysis
To analyze the data in regard to our hypotheses, we grouped the subjects according to their performances on the FRT or JLO tests, and then analyzed the neuroanatomical results. The lesion analysis followed an approach that we have used previously with comparable datasets (Adolphs et al., 2000; Damasio et al., 2004; Tranel et al., 2001, 2003), with a refinement modeled on recent developments described by Rudrauf et al. (2008). Specifically, we contrasted the lesions of the group of participants who were in the impaired range on each test, with the lesions of the group of participants who were in the unimpaired range on each test (as defined above). Thus, neuropsychological performance (FRT or JLO) served as the independent variable, and lesion overlap (according to proportional MAP-3) served as the dependent variable.
The proportional MAP-3 (hereafter referred to as PM3) statistic is calculated as follows. For a given sample of N participants, at a given brain voxel v, the PM3 is the proportion of participants with a lesion and a deficit (NLD) relative to all participants with a deficit (ND), minus the proportion of participants with a lesion and no deficit (NL~D) relative to the all participants with no deficit (N~D). Thus, at a given voxel, the lesion proportion difference map PM3 is defined by the following equation:
It can be noted that this calculation takes into account, at any given brain voxel v, the number of participants with a lesion and a deficit relative to the number of participants with a deficit, and the number of participants with a lesion and no deficit relative to the number of participants with no deficit. An extended presentation of this method is presented in Rudrauf et al. (2008), but for current purposes, suffice it to say that as the value of PM3 increases, the evidence that there is a relationship between the presence of a lesion and the presence of an impairment becomes stronger, i.e., for that voxel, a larger proportion of the impaired participants have a lesion at that voxel (“true positives,” as it were), and a larger proportion of the unimpaired participants do not have a lesion at that voxel (“true negatives,” as it were).
The PM3 map was constructed for all voxels in the brain, and then color-coded so that stronger lesion-deficit relationships are coded by “warmer” colors (with the highest set for red, which is at a proportion difference outcome of +0.2 or higher; see Figure 1 and Figure 2), and weaker lesion-deficit relationships are coded by “cooler” colors (with the lowest set for blue, which is at a proportion difference outcome of −0.2 or lower). The color codes for the PM3 are displayed on the surfaces of the right and left hemispheres, and lateral, inferior, and superior perspectives are rendered in the relevant Figures. In this study, we interpret proportion difference values of +0.2 or higher as indicative of a specific lesion-deficit relationship, although at the same time, it should be noted that the PM3 maps we present are maps of descriptive, as opposed to inferential, statistics. PM3 maps were calculated for the FRT and JLO tests, respectively.
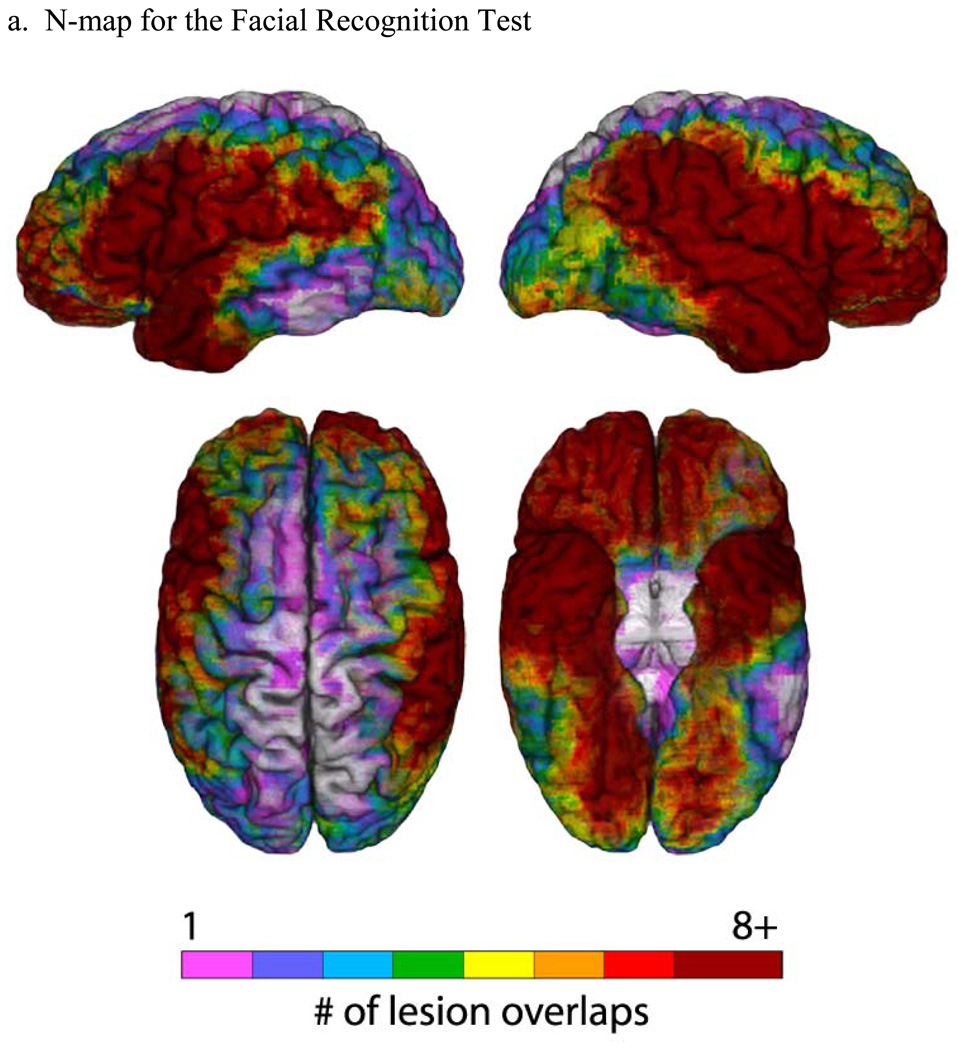
Figure 1. Lesion maps for the Facial Recognition Test.
(a) An N-map, showing the number of lesion overlaps in the entire sample of participants who performed the FRT (overlaid on a reference brain). The colorbar indicates different degrees of lesion overlap, from 1 up to 8, with numbers higher than 8 all coded to dark red. (b) PM3 (lesion proportion difference) map, where the PM3 results are overlaid on a reference brain. Positive values (colorbar) indicate a greater proportion of participants with a lesion and a deficit among those with a deficit, compared to the proportion of participants with a lesion and no deficit among those with no deficit. Negative values (colorbar) indicate a lower proportion of participants with a lesion and a deficit among those with a deficit, compared to the proportion of participants with a lesion and no deficit among those with no deficit. For both (a) and (b), the color-coded outcomes are rendered on left lateral (upper left), right lateral (upper right), superior (bottom left), and inferior (bottom right) hemispheric perspectives.
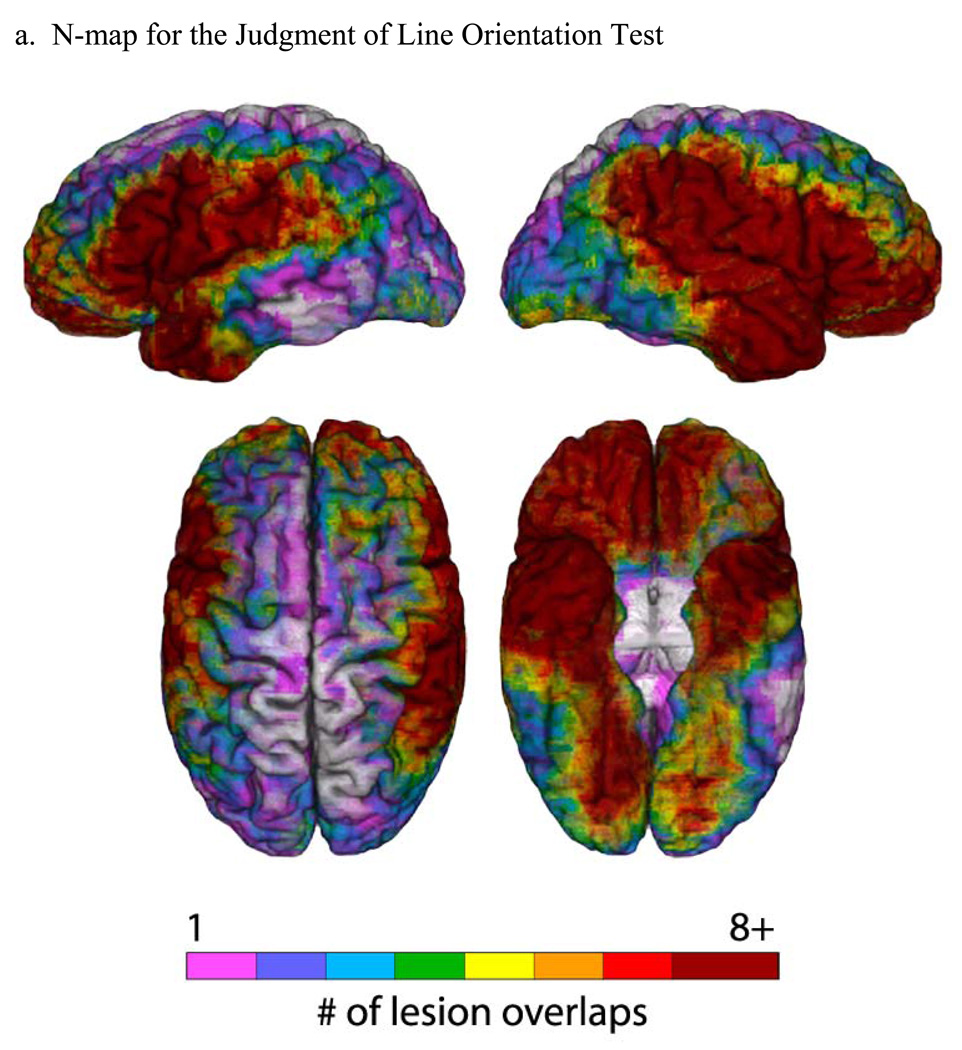
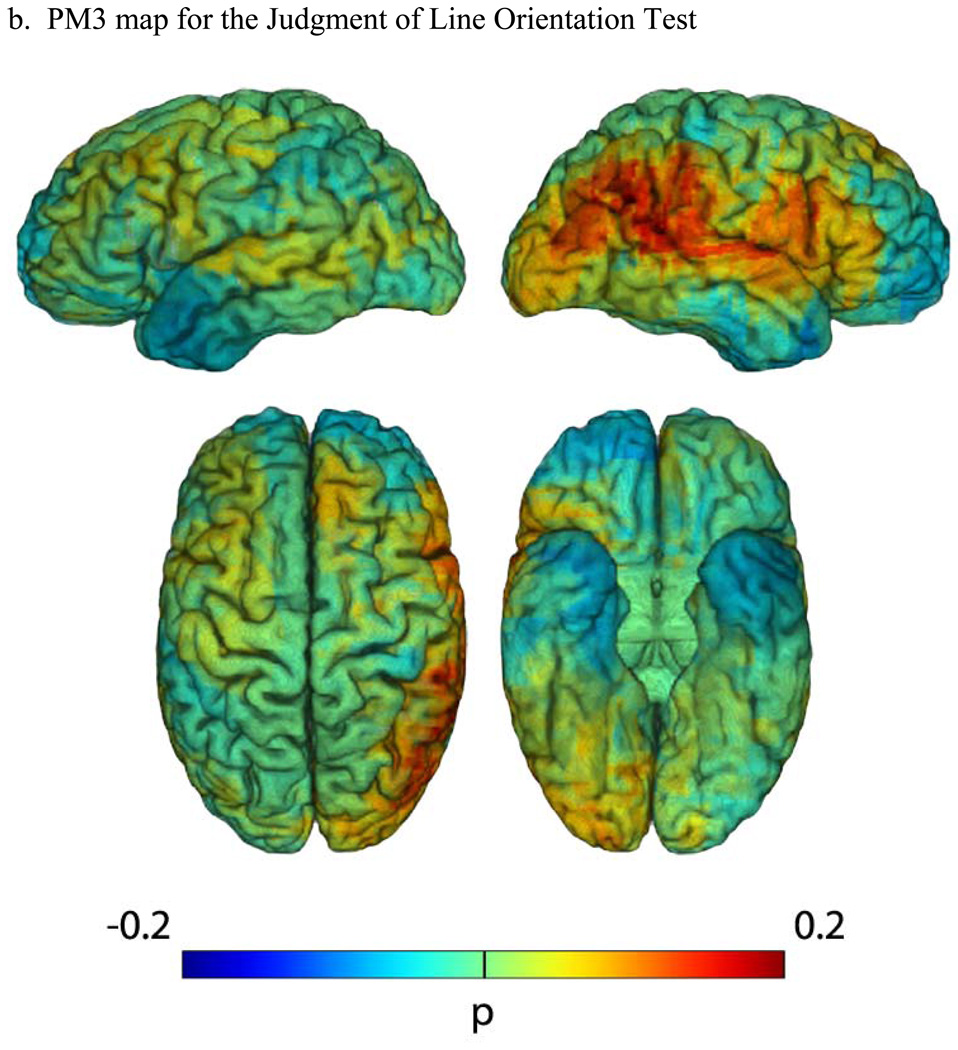
Figure 2. Lesion maps for the Judgment of Line Orientation Test.
(a) An N-map, showing the number of lesion overlaps in the entire sample of participants who performed the JLO test (overlaid on a reference brain). The colorbar indicates different degrees of lesion overlap, from 1 up to 8, with numbers higher than 8 all coded to dark red. (b) PM3 (lesion proportion difference) map, where the PM3 results are overlaid on a reference brain. Positive values (colorbar) indicate a greater proportion of participants with a lesion and a deficit among those with a deficit, compared to the proportion of participants with a lesion and no deficit among those with no deficit. Negative values (colorbar) indicate a lower proportion of participants with a lesion and a deficit among those with a deficit, compared to the proportion of participants with a lesion and no deficit among those with no deficit. For both (a) and (b), the color-coded outcomes are rendered on left lateral (upper left), right lateral (upper right), superior (bottom left), and inferior (bottom right) hemispheric perspectives.
RESULTS
Neuropsychological Test Performances
As a first consideration, we present the FRT and JLO performances for different subgroups of participants, and verify the classification of participants as impaired or unimpaired as described above.
Facial Recognition Test
For the FRT, the impaired group had an average score of 36.7 (SD = 3.1), and the unimpaired group had an average score of 45.6 (SD = 3.1) (Table 2). These means were significantly different in a t-test: t(199) = 16.0, p < .001; mean difference = 8.9, 95% Confidence Interval of the Difference = 7.8 to 10.0. (We report this contrast for the sake of completeness, acknowledging that the a priori classification of the participants virtually guarantees that the groups will have significantly different scores.) For the 38 impaired patients, 12 had left hemisphere lesions (7 men, 5 women), 17 had right hemisphere lesions (14 men, 3 women), and 9 had bilateral lesions; for the 163 unimpaired patients, 79 had left hemisphere lesions (46 men, 33 women), 60 had right hemisphere lesions (36 men, 24 women), and 24 had bilateral lesions. Overall, there were 11 women and 27 men in the impaired group, and 72 women and 91 men in the unimpaired group (see Table 1). We ran Chi square tests on the gender distributions for the left hemisphere and right hemisphere subgroups, and the outcomes were nonsignificant for both the impaired (p = 0.15) and unimpaired (p = .83) groups, indicating statistically comparable gender representation in the impaired and unimpaired subgroups for each hemisphere.
Table 2.
Performances on the Facial Recognition Test and Judgment of Line Orientation Test
| Test/Subgroup | N | Score | Lesion Side | |
|---|---|---|---|---|
| FRT | Impaired | 38 | 36.7 (3.1) | 12 LH; 17 RH; 9 BL |
| Unimpaired | 163 | 45.6 (3.1) | 79 LH; 60 RH; 24 BL | |
| JLO | Impaired | 23 | 16.3 (4.1) | 7 LH; 12 RH; 4 BL |
| Unimpaired | 158 | 26.4 (3.2) | 68 LH; 63 RH; 27 BL | |
N = number of participants; Score is mean score, number correct (SD in parentheses); LH = left hemisphere, RH = right hemisphere, BL = bilateral. For both the FRT and JLO tests, the impaired and unimpaired groups had significantly different performances (see text for details).
In the entire sample of 201 patients, men and women performed similarly and not significantly different statistically: for men, M = 43.5, SD = 4.8; for women, M = 44.5, SD = 4.4 (p = .132 in a t-test).
We also looked at the FRT performances as a function of hemispheric side of lesion (Table 3). As the data in the Table show, the lowest mean FRT score was in the bilateral group, followed by the right hemisphere group, with the left hemisphere group performing the best. The main effect of group was significant in a one-way ANOVA (F(2,198) = 4.76, p = .01), but follow-up tests indicated that the only pair-wise contrast that was significant was between the left hemisphere group versus the bilateral group (mean difference = 2.79, p = .009, 95% Confidence Interval of the Difference = .54 to 5.03). The right hemisphere group did not differ significantly from the left hemisphere group, even though the right hemisphere group did have a slightly lower mean score (p = .249). The three groups were not significantly different on age or education (p’s > .19).
Table 3.
Performances on Facial Recognition Test and Judgment of Line Orientation Test as a function of hemispheric side of lesion, for all participants
| Test: Hemisphere | N | Test Score | Age | Education |
|---|---|---|---|---|
| FRT: Left | 91 | 44.9 (4.1) | 51.4 (14.9) | 12.7 (2.2) |
| FRT: Right | 77 | 43.6 (4.9) | 50.1 (16.0) | 13.3 (2.4) |
| FRT: Bilateral | 33 | 42.1 (5.0) | 54.9 (13.5) | 12.6 (2.9) |
| JLO: Left | 75 | 25.8 (3.9) | 50.9 (15.5) | 12.8 (2.2) |
| JLO: Right | 75 | 24.6 (5.0) | 49.9 (16.4) | 13.2 (2.4) |
| JLO: Bilateral | 31 | 24.5 (5.6) | 54.7 (13.8) | 12.7 (2.9) |
Test score is the mean number correct (SD in parentheses); Age and Education are means, in years (SDs in parentheses)
We also looked at FRT performances in the subset of impaired participants (overall N = 38), as a function of hemisphere side of lesion (Table 4). As might be expected, the left hemisphere group participants had the least impaired average score (37.5), followed by the right hemisphere participants (36.9) and then the bilateral participants (35.2). The between-group differences were not statistically significant, though, per one-way ANOVA.
Table 4.
Performances on Facial Recognition Test and Judgment of Line Orientation Test as a function of hemispheric side of lesion, for Impaired participants
| Test: Hemisphere | N | Test Score |
|---|---|---|
| FRT: Left | 12 | 37.5 (2.5) |
| FRT: Right | 17 | 36.9 (3.5) |
| FRT: Bilateral | 9 | 35.2 (2.9) |
| JLO: Left | 7 | 18.0 (1.5) |
| JLO: Right | 12 | 16.2 (4.2) |
| JLO: Bilateral | 4 | 13.8 (6.1) |
Test score is the mean number correct (SD in parentheses).
Judgment of Line Orientation Test
For the JLO, the impaired group had an average score of 16.3 (SD = 4.1), and the unimpaired group had an average score of 26.4 (SD = 3.2) (Table 2). These means were significantly different in a t-test: t(179) = 13.5, p < .001; mean difference = 10.0, 95% Confidence Interval of the Difference = 8.6 to 11.5. (Again, we report this contrast for the sake of completeness, acknowledging that the a priori classification of the participants virtually guarantees that the groups will have significantly different scores.) For the 23 impaired patients, 7 had left hemisphere lesions (3 men, 4 women), 12 had right hemisphere lesions (6 men, 6 women), and 4 had bilateral lesions; for the 158 unimpaired patients, 68 had left hemisphere lesions (42 men, 26 women), 63 had right hemisphere lesions (41 men, 22 women), and 27 had bilateral lesions. There were 13 women and 10 men in the impaired group, and 62 women and 96 men in the unimpaired group (see Table 1). We ran Chi square tests on the gender distributions for the left hemisphere and right hemisphere subgroups, and the outcomes were non-significant for both the impaired (p = 0.76) and unimpaired (p = .69) groups, indicating statistically comparable gender representation in the impaired and unimpaired subgroups for each hemisphere.
In the entire sample of 181 patients, men outperformed women by a small but statistically significant margin: for men, M = 25.7, SD = 4.5; for women, M = 24.2, SD = 4.9 (t(179) = 2.1, p < .05; mean difference = 1.5, 95% Confidence Interval of the Difference = .12 to 2.90). This difference, albeit small, is interesting to compare to the normative observations published in the Benton Manual (obtained from what the authors call a group of “normal subjects or control patients”). Specifically, the men in the Benton Manual group, with scores ranging from 25.3 to 25.7 in different age brackets, performed almost identically to the men in our current study (25.7), whereas the women in the Benton Manual group, with scores ranging from 25.2 to 25.8, outperformed the women in our study (24.2) (remember that in all cases, the JLO raw scores have been age and gender corrected). This, even after the Benton Manual correction for gender, we observed a sex-related effect on the JLO test performance, with men outperforming women by a small margin.
We also looked at JLO performances as a function of hemispheric side of lesion (Table 3). As the data in the Table show, the bilateral group and right hemisphere group had very similar scores, and the left hemisphere group performed somewhat better. The main effect of group was not significant in a one-way ANOVA, however (p = .243). The three groups were not different in terms of age and education (p’s > .35).
We also looked at JLO performances in the subset of impaired participants (overall N = 23), as a function of hemisphere side of lesion (Table 4). Similar to the pattern we observed for the FRT, the left hemisphere group participants had the least impaired average score (18.0), followed by the right hemisphere participants (16.2) and then the bilateral participants (13.8). The between-group differences were not statistically significant per one-way ANOVA. It is interesting, however, that for both FRT and JLO, when failure occurs it tends to be more severe with bilateral lesions and least severe with left hemisphere lesions, with right hemisphere lesions falling somewhere in between.
Failure on both the Facial Recognition Test and Judgment of Line Orientation Test
One additional question of interest in regard to the behavioral results was the extent to which participants would evidence impairments on both the FRT and JLO tests. In our sample, there were 156 patients who had taken both tests. Of this group, there were 17 who were impaired on one or the other of the tests (11 on FRT, 6 on JLO), and 5 who were impaired on both tests. In the 5 patients who failed both tests, 2 had bilateral lesions, 2 had right hemisphere lesions, and 1 had a left hemisphere lesion. The numbers here are probably too small to yield reliable results, but one conclusion that can be drawn from these data is that failure on both the FRT and JLO tests in chronic brain-damaged patients is rather rare, occurring with a frequency of 5/156 (or about 3%) in our sample. And failure on both tests would appear to be extremely rare in the setting of a single, unilateral left hemisphere lesion, as we turned up only 1 patient with such a lesion who failed both tests, in the sample of 156 total patients. However, it is important to interpret this outcome in the context of our chronicity data (reported earlier, and presented in Table 1), which show that our patients were administered the FRT and JLO tests some two to three years post lesion onset, on average, which is well into the chronic recovery epoch. It seems quite possible, and even likely, that more acute assessment, e.g., within the first three months after lesion onset, would yield a higher rate of test failure.
Neuroanatomical Results
The results from the neuroanatomical analyses are presented in Figure 1 and Figure 2, for the FRT and JLO tests, respectively. In both Figures, the first part (a) is an “N map” that shows the lesion overlaps for the entire sample of participants who took the test (including both unilateral and bilateral cases). These maps provide information about the lesion sampling for each test in our study. As Figure 1a and Figure 2a show, there is good sampling in the regions of the middle cerebral artery territory, anterior temporal lobe, and orbital prefrontal cortex in both hemispheres. Areas that are fairly underrepresented include the superior-mesial aspects of both hemispheres, and the lateral inferotemporal region, especially more posteriorly and especially on the left. Where there is low lesion coverage, we simply cannot reach conclusions regarding whether or not those areas might be critical to performance on the FRT and JLO tests, and this is a limitation of the study. Importantly, there is good lesion sampling of the parietal regions, especially on the right. The lesion sampling is fairly similar for the two tests. There is no indication in the lesion sampling distributions that we systematically over- or under-represented either smaller or larger lesions in either the left or right hemispheres, and the proportional MAP-3 calculation, combined with consideration of the lesion sampling distribution, allows us to exclude lesion volume as an explanation for the lesion-deficit relationships we obtained.
Facial Recognition Test
The PM3 results provide clear support for our hypothesis that failure on the FRT would be associated with right parietal and right occipitotemporal lesions (Figure 1b). The lateral right hemisphere depiction shows that the highest lesion proportion difference value (0.2) is in the posterior-inferior parietal region (specifically, in the angular gyrus), with extension into the adjacent lateral superior occipital cortices and the superior temporal gyrus. In the inferior view, it can be seen that there is also a high lesion proportion difference in the inferior occipitotemporal region, in what corresponds to the fusiform gyrus and appears to encompass the so-called “fusiform face area” that has been reliably identified in functional imaging studies (e.g., Gauthier et al., 1999; Kanwisher et al., 1997; Puce et al., 1996). Finally, there is a small area of high lesion proportion difference in the right inferior frontal region of the precentral gyrus, at the foot of the motor/premotor strips (the motor face area). There are no areas of notably high PM3 values in the left hemisphere. Overall, the results suggest that impairment on the FRT is most strongly associated with the right inferior posterior parietal and inferior occipitotemporal regions.
Judgment of Line Orientation Test
The PM3 results (Figure 2b) provide clear support for our hypothesis that failure on the JLO test would be associated with right parietal and right occipitoparietal lesions. The lateral right hemisphere depiction shows that the highest values of lesion proportion difference (0.2) are in the posterior parietal region (specifically, the angular gyrus and posterior supramarginal gyrus), with extension into the adjacent lateral superior occipital cortex and the superior temporal gyrus. There are no other areas of notably high PM3 values for JLO, in either the right or left hemispheres. There is no evidence of involvement of the inferior occipitotemporal regions. The overlap in the right inferior posterior parietal region is similar to that found in this region for FRT.
Unilateral and bilateral lesions
An issue that is pertinent to the consideration of the results for both tests is the inclusion of participants with bilateral lesions. (The participants with unilateral lesions are straightforward, as there is only one lesion that could be contributing to a performance deficit.) The bilateral lesions, by themselves, cannot adjudicate the critical contribution from either the left-sided or right-sided lesion to a performance deficit. When analyzed using the approach of proportional MAP-3 as described above, though, these cases add power to the analyses and help sharpen the lesion-deficit relationships and inferences that can be derived. In fact, for both the FRT and JLO tests, the lesion-deficit findings revealed in the proportional MAP-3 results suggest that it is the right hemisphere lesion components, and not the left hemisphere ones, that are contributing to test failure (a more detailed consideration of these issues is presented in Rudrauf et al., 2008). In all views of the left hemispheres (Figure 1b and Figure 2b), there is no indication of notable lesion proportion difference associated with defective FRT or JLO performance. A caution here, though, is that in bilateral cases who failed the FRT or JLO tests, it could still be the fact that the right hemisphere component of the lesion would not have produced a defect by itself, but when combined with a left-sided lesion (perhaps thereby weakening potential compensatory mechanisms), did lead to a deficit. And it should be clear in any event that we are not arguing that the left hemisphere is unimportant for performance on these tasks—we are simply making the point, which recapitulates the early literature from Benton and colleagues (summarized in Benton et al., 1994), that right hemisphere damage is more frequently associated with FRT and JLO test failure.
DISCUSSION
For two of the classic Benton tests, the Facial Recognition Test and the Judgment of Line Orientation test, we found that impaired performance was associated with discrete neuroanatomical loci, which lined up well with the traditional lore about the “localization” of these tests. Specifically, failure on the FRT was associated with damage to the right inferior-posterior parietal region (specifically, in the angular gyrus) and extending into lateral superior occipital gyri, and with damage in the mesial inferior occipitotemporal region in the territory of the fusiform gyrus. (There were minor additional neural correlates of impaired FRT performance in the right inferior precentral gyrus (motor face area) and in the right superior temporal gyrus.) Failure on the JLO test was associated with damage to the right posterior parietal region (specifically, in the angular gyrus and posterior supramarginal gyrus) and occipitoparietal region (specifically, extending into the lateral superior occipital gyri). These findings are, in fact, remarkably consistent with the traditional teaching about the localization value of these tests (e.g., Benton et al., 1994; Lezak et al., 2004), but they add some empirical teeth to these notions, using detailed modern lesion analysis techniques. It is also intriguing that the two tests seem to be a bit differentially skewed to the classic “what” (for FRT) and “where” (for JLO) visual processing streams, respectively, which makes sense given the nature of the stimuli and the putative processing requirements of the tests (cf. Goodale & Milner, 1992; Ungerleider & Mishkin, 1982)
For the FRT, the area of high lesion proportion difference in the right fusiform gyrus (in the vicinity of the fusiform face area, or FFA) is intriguing and warrants a comment. The FFA is very reliably activated by face stimuli in functional imaging studies (e.g., Gauthier et al., 1999; Kanwisher et al., 1997; Puce et al., 1996). It is interesting that damage to this area on the right is reliably associated with impaired discrimination of faces, as suggested by our results. In fact, our findings can be taken as further evidence that the FFA is strongly linked to face processing (even if not exclusively so, a debate that is beyond the scope of the current discussion; see Gauthier et al., 1999; Kanwisher, 2000). The current findings also suggest that the right FFA, and not the left, is more critical for face processing. This outcome fits with the general notion that face processing, including recognition of facial identity, is preferentially performed by right hemisphere structures, even if there are some important contributions from left hemisphere structures (e.g., Damasio et al., 1990).
The motor/premotor finding in our study is also intriguing, and although we did not have an a priori hypothesis about this, it is tempting to interpret this finding in the general context of mirror neuron theory (e.g., Rizzolatti & Craighero, 2004). For example, this finding fits with the notion that to solve problems that involve body parts or more complex “theory of mind” demands, there is utilization of brain regions that are dedicated to mapping these parts in the observer. In other words, it could be that the perceptual matching of unfamiliar faces on the FRT would be facilitated by activation of the motor face area, akin to the manner in which activation of specific body part sensorimotor maps occurs during perceptual tasks involving others’ body parts (e.g., Iacoboni et al., 1999). This idea could be tested using a functional imaging approach. In any event, this line of reasoning—which is admittedly speculative—could help explain why failure on FRT is associated with damage in the motor face region.
One other issue that warrants a comment is in regard to the similarities and differences between the FRT and JLO tests, vis-à-vis neuroanatomical correlates. Our study revealed some clear similarities, with the right angular gyrus in particular standing forth as a common neuroanatomical correlate, along with the posteriorly adjacent lateral superior occipital gyri (compare Figure 1b and Figure 2b). This commonality is not unexpected, given that both tests place demands on visuoperceptual processes, especially ones that are not easily amenable to verbal mediation. The tests also had some interesting differences, however. The FRT in general tends to line up with regions that are more known for playing a role in face processing per se, especially the right inferior occipitotemporal (fusiform gyrus) region. The JLO test, on the other hand, tends to line up somewhat more with “dorsal stream” processing systems, including the posterior sector of the supramarginal gyrus, regions that are more strongly connected to spatial functions per se. As an oversimplification, one could think of the FRT as being more of an “occipitotemporal” test, and JLO as being more of an “occipitoparietal” test. Again, this harkens back to the classic distinction between ventral “what” and dorsal “where” visual processing streams, as noted earlier.
There are some limitations in our study. Perhaps the main one is the limitation imposed by lesion coverage. In a human lesion sample, needless to say, we do not have formal experimental control over the locations of the lesions, and there are areas in the brain that are simply not sampled very frequently by naturally-occurring or surgically-induced lesions. As noted earlier, such areas in the current sample included the high, mesial aspects of both hemispheres, and the lateral inferotemporal region especially posteriorly and especially in the left hemisphere. We cannot comment on whether or not these areas might be critical for FRT and JLO performance. Neither of these general territories, though, has traditionally been associated with FRT and JLO performance, and these regions have not typically been purported to subserve the types of functions required by the FRT and JLO tests, so we doubt that we are missing any very important messages here. And it is important to remember that we do have good sampling of the main regions targeted by our hypotheses, namely, the right parietal, occipitoparietal, and occipitotemporal regions. We should also mention that our findings should not be taken to mean that left hemisphere structures are irrelevant for performing the FRT and JLO tests. In fact, in our sample (like what has been reported in nearly all previous patient samples), some patients with left hemisphere damage did fail the FRT and/or JLO tests, although the severity of their impairment tended to be less than that of patients with right hemisphere lesions (see Table 4). Thus, we would echo conclusions from previous work, which have noted that left hemisphere structures could contribute to FRT and JLO performance; however, we would also emphasize that the dominant—or perhaps what could be termed “preferred”—systems for performing these tests appear to be in right hemisphere.
It is also possible that our findings will require further refinement as a function of the attribute variable of gender. A detailed consideration of gender effects was beyond the scope of the current study, and we plan to follow up on this issue in future work. However, it seems quite plausible that sex-related effects on the FRT and JLO tests, and on the lesion-deficit relationships for these tests, could be non-trivial. Both tests make demands on cognitive processes (e.g., spatial perception, visuospatial analysis) that have stood out as some of the ones that often yield reliable and sizeable sex-related differences, at least at population level (e.g., see meta-analyses by Linn & Petersen, 1985; Voyer, Voyer, & Bryden, 1995), and also, these types of processing may be lateralized differently in men and women (e.g., Voyer, 1996).
Finally, it should be clear that our study does not speak formally to issues of sensitivity and specificity in regard to the lesion-deficit relationships we uncovered, and we would caution against over-interpreting these findings. Our data do not imply that all patients with damage to right inferior parietal (or occipitotemporal or occipitoparietal) structures will manifest impairments on FRT or JLO, and nor do our data imply that every patient who failed FRT or JLO had damage in the right inferior parietal (or occipitotemporal or occipitoparietal) region. The lesion-deficit relationships we observed are simply not that rigid or specific (and lesion-deficit relationships hardly ever are, regardless of the functions and structures under consideration).
In sum, we used a new lesion analysis method to investigate the neuroanatomical correlates of performance on Benton’s Facial Recognition Test and Judgment of Line Orientation test, and found that, consistent with clinical lore, the tests have good localization value that points to right inferior parietal and nearby temporal and occipital structures as being important for performance on these tests. These results support the clinical application of these tests as good measures of right hemisphere functioning, especially in the inferior parietal, occipitoparietal, and occipitotemporal sectors that have been associated with visuoperceptual discrimination and visuospatial judgment.
Acknowledgments
Supported by NINDS P01 NS19632 and NIDA R01 DA022549
Footnotes
We acknowledge that patients can continue to show recovery after the first 3 months following lesion onset, in both neuroanatomical and neuropsychological status. However, such recovery tends to be modest, and we have found that the 3-month demarcation is satisfactory for separating the more rapid and substantial changes in the acute epoch from the slower and more modest changes in the chronic epoch.
References
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke. Archives of Neurology. 1990;47:397–405. doi: 10.1001/archneur.1990.00530040039017. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hannay HJ, Varney NR. Visual perception of line direction in patients with unilateral brain disease. Neurology. 1975;25:907–910. doi: 10.1212/wnl.25.10.907. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KdeS, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2nd edition. New York: Oxford University Press; 1994. [Google Scholar]
- Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: A clinical test. Archives of Neurology. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Cortex. 1968;4:344–358. [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annual Review of Neuroscience. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P, Spinnler H. The performance of patients with unilateral brain damage on face recognition tasks. Cortex. 1968;4:17–34. [Google Scholar]
- De Renzi E, Spinnler H. Facial recognition in brain-damaged patients. Neurology. 1966;16:145–152. doi: 10.1212/wnl.16.2_part_1.145. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal, visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neuroscience. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Archives of General Psychiatry. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- Hamsher K, Capruso D, Benton A. Visuospatial judgment and right hemisphere disease. Cortex. 1992;28:493–496. doi: 10.1016/s0010-9452(13)80157-8. [DOI] [PubMed] [Google Scholar]
- Hamsher KdeS, Levin HS, Benton AL. Facial recognition in patients with focal brain lesions. Archives of Neurology. 1979;36:837–839. doi: 10.1001/archneur.1979.00500490051008. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Falgout JC, Leli DA, Katholl CA, Halsey JH, Wills EL. Focal right temporo-occipital blood flow changes associated with judgment of line orientation. Neuropsychologia. 1987;25:755–763. doi: 10.1016/0028-3932(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Hécaen H, Albert ML. Human neuropsychology. New York: Wiley; 1978. [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, Reiss AL. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cerebral Cortex. 2004;14:174–180. doi: 10.1093/cercor/bhg116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. The Judgment of Line Orientation Test: A clinical evaluation. M. Psychology Thesis. Australia: University of Newcastle; 1982. [Google Scholar]
- Levin HS, Hamsher KD, Benton AL. A short form of Facial Recognition for clinical use. Journal of Psychology. 1975;91:223–228. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th edition. New York: Oxford University Press; 2004. [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Development. 1985;56:1479–1498. [PubMed] [Google Scholar]
- Mehta Z, Newcombe F. A role for the left hemisphere in spatial processing. Cortex. 1991;27:153–167. doi: 10.1016/s0010-9452(13)80121-9. [DOI] [PubMed] [Google Scholar]
- Mehta Z, Newcombe F, Damasio H. A left hemisphere contribution to visuospatial processing. Cortex. 1987;23:447–461. doi: 10.1016/s0010-9452(87)80006-0. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Face-sensitive regions in extrastriate cortex studied by functional MRI. Neurophysiology. 1996;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Püschel J, Zaidel E. The Benton-Van Allen Faces: A lateralized tachistoscopic study. Neuropsychologia. 1994;32:357–367. doi: 10.1016/0028-3932(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski T. Thresholding lesion overlap difference maps: Application to category-related naming and recognition deficits. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2007.12.033. (in press; available on-line at the Neuroimage website 12/27/07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Pearlson GD, Anthony JC, Yates KO. Determinants of Benton Facial Recognition Test performance in normal adults. Neuropsychology. 2001;15:405–410. doi: 10.1037//0894-4105.15.3.405. [DOI] [PubMed] [Google Scholar]
- Sperry R. Some effects of disconnecting the cerebral hemispheres. Science. 1982;217:1223–1226. doi: 10.1126/science.7112125. [DOI] [PubMed] [Google Scholar]
- Trahan D. Judgment of line orientation in patients with unilateral vascular lesions or severe head trauma. Journal of Clinical and Experimental Neuropsychology. 1991;13:23–24. [Google Scholar]
- Trahan D. Relationship between facial discrimination and visual neglect in patients with unilateral vascular lesions. Archives of Clinical Neuropsychology. 1997;12:57–62. [PubMed] [Google Scholar]
- Trahan D. Judgment of Line Orientation in patients with unilateral cerebrovascular lesions. Assessment. 1998;5:227–235. doi: 10.1177/107319119800500303. [DOI] [PubMed] [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. New York: Taylor and Francis; 2007. pp. 27–39. [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR. A neural basis for the retrieval of words for actions. Cognitive Neuropsychology. 2001;18:655–670. doi: 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Damasio H, Adolphs R, Damasio AR. Neural correlates of conceptual knowledge for actions. Cognitive Neuropsychology. 2003;20:409–432. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle D, Goodale M, Mansfield R, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Voyer D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality. 1996;1:51–83. doi: 10.1080/713754209. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Warrington E, James M. An experimental investigation of facial recognition in patients with unilateral cerebral lesions. Cortex. 1967;3:317–326. [Google Scholar]
- Warrington E, Rabin P. Perceptual matching in patients with cerebral lesions. Neuropsychologia. 1970;8:475–487. doi: 10.1016/0028-3932(70)90043-6. [DOI] [PubMed] [Google Scholar]
- Wasserstein J, Barr WB, Zappulla R, Rock D. Facial closure: interrelationship with facial discrimination, other closure tests, and subjective contour illusions. Neuropsychologia. 2004;42:158–163. doi: 10.1016/j.neuropsychologia.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Wasserstein J, Zappulla R, Rosen J, Gerstman L. Evidence for differentiation of right hemisphere visual-perceptual functions. Brain and Cognition. 1984;3:51–56. doi: 10.1016/0278-2626(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Zangwill OL. Cerebral dominance and its relation to psychological functions. Edinburgh, United Kingdom: Oliver & Boyd; 1960. [Google Scholar]