Abstract
Background
Antiretroviral treatment (ART) initiatives have now been established in many sub-Saharan African countries showing early benefits. To date, few results are available concerning long-term clinical outcomes in these treatment programs.
Methods
Response to ART is described in the first HIV–1C infected adults enrolled in the Botswana ART program in 2002. Data analysis was conducted on available longitudinal data up to April 1st, 2007.
Results
633 severely immunodeficient patients with a median CD4+ cell count of 67 cells/mm3 were initiated on NNRTI-based combination ART and followed for a median of 41.9 months. The median CD4+ increases were 169 cells/mm3, 302 cells/mm3, and 337 cells/mm3 at 1, 3, and 5 years, respectively. The percentages of patients with a viral load of less than 400 copies/mL at 1, 3, and 5 years were 91.3%, 90.1%, and 98.3%, respectively. 75% of patients did not miss a single, or missed only one, monthly ART pick-up per year with a mean pick-up rate of 92.5%. The Kaplan-Meier survival estimates (95% CI) at 1, 3, and 5 years were 82.7% (81.2%, 84.3%), 79.3% (77.6%, 81.0%), and 79.0% (77.3%, 80.7%), respectively. At six months, the risk of treatment modification for anemia was 6.94% (5.9%, 8.0%) for cutaneous hypersensitivity reactions, 1.3% (0.8%, 1.7%), and 1.1% (0.7%, 1.6%) for hepatotoxicity.
Conclusions
This initial group of adults on ART in Botswana had excellent sustained immunologic, virologic, and clinical outcomes for up to five years of follow-up with low mortality among those surviving into the second year of antiretroviral treatment.
Keywords: HIV/AIDS, Africa, antiretroviral therapy, Botswana, public sector
INTRODUCTION
Botswana was the first country in Africa to offer large-scale public antiretroviral treatment (ART) to qualifying citizens. From its inception in January 2002, the Botswana national ART program, commonly referred to as the “MASA program” with MASA meaning “new dawn” in Setswana, has significantly scaled up its efforts. The program currently has more than 90,000 persons receiving ART at 32 designated sites and associated satellite clinics throughout the country [1].
In recent years, other ART initiatives have been established in neighboring sub-Saharan African countries, and the preliminary positive benefits of ART are well documented [2] [3] [4] [5] [6] [7] [8]. These outcomes demonstrate excellent immunologic and virologic responses coupled with overall ART adherence rates that are comparable to outcomes reported in industrialized countries. However, mortality rates, especially during the first six months after ART initiation, are substantially higher in developing countries when compared to mortality rates in industrialized nations [9]. These high early mortality rates are associated with severe immunodeficiency [2], a wide spectrum of co-morbid conditions including poor or reduced nutritional status, anemia, tuberculosis, and other opportunistic infections among adults initiating ART in sub-Saharan Africa [3] [4] [10]. In addition, the reports of high rates of early losses to follow-up (LFU) among those initiated onto ART in southern Africa are concerning and require more efficient patient tracking and monitoring systems [11]. In general, these ART regimens appear to be well tolerated, however, the possibility of higher than expected rates of ARV-associated toxicities, when compared to developed country ART-treated cohorts, warrants more in-depth and long-term study. These toxicities include lactic acidosis [11] [12], nevirapine cutaneous hypersensitivity reactions [12] [13], and lipodystrophy [13] [14] [15].
To date, though, very few studies have reported long-term outcomes among patients on ART in the region [8]. We report herein five-year outcomes among the first 633 patients initiated on public ART as part of Botswana’s MASA program.
METHODS
Patient Population
Princess Marina Hospital (PMH), in the capital city of Gaborone, is the largest referral hospital in the country and serves a population of approximately 800,000 persons residing primarily in southern Botswana. The overwhelming majority of adults and children in Botswana receive health care free-of-charge via the public health care system. Only a small fraction of the population receives their healthcare in the private sector.
The Infectious Disease Care Clinic (IDCC) of PMH was established in August 2001 and the first group of patients on ART in Botswana received longitudinal care at this clinic [3,17]. On January 21, 2002, the IDCC became the first public sector ART site of Botswana’s MASA program, providing care to patients from a large geographic catchment area. Between May 2002 and December 2004 thirty-one additional MASA sites were established countrywide. All adult patients with an AIDS-defining illness and/or a CD4+ cell count of less than 200 cells/mm3 were offered ART, according to existing national Botswana Guidelines on Antiretroviral Treatment [16] .
For this analysis, the study population consisted of all HIV-1 infected, ART-naïve adults who registered at the PMH IDCC between January 21, 2002 and August 7, 2002.
Treatment Regimens
The Botswana ART guidelines [18] for adults recommend two nucleoside reverse transcriptase inhibitors (NRTIs), zidovudine/lamivudine, plus one non-nucleoside reverse transcriptase inhibitor (NNRTI), usually efavirenz or for women of reproductive potential, nevirapine. Zidovudine and lamuvidine were given initially as co-formulated Combivir™ and once available, as co-formulated Lamzid™, which was taken twice daily. Following a 14-day lead-in period of 200 mgs OD, nevirapine was maintained at 200 mgs BD . The efavirenz dose was 600 mgs/day, given as three 200 mg tablets and, once available, as a single 600 mg tablet. Therefore, total ARV pill counts ranged from 3 tablets/day for EFV-based HAART regimens and 4 tablets/day for NVP-based HAART regimens. Patients with pre-existing significant anemia were offered stavudine instead of zidovudine.
Patients experiencing virologic failure on first-line regimens were switched to protease inhibitor (PI)-based HAART (initially with nelfinavir, and later changed to lopinavir/ritonavir) with two new NRTI’s (didanosine plus stavudine or when available tenofovir or abacavir plus lamivudine). Genotypic resistance testing was reserved for second-line failures and/or complicated first-line switches requiring resistance testing, i.e. pregnancy with ongoing viremia.
Patient Visits
At the initial clinic visits, all patients had a comprehensive history taken and received a physical examination. When clinically indicated, chest radiography and sputum microscopy for acid-fast bacilli smears were done to rule out active pulmonary tuberculosis. The following baseline laboratory tests were performed: chemistry, hematology, CD4+ cell count, plasma HIV-1 RNA level, hepatitis B surface antigen, and syphilis serology. Serology testing and tracking of results, however, was not routinely done in the early days of the national ARV treatment programme . Patients with an active opportunistic infection (OI) were treated in accordance with national standards. OI prophylaxis was offered in the form of six-month isoniazid/ pyridoxine (B6) to all persons documented to be HIV-1 infected and cotrimoxasole (PCP prophylaxis) for all adults having a CD4+ cell count of less than 200 cells/mm3.
All eligible patients were initiated on ART within two weeks of initial registration, allowing for OI screening and review of baseline laboratory investigations. Nevirapine-treated patients had liver function testing and a scheduled clinical visit prior to dose escalation at two weeks. CD4+ cell counts and plasma viral load levels were initially obtained three-monthly and after November 2006, six-monthly. Chemistry and hematology were obtained at month-1 and 3 and then at three month intervals. According to 2005 guidelines safety labs including chemistry, hematology, lipase, non-fasting or fasting glucose, lipids, and lactate were done on an as-needed basis.
A confirmatory test was performed for patients with detectable plasma HIV-1 RNA levels (>400 copies/mL). These patients received additional adherence counseling and education. In cases of confirmed virologic failure, patients were switched to second-line PI-based regimen.
All clinical visits within the first year were conducted by physicians trained in HIV care via the National AIDS Training Program, KITSO [17]. Clinically stable patients were subsequently managed by trained nurses. Since 2005, 103 patients deemed “stable” on their first-line ART regimen beyond one year were transferred to private physicians for care as part of the “public-private partnership” (PPP) arrangement.
Patient Education
At the time of ART initiation, all adult patients were required to have a self-appointed adherence assistant, who would receive adherence education and counseling. These adherence assistants were trained to help with ART adherence and toxicity recognition. All patients returned monthly to the PMH IDCC pharmacy for ARV medication refills, during which time pill counts were performed and patients received further counseling and education.
Laboratory Methods
Plasma HIV-1 RNA levels were quantified using the Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostics Systems, Branchburg, NJ) with a lower limit of detection of 400 copies/mL. CD4+ cell counts were determined using the FACSCalibur™ flow cytometer (Becton Dickenson, San Jose, CA, USA) with CD3/4/8/45 Multiset reagents.
Data Collection
The MASA program documentation system has evolved from facility-based paper records of clinical, laboratory, and pharmacy data to an electronic integrated patient management system with direct computer entry of clinical and pharmacy information and transfer of data to a centrally maintained database in the Ministry of Health. Using SAS software, a comprehensive electronic analysis database was created that incorporated data from the following: (i) data abstracted from paper-based clinical records, (ii) data from the centralized Ministry of Health electronic database, and (iii) data from the Botswana PPP. For all patients who did not return for follow-up, an attempt was made to obtain information on visits by direct phone contact, home visits, or cross-checking with other ART sites.
Statistical Analysis
Data from all patients are included in analyses, irrespective of adherence to or change in ART regimen. Length of follow-up varied because of death, loss to follow-up (LFU), and transfers to other MASA clinics. Observations were censored at April 1, 2007 or at the time of LFU or transfer. Time to virologic failure and drug changes were censored at the date of the last clinic visit. Time to laboratory marker events was censored at the date of the last laboratory specimen. Kaplan-Meier estimates and 95% confidence intervals (CI) based on the Greenwood formula were used to describe time-to-events distribution. Log-rank tests were used to compare time-to-event after ART initiation across groups. Likelihood ratio tests from Cox proportional hazard regression models were used to compare time-to-event after ART initiation for continuous variables and multiple outcomes predictors.
For analyses involving CD4+ cell counts and plasma HIV-1 RNA, data were grouped into intervals around the ideal time points. Counting time from treatment initiation, non-overlapping intervals centered at each evaluation time point were created and the measure closest was selected, so that each patient contributed to each interval either one observation or none. CD4+ cell graphs show median changes with interquartile ranges. HIV-1 RNA graphs display percentages with 95% CI’s as calculated using the method of Agresti and Coull . Pre-treatment values were measured no more than three months before ART initiation. Patient data were censored at the event of transfer to another public MASA site. We assumed that censoring at transfer was non-informative.
Univariate analyses were performed to identify predictors of death and LFU using a log rank test. The following baseline characteristics were included in the analysis: gender, WHO staging, CD4+ cell count, viral load (less than, greater than, or equal to 200,000 copies/mL), active TB, age (<35 years, 35–45 years, and >45 years), initial ART regimen, and hemoglobin <8.0 grams/dL. To adjust for potential bias arising from LFU, we identified predictors of both death and loss to follow-up. Two variables, anemia and WHO clinical stage 3/4 disease, predicted both death and LFU. Therefore, we performed Kaplan-Meier analyses, stratified by all combinations of predictor variables. Our results from stratified Kaplan-Meier estimates differed from the non-stratified Kaplan-Meier estimates by less then 0.35%, implying low bias from the assumption of non-informative censoring. All values and figures reported are generated from non-stratified analyses.
Ethics
Ethical approval was obtained from the Botswana Ministry of Health’s Health Research Development Committee and the Harvard School of Public Health’s Human Subjects Committee.
RESULTS
Between January 21, 2002 and August 7, 2002, 871 adult patients were registered at the PMH IDCC. 633 ARV-naïve adults were initiated on ART, with the remaining 238 not initiating ART for the following reasons: 131 were already receiving ART via the private sector, 42 died prior to ART initiation, 38 were lost to follow-up before initiating ART, and 27 patients were not yet eligible by clinical and/or immunologic criteria to receive ART [16].
Baseline characteristics of the 633 ARV-naïve patients are shown in Table 1. The median age was 34.8 years [IQR 30.2 – 41.3]; 60.0% were female. At baseline, the vast majority of patients had advanced HIV disease, as evidenced by their WHO clinical stage, the number of recent or active OIs, and CD4+ cell count and plasma viral load values. The most common first-line ART regimens were zidovudine/lamuvidine plus either efavirenz (50.6%; n=320) or nevirapine (44.4%; n=281).
Table 1.
Baseline characteristics and Initial Treatment of first national program patients initiated on HAART between January and July 2002 (N=633)
| Age, Median (IQR)in years | 34.8 (30.2 – 41.3) |
| Female, Number (%) | 378 (59.88) |
| Weight, mean (kg) | 52.3 |
| BMI, mean | 18.8 |
| BMI< 18.5, Number (%) | 219 (35) |
| CD4 cell count, Median (IQR) | 67 (28–127) |
| HIV-1 plasma RNA, Median (copies/mL) | 444,000 (173,000–750,000) |
| Karnofsky score | 80.4 |
| Mean ≤60, Number (%) | 55 (9) |
| Hemoglobin, Mean, g/dl | 10.7 |
| Hemoglobin <8 g/dL, Number (%) | 62 (9.8) |
| WHO stage at entry, Number (%) | 606 (95.5) |
| I | 18 (3) |
| II | 74 (12) |
| III | 271 (43) |
| IV | 243 (38) |
| Opportunistic Infections n, (%) | |
| Wasting Syndrome | 407 (64) |
| Pulmonary TB | 244 (39) |
| Extrapulmonary TB | 42 (7) |
| Herpes Zoster | 110 (17) |
| Papular pruritic dermatitis/eosinophilic folliculitis | 106 (17) |
| Chronic diarrhea | 103 (16) |
| Esophageal candidiasis | 48 (8) |
| Kaposis’s sarcoma | 46 (7) |
| Pneumocystis jiroveci pneumonia (PJP) | 39 (6) |
| Cryptococcal Meningitis | 33 (5) |
| AIDS Dementia | 29 (5) |
| Cytomegalovirus (CMV) retinitis | 14 (2) |
| Initial ART regimen n, (%) | |
| CBV / NVP | 281(44.4) |
| CBV / EFV | 320 (50.6) |
| d4T / 3TC / NVP | 15 (2.4) |
| d4T / 3TC / EFV | 11(1.7) |
| d4T / ddI / NVP | 2 (0.3) |
| d4T / ddI / EFV | 4(0.8) |
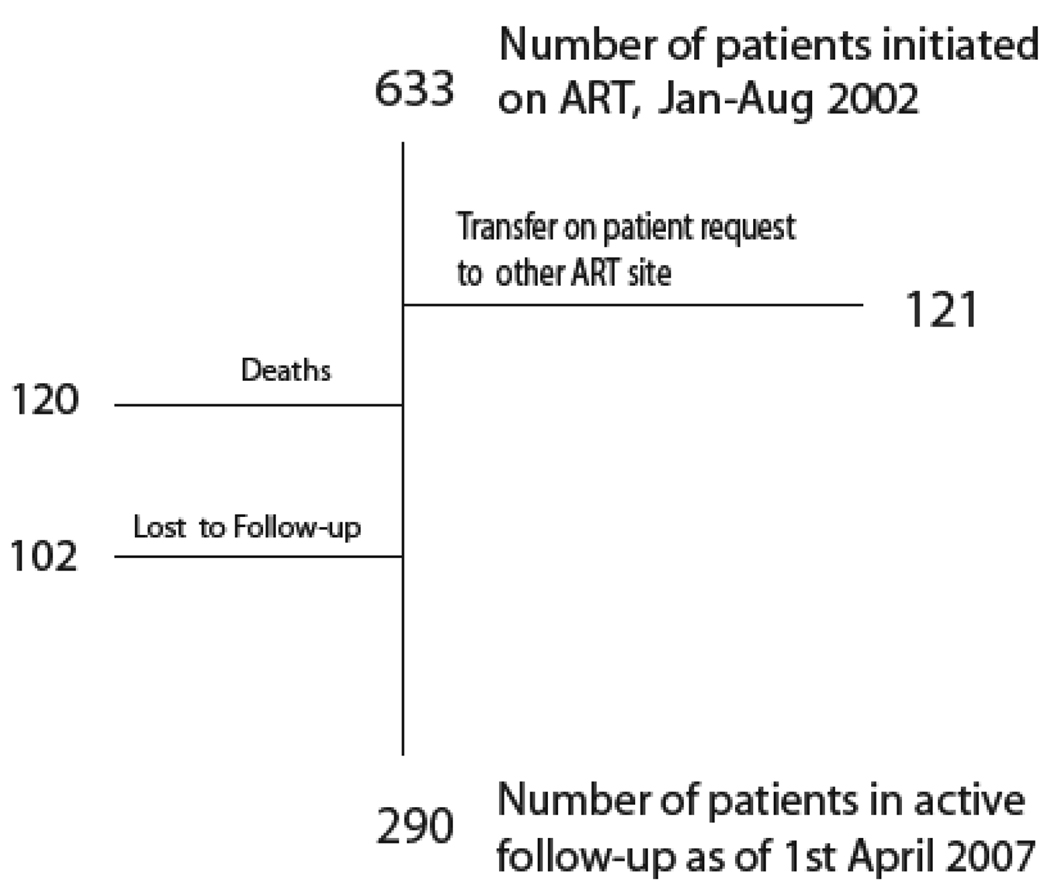
The median duration of follow-up was 41.9 months [IQR 8.3 – 56.9 months], calculated as time to either a) death, b) transfer to non-PMH MASA site, c) LFU, or, d) April 1, 2007 (Figure 1). The total duration of follow-up was 1822.2 patient-years. The median duration of follow-up for the 290 patients alive and known to be receiving treatment as of April 1, 2007 was 57.2 months [IQR 55.9 – 58.8 months]. The Kaplan-Meier estimates of LFU for all 633 patients at 1, 3, and 5 years were 8.9% (CI 7.7%, 10.1%), 15.4% (CI 13.8%, 17.1%), and 21.8% (CI 19.9%, 23.8%).
Figure 1.
Status of first group of adults initiated on ART Botswana national antiretroviral treatment program
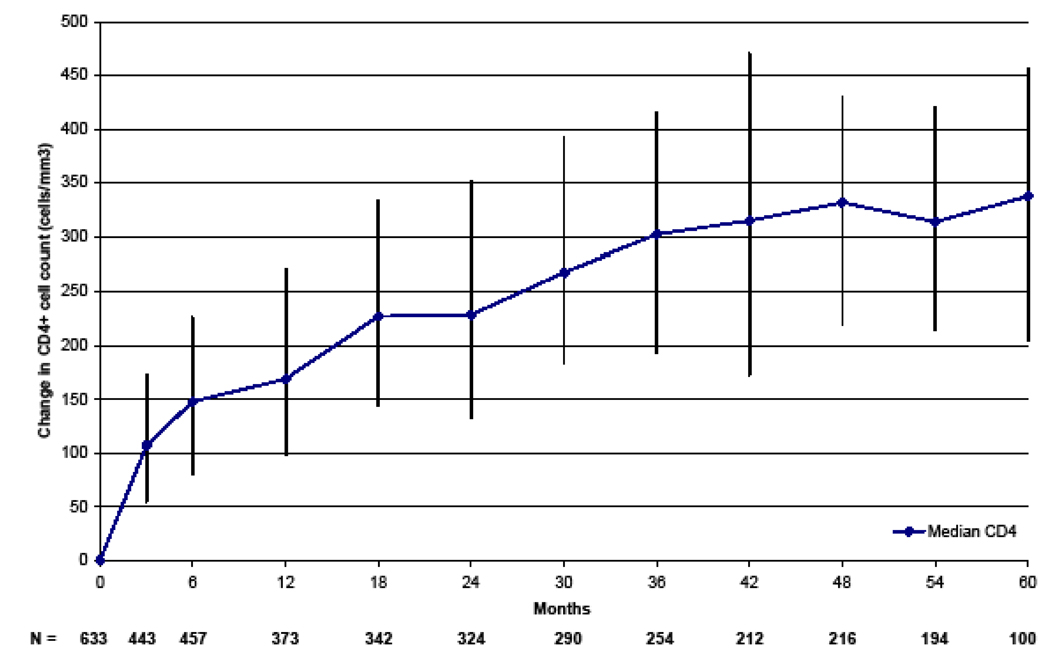
The median CD4+ cell count increases were 169, 302, and 337 cells/mm3 at 1, 3, and 5 years (Figure 2). For subjects with a baseline CD4+ cell count of less than 50 cells/mm3, the median CD4+ cell count increases were 200, 315, and 367 cells/mm3 at 1, 3, and 5 years, respectively. For subjects with a baseline CD4+ cell count of greater than 50 cells/mm3, the median CD4+ cell count increases were 162, 298, and 326 cells/mm3 at 1, 3, and 5 years, respectively. The one-year median CD4+ increase in the group of patients initiated on ART with baseline CD4+ <50 cells/mm3 was 200 versus 162 cells/ mm3 in the group initiated with a CD4+ of 50–200 cells/mm3 (p=0.037). By 4 years, there were no longer any significant differences in absolute CD4+ cell count increases between the groups (p=0.81)
Figure 2.
