Abstract
Gadolinium is widely known by all practitioners of MRI but few appreciate the basic solution chemistry of this trivalent lanthanide ion. Given the recent linkage between gadolinium contrast agents and nephrogenic systemic fibrosis, some basic chemistry of this ion must be more widely understood. This short primer on gadolinium chemistry is intended to provide the reader the background principles necessary to understand the basics of chelation chemistry, water hydration numbers, and the differences between thermodynamic stability and kinetic stability or inertness. We illustrate the fundamental importance of kinetic dissociation rates in determining gadolinium toxicity in vivo by presenting new data for a novel europium DOTA-tetraamide complex that is relatively unstable thermodynamically yet extraordinarily inert kinetically and also quite non-toxic. This, plus other literature evidence forms the basis of the fundamental axiom that it is the kinetic stability of a gadolinium complex, not its thermodynamic stability, that determines its in vivo toxicity.
Keywords: gadolinium MRI contrast agents, nephrogenic systemic fibrosis, thermodynamic and kinetic principles
The lanthanide series of elements are often overlooked in teachings of fundamental chemistry (1) because they fall into a category called “hard” acids where bonding with other elements is considered largely ionic or electrostatic. This makes the coordination chemistry less predictable and therefore less interesting to teach compared to the transition metal ions where ligand field effects play a significant and predictable role. The most common oxidation state for the lanthanide elements is +3 and, for all practical purposes, this is the only important ionic form found in the types of aqueous complexes we will consider here. The ionic radii of the trivalent lanthanide cations range from 1.1A for La3+ to 0.85Å for Lu3+ (2) while Gd3+, sitting exactly in the center of the lanthanide series, has an ionic radius of 0.99Å, very nearly equal to that of divalent Ca2+. This is one of the reasons why Gd3+ is so toxic in biological systems - Gd3+ can compete with Ca2+ in all biological systems that require Ca2+ for proper function and, in doing so, the trivalent ion binds with much higher affinity. When bound to a Ca2+ binding enzyme, lanthanide ion replacement often alters the kinetics of the biological process catalyzed by that enzyme (3).
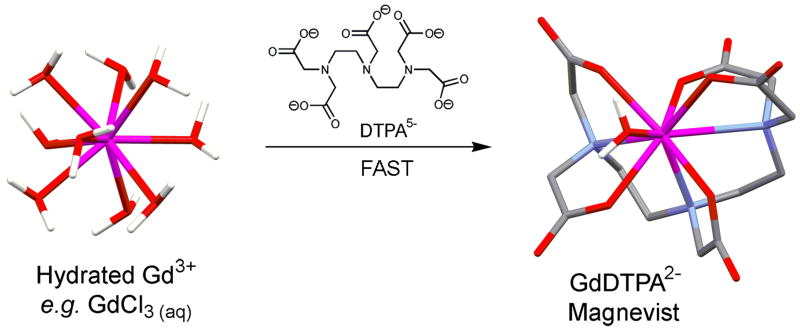
The trivalent lanthanide cations also differ from the more common transition metal ions in having usually large coordination numbers, typically 8-10. Gd3+ exists in aqueous solution below pH ∼6 as a hydrated “aqua” ion with ∼8-9 inner-sphere water molecules (see Figure 1) each contributing a lone pair of electrons to its coordination sphere. Water molecules are quite polar so these “inner-sphere” water molecules each contribute a small amount of electron density to the highly charged Gd3+ ion thereby reducing the effective charge on the central ion. Although the preferred geometry of Gd(H2O)83+ in solution likely approximates a square antiprism with the gadolinium ion situated between two planes each containing four water molecules on the corners of a square with the two planes staggered 45° with respect to each other. The entire system is quite dynamic with any one water molecule residing in the inner coordination sphere for only about 1 ns (4). Water exchange between an inner-sphere coordination site and bulk water occurs via an associative pathway with an additional water molecule “squeezing” into the coordination sphere to form a 9-coordinate intermediate followed by rapid dissociation of one of the existing water molecules to reform the Gd(H2O)83+ structure. Upon chelation by an organic ligand such as those used in clinically approved MRI contrast agents, most of the water molecules are displaced from the inner-sphere of the Gd3+ ion by the more basic donor atoms of the ligand, typically amines (N) or carboxylates (O). A typical ligand used to create a MRI contrast agent has eight donor atoms so, upon chelation, only a single water molecule remains in the inner coordination sphere of the Gd3+. This single water binding site is important for MRI contrast because it allows, through chemical exchange, a large number of water molecules to experience the Gd3+ each second. Interestingly, it wasn't until in early 90's that we learned that this single Gd3+-bound water molecule in agents such as GdDTPA2- and GdDOTA- exchanges about 200-fold more slowly (5) than do the water molecules in the aqua ion, Gd(H2O)83+. This moderately slow water exchange has consequences in limiting the relaxation efficiency or relaxivity of Gd3+-based MRI contrast agents (6, 7) in certain circumstances.
Figure 1.
Molecular stick models of Gd(H2O)83+ and Gd(H2O)DTPA2-. Note the one inner-sphere water molecule in Gd(H2O)DTPA2- that is important for MRI contrast. Mixing of DTPA with Gd3+ results in fast formation of a ML complex because of the DTPA ligand is flexible and can “wrap around” the Gd3+ ion very quickly. Water exchange is fast so removal of water molecules from the inner coordination sphere of the Gd3+ does not hinder the rate at which the complex forms.
