Abstract
Quantitation of iAs and its methylated metabolites in biological samples provides dosimetric information needed to understand dose-response relations. Here, methods are described for separation of inorganic and mono-, di-, and trimethylated arsenicals by thin layer chromatography. This method has been extensively used to track the metabolism of the radionuclide [73As] in a variety of in vitro assay systems. In addition, a hydride generation-cryotrapping-gas chromatography-atomic absorption spectrometric method is described for the quantitation of arsenicals in biological samples. This method uses pH-selective hydride generation to differentiate among arsenicals containing trivalent or pentavalent arsenic.
Keywords: arsenic, methylated arsenicals, [73As], thin layer chromatography, hydride generation-cryotrapping-gas chromatography-atomic absorption spectrometry, pH-selective hydride generation, arsenic oxidation state
Introduction
Because iAs is extensively converted to methylated metabolites in a series of enzymatically catalyzed reactions, any exposure to iAs is actually a simultaneous exposure to inorganic and methylated arsenicals. Although measurements of concentrations of total As in tissues and excreta after exposure to iAs provide some dosimetric information, vital information will be lost if concentrations of all metabolites are not determined. This analytical issue is especially salient because it is likely that the adverse health effects associated with chronic exposure to iAs are mediated by MAsIII or DMAsIII, active intermediates formed during methylation reactions. In this section, two methods are described for the determination of iAs and its methylated metabolites in biological samples. The first procedure uses thin layer chromatography (TLC) to separate and quantify inorganic and methylated arsenicals. However, this approach does not provide information about oxidation state of As present in arsenicals. In the second method, pH-dependent differences in generation of arsines between As in the trivalent and pentavalent oxidation states are exploited to selectively generate and quantify arsenicals on the basis of the oxidation state of As.
Basic Protocol 1
Protocol - Analysis of arsenical metabolites by thin layer chromatography
Overview
Quantitative determination of iAs and its methylated metabolites is an essential tool in the study of the fate and effects of arsenicals in cells and organisms. TLC is a relatively inexpensive high-throughput method for quantitation of these arsenicals. We have routinely used TLC to separate and quantify radiolabeled metabolites that are products of enzymatically catalyzed reactions. Here, iAsIII labeled with the radionuclide, 73As, is added as a labeled substrate to in vitro systems containing recombinant AS3MT, to cultured mammalian cells, or to subcellular fractions prepared from animal or human cells or tissues. Addition of stable iAsIII as a substrate makes it possible to determine the concentration dependency of rates of methylation reactions. Although the TLC method was developed specifically for analysis of radiolabeled arsenicals, it could be combined with other detection systems for analysis of non-radioactive arsenicals.
Ion exchange TLC is performed on polyethyleneimine (PEI)-cellulose plates using either an isopropanol-acetic acid-water or an acetone-acetic acid-water mobile phase. Separation of As species on PEI-cellulose plates developed in the isopropanolacetic acid-water system is a relatively slow process; a 20-cm plate may take up to 5 hours to develop. Notably, the relatively slow migration of the isopropanol-acetic acid-water mobile phase provides excellent chromatographic resolution. In contrast, TLC separation using acetone-acetic acid-water mobile phase is faster, but there is a loss of resolution of different arsenicals. Both solvent systems efficiently separate iAsIII, iAsV, MAsV, and DMAsV. In contrast, only the isopropanol-acetic acid-water mobile phase resolves TMAsO (Waters et al., 2004).
Mixtures of arsenicals in aqueous solutions can be analyzed directly. In contrast, biological samples in which arsenicals are commonly bound to low- or high-molecular weight compounds (e.g., urine, tissues, or cells) require preparation before analysis. These samples are treated with an acidic solution of cuprous chloride to displace arsenicals bound to thiols in biological matrices. Liberated arsenicals are then separated from denatured proteins by centrifugation or ultrafiltration. This treatment releases at least 90% of arsenicals that are bound in biological matrices (e.g., urine, tissue homogenates, and cell lysates). These extracts are treated with hydrogen peroxide to convert all arsenicals to the pentavalent oxidation state. Conversion to pentavalency simplifies separation of arsenical species by TLC. However, this procedure cannot be used if oxidation state-specific analysis of arsenicals is desired.
Materials list
1. Materials
a. Baker-flex PEI-F cellulose TLC plates (20×20 cm) with fluorescent indicator- (J.T. Baker, Phillipsburg, NJ) - Polyester backed PEI-cellulose plates that are also commonly used for analysis of nucleotides or amino acids are available from various manufacturers, including Sigma-Aldrich, J.T. Baker or Sorbent Technologies, Inc. This laboratory routinely uses PEI-cellulose plates with a fluorescent indicator (PEI-F cellulose) obtained from J.T. Baker. Although predevelopment of PEI- or PEI-F-cellulose plates in water is generally recommended by manufacturers, it is not required for analysis of radioactive arsenicals.
b. Microcon concentrators with a nominal molecular weight cutoff of 10 kDa - Microconcentrators are designed to separate by centrifugation the solvent phase from large molecules in solution. The principle of operation involves centrifugation of a protein-containing solution in an apparatus that contains a membrane with controlled pore size that allows passage of molecules of a given size but prevents passage of larger molecules. Migration of solvent and smaller molecules through the membrane into a reservoir leaves behind a retentate enriched in molecules larger than the nominal molecular weight cutoffs for the filter and depleted in solvent and smaller molecules. In most applications, mircroconcentrators are used to enrich proteins present in dilute solutions. In this application, microconcentrators are used to separate proteins from liberated arsenicals as a preparatory step for TLC. Filters with nominal molecular weight cutoffs of 3 to 100 kDa are commercially available. Because the time needed to filter samples increases as a function of the nominal molecular weight cutoff of the membrane, it is desirable to use a microcentrator with relatively high molecular weight cutoff. In the work described here, a microconcentrator with a nominal molecular weight cutoff of 10 kDa was used. This product is currently marketed as the Microcon YM-10 Centrifugal Filter Unit (Millipore, Billerica, MA).