Median (IQR) CD4+ Cell Count Increase From ART initiation
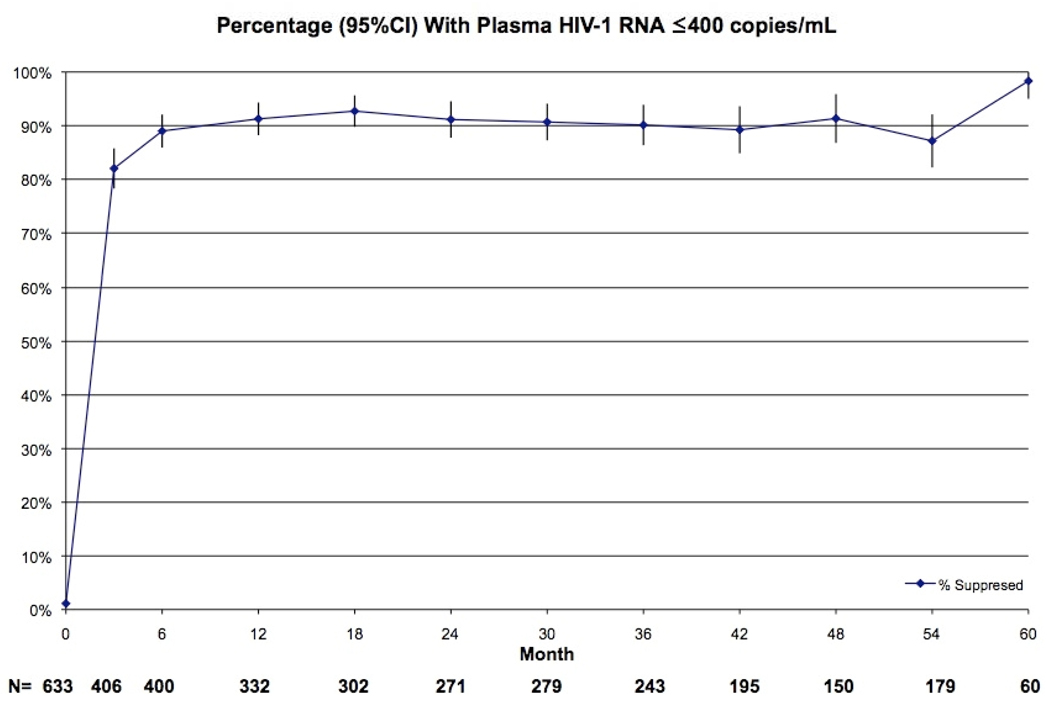
The percentages of patients with undetectable viral load levels at 1, 3, and 5 years were 91.3%, 90.1%, 98.3%, respectively (Figure 3).
Figure 3.
Percentage (95%CI) With Plasma HIV-1 RNA ≤400 copies/mL
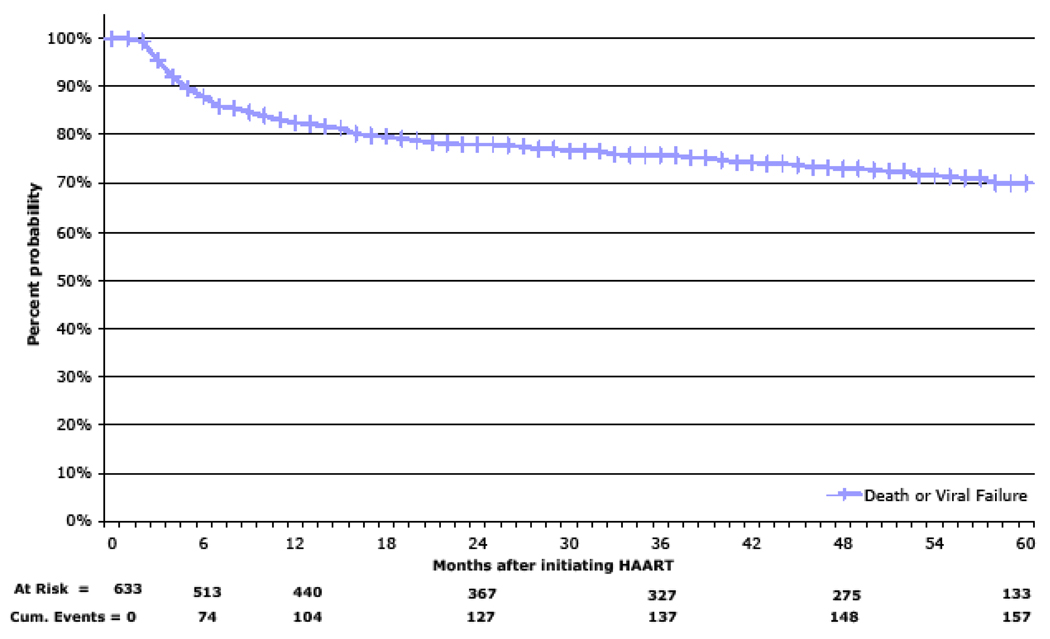
During follow-up, 47 patients experienced virologic failure. The 1, 3, and 5-year Kaplan-Meier estimates of death or virologic failure were 17.6% (CI 16.0%, 19.1%), 22.1% (CI 20.3%, 23.8%) and 30.1% (CI 28.0%, 32.2%), respectively (Figure 4).