The kinetics of ML complex formation
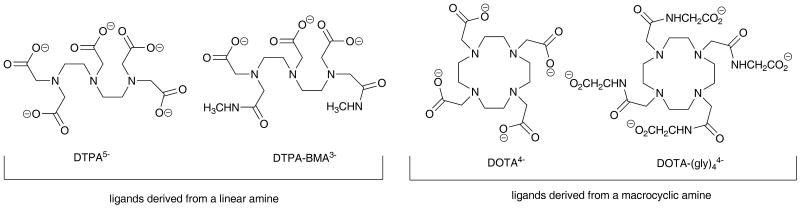
The rate at which a ligand (L) forms a complex Gd(H2O)83+ depends upon many factors including pH, temperature, concentration of reactants and, interestingly, whether the ligand is derived from a linear or macrocyclic poly-amine. Given that pH, temperature and concentration can all be controlled, the one variable among these factors that remains is the structure of the free ligand. Why is the structure of the ligand so important and how does this impact our discussion of thermodynamics and kinetics? Let's contrast and compare data for two of the most common ligands - one derived from a linear amine (DTPA) and another derived from a cyclic amine (DOTA), Figure 2. DTPA is derived from the linear amine, diethylenediamine, by addition of five acetate groups. The resulting ligand is octadentate with three nitrogen donor atoms and five carboxylate oxygen donor atoms. The Gd3+ aqua ion is first attracted to the negatively charged carboxyl groups on DTPA just by electrostatic attraction but the multidentate ligand is conformationally flexible so, once the Gd3+ ion is in the vicinity of the ligand, it rapidly wraps around the Gd3+ aqua ion and displaces all but one inner-sphere water molecule (Figure 1). The time required to fully form the final thermodynamically stable product, GdDTPA, does depend to some extent on the number of protons on the ligand (hence, the pH) but in general the complex is fully formed rather quickly, on the order of a few hundred milliseconds to several seconds. This is typical of other ligands derived from diethylenetriamine as well, such as DTPA-BMA.
Figure 2.
Chemical structures of the four ligands examined in this paper. Two are derived from linear amines (DTPA and DTPA-BMA) and two are derived from macrocyclic amines (DOTA and DOTA-(gly)4).
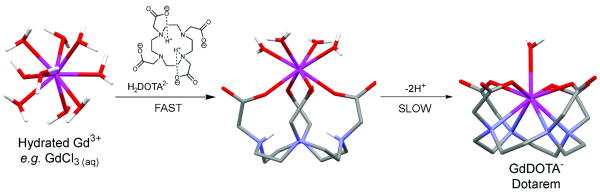
Why do ligands derived from a macrocyclic amine differ in their kinetic behavior? Let's consider DOTA as our example macrocyclic ligand. DOTA is derived from the macrocyclic tetraamine, cyclen, by addition of four acetate side arms. The resulting ligand is also octadentate consisting of four nitrogen donor atoms and four carboxylate oxygen donor atoms. Again, the Gd3+ aqua ion is first attracted to the negatively charged carboxyl groups on DOTA by electrostatic attractions and the first product formed is a relatively stable intermediate wherein the four carboxylates are coordinated to the Gd3+ ion and 4-5 water molecules remain in the coordination sphere (Figure 3). But in this case, the macrocyclic amine portion of the molecule is conformationally rigid so it takes more time for the Gd3+ ion to be completely encapsulated by the ligand. Also, the macrocyclic amines tend to be more basic than the amines in a linear structure and any protons left in the macrocyclic cavity must first dissociate from the amine before the Gd3+ can enter. Thus, the time required to fully form the thermodynamically stable product, GdDOTA, is considerably longer, on the order of many minutes to a few hours, depending upon pH. This is generally true of other macrocyclic ligands as well, including DOTA-(gly)4.
Figure 3.
Molecular stick models of Gd(H2O)83+ and Gd(H2O)DOTA- and the kinetic intermediate, Gd(H2O)4DOTA-. Although the intermediate, Gd(H2O)4DOTA-, is formed quickly upon mixing DOTA with Gd3+, it takes considerable time for this intermediate to rearrange into the thermodynamically stable product, Gd(H2O)DOTA- (Dotarem).
Thermodynamic stability constants. Are they really all that important?
Much emphasis has been focused on the stability of GdL complexes and its relationship to toxicity. The thermodynamic stability of a complex simply describes the concentrations of all species present in solution at equilibrium as given by the following equations:
| [1] |
| [2] |
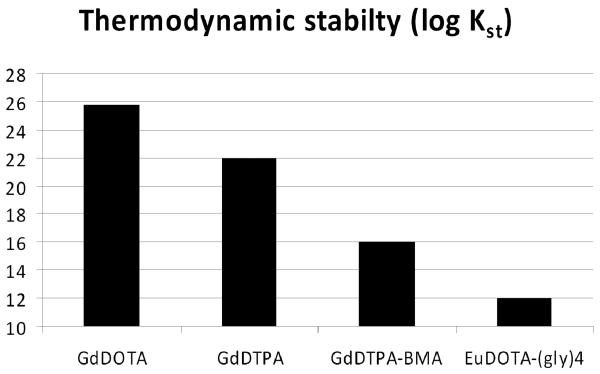
One can see by inspection of equation 1 that the Gibbs free energy of this equilibrium process will have a large favorable entropy term due to release of seven of the eight inner-sphere water molecules from the Gd3+. This entropy contribution is referred to as the “chelate effect”. Also, we have seen that the metal ion-ligand interaction must have a large electrostatic component that contributes an additional favorable enthalpy term so the overall free energy change becomes quite favorable. As a general rule of thumb, the Gd3+ aqua ion forms the most stable complexes with ligands having the most basic donor atoms. Some thermodynamic data (8-11) for the four ligands shown in Figure 2 are summarized in Table 1.
Table 1.
Ligand protonation constants (L) and thermodynamic stability constants (GdL).
| Ligand protonation constants | DTPA | DTPA-BMA | DOTA | DOTA-(gly)4 |
|---|---|---|---|---|
| log K1 | 10.2 | 9.4 | 12.60 | 9.19a |
| log K2 | 8.6 | 4.5 | 9.70 | 6.25a |
| log K3 | 4.2 | 3.4 | 4.50 | 4.08 |
| log K4 | 2.9 | 2.0 | 4.14 | 3.45 |
| Σ log K1-4 | 25.9 | 19.3 | 30.94 | 15.44a |
| Thermodynamic stability constants (log KGdL) | 22.46 (8) | 16.85 (9) | 24.7 (10) | 14.54 (11) |
| Conditional stability constant at pH 7.4 (log Keff) | 18.4 | 14.8 | 17.2 | 12.7 |
Only the first two protonations (macrocyclic amine protonations) are considered because the remaining protonations occur at extended groups (glycinate carboxyls) that do not bind with the metal ion.
These data illustrate some common themes. First, the two most basic atoms in ligands such as these are always amines (represented by the first two protonation constants, log K1 and log K2) and, second, linear amines are typically less basic than macrocyclic amines. This is clearly evident in that the macrocyclic-derived ligand, DOTA, has the most basic nitrogen (highest log K1), followed by DTPA, followed by the bis-amide of DTPA (DTPA-BMA), and the tetra-amide of DOTA (DOTA-(gly)4). This trend also shows that amine groups with amide-containing side-chains (DTPA-BMA and DOTA-(gly)4) are considerably less basic than amine groups with acetate side-chains (DTPA and DOTA). These apparently small differences in log K1 and log K2 values, however, have a significant impact on the thermodynamic stabilities of the resulting GdL complexes. Unlike the relatively small variations in the log K1 and log K2 values for the ligands, the log Kst values for the complexes vary by over 10 orders of magnitude (Table 1). Linear correlations between the sum of ligand protonation constants (Σ log K1-n) and thermodynamic stabilities (log Kst) have been noted many times over the years (12) and the four ligands described here follow that trend nicely. Of course, certain assumptions must be made about how many protonations constants to include in the term Σ log K1-n for each ligand (see footnote to Table 1) but the general theorem that ligands having more basic donor atoms form the most stable complexes certainly holds true. Given the low value of log Kst for GdDOTA-(gly)4, one would then be tempted to conclude that this system would not be stable enough for in vivo applications. However, as we shall see from data presented below, this is not the case. In fact, the later system is extraordinarily kinetically inert toward dissociation and it is this chemical feature that plays a critical role in reducing in vivo toxicity.
The stability constant is widely used to compare contrast agents because it reduces comparisons to a single convenient number. From Table 1, it is clear that these different ligands have different basicity and thus different affinities for the proton. The equilibrium described by equation 1 is valid under conditions when the ligand is entirely deprotonated but, at physiological pH values, the ligand will be partially protonated so one can argue that a better way to compare ML stabilities is to use conditional stability constants, defined by equations 3 and 4.
| [3] |
| [4] |
The calculated conditional constants (log Keff) at pH 7.4 are also given in Table 1. Because DOTA is such a basic ligand, there is strong competition for protons at pH 7.4 and log Keff is 7 orders of magnitude lower for GdDOTA at pH 7.4 than the thermodynamic stability constant. Consequently, GdDTPA has a more favorable binding constant than does GdDOTA at pH 7.4. For less basic ligands like GdDTPA-BMA and DOTA-(gly)4, the conditional constant is closer to the thermodynamic stability constant because there is less competition by available protons at pH 7.4. Another consequence of equation 3 is that as the pH is lowered, the concentration of protons is increased and the equilibrium in equation 3 is pushed to the left. Thus for GdDTPA, log Keff is 18.4 at pH 7.4 while at pH 4, log Keff = 11.2. Stronger acid conditions clearly results in lower complex stability.
In biological media, there are additional competitors besides the proton. For instance endogenous ions like zinc, copper, and iron form very stable complexes with these ligands. At the same time, gadolinium has a high affinity for phosphate, citrate, and carbonate ions and will bind to proteins like serum albumin. In a widely cited paper, Cacheris et al. (9) built a model taking into account a range of equilibrium constants and argued that GdDTPA-BMA had a high LD50 (low acute toxicity) because the DTPA-BMA ligand had a high selectivity for Gd3+ compared to other endogenous ions like Zn2+. Cacheris et al. (9) proposed a “selectivity factor” that takes into account the stability constants of the Ca2+, Zn2+, and Cu2+ complexes as well as the pH. On the basis of their calculations, GdDTPA-BMA is expected to release half as much of its Gd3+ in vivo as compared with GdDTPA (9). However animal experiments have consistently demonstrated that more Gd3+ is retained in bone and other organs when the animals are given GdDTPA-BMA than when GdDTPA is administered (13-15). Clearly there are additional factors to consider besides thermodynamic stability. Models like the “selectivity factor” are based on the assumption that the system is either at thermodynamic equilibrium or will eventually reach thermodynamic equilibrium – likely a poor assumption in most cases where agents are cleared from the body relatively quickly. Clearly, the kinetics of metal dissociation or metal exchange is much more important in determining long term toxicity. Animal studies and increasingly the experience with NSF patients support the notion that kinetic inertness is the most critical factor.
An informative comparision is GdDTPA versus the macrocyclic complex, GdHP-DO3A (aka gadoteridol or Prohance). At pH 7.4, the conditional stability constant is slightly higher for GdDTPA (log Keff = 18.4 vs 17.2 for GdHP-DO3A) but the rate of acid assisted Gd3+ dissociation is 20× faster for GdDTPA (12). This appears to correlate with a small but measurable amount of Gd3+ deposited in the bone of mice 14 days after GdDTPA administration, but no detectable Gd3+ after GdHP-DO3A administration (16). In a very recent paper, Sieber, et al. measured Gd3+ deposited in the skin of rats after 4 weeks of daily high dose (2.5 mmol/kg) administration of Gd-based contrast agents (14). In that study, gadoteridol was not used but rather gadobutrol (Gadovist), a similar macrocyclic complex with slightly lower stability but greater kinetic inertness (17) than gadoteridol was used. Predictably, there was significantly less Gd3+ in the skin or femur of rats that received gadobutrol compared to those that received GdDTPA. These examples point to the importance of kinetic inertness as a predictor of Gd3+ loss. Indeed, there appears to be a much lower incidence of NSF among patients who received GdHP-DO3A (18), likely reflecting its kinetic inertness.
Transmetallation of gadolinium chelates by Zn2+
Much attention has been paid to the potential of Zn2+ to react with a gadolinium contrast agent and displace the gadolinium. Such exchange of one metal for another is termed transmetallation. Of the endogenous metal ions, Na+, K+, Mg2+, and Ca2+, all form very weak complexes with the chelators used in contrast agents and are thermodynamically disfavored from such transmetallation reactions. The order of affinity of contrast agent chelators for other endogenous ions is Fe3+ > Cu2+ > Zn2+. However plasma iron is tightly regulated by transferrin and the serum copper concentration is fairly low (<10 μM) so more attention has been paid to zinc, present at up to 50 μM in serum. For the acyclic compounds GdDTPA and GdDTPA-BMA at pH > 4.5, the transmetallation kinetics are directly proportional to the Zn2+ (or Cu2+) concentration indicating that the transmetallation reaction occurs via direct attack of a Zn2+ ion on the GdDTPA complex (19, 20). It was found that GdDTPA is about 10 times more reactive than GdDTPA-BMA with respect to the rate of Zn2+ transmetallation. Contrary to these results, transmetallation of the macrocyclic complexes GdDOTA and GdHP-DO3A are independent of Zn2+ concentration. These reactions depend on the rate of acid-assisted dissociation of the complex and are much slower than for the acyclic complexes.
Laurent and colleagues (21, 22) tested all approved gadolinium-based contrast agents under a standard set of conditions involving 2.5 mM gadolinium complex, 2.5 mM Zn2+, and phosphate buffer. They measured the increase in proton relaxation time (T1) which occurs when a gadolinium complex is transmetallated by Zn2+ and the released Gd3+ precipitates as GdPO4. The macrocyclic complexes showed no reaction after 4 days. For the acyclic compounds, the order of stability found was MS-325 > GdEOB-DTPA > GdBOPTA > GdDTPA ≫ GdDTPA-BMA. Puttagunta et al. (23) showed that Zn2+ levels were elevated in the urine of subjects administered GdDTPA and GdDTPA-BMA but not in subjects administered GdHP-DO3A. However it was noted that the amount of Zn2+ in the urine was less than the amount of excess ligand present in the formulated contrast agents. Thus the elevated Zn2+ was likely a result of transmetallation of the weak CaDTPA-BMA complex that is present in the Omniscan formulation. Thus, while it is clear that acyclic gadolinium contrast agents can undergo transmetallation reactions with Zn2+ in a test tube, it is not clear what role, if any, Zn2+ plays in transmetallation of gadolinium contrast agents in vivo.
The kinetics of ML complex dissociation
Based on the observations summarized above, the most important chemical feature that determines the toxicity of such Gd-complexes appears to be the rate of dissociation of the complex in vivo. As discussed above, it takes substantially more time for a macrocyclic poly-amine ligand to equilibrate with Gd3+ to form a thermodynamically stable product than it does for a linear poly-amine ligand to do the same. The basic chemical principle of microscopic reversibility states that for any reversible reaction, the mechanism in one direction must be exactly the reverse of the mechanism in the other direction. This means that Gd3+ dissociates from a fully-formed equilibrium structure such as GdDTPA or GdDOTA by exactly the reverse of the mechanisms illustrated in Figures 1 and 3. Hence, it is logical that Gd3+ can escape from DTPA much more quickly than Gd3+ can escape from DOTA. Numerous detailed kinetic studies have been published about the rates of formation and dissociation of both complexes but one simple test to evaluate how quickly Gd3+ is released from any chelated form is simply to add the complex to strong acid and measure the appearance of Gd3+ with time. Wedeking, Kumar and Tweedle (16) reported years ago that the rates of dissociation of various 153Gd-chelates in 0.1M HCl most closely paralleled the amount of 153Gd found in liver and bone of mice at 7 days. It is well known that any 153Gd3+ that dissociates from a ligand in vivo is most likely to accumulate in those tissues. The in vivo tissue distribution and excretion of various Gd3+-ligands from mice was not predicted by the log P value or charge of the complex. While thermodynamic and conditional equilibrium constants demonstrated some ability to discriminate among the complexes with respect to residual Gd3+, it was the rates of dissociation of the complexes in acid that most accurately predicted in vivo dissociation.
An illustration of the relative importance of kinetic stability of each contrast agent is to consider a system where the contrast agent is dissolved in a solution containing phosphate anions at physiological pH. Even chelates with very high thermodynamic stability constants will equilibrate to form some free chelate and unchelated gadolinium (so called “free gadolinium”). Since gadolinium phosphate is insoluble, any unchelated gadolinium will precipitate as insoluble gadolinium phosphate. The system will react according to Le Chatelier's Principle to establish a new equilibrium to replenish the “free gadolinium” until the phosphate anions are almost all precipitated from the solution. The rate at which this happens is determined by the rate of dissociation of Gd3+ from each ligand. A contrast agent with a relatively low thermodynamic stability constant and relatively slow rate of dissociation will produce less solid gadolinium phosphate per unit time than an agent with a high thermodynamic stability constant and a relatively fast rate of dissociation. These kinds of considerations are important if the precipitation of solid gadolinium phosphate initiates a sequence of events that leads to toxicity.
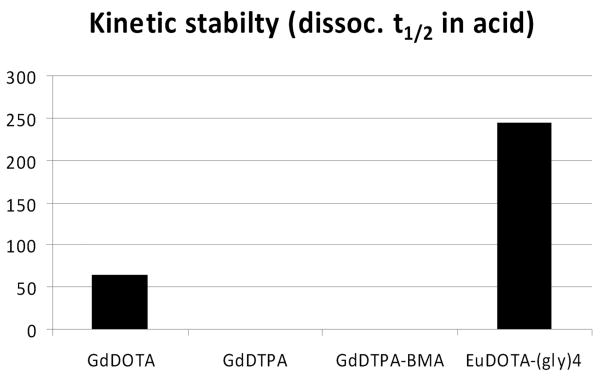
Given all of this prior knowledge, can we then use kinetic dissociation rates to predict the in vivo safety of new imaging agents such as GdDOTA-(gly)4 or EuDOTA-(gly)4? The acid catalyzed dissociation rates of these new complexes are compared with those of GdDTPA, GdDTPA-BMA and GdDOTA are given in Table 2 (11, 24). These data illustrate one very important point - the complex with the lowest thermodynamic stability, GdDOTA-(gly)4 (2.3 log K units smaller than that of GdDTPA-BMA) is also the complex that dissociates most slowly in acid, about 25-fold slower than GdDOTA. While this does not prove in vivo safety, this information encouraged us to proceed with further animal toxicity and biodistribution evaluations of EuDOTA-(gly)4-.
Table 2.
Dissociation rates of complexes in strong acid (pH = 1).
| Dissociation rates in 0.1M HCl (25°C) |
kobs (s-1) | t½ |
|---|---|---|
| GdDTPA2- | 1.2 × 10-3 (24) | 9.6 min |
| GdDTPA-BMA | > 2 × 10-2 (24) | < 34 s |
| GdDOTA- | 2.1 × 10-5 (24) | 9.2 hr |
| EuDOTA-(gly)4- | 8.1 × 10-6 (11) | 237 hr |
Note that data for EuDOTA-(gly)4- is reported here rather than GdDOTA-(gly)4-; Eu3+ and Gd3+ are immediate neighbors in the lanthanide series so share practically identical chemistries. The Eu3+ complex was evaluated because of our interest in using it as a PARACEST agent for molecular imaging.
Pharmacokinetics, toxicity and biodistribution of EuDOTA-(gly)4- in rats
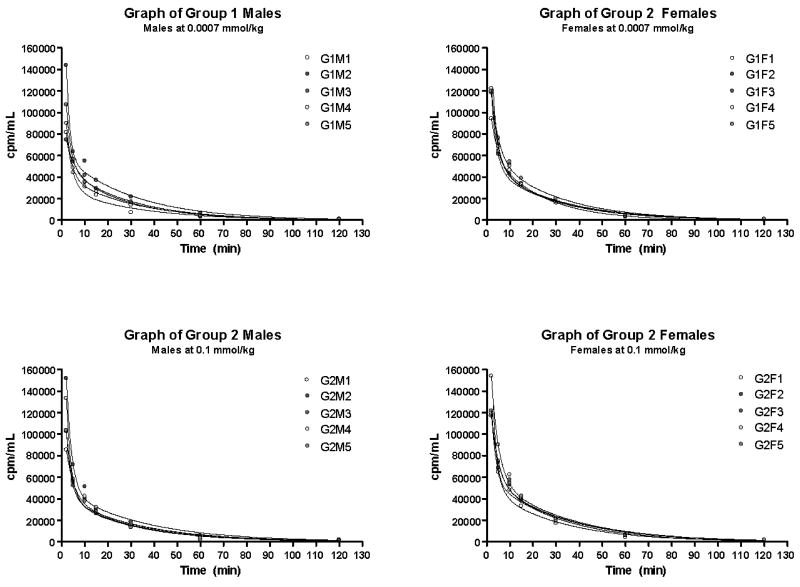
To evaluate the efficacy of this compound further, a 100 μL of stock solution of EuDOTA-(gly)4- doped with tracer levels of 177LuDOTA-(gly)4- was injected into rats weighing between 230-250 g via a jugular catheter. The total radioactive dose was 100 μCi. At various time points, a sample of blood was withdrawn and counted and, after 2 h, the animals were sacrificed for analysis of the tissue biodistribution. Typical pharmacokinetic curves for five animals is shown in Figure 4. Analysis of these time dependent data gave an average elimination rate constant of 0.036 ± 0.005 min-1 which corresponds to an elimination half-live of 20 min. Increasing the total drug concentration in the injection solution from 2 mM to 250 mM had no effect on the elimination rates. These data shown that EuDOTA-(gly)4- is completely eliminated from blood by 2 hours post IV dosing.
Figure 4.
Pharmacokinetic curves for elimination of EuDOTA-(gly)4- from the blood of rats (male and female, n = 5 in each of four groups) showing no differences between low (0.7 μmol/kg) and high (0.1 mmol/kg) doses.
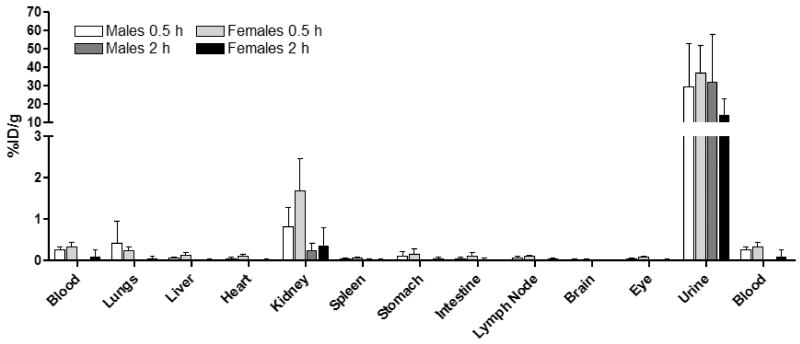
An analysis of residual 177LuDOTA-(gly)4- in tissue showed that the compound was mainly in blood, kidney and urine at 30 minutes (Figure 5). At 2 hours, the amount remaining in these organs had dropped significantly consistent with rapid excretion from the body via renal filtration with little apparent accumulation in any of the organs tested. These data are quite similar to results obtained for other macrocyclic-based ligand systems such as GdDOTA-.
Figure 5.
Biodistribution of EuDOTA-(gly)4- at 30 minutes and 2 hours. The percent initial dose per gram of tissue (%ID/g) is plotted for each tissue. Error bars represent standard deviations.
Finally, the chronic toxicity of EuDOTA-(gly)4- was evaluated in three groups of rats, ten rats in each group. Group 1 received a single dose of 0.1 mmol/kg via tail vein injection, group 2 received a single dose 0f 0.5 mmol/kg and group 3 received a single dose of 1.0 mmol/kg. The animals were monitored for 14 days for mortality, daily clinical observations, body weight, and overall food and water consumption. The compound was well tolerated in all cases – none of the animals died. At the high dose (1.0 mmol/kg), an increase in the number of polymorphonuclear leukocytes and a corresponding decrease in the number of lymphocytes were noted in four animals. In addition, the presence of protein and ketones in the urine of animals from all dosage groups was also observed and local tail necrosis was noted in the gross necropsy as well as in the histopathologic analysis. None of these indications appeared to have an overall effect on the health of the animals. Also, clinical chemistries were within normal ranges for all parameters analyzed. Thus, doses of EuDOTA-(gly)4- up to 1.0 mmol/kg was not associated with any overt toxicity. These combined data suggest that the key feature for in vivo safety of lanthanide complexes such as these is not thermodynamic stability but rather kinetic inertness.
Are all macrocyclic complexes stable and inert?
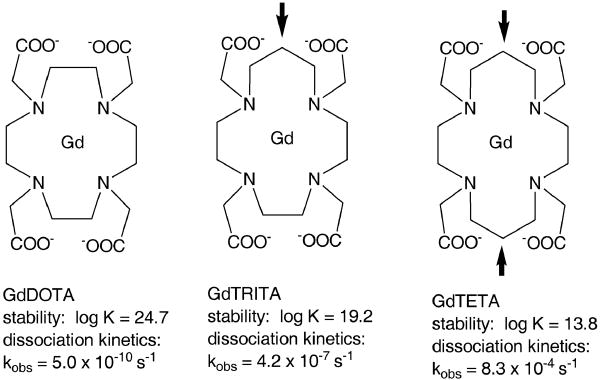
Although the approved macrocyclic gadolinium-based contrast agents are more inert than the approved linear compounds, it is not necessarily true that all macrocyclic complexes are more inert/stable than their linear analogs. For instance Varadarajan et al. (25) described five macrocyclic versions of GdDTPA-BMA, but four of these have lower stability constants than GdDTPA-BMA itself. Another example is the effect of adding one or two CH2 groups to the DOTA macrocycle to give the compounds TRITA and TETA, respectively (Figure 6). Here the stability constants drop by 5 (TRITA) and 10 (TETA) orders of magnitude compared to GdDOTA, i.e. GdTETA is 10 billion times less stable than GdDOTA (26). Nor does the macrocycle necessarily confer kinetic stability. The dissociation rate of Gd3+ from TRITA is 1000 times higher than from DOTA and the dissociation rate from TETA is 1,000,000 times faster than from DOTA (27).
Figure 6.
Modifying the DOTA ligand by introducing one (TRITA) or two (TETA) additional CH2 units (arrows) dramatically lowers the thermodynamic stability of the Gd complex and increases its metal dissociation rate constant. The stability data were reported in (26) and the kinetic data in (27).
Role of Models and Physical Measurements in Contrast Agent Research
In much of scientific research, we make use of models to predict behavior in complex systems. Measurements are made under controlled conditions and, after making several assumptions, the results are extrapolated to predict behavior in a more complex system. These models are only as good as how well they predict behavior in the clinical setting. As we have pointed out, a model solely relying on thermodynamic stability constants and stressing the thermodynamic selectivity of a ligand for Gd3+ over Zn2+ is not supported by the body of animal data and, increasingly, NSF data. It is apparent that kinetic inertness is a useful predictor of Gd3+ deposition in vivo. However relying on a single physical measurement to predict in vivo behavior can be naïve. For instance McMurry et al. (28) studied a series of eight DTPA-based ligands as chelators for 90Y for radiotherapy. As in MRI, in metal-based radiotherapy it is critical that the metal not dissociate from the chelator. In their study, they found that the compound with the slowest acid dissociation rate and 2nd highest stability constant was the least stable in biological settings: it released the most metal when incubated in serum, and it deposited the most metal in bone when administered to mice. This example underscores the point that while modeling, physical chemistry measurements, and animal studies may be useful for selecting compounds to take forward, the ultimate standard is how the compound behaves in humans.
Conclusions
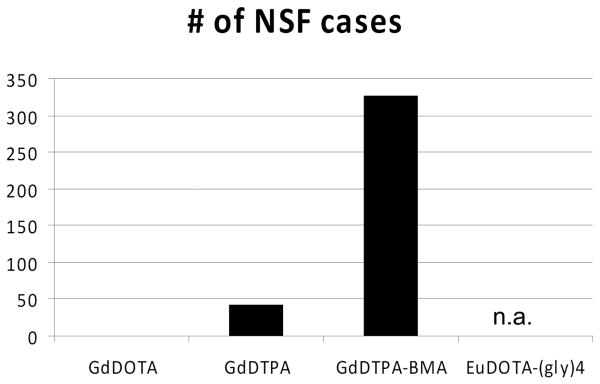
The chemistry of the gadolinium aqua ion (Gd3+) is often overlooked in standard teachings of chemistry but has become an important focal point of the MRI contrast agent world. Gd3+ forms stable complexes with a wide variety of organic ligands having high coordination numbers. This “hard” ion prefers to bind with “hard” ligand donor atoms. Negatively charged oxygen atoms such as those in carboxylate ligands are preferred although all clinically approved contrast agents contain both nitrogen and oxygen donor atoms as part of the ligand structure. The importance of thermodynamic stability constants in determining the biological toxicity of these agents has been over-emphasized in the past while the greater importance of kinetics has been largely overlooked despite early observations that the dissociation rates of these complexes in strong acid “most accurately predicted in vivo dissociation” (16). The importance of dissociation kinetics is highlighted by a comparison some of the fundamental chemical features of the four complexes shown in Figure 2 with their incidence in involvement of NSF (Figure 7). Although no clinical data is available for EuDOTA-(gly)4-, this complex was included here to illustrate the importance of kinetics in determining biological toxicity. Preliminary chronic toxicity and biodistribution data for this compound show that it is well tolerated in rats even at doses ten-fold higher than the clinically accepted dose of a Gd-based contrast agent and that it is rapidly excreted from normal health rats. Based on the comparisons shown in Figure 7, one would predict that the least thermodynamically stable complex, EuDOTA-(gly)4-, may in fact be the safest to use in animals with compromised kidney function because of its kinetic inertness. The central role a macrocyclic ligand structure plays in determining the kinetic inertness of a complex cannot be over-emphasized. Clearly, these features should be kept in mind during the development of any future lanthanide-base imaging agents.
Figure 7.
Plots of thermodynamic stability constants, dissociation half-times in acid, and incidence of NSF for two linear amine-based agents and two macrocyclic amine-based agents. EuDOTA-(gly)4- is not clinically approved so data on the incidence of NSF for this compound are not available. Incidence data for the other agents were reported in (29).
Some notes on nomenclature
The literature can be confusing because of the different names used to describe contrast agents. When new compounds are first synthesized they are given a short descriptive name by the chemist, e.g. gadolinium diethylenetriaminepentaacetate (GdDTPA), or a code number like MS-325. There are systematic ways to name compounds but these are rarely used because the names become cumbersome, e.g. the systematic name for MS-325 is ((trisodium-{(2-(R)-[(4,4-diphenylcyclohexyl)phosphonooxymethyl] diethylenetriaminepentaacetato) (aqua)gadolinium(III)}. For compounds being developed for clinical use, companies apply to regulatory agencies to approve a generic name for the compound. The GdDTPA complex is a dianion (2-) and it is typically prepared as the N-methylglucammonium (NMG) salt. The generic name for this compound is gadopentetate dimeglumine: gadopentetate for the GdDTPA part, dimeglumine for the 2 NMG required to balance the charge. The formulated product of GdDTPA is sold as Magnevist. This refers to the solution of (NMG)2[Gd(DTPA)] in the vial along with any other excipients, including any excess ligand that may be present. These names are used interchangeably in the scientific and medical literature. In Table 3, the chemical or code names of gadolinium contrast agents are listed along with their generic names and their trade names.
Table 3.
Chemical, generic, and product (trade) names for gadolinium-based contrast agents approved for use in the USA and/or the European Union.
| Chemical or Code Name | Generic Name | Product Name |
|---|---|---|
| Gd-DTPA | Gadopentetate Dimeglumine | Magnevist® |
| Gd-DOTA | Gadoterate Meglumine | Dotarem® |
| Gd-DTPA-BMA | Gadodiamide | Omniscan® |
| Gd-HP-DO3A | Gadoteridol | Prohance® |
| Gd-DO3A-butrol | Gadobutrol | Gadovist® |
| Gd-DTPA-BMEA | Gadoversetamide | Optimark® |
| Gd-BOPTA | Gadobenate Disodium | Multihance® |
| Gd-EOB-DTPA | Gadoxetate Disodium | Primovist® |
| MS-325 | Gadofosveset Trisodium | Vasovist® |
Acknowledgments
We wish to thank the NIH (CA-126608, RR-02584 and EB-04582) and the Robert A. Welch Foundation (AT-584) for partial support of this work.
References
- 1.McMurry JE, Fay RC. Chemistry. Englewood Cliffs, NJ: Prentice Hall; 2003. [Google Scholar]
- 2.Shannon RD, Prewitt CT. Revised values of effective ionic radii. Acta Cryst. 1970;B26:1046–1048. [Google Scholar]
- 3.Darnall DW, Birnbaum ER. Lanthanide ions activate alpha-amylase. Biochemistry. 1973;12:3489–3491. doi: 10.1021/bi00742a021. [DOI] [PubMed] [Google Scholar]
- 4.Micskei K, Powell DH, Helm L, Brucher E, Merbach AE. Water exchange on gadolinium (aqua)(propylenediamine tetraacetate) complexes [Gd(H2O)8]3+ and [Gd(PDTA)(H2O)2]- in aqueous solution: a variable-pressure, -temperature and -magnetic field oxygen-17 NMR study. Magnetic Resonance in Chemistry. 1993;31:1011–1020. [Google Scholar]
- 5.Micskei K, Helm L, Brucher E, Merbach AE. Oxygen-17 NMR study of water exchange on gadolinium polyaminopolyacetates [Gd(DTPA)(H2O)]2- and [Gd(DOTA)(H2O)]- related to NMR imaging. Inorganic Chemistry. 1993;32:3844–3850. [Google Scholar]
- 6.Merbach AE, Toth E, editors. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. 2001. [Google Scholar]
- 7.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 8.Smith RM, Martell AE. Critical stability constants, enthalpies and entropies for the formation of metal complexes of aminopolycarboxylic acids and carboxylic acids. Science of The Total Environment. 1987;64:125–147. [Google Scholar]
- 9.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magnetic Resonance Imaging. 1990;8:467–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 10.Cacheris WP, Nickle SK, Sherry AD. Thermodynamic study of lanthanide complexes of 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA) and 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) Inorganic Chemistry. 1987;26:958–960. [Google Scholar]
- 11.Baranyai Z, Brücher E, Ivanyi T, Király R, Lazar I, Zekany L. Complexation Properties of H4dotagl. Equilibrium, Kinetic and Relaxation Behavior of the lanthanide(III) complexes. Helv Chim Acta. 2005;88:604–617. [Google Scholar]
- 12.Kumar K, Chang CA, Tweedle MF. Equilibrium and kinetic studies of lanthanide complexes of macrocyclic polyamino carboxylates. Inorganic Chemistry. 1993;32:587–593. [Google Scholar]
- 13.Tweedle M, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterater and gadodiamide in mice and rats. Investigative Radiology. 1995;30:372–380. doi: 10.1097/00004424-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sieber M, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. European Radiology. 2008;18:2164–2173. doi: 10.1007/s00330-008-0977-y. [DOI] [PubMed] [Google Scholar]
- 15.Sieber M, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: A possible role for gadolinium-based contrast media. Investigative Radiology. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 16.Wedeking P, Kumar K, Tweedle MF. Dissociation of gadolinium chelates in mice: Relationship to chemical characteristics. Magnetic Resonance Imaging. 1992;10:641–648. doi: 10.1016/0730-725x(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 17.Tóth É, Király R, Platzek J, Radüchel B, Brücher E. Equilibrium and kinetic studies on complexes of 10-[2,3-dihydroxy-(1-hydroxymethyl)-propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate. Inorganica Chimica Acta. 1996;249:191–199. [Google Scholar]
- 18.Reilly RF. Risk for Nephrogenic Systemic Fibrosis with Gadoteridol (ProHance) in Patients Who Are on Long-Term Hemodialysis. Clin J Am Soc Nephrol. 2008;3:747–751. doi: 10.2215/CJN.05721207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarka L, Burai L, Brücher E. The Rates of the Exchange Reactions between GdDTPA2- and the Endogenous Ions Cu2+ and Zn2+: A Kinetic Model for the Prediction of the In Vivo Stability of GdDTPA2- Used as a Contrast Agent in Magnetic Resonance Imaging. Chemistry - A European Journal. 2000;6:719–724. doi: 10.1002/(sici)1521-3765(20000218)6:4<719::aid-chem719>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Sarka L, Burai L, Király R, Zékány L, Brücher E. Studies on the kinetic stabilities of the Gd3+ complexes formed with the N-mono(methylamide), N′-mono(methylamide) and N,N″-bis(methylamide) derivatives of diethylenetriamine-N,N,N′,N″,N″-pentaacetic acid. Journal of Inorganic Biochemistry. 2002;91:320–326. doi: 10.1016/s0162-0134(02)00418-x. [DOI] [PubMed] [Google Scholar]
- 21.Laurent S, Elst LV, Copoix F, Muller RN. Stability of MRI paramagnetic contrast media: A proton relaxometric protocol for transmetallation assessment. Investigative Radiology. 2001;36:115–122. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Laurent S, Elst LV, Muller RN. Comparative study of the physiochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media and Molecular Imaging. 2006;1:128–137. doi: 10.1002/cmmi.100. [DOI] [PubMed] [Google Scholar]
- 23.Puttagunta NR, Gibby WA, Smith GT. Human in vivo comparative study of zinc and copper transmetallation after admininstration of magnetic resonance imaging contrast agents. Investigative Radiology. 1996;31:739–742. doi: 10.1097/00004424-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Brücher E. Kinetic Stabilities of Gadolinium(III) Chelates Used as MRI Contrast Agents. Topics in Current Chemistry. 2002;221:103–122. [Google Scholar]
- 25.Varadarajan JA, Crofts SP, Carvalho JF, et al. The synthesis and evaluation of macrocyclic gadolinium-DTPA-bis(amide) complexes as magnetic resonance imaging contrast agents. Investigative Radiology. 1994;29:S18–S20. doi: 10.1097/00004424-199406001-00007. [DOI] [PubMed] [Google Scholar]
- 26.Clarke ET, Martell AE. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorganica Chimica Acta. 1991;190:37–46. [Google Scholar]
- 27.Balogh E, Tripier R, Ruloff R, Toth E. Kinetics of formation and dissociation of lanthanide(III) complexes with the 13-membered macrocyclic ligand, TRITA. Dalton Transactions. 2005:1058–1065. doi: 10.1039/b418991d. [DOI] [PubMed] [Google Scholar]
- 28.McMurry TJ, Pippin CG, Wu C, et al. Physical parameters and biological stability of yttrium(III) diethylenetriaminepentaacetic acid derivative conjugates. J Med Chem. 1998;41:3546–3549. doi: 10.1021/jm980152t. [DOI] [PubMed] [Google Scholar]
- 29.Leiner T. Nephrogenic systemic fibrosis: Safety update. International Society for Magnetic Resonance in Medicine; Toronto, CA: 2008. [Google Scholar]