2. Chemicals and reagents
a. Isopropanol - HPLC grade or equivalent
b. Acetic acid, glacial - Analytical grade or equivalent
c. Acetone - HPLC grade or equivalent
High grade chemicals should be used for preparation of all reagents used in TLC separations described here. This is especially important if one intends to use the TLC methods to separate stable (i.e., nonradiolabeled) arsenicals. The two mobile phases described here contain isopropyl alcohol (isopropanol), glacial acetic acid, acetone, and distilled/deionized water. Mobile phases should be prepared shortly before TLC separation by mixing isopropanol, acetic acid, and water (10:1:2.5) or acetone, acetic acid, and water (2:1:1). A 0.2 M CuCl solution in 0.2 N HCl is used for extraction of arsenicals from biological samples. Hydrogen peroxide (30%) is needed for oxidation of extracted samples.
3. Equipment
a. Glass TLC tank
b. Chemical fume hood
c. Dry bath incubator
d. Benchtop centrifuge
e. Phosphorimager or Radio-TLC scanner
A glass TLC development tank with a lid and a chemical hood are needed for TLC plate development. A dry bath incubator or a similar heating device reaching at least 100°C is required for sample extraction. A benchtop centrifuge is needed for separation of denatured proteins from soluble extracts containing free arsenicals and for use of microconcentrators. A phosphorimager or a radio-TLC scanner is needed for detection and quantification of radioactive As species on developed TLC plates.
Note: Additional materials, chemicals or equipment will be needed for TLC analysis of non-radioactive arsenicals.
Protocol steps
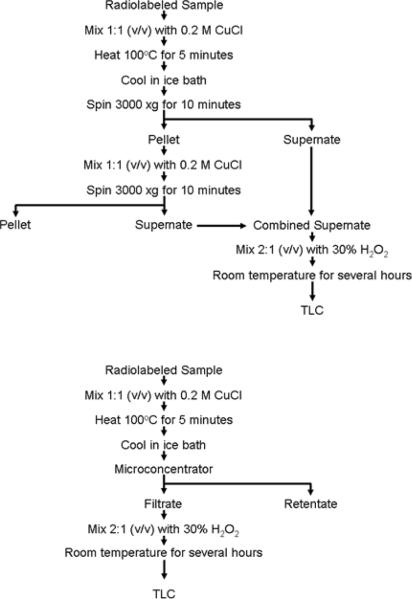
Figure 1 shows the steps in sample preparation for analysis of radiolabeled arsenicals by TLC using two methods for liberation of bound arsenicals.
Figure 1.
Schemes for treatment of samples to liberate protein bound arsenicals. a). Method using centrifugation to prepare supernate fraction. b). Method using microconcentrator to separate liberated arsenicals from proteins.
1. Liberation of arsenicals bound to biological matrices
There are two methods for the treatment of samples to liberate bound arsenicals
a. Centrifugation method
i. Mix equal volumes of sample and 0.2 M CuCl in 0.2 N HCl in a heat-resistant microcap tube. Some samples (e.g., tissue homogenates or cell lysates) can be prepared directly in 0.2 M CuCl in 0.2 N HCl.
ii. Secure the lid with a lid lock or a clip and incubate at 100°C for 5 minutes.
iii. Cool samples in an ice bath and centrifuge at 3,000 × g for 10 minutes at room temperature to separate supernate and pellet.
iv. Transfer supernate containing liberated arsenicals to a new tube. Resuspend pellet in the original tube in a small volume of 0.2 M CuCl solution in 0.2 N HCl and repeat centrifugation. Combine supernates from both centrifugation steps as the extract.
Treatment with Cu is not necessary for aqueous solutions or for samples that do not contain As-ligand complexes or other interferants that might affect TLC separation.
b. Microconcentration method
i. Mix equal volumes of the sample and 0.2 M CuCl in 0.2 N HCl in a heat-resistant microcap tube. Some samples (e.g., tissue homogenates or cell lysates) can be prepared directly in 2 M CuCl in 0.2 N HCl.
ii. Secure the lid with a lid lock or a clip and incubate it at 100°C for 5 minutes. Cool samples in an ice water bath and transfer sample to upper chamber of a microconcentrator apparatus.
iii. Centrifuge microconcentrator as directed by manufacturer to separate retentate and filtrate. Filtrate contains arsenicals liberated by Cu treatment.
Selection of an appropriate method for sampling handling during Cu treatment depends on the amount of protein present in the sample. For samples with high protein contents, centrifugation after treatment with Cu and heat will yield discernable pellets that can easily be manipulated. For samples with low protein contents, pellets collected after Cu treatment and heating may be quite small, making it difficult to assure that only the supernatant fraction is collected for further processing. In this case, retention of proteins on the membrane of the microconcentrator simplifies processing of the protein-free fraction produced by Cu treatment and heating.
2. Oxidation of arsenicals in cuprous chloride extracts
a. Prepare 0.5 ml microcap tubes for use in oxidation step by piercing the cap with 18 gauge needle to provide a vent.
b. Mix 50 μl of the extract with 25 μl of 30% H2O2 in a 0.5-ml microcap tube with a vented lid.
c. Let samples stand at room temperature for several hours or overnight to convert arsenicals in the extract to pentavalency. Oxidized samples can be stored in a refrigerator for several days before analysis by TLC.
3. TLC separation
a. With a soft lead pencil draw a starting line 2 cm from the edge of the PEI-cellulose plate, taking care not to scratch the absorbent layer.
b. On the start line, mark 12 points spaced by 1.5 cm apart, starting 1.75 cm from the left edge, as the points for loading of samples.
c. Use a micropipettor with a narrow tip or a capillary tube to apply an aliquot (0.5 to 1 μl) of an oxidized sample at a loading point on the start line. Let applied samples dry at room temperature. To add more material, the application and drying steps can be repeated for each sample.
Applying more than 2 μl of Cu-treated sample degrades TLC separation. Appropriate arsenical standards should be also applied individually or in a mixture. Separating more than 12 samples on a 20-cm wide TLC plate is not recommended
d. Develop TLC plate with either of the two mobile phases in a sealed development tank which is placed in a chemical fume hood.
During development in either mobile phase, Cu from the CuCl reagent forms blue arrow-shaped spots at the origin.
e. Stop TLC when the mobile phase front is ~ 2.5 cm from the upper edge of the plate.
f. Dry TLC plate in chemical fume hood at room temperature.
4. Detection of arsenicals on TLC plates
Phosphorimaging and γ/β-TLC scanners equipped with supporting software provide effective tools for detection and quantification of radiolabeled arsenicals. Sensitivities of these techniques for detection of 73As are comparable. In general, samples containing as little as 100 to 1,000 cpm of 73As can be reliably detected and quantified. Alternatively, sections of the TLC absorbent layer scraped from the polyester backing can be radioassayed, using a γ-counter or liquid scintillation counter. Scraping the TLC absorbent layer could also be used to provide material for analysis of stable arsenicals separated by TLC. In cases where stable arsenicals are separated by TLC, samples of the absorbent layer could be analyzed by atomic absorption, fluorescence, or emission spectrometry or by inductively coupled plasma-mass spectrometry.
Alternate Protocol
Protocol - Analysis of arsenical metabolites by hydride generation-atomic absorption spectrophotometry
Overview
Hydride generation-atomic absorption spectrophotometry (HG-AAS) converts arsenicals into volatile hydride species (arsines) which are quantified by atomic absorption spectrometry. Sodium borohydride (NaBH4) is commonly used to convert arsenicals to arsines. HG-AAS has long been used for analyses of total As in environmental and biological samples. Combining hydride generation with an adequate technique for separation of arsine and methyl-substituted arsine species has made HGAAS an effective tool for studies of As metabolism. The common method used for separation of arsine species involves trapping arsines at low temperature (cryotrapping) followed by thermal separation. Typically, arsines are cryotrapped in a U-tube containing an inert (glass wool) material or a reactive absorbent which is immersed in liquid nitrogen. The U-tube is removed from liquid nitrogen and heated to produce a temperature gradient. Under these conditions, cryotrapped arsine species are liberated from the U-tube on the basis of boiling points (−55°C, arsine; 2°C, methylarsine; 35.6°C, dimethylarsine, and 70°C, trimethylarsine). Based on this desorption, each arsine species is swept in a stream of inert gas from the U-tube into the detector of an atomic absorption spectrometer. This approach is eminently suitable for determination of As species in aqueous solutions or biological fluids with low protein contents (e.g., urine).
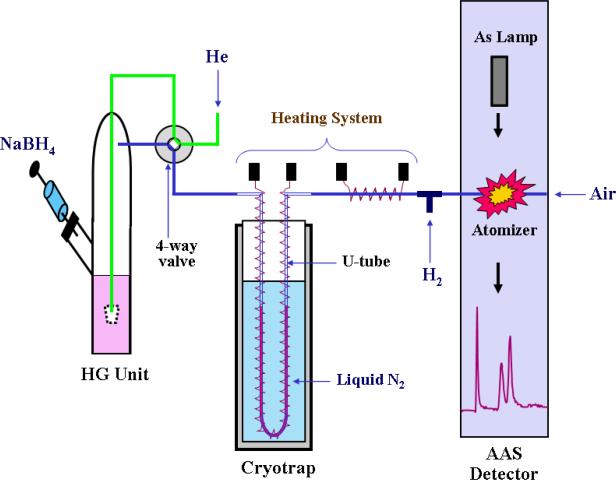
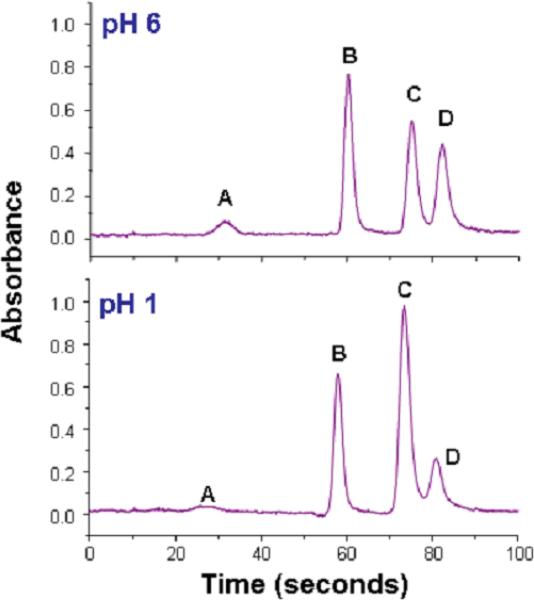
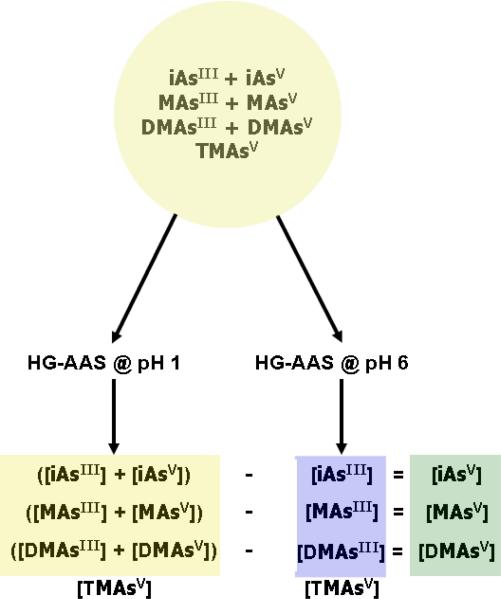
The assay described in this protocol involves pH-selective generation of arsines in a reaction vessel, sweeping of arsines into a cryotrap, a U-tube filled with an absorbent that is immersed in liquid nitrogen, thermal release of trapped arsines from the cryotrap, and sweeping of eluted arsines into the AAS detector. A schematic of this HG-CT-AAS system previously described by Devesa and associates (2004) is shown in Figure 2. Using this system, a complete base-line separation of arsine, methylarsine, dimethylarsine and trimethylarsine can be achieved in less than 90 seconds (Figure 3). This pH-selective method for generation of arsines can be used in conjunction with HGAAS to provide information on the distribution of various As species in biological samples. Because this method does not use extraction procedures or chemical digestion that may change the oxidation state of As, it can be used to quantify iAsIII, iAsV, MAsIII, MAsV, DMAsIII, DMAsV, and TMAsO in relatively complex biological matrices (Del Razo et al., 2001; Devesa et al., 2004). This approach requires hydride generation at two pH values. In the first analysis, arsines are generated at pH 1, a condition that generates arsines for all inorganic, methylated, dimethylated, and trimethylated arsenicals. In the second analysis, arsines are generated at pH 6. Under these conditions, arsines are generated from arsenicals containing trivalent As and TMAO. The scheme for analysis is summarized in Figure 4.
Figure 2.
Schematic for system used for hydride generation with cryotrapping interfaced with atomic absorption spectrometer.
Figure 3.
Speciation analysis of As in an in vitro reaction mixture containing 100 mM phosphate buffer (pH 7.4), 1 mM TCEP, 1 mM S-adenosylmethionine, 5 μg recombinant rat As3mt, and 1 μM iAsIII. Mixture was incubated at 37°C for 20 minutes
Figure 4.
Scheme for the determination of arsenicals on the basis of oxidation state of arsenic based on the pH selective method of hydride generation-atomic absorption spectrometry.
Materials List
1. Materials
Cryotrap assembly
a. 30-cm long borosilicate glass U-tube (4 mm i.d. and 6 mm o.d.) - The dimensions of the U-tube used in any cryotrapping procedure will be determined by the design of the apparatus as well as the gas flow requirements of the atomic absorption spectrometer. U-tubes used in this laboratory are custom made by a local glassblowing shop. Commercially available or custom-made U-tubes can be used. Notably, retention times and chromatographic resolution are affected by the length and internal diameter of the U-tube as well as by the characteristics of each packed U-tube. It is useful to track the performance of each packed U-tube to determine differences in performance among U-tubes and to determine the useful life for each packed U-tube.
b. Chromosorb WAW-dimethyldichlorosilane 46/60 with 15% OV-3 (Supelco, Inc., Bellefonte, PA)
c. Rejuv-8 silylating reagent (Sigma)
d. 1.6 Ω ft−1 Ni80/Cr20 wire (Omega Engineering, Stamford, CT)
e. Silanized glass wool
f. 1-liter Dewar flask
g. 4-way teflon valve
h. 3-way connector for carrier gas lines
i. Borosilicate tubing (6 mm i.d.)
2. Chemical reagents and gases
a. NaBH4 (EM Science, Gibbstown, NJ)
b. NaOH
c. HCl
d. Trizma HCl tris(hydroxymethyl)aminomethane hydrochloride
e. Antifoam B silicone emulsion (J. T. Baker, Inc., Phillipsburg, NJ)
f. Hydrogen – high purity gas in cylinders or from a hydrogen generator
g. Helium – high purity gas in cylinder
h. Deionized water (18 MΩ cm−1 or better)
i. Liquid nitrogen
j. As calibration standards
aa. sodium arsenate (96% pure, Sigma)
bb. sodium arsenite (99% pure, Sigma)
cc. sodium methylarsonate (monosodium acid methane arsonate, 98% pure, Chem Service, West Chester, PA)
dd. dimethylarsinic acid (98% pure, Strem Chemicals, Inc., Newburyport, MA).
Methylated trivalent arsenicals and TMAO are not commercially available. Previous studies have used oxomethylarsine (CH3AsIIIO), iododimethylarsine [(CH3)2AsIII], and trimethylarsine oxide synthesized by Professor William R. Cullen (University of British Columbia, Vancouver, Canada).
3. Equipment
a. Computerized atomic absorption spectrometer equipped with an air/hydrogen flame unit - A Perkin Elmer Model 5100 atomic absorption spectrometer has been used in this laboratory for many years.
b. As electrodeless discharge lamp
c. Manual HG system
d. Power source with a 30- to 60-volt output for heating the U-tube
e. Gas flow controllers (e.g., (FMA-2400 or 2600 Series, Omega Engineering, Stamford, CT, USA)
f. Timer
g. Miscellaneous stoppers, valves and tubing for carrier gas lines.
1. Preparation of the U-tube
a. Wrap the U tube with ~2 meters of the 1.6 Ω ft−1 Ni80/Cr20 wire; leave sufficiently long ends for connection to a power source.
b. Fill the U-tube loosely with 2.9 g of Chromosorb WAW-dimethyldichlorosilane and secure adsorbent at both ends with a plugs of silanized glass wool.
c. Inject 25 μl of Rejuv-8 silylating reagent into the U-tube, connect it to a helium tank, and condition the U-tube in a stream of helium (40 ml≅min−1) at room temperature for 24 hours.
2. Cryotrap unit assembly
a. Using borosilicate tubing, connect one end packed and conditioned U tube to the HG reaction vessel though 4-way valve as shown in Figure 2. Connect other end of U-tube to 3-way connector.
b. Connect 3-way connector to atomizer in spectrometer and to hydrogen source.
c. Adjust H flow at 90 ml min−1, using a flow controller.
d. Connect sparger in HG reaction vessel to He tank through the 4-way valve (Figure 2) and adjust He flow to 120 ml min−1, using a flow controller.
e. Connect ends of Ni80/Cr20 wire to power source.
f. Insert U-tube into Dewar flask filled with liquid nitrogen. Make sure the section of the U-tube filled with adsorbent is completely submerged in liquid nitrogen.
g. Turn on H/air heating unit in the spectrometer.
3. Sample preparation
Aqueous solutions and urine samples or simple biological matrices (e.g., cell lysates or tissue culture media) can be analyzed directly without prior treatment. More complex biological matrices (e.g., tissue homogenates) must be digested in a concentrated phosphoric acid at 90°C overnight (Del Razo et al., 2001; Devesa et al., 2004). However, because acid digestion oxidizes all trivalent arsenicals to pentavalency, samples prepared by this method cannot be used for determination of the original oxidation state of As in arsenicals. In cases where the oxidation state of arsenic can be preserved during sample preparation, then the pH at which hydride generation is performed can be controlled. With generation at pH 1, compounds containing arsenic present either in the trivalent or pentavalent oxidation state are converted to the corresponding arsines which can be cryotrapped, chromatographed, and detected by atomic absorption spectrometry. With generation at pH 6, only compounds containing arsenic in the trivalent oxidation state are converted to the corresponding arsines which can be cryotrapped, chromatographed, and detected by atomic absorption spectrometry. This pH-selective method allows estimation of the concentrations of arsenicals containing trivalent arsenic and of arsenicals containing pentavalent arsenic.
Protocol Steps
1. HG at pH 1
a. Prepare the following solutions for hydride generation at pH 1:
4% NaBH4 in 0.02 M NaOH
6 N HCl, and 1% antifoam emulsion in deionized water.
b. Add 7 ml of deionized water, 1 ml of 6 N HCl, and sample solution (0.5 to 5 ml) into HG reaction vessel.
c. Insert sparger into reaction vessel and seal.
d. Turn 4-way valve to start a flow of He through the reaction vessel into the U-tube which is immersed in liquid nitrogen.
e. Start timer. After exactly 50 seconds, inject 1 ml of 4% NaBH4 solution in 0.02 M NaOH through the silicone rubber septum of the HG reaction vessel. Inject NaBH4 solution slowly over a 50-second time interval.
f. Run HG reaction for additional 170 seconds while purging HG vessel with He.
g. Turn 4-way valve to deliver He directly to U-tube (bypassing the HG reaction vessel).
h. Remove U-tube from liquid nitrogen. Heat U-tube for 90 seconds by applying 30-V current to the heating wire that is wrapped around the U-tube (under these conditions temperature inside the U-tube reaches approximately 80°C).
i. Simultaneously, switch the spectrometer to reading mode. Save sample reading in a file for further analysis.
2. HG at pH 6
a. Prepare the following solution for hydride generation at pH 6.
2.5 M tris HCl solution (pH 6) in deionized water.
b. Add 7 ml of deionized water, 1 ml of 2.5 M tris HCl (pH 6), 1 ml of 1% antifoam emulsion, and sample to HG reaction vessel.
c. Insert sparger into reaction vessel and seal.
d. Turn 4-way valve to start a flow of He through reaction vessel into U-tube which is immersed in liquid nitrogen.
e. Start timer and inject 1 ml of NaBH4 solution. For HG at pH 6, the injection of the NaBH4 solution should take approximately 50 seconds.
f. Run HG reaction for additional 170 seconds while purging HG vessel with He.
g. Turn 4-way valve to deliver He directly to U-tube (bypassing the HG reaction vessel).
h. Remove U-tube from liquid nitrogen. Heat U-tube for 90 seconds by applying 30-V current to the heating wire that is wrapped around the U-tube (under these conditions temperature inside the U-tube reaches approximately 80°C).
i. Simultaneously, switch the spectrometer to reading mode. Save sample reading in a file for further analysis.
3. Cleaning the hydride generation system
Remove sparger from the reaction vessel, turn the 4-way to the original position, and discard the contents of the reaction vessel to an appropriate receptacle for chemical waste. Rinse the HG reaction vessel with 20 ml of deionized water before use in the next analysis. Run a blank HG reaction to assure that there is no residual As in the system. For this blank determination, the sample is replaced with deionized water; all other reagents and procedures follow those described in preceding paragraphs.
4. AAS signal analysis
Analyze the AAS signal using software supplied with the spectrometer. Use peak area to quantify As in peaks of arsines separated thermally during the heating of the cryotrap. Calculate the amounts of As in the peaks, using calibration curves.
5. Standard solutions for calibration
Prepare a stock solution containing 1000 ppm of As for each of the As standards (iAsV, iAsIII, MAsV, MAsIII, DMAsV, DMAsIII, TMAO). Use deionized water for all stock solutions except for iododimethylarsine, which must be prepared in ethanol. Prepare 0.1 ppm As stock solutions by a serial dilution of the 1000 ppm As stock solutions in deionized water. Prepare a standard mixture “A” of the pentavalent arsenicals and TMAO by mixing equal amounts of the 0.1 ppm As stock solutions of iAsV, MAsV, DMAsV and TMAO. Prepare a standard mixture “B” of the trivalent arsenicals and TMAO by mixing equal amounts of the 0.1 ppm As stock solutions of iAsIII, MAsIII, DMAsIII, and TMAO.
Note: Methylated trivalent arsenicals are liable to oxidation in aqueous solutions. Therefore, the standard solutions of oxomethylarsine and iododimethylarsine must be prepared shortly before use in calibration.
6. Calibration
Run HG-CT-AAS assays at pH 1 in which the mixture in the reaction vessel contains 5, 25, 100, 200 or 800 μl of the standard mixture “A”. Run HG-CT-AAS assays at pH 6 in which the mixture in the reaction vessel contains 5, 25, 100, 200 or 800 μl of the standard mixture “B”. Additions of these volumes of standards to the reaction vessel correspond to 0.5, 2.5, 10, 20 and 80 ng of As for each standard. Repeat each measurement twice and use the average value for the construction of the corresponding calibration curves “A and “B”. Use calibration curves to determine the contents of arsenicals in analyzed samples.
Commentary
Background Information
Basic Protocol 1
The early use of in vitro assay systems to study the methylation of inorganic arsenic (Hirata et al., 1989; Buchet and Lauwerys, 1985) necessitated the development of methods for the detection of iAs and its methylated metabolites at relatively low concentrations. Because atomic absorption or emission methods then available lacked sufficient sensitivity, the use of radiotracer methods for the study of iAs metabolism was an attractive alternative. The radionuclide [73As] was commercially available and a method for the chemical reduction of arsenic (AsV) acid to arsenous (AsIII) acid had been published (Reay and Asher, 1977). The relatively high throughput of the TLC method made it possible to quickly evaluate the factors that controlled in vitro methylation and aided in the purification of arsenic (+3 oxidation state) methyltransferase.
Alternate Protocol 1
The evolution of hydride generation methods for use in conjunction with atomic absorption spectrometry that has occurred since the pioneering work of Braman and Foreback (1973) and Crescelius (1977) has lead to the development of highly sensitive and specific analytical methods. In particular, the pH-selective generation of arsenicals based on the oxidation state of arsenic has the potential of providing useful information about the intracellular metabolism of arsenic. One limitation of this method is the low rate of generation of arsines from thioarsenicals. Other approaches will be required to detect and quantify these species.
Troubleshooting
Basic Protocol 1
A particular issue in TLC is variability in the coating applied to commercially prepared plates. Variation in physical characteristics of the absorbent layer (e.g., the size and density of cellulose fibers) affects separation and resolution of arsenicals. Our experience over many years shows that these properties often vary widely among different lots of PEI-cellulose plates. Screening different production lots of TLC plates can be used to select those with desirable separatory properties. Mobile phases can also be modified to optimize separation on a particular lot of TLC plates. However, optimization of mobile phase on a lot by lot basis can be time consuming. With PEI-cellulose plates, an isopropanol-acetic acid-water mobile phase provides better separation than does an acetone-acetic acid-water mobile phase but requires more time for plate development. Increasing the relative amount of acetic acid in isopropanol-acetic acid-water mobile phase increases mobilities of iAsV and MAsV and increases lateral diffusion. Tradeoffs between separation times and resolution should be considered in analytical use of TLC; significant experimental effort may be needed to optimize chromatography conditions.
If this TLC method is to be applied for separation of non-radiolabeled arsenicals in CuCl-treated samples, special attention is required to deal with the presence of Cu in samples. Under the chromatographic conditions described here, Cu co-migrates with iAsV. However, Cu can be removed from extracts by addition of concentrated oxalic acid. This procedure produces Cu oxalate which precipitates out of the extracts and can be easily removed by low speed centrifugation of treated extracts.
Other common problems in TLC separation of arsenical metabolites are summarized in Table 1.
Table 1.
Troubleshooting guide for analysis of arsenical metabolites by TLC
| Problem | Cause | Solution |
|---|---|---|
| Incomplete separation of As species | Incorrect mobile phase composition | Make sure all components of the mobile phase are mixed in the correct proportions |
| Old mobile phase | Use only fresh mobile phase prepared from fresh high-grade components | |
| Flaws in thin layer coating | Use TLC plates with homogenous and uniform layers of ion exchanger | |
|
| ||
| Front of mobile phase dries before reaching top of TLC plate | Chromatographic chamber not properly sealed | Seal lid of chromatographic chamber using petroleum jelly or similar sealant or with parafilm |
|
| ||
| No As species detected after separation on TLC plate | Applied radioactivity below detection limit of detection system | Increase volume of sample on TLC plate or concentrate samples before CuCl extraction |
| Use radiolabeled As with higher specific radioactivity | ||
Alternate Protocol 1
This section focuses on aspects of hydride generation and cryotrapping that are common to all systems that use this approach to determination of arsenicals by generation of arsines and thermal chromatography of the arsines. There are many aspects of troubleshooting for these assays that will be related to the specifications and operating conditions of the atomic absorption spectrophotometer that is used for detection of arsines. Rectification of problems related to performance of specific atomic absorption spectrophotometer is best achieved by consultation with the manufacturers. Therefore, the following paragraphs emphasize identification of problems related to hydride generation and cryotrapping and potential solutions to these problems.
Contamination of reagents with As is a potential source of error in the determination of arsenicals by hydride generation-atomic absorption spectrometry. In our experience, the reagents most likely to contain contaminating As are phosphoric acid and sodium borohydride. Even products designated as “Analytical grade” or its equivalent have been intermittently found to contain high levels of As. To minimize the potential of contamination, we routinely use Ultrex grade phosphoric acid from J.T. Baker (Plainview NJ) and sodium borohydride from EMD Chemicals (Gibbstown, NJ).
Many factors may affect retention times for arsines generated in the HG reactions and trapped in CT. These include size (length and internal diameter) of the U-tube, the volume and chemical properties of the adsorbent packing material, the temperature gradient used in the heating step, and the flow rates for carrier gases. Therefore, when one of these variables is modified, retention times for each arsine must be reexamined with appropriate As standards. Identities of arsines in peaks generated from samples should be confirmed by analyzing sample aliquots spiked with small amounts of individual As standards.
“Memory” in HG systems refers to the persistence of peaks in chromatograms that are due to carryover between analytical runs. The retention of arsines on glass or plastic surfaces in the HG-CT-AAS system is the apparent source of most “memory” problems. For example, small amounts of dimethylarsine are often found in blanks run between samples. If a “memory” arsine signal is detected in a blank run, then blank run must be repeated until the “memory” signal disappears. In our experience, an especially persistent “memory” signal can be eliminated by replacement of the U-tube and all parts of tubing connecting the HG and CT units within the spectrometer. All glass components of the system removed must be thoroughly washed before reuse.
Water condensation in tubing is another common problem that can interfere with a proper chromatographic separation of arsines. A common site for condensation is tubing connecting the outlet of the U-tube to the atomizer in the AAS. In our experience, using a resistance wire to heat this section of the gas flow pathway during the heating step minimizes condensation.
Standard reference materials with certified or reference values for individual arsenicals of interest are not available. For example, the currently available standard reference material for toxic elements in human urine (SRM 2670a - Toxic Elements in Urine (Freeze-Dried), National Institute of Standards and Technology) provides no certified values for the low or high level concentrations of As in urine. Indeed, only a reference value is provided for the high level concentration of As which is attained by the addition of iAs to the urine. Human urine prepared by the National Institute of Environmental Studies in Japan has certified values for arsenobetaine, dimethylAs, and total As (CRM, NIES CRM No. 18 Human Urine) (Yoshinaga et al., 2000). The Interlaboratory Comparison Program for Metals in Biological Matrices and the Quebec Multielement External Quality Assessment Scheme of the Centre de Toxicologie du Quebec, (Intstitut National de Sante Publique, Quebec City, Quebec, Canada, www.inspq.qc.ca/ctq/default.asp?Page=1&Lg=en) also provides human urine for assessment of arsenic contents. There is a pressing need for a standard reference material that is certified for the levels of inorganic, methylated, dimethylated, and trimethylated arsenicals. Ideally, there would be a standard reference material that is certified for these arsenicals on the basis of the oxidation of As in each species. However, problems with the preservation of the oxidation state of As in stored samples may make it impossible to prepare the latter. As an alternative approach in the absence of these standards, it is imperative that analyses be properly calibrated using well-characterized As standards prepared in the laboratory for use in quality assurance and control. It is useful to determine independently the total As content of samples. Comparison of the total As content with the sum of As species determined by HG-CT-AAS provides useful information about the recovery of As. Discrepancies between the two analytical values may indicate the presence of As species that were not detected by HG-CT-AAS.
The speciation analysis of As using the manual HG-CT-AAS system described here is a relatively laborious procedure with limited throughput. In addition, the detection limits for all relevant As species are generally higher for HG-CT-AAS systems equipped with conventional atomizers (140 to 400 pg) than are detection limits reported for systems using inductively coupled plasma-mass spectrometry (ICP-MS) for As detection. Hernandez and associates (Hernandez et al., 2008) and Matousek and associates (Matousek et al. 2008) have described a modified HG-CT-AAS system in which HG and cryotrapping steps that are usually performed manually are fully automated and in which the conventional atomizer is replaced with a multiple microflame quartz tube atomizer (multiatomizer). The throughput of the method increased with the automated system and detection limits significantly improved, ranging from 8 to 20 pg.
Anticipated Results
Basic Protocol 1
Table 2 shows relative mobilities (RF) for authentic radiolabeled standards of inorganic, methylated, and dimethylated arsenicals separated on PEI-cellulose plates using the two solvent systems described above. Treatment of samples with Cu markedly affected RF's for iAsV but had little effect on the mobility of other arsenicals. In early studies with the in vitro methylation system or with recombinant rat As3mt, we routinely used an acetone-acetic acid-water mobile phase for development of TLC because this system quickly yielded reproducible separations. Cloning of the rat As3mt gene and expression of the recombinant protein permitted new work on the characteristics of the reactions catalyzed by this enzyme. Figure 5 shows a phosphoimager analysis of a TLC separation of radiolabeled arsenicals from reaction mixtures containing recombinant rat As3mt, AdoMet, a thioredoxin/thioredoxin reductase/NADPH coupled system, and radiolabeled [73As]- iAsIII. Using an acetone-acetic acid-water mobile phase yielded well defined spots for inorganic and methylated arsenicals but the spot for dimethytlated arsenicals was often diffuse and attenuated along the axis of solvent migration. Using an isopropanol-acetic acid-water mobile phase yields four distinct spots on the TLC plate. Three of these can be identified by co-migration with authentic radiolabeled standards for iAs, MAs, and DMAs. Identifying the fastest migrating spot in the isopropanol-acetic acid-water mobile phase as TMAO involved use of HG-AAS and ICP-MS to confirm its identity (Waters et al., 2004).
Table 2.
Effect of treatment with cuprous chloride on relative mobilities (RF) for arsencials separated by TLC on PEI-cellulose TLC plates using an acetone, acetic acid, and water (2:1:1) mobile phase or an isopropanol, acetic acid, and water (10:1:2.5) mobile phase.
| Arsenical | Acetone- acetic acid - water | Isopropanol- acetic acid - water | ||
|---|---|---|---|---|
| Untreated | Cu-treated | Untreated | Cu-treated | |
| Arsenate | 0.02 | 0.16 | 0.01 | 0.15 |
| Arsenite | 0.33 | 0.34 | 0.48 | 0.49 |
| Methylarsonic acid | 0.49 | 0.53 | 0.33 | 0.38 |
| Dimethylarsinic acid | 0.91 | 0.92 | 0.75 | 0.74 |
Figure 5.
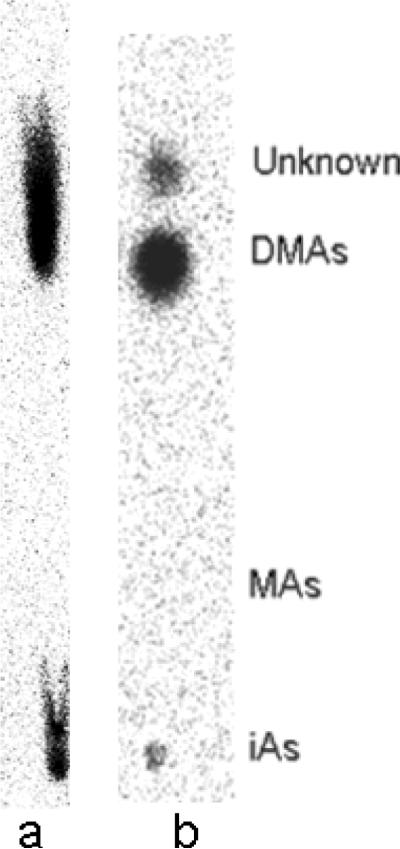
Effect of mobile phase on resolution of radiolabeled arsenicals by TLC. Aliquots of reaction mixtures containing 5 μg of recombinant rat As3mt, 1 mM S-adenosylmethionine, thioredoxin-thioredoxin reductase-NADPH coupled system, and 1 μM [73As]-arsenite incubated for 120 minutes at 37°C separated on PEI-cellulose TLC plates using (a) an acetone, acetic acid, and water (2:1:1) mobile phase or (b) an isopropanol, acetic acid, and water (10:1:2.5) mobile phase. Positions for the migration of authentic standards of arsenate, methylarsonic acid, and dimethylarsinic acid shown. The unknown species separated with the isopropanol, acetic acid, and water mobile phase was identified as trimethylarsenic by HG-CT-AAS and ICP-MS.
Alternate Protocol 1
Using pH selective generation of arsines from biological samples provides information that allows estimation of the concentrations of arsenicals based on the oxidation state of As. In this approach, hydride generation at pH 6 allows quantitation of inorganic, methylated, and dimethylated arsenicals containing trivalent As and of TMAO which contains pentavalent As. Analysis at pH 1 allows quantitation of inorganic, methylated, and dimethylated arsenicals that contain trivalent As. In practice, we do not quantify TMAO on the basis of results obtained with HG at pH 1 but rather use data from HG at pH 6. Given these analytical results, one can estimate the concentration of inorganic, methylated, and dimethylated arsenicals containing pentavalent As by the difference in the estimated concentration of each arsenical species at pH 1 and pH 6.
Time Considerations
Basic Protocol 1
Time Considerations
The time required for the extraction of samples with CuCl depends on the number of samples; the extraction of 12 samples needed to fill one TLC plate can be completed in less than 1 hour. We recommend that oxidation of CuCl-treated samples with H2O2 be carried out overnight to ensure a complete conversion of trivalent arsenicals to pentavalency. Once samples have been treated with CuCl, they can be stored one to two days at 4°C before oxidation with H2O2. Similarly, CuCl-treated and H2O2-oxidized samples can be stored for one or two days at 4°C before TLC separation. Longer periods of storage may result in changes in the chemical characteristics of As metabolites (e.g., apparent demethylation of methylated arsenicals). Application of 12 samples on one TLC plate (2 μl/sample) takes about 30 minutes, including drying. The time needed for an optimal separation of As species on the TLC plate depends mainly on the mobile phase used for elution: 1.5 to 2 hours for the acetone-acetic-acid-water phase and 4 to 5 hours for the isopropanol-acetic acid-water phase. However, a satisfactory separation can be achieved with a shorter elution time, especially for samples separated in the isopropanol-acetic acid-water phase which restricts samples diffusion. The time needed for detection of radiolabeled arsenicals by phosphorimaging depends strictly on the amount of radioactivity associated with each of these species. To obtain an optimal signal on phosphorimaging plates, screening as short as 1 to 5 minutes may be sufficient for highly radioactive samples. However, imaging may take several days for samples containing low radioactivity. The instrumental analysis of non-radioactive arsenicals collected from TLC plates may take hours or days, depending on the analytical method chosen.
Alternate Protocol 1
Time Considerations
Setup and preparation of calibration samples and the initial calibration of the HG-AAS system will probably require a full day's effort. pH-selective analysis of 20 to 30 samples with duplicate analysis of each sample and inclusion of repeated analysis of calibration samples will typically require at least 8 hours. Hence, for analysis of 20 to 30 samples at pH 1 and 6 will require about 16 hours. If samples require digestion in phosphoric acid before HG-AAS, then an additional 12 hours (overnight) will be required.
Footnotes
DISCLAIMER - This manuscript has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Literature Cited
- Braman RS, Foreback CC. Methylated forms of arsenic in the environment. Science. 1973;182:1247–1249. doi: 10.1126/science.182.4118.1247. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R. Study of inorganic arsenic methylation by rat liver in vitro: relevance for the interpretation of observations in man. Arch. Toxicol. 1985;57:125–129. doi: 10.1007/BF00343122. [DOI] [PubMed] [Google Scholar]
- Crecelius EA. Changes in the chemical speciation of arsenic following ingestion by man. Environ. Health Perspect. 1977;19:147–150. doi: 10.1289/ehp.7719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, Styblo M, Cullen WR, Thomas DJ. Determination of trivalent methylated arsenicals in biological matrices. Toxicol. Appl. Pharmacol. 2001;174:282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- Devesa V, Del Razo LM, Adair B, Drobna Z, Waters SB, Hughes MF, Styblo M, Thomas DJ. Comprehensive analysis of arsenic metabolites by pH-specific hydride generation atomic absorption spectrometry. J. Anal. At. Spectrom. 2004;19:1460–1467. [Google Scholar]
- Hernandez-Zavala A, Matousek T, Drobna Z, Walton F, Adair BM, Dedina J, Thomas DJ, Styblo M. Speciation of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer. J. Anal. At. Spectrom. 2008;23:342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Mohri T, Hisanaga A, Ishinishi N. Conversion of arsenite and arsenate to methylarsenic and dimethylarsenic compounds by homogenates prepared from livers and kidneys of rats and mice. Appl. Organomet. Chem. 1989;3:335–341. [Google Scholar]
- Matoušek T, Hernández-Zavala A, Svoboda M, Langerová L, Adair BM, Drobná Z, Thomas DJ, Stýblo M, Dĕdina J. Oxidation state specific generation of arsines from methylated arsenicals based on L- cysteine treatment in buffered media for speciation analysis by hydride generation - automated cryotrapping - gas chromatography- atomic absorption spectrometry with the multiatomizer. Spectrochim. Acta Part B: Atomic Spectroscopy. 2008;63:396–406. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay PF, Asher CJ. Preparation and purification of 74As-labeled arsenate and arsenite for use in biological experiments. Anal. Biochem. 1977;78:557–560. doi: 10.1016/0003-2697(77)90117-8. [DOI] [PubMed] [Google Scholar]
- Styblo M, Delnomdedieu M, Hughes MF, Thomas DJ. Identification of methylated metabolites of inorganic arsenic by thin-layer chromatography. J. Chromat. B. 1995;668:21–29. doi: 10.1016/0378-4347(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Styblo M, Hughes MF, Thomas DJ. Liberation and analysis of protein-bound arsenicals. J. Chromat. B. 1996;677:161–166. doi: 10.1016/0378-4347(95)00490-4. [DOI] [PubMed] [Google Scholar]
- Waters SB, Devesa V, Fricke M, Creed J, Styblo M, Thomas DJ. Glutathione modulates recombinant rat arsenic (+3 oxidation state) methyltransferase-catalyzed formation of trimethylarsine oxide and trimethylarsine. Chem. Res. Toxicol. 2004;17:1621–1629. doi: 10.1021/tx0497853. [DOI] [PubMed] [Google Scholar]
- Yoshinaga J, Chatterjee A, Shibata Y, Morita M, Edmonds JS. Human urine certified reference material for arsenic speciation. Clin. Chem. 2000;46:1781–1786. [PubMed] [Google Scholar]