Figure 4.
Survival Estimate for Death or Viral Failure
120 of the 633 patients died during follow-up. The Kaplan-Meier survival estimates at six months, 1, 3, and 5 years were 87.8% (CI 86.5%, 89.1%), 82.7% (CI 81.2%, 84.3%), 79.3% (CI 77.6%, 81.0%), and 79.0% (CI 77.3%, 80.7%), respectively. The Kaplan-Meier 1, 3, and 5-year survival estimates for patients with baseline CD4+ cell counts of less than 50 cells/mm3 were 74.8% (CI 71.9%, 77.6%), 71.1% (CI 68.1%, 74.1%), and 70.5% (CI 67.4%, 73.5%) versus 87.9% (CI 86.1%, 89.6%), 84.5% (CI 82.5%, 86.5%), and 84.5% (CI 82.6%, 86.5%) for patients with baseline CD4+ cell counts of greater than or equal to 50 cells/mm3. At year 1, there was a 2.3-fold (CI 1.6, 3.4) higher mortality rate among patients initiating ART with a baseline CD4+ cell count of less than 50 cells/mm3 compared to those initiating ART with baseline CD4+ cell counts of 51–200 cells/mm3 (p=0.0001). Advanced immunosuppression (i.e. CD4+ cell count of less than 50 cells/mm3; log-rank p<0.001)), WHO clinical stage 3/4; (log-rank p=0.003), and baseline hemoglobin <8.0 grams/dL (log-rank p=0.0087) were all significant predictors of mortality. Autopsy data were not available for the majority of deaths. Clinical records and verbal autopsy information, however, identified presumptive causes of deaths in 71 (59.2%) patients. The most common causes of death were advanced AIDS with wasting syndrome (34.2%) and pulmonary tuberculosis (6.7%).
Serious, potentially life-threatening clinical and/or laboratory events occurring within the first six months following ART initiation included anemia, cutaneous hypersensitivity reactions, and grade 3/4 hepatotoxicity. At six months, the risk of treatment modification for anemia was 6.9% (CI 5.9, 8.0), 1.3% (CI 0.8, 1.7) for cutaneous hypersensitivity reactions, and 1.1% (CI 0.7, 1.60) for hepatotoxicity. Overall, 13 patients had a regimen changed for reasons of lipodystrophy, with the majority of these switches among stavudine-treated patients. Other reasons for treatment modification during the five years of follow-up included temporary interruption of EFV supply, pregnancy, and the development of tuberculosis.
Adherence was measured by the individual patient’s frequency of attended medication refill visits (percent of attended visits vs. scheduled visits). Using this method, adherence could only be calculated beginning in July 2002 when the computerized pharmacy record system was introduced. The mean overall monthly pharmacy refill rate was 92.5% (CI 91.2%, 93.6%). 73.7% (CI 70.1%, 77.4%) of all patients never missed or missed only one refill visit per year, i.e. were adhering to more than 90% of refill visits, while 11.3% (CI 7.9%, 14.8%) of patients missed two or more visits per year. Nearly half, 49.6% (CI 45.4%, 53.8%), of our patients did not miss any refill visits over the entire reporting period. There were no significant differences in adherence rates by gender (p=0.11).
DISCUSSION
We herein report 5 year outcomes of the first group of ART-treated adults in Botswana’s public national ARV treatment program. A striking feature of this cohort is the severity of immunodeficiency at the time of ART initiation. Early treatment cohorts in other resource-limited countries were selected in part by financial [19] and/or social criteria [4] in addition to the degree of immunodeficiency, Botswana offered public ART to all qualifying persons [20]. For the first six months of 2002, the PMH IDCC in Gaborone was the only ARV treatment site countrywide. The selection of patients, therefore, was heavily biased by “disease severity”, as evidenced by the median CD4+ and viral load values, the high percentage of patients with active or recent OIs and, advanced WHO clinical stage disease [21].
The vast majority of ART-treated adults exhibited excellent immune recovery with sustained CD4+ cell gains persisting beyond five years on ART. While the gain in CD4+ cells observed in this severely immunodeficient population is similar to that reported among adults initiating ART at higher CD4+ cell counts [22] [23], the high mortality rate in this population might have seriously biased this result and highlights the importance of earlier ART initiation.
A paucity of data is available describing the long term virologic outcomes among ART-treated patients in resource-limited settings. The high virologic suppression rate among patients still receiving care in our cohort is encouraging, especially in light of underlying public health concerns that indiscriminate prescription of ART in resource-limited settings could generate widespread HIV-1 drug resistance. The virologic failure rate is an underestimate considering the high LFU and death rates, but within our cohort, the vast majority of patients alive and continuing ART maintained excellent virologic suppression. Importantly, however, measures to minimize and monitor emergence of treatment-associated drug resistance remain essential [24].
Our adjusted mortality rates showed similarly high early mortality rates when compared to other cohorts of adults initiating ART with severe baseline immunodeficiency [2] [9] [25] [26] [27]. Mortality in this cohort was highest in the first year, with 50% occurring in the first three months and approximately 86% of all deaths occurring within the first year. The majority of deaths were due to advanced AIDS, with only a small fraction attributed to ARV-related toxicities. In addition to the high early on-treatment mortality, another concern is the significant number of patients who were qualified to receive treatment but died before ART could be initiated and indicates that a swift and decentralized plan for the roll-out of ART programs in high-prevalence countries is urgently needed [28] [29].
Botswana is one of the few sub-Saharan countries to choose zidovudine-based ART for first line treatment [4] [18] [30]. Overall rates of early treatment-modifying toxicities were low, but higher rates of grade 3/4 anemia following ART initiation occurred in our cohort of severely immunodeficient adults when compared to healthier patient cohorts [30]. These high rates of anemia are of significant concern for rural Botswana and other sites in the region where blood supplies are limited, and alternatives, including better tolerated NRTI-backbones for first-line ART, should be considered. Of note, tenofovir-based first-line ART is now offered in Botswana, Zambia and Nigeria.
The good initial ART adherence rates found among early African cohorts [31] are also demonstrated in our long-term cohort. Even into the fifth year on ART, overall patient adherence rates did not wane. As evidenced by high rates of virologic suppression (90% or greater), a low virologic failure rate (<10%), and excellent monthly ARV medication refill rates, sustained adult ART adherence is possible. There was no difference in ARV medication adherence rates when analyzed by gender, although a separate clinical trial done in this same setting did show poorer adherence rates among men [14]. Long-term follow-up, including studies evaluating socio-behavioral aspects of adherence, is still needed and continued diligence in terms of adherence counseling and education is warranted. We abstained from investigating predictors of virologic failure in our cohort due to the high numbers of death which would bias such analysis, as death informatively censors virologic failure.
Limitations of our analyses include the high number of patients who were LFU or transferred to another ART site following the countrywide roll-out of ART. Transfers to other sites mainly occurred to facilitate patient care in newly established sites in closer geographic proximity to the patient’s home. There were no significant baseline characteristic differences between transfers and non-transfers. We, therefore, assume that patients who were censored at transfer have similar clinical course after censoring compared to patients who were not censored. Although the LFU rate in our cohort compares favorably with those from other sub-Saharan cohorts [32] [33] [34], it potentially biased the survival analysis. The number of deaths between three and five years of follow-up is likely to be underestimated since a significant number of patients classified as LFU have probably died [11]. The clinical event analysis was also limited due to the varying levels of detail available in the medical records and the reliance on patient self-reporting. Laboratory and pharmacological data, however, were ascertained via multiple systematic crosschecks.
In summary, we present the first five-year ART outcomes from a severely immunodeficient cohort of HIV-1 infected adults receiving treatment in the public sector in southern Africa. The high early mortality rate overshadows excellent immunologic and virologic outcomes and overall ARV medication adherence rates of greater than 90%. Outcomes data from these 633 ART-treated adults represent one of the longest follow-up time periods of any public ART program in Africa. This five-year data provides important information for clinicians and policymakers in the region as they begin to evaluate and plan for the future needs of their own rapidly expanding programs.
ACKNOWLEDGEMENTS
Additional authors: Charles Snyman1,2, Geoffrey Tirelo2, Loeto Mazhani2, Segolame Ramotlhwa2, Howard Moffat2, Joseph Makhema1
1Botswana-Harvard Partnership; 2Botswana Ministry of Health; 3Botswana-Harvard Partnership – PEPFAR; 4Harvard School of Public Health
The authors thank the patients who were the first group of adults to enroll for care and treatment as part of Botswana’s public national ARV treatment program. The authors are also grateful to the Adult Antiretroviral Treatment and Drug Resistance (“Tshepo”) study staff of the Botswana-Harvard School of Public Health AIDS Initiative Partnership for HIV Research and Education (BHP), the entire Infectious Disease Care Clinic staff of Princess Marina Hospital, as well as the Administration of Princess Marina Hospital, who provided the care and support for this first group of publicly ART-treated adults in Botswana. The authors thank Joy Phumaphi, former Minister of Health, and the entire Ministry of Health Botswana and the MASA Program operations team for having the vision and resolve to begin the national ARV treatment program, the Bristol-Myers Squibb’s “Secure the Future” program for supporting the creation of the initial laboratory and clinical infrastructure and the ‘African Comprehensive HIV/AIDS Partnerships’ (ACHAP) for supporting the overall MASA program from its inception to the present. The authors also acknowledge and thank Erika Färdig (Harvard School of Public Health, Boston, MA) for her editorial assistance.
This work was also supported by a grant from the National Institutes of Health (K23-AI073141 for C. William Wester). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Description of the role of each of the authors:
Conceptualisation of the MS: Bussmann, Wester, Ndwapi and Marlink,
Patient care:, Wester, Bussmann, Ndwapi, Gaolathe, Avalos, Tirelo,
Data collection and management: Bussmann, Wester, Snyman, Puvimanasinghe, Grundmann
Analysis: De gruttola, Grundmann, Bussmann
MS Writing: Bussmann, Wester, Grundmann, Marlink
Funding: Marlink, Essex
Program support: Seipone, Moffat, Mazonde, Makhema, Mazhani, Ramotlhwa
Laboratory testing: Mine
MS revision: All authors reviewed the MS
Please note: We feel that an extended author list should be justified for the following reasons (i) this a study within a national ART program with a large number of contributors and (ii) the long period of follow up involved an additional number of collaborators.
REFERENCES
- 1.Puvimanasinghe J. MASA Report. Gaborone: Ministry of Health, Botswana; 2007. [Google Scholar]
- 2.Stringer JS, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 3.Wester CW, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40(3):336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 4.Coetzee D, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 5.Weidle PJ, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360(9326):34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferradini L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins C, et al. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45(3):304–310. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 8.Etard JF, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. Aids. 2006;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 9.Braitstein P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Wood R. Incidence of tuberculosis during highly active antiretroviral therapy in high-income and low-income countries. Clin Infect Dis. 2005;41(12):1783–1786. doi: 10.1086/498308. [DOI] [PubMed] [Google Scholar]
- 11.Wester CW, et al. Higher-Than-Expected Rates of Lactic Acidosis Among: Preliminary Results from a Large Randomized Clinical Trial. J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e3181568e3f. [DOI] [PubMed] [Google Scholar]
- 12.Boulle A, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–760. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 13.Bussmann H, et al. HAART Outcomes among HIV-1C-infected Adults in Botswana: Interim Results from a Randomized Clinical Trial; 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles: 2007. [Google Scholar]
- 14.Pujari SN, et al. Lipodystrophy and dyslipidemia among patients taking first-line, World Health Organization-recommended highly active antiretroviral therapy regimens in Western India. J Acquir Immune Defic Syndr. 2005;39(2):199–202. [PubMed] [Google Scholar]
- 15.van Griensven J, et al. High prevalence of lipoatrophy among patients on stavudine-containing first-line antiretroviral therapy regimens in Rwanda. Trans R Soc Trop Med Hyg. 2007;101(8):793–798. doi: 10.1016/j.trstmh.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 16.MoH B. Guidelines on Antiretroviral Treatment, 2005 version. Gaborone: Ministry of Health, Botswana; 2005. [Google Scholar]
- 17.Philip Bussmann CR, Ndwapi Ndwapi, Baxter Daniel, Bussmann Hermann, Wester C William, Ncube Patricia, Avalos Ava, Mine Madisa, Mabe Elang, Burns Patricia, Cardiello Peter, Makhema Joseph, Marlink Richard. Strengthening Healthcare Capacity Through a Responsive, Country- Specific, Training Standard: The KITSO AIDS Training Program's Support of Botswana's National Antiretroviral Therapy Rollout. The Open AIDS Journal. 2008;2:10–16. doi: 10.2174/1874613600802010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anabwani G, Jimbo W. Botswana Guidelines on Antiretrviral Treatment. Gaborone: Ministry of Health; 2002. [Google Scholar]
- 19.Laurent C, et al. The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. Aids. 2002;16(10):1363–1370. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 20.Wester CW, et al. Establishment of a public antiretroviral treatment clinic for adults in urban Botswana: lessons learned. Clin Infect Dis. 2005;40(7):1041–1044. doi: 10.1086/428352. [DOI] [PubMed] [Google Scholar]
- 21.Keiser O, et al. Time Trends in Demographic and Clinical Characteristics of Patients Starting ART in Lower-income Countries: The ART-LINC Collaboration of IeDEA; 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, US: 2008. [Google Scholar]
- 22.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 23.Apornpong T, et al. Normalization of CD4 Count in Thai PAtients Started NNRTI-based Regimens at Low NAdir CD4 Count; 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, US: 2008. [Google Scholar]
- 24.Jordan MR, et al. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13 Suppl 2:15–23. [PubMed] [Google Scholar]
- 25.Lawn SD, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19(18):2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 26.Moh R, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. Aids. 2007;21(18):2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 27.Alamo S, et al. 2-Year Virologic OUtcomes of an Alternative AIDS Care Model: Evaluation of a Peer Health Worker and Nurse-staffed Community-based Program in Uganda; 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, US: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, et al. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43(6):770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 29.Bassett I, et al. Loss to Care and Death before ART: Patients Eligible for Treatment Who Do Not Make It in Durban, South Africa; 15th Conference on Retroviruses and Opportunistic Infections; Boton, MA, US: 2008. [Google Scholar]
- 30.Hoffmann CJ, et al. Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events. Aids. 2008;22(1):67–74. doi: 10.1097/QAD.0b013e3282f2306e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coetzee D, et al. Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. Aids. 2004;18 Suppl 3:S27–S31. doi: 10.1097/00002030-200406003-00006. [DOI] [PubMed] [Google Scholar]
- 32.Nash D, et al. Characteristics of Facilities and Programs Delivering HIV Care and Treatment Services are Associated with Loss to Follow-up Rates in Programs from 7 Sub-Saharan African Countries; 15th Conference on Retroviruses and Opportunistics Infections; Boston, MA, US: 2008. [Google Scholar]
- 33.Geng E, et al. What becomes of the Defaulters? A Sampling-based Approach to Determine Outcomes of Patients Who Become Lost To Follow-up in ART Scale-up Programs in Africa; 15th Conference ob Retroviruses and Opportunistic Infections; Boston, MA, US: 2008. [Google Scholar]
- 34.Wang B, et al. Loss to Follow-up in Community Clinics in South Africa: Role of CD4 Count, Gender, and Pregnancy; 15th Conference on Retroviruses and Opportunistic Infections; Bosotn, MA, US: 2008. [Google Scholar]