ABSTRACT
Assessment of outcomes after craniofacial surgery for skull base tumors poses unique challenges because of the rarity of the problem and heterogeneity in clinical behavior of these tumors. Collaborative studies of outcome provide an opportunity for meaningful analysis of not just tumor-related outcome, but also quality of life after treatment in these patients. This article introduces the use of a Web-based data collection method that can function as a collaborative registry and a tool for collection of quality of life data.
Keywords: Skull base neoplasms/surgery, questionnaires, neoplasms/psychology, international cooperation, software design
Craniofacial surgery (CFS) is a well-established modality for treatment of patients with tumors of the skull base. These tumors are relatively rare so that only a few selected centers have the surgical expertise and perioperative support and infrastructure needed for successful outcome after CFS. A wide variety of tumors with diverse biological characteristics affect the skull base, and this heterogeneity in clinical behavior creates additional problems in analysis of outcomes. Outcomes data are consequently difficult to accumulate for any single institution. The problem of outcomes analysis in rare diseases has been addressed by collaborative national and international data registries that have effectively studied disease behavior and reported outcomes of treatment in other diseases.1,2,3
An International Collaborative Study Group comprising 17 institutions was formed under the leadership of Dr. Jatin P. Shah in 1996 to collect data on patients undergoing CFS with the aim of reporting outcomes of CFS based on a large patient cohort.4 Participating investigators filled out a multi-item questionnaire that included patient-, tumor-, and treatment-related information. These data were collected retrospectively by each investigator and entered on paper forms that were then submitted to the central analyzing office for entry into a computerized database, compilation, statistical analysis, and reporting. During collation and analysis of data for this collaborative study, the need for significant improvement in the quality and uniformity of reporting certain patient- and tumor-related variables became evident.
The major limitation of paper data collection forms in terms of quality of data includes difficulty in interpretation of handwritten data and the potential for error in transferring data to a computer database. Other issues pertain to logistics and include costs for paper supplies, postage, duplication of data collection effort, and delay in response. Audit and quality control of collected data are also cumbersome because of the potential difficulty in identifying inconsistencies and the considerable effort and communication required between the contributor and central analyzing office to resolve discrepancies. With the widespread availability and use of the Internet, Web-based data collection has become an attractive option for collaboration across institutions.5,6 It is easy to see how this electronic approach can overcome some of the obvious limitations of paper forms, but the major advantage is that the interface can be programmed to perform certain quality control measures at the point of data entry. For instance, conditional alerts can be incorporated to warn against inconsistent data entry. Automatic e-mail alerts and reminders can also be programmed for housekeeping and maintaining follow-up information. The Internet has therefore become a valuable tool in data collection for multi-institutional clinical research.7,8,9
METHODS AND RESULTS
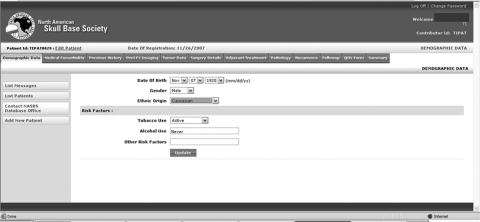
In 2004, the North American Skull Base Society (NASBS) database committee embarked upon the creation of a Web-based registry of patients treated with CFS for skull base tumors. The process has been arduous largely due to logistic and financial hurdles, but also because of the complex issues involved in design and programming. A prototype is now in place and should be available for use in the near future after approval by the NASBS. The NASBS database has a modular design to allow future modifications and refinement. Patient-, tumor-, and treatment-related information is accessible via tabs (Fig. 1) that expand to reveal subforms (Fig. 2). Obvious issues that need to be considered include protection of patient health information.10 Web-based patient data registries have been shown to be secure,8 and the protection of patient health information is imperative to the success of any collaborative research effort. The NASBS database is designed to be used with anonymous identifiers and appropriate data encryption and secure storage (Fig. 3).
Figure 1.
Patient-, tumor-, and treatment-related information is accessible via tabs on the data entry screen of the Web-based database.
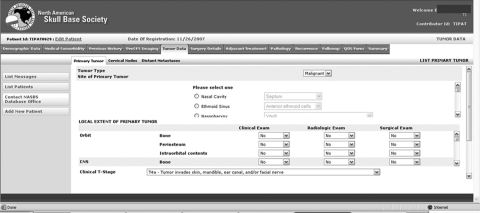
Figure 2.
Detailed information can be entered by expanding the relevant subforms.
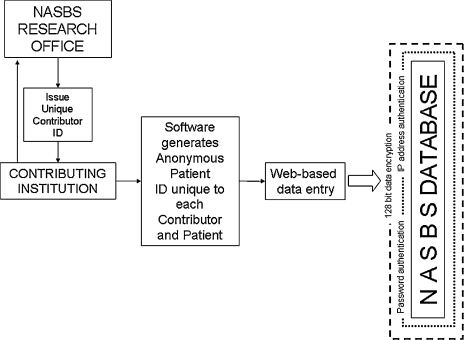
Figure 3.
Security features for a Web-based database should include the use of anonymous patient identifiers, password protection, appropriate data encryption, and secure storage.
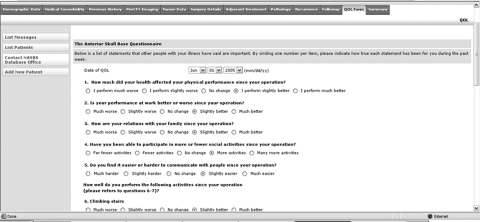
The importance of analyzing and understanding the quality of life (QOL) in patients undergoing CFS is highlighted in this issue of the journal. The Web-based NASBS database will provide a unique opportunity to collect QOL information on a large cohort of patients. A previously validated QOL questionnaire11 has been included on the database as a tab (Fig. 4) that opens a questionnaire, which can be used for either single-use cross-sectional entry or for prospective longitudinal data collection.
Figure 4.
The database includes a previously validated QOL questionnaire that is accessible from a tab on the data entry screen.
DISCUSSION
Data collection using this Internet-based tool can be expected to generate reliable QOL data that can be accurately correlated with patient, tumor, and treatment data. The success of this effort will obviously depend on the enthusiasm and compliance of the contributors. Despite the numerous advantages of Web-based collection of QOL information, significant challenges exist in adapting existing QOL instruments to the Web-based format.12 Other registry-type databases have proved unsustainable for lack of adequate long-term participation.13 Incentives favoring data collection, analysis, and reporting will need to be built into the system.
Because there seems to be no direct incentive for busy clinicians to invest time and effort in prospectively collecting QOL information after CFS for skull base tumors, an alternative approach for the future might be to directly involve patients in this effort. Computerized patient-reported QOL and symptom reporting has been tested and found effective in patients treated for other cancers.14 Although some studies have reported a high level of acceptability for computerized assessment in patients of all backgrounds and abilities,15,16 a significant concern for this approach may be the ability of patients undergoing CFS to use a computer and access the Internet. This population of patients presents unique challenges not just in analysis of treatment-related outcomes, but also for assessment of posttreatment function and QOL. With accumulating experience in the use of technology and the Internet for QOL assessment, it will be interesting to study patients' perceptions of their QOL and how clinicians are able to integrate this information in clinical practice with the aim of improving outcomes.
REFERENCES
- Federici A B, Rand J H, Bucciarelli P, et al. Subcommittee on von Willebrand Factor Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84:345–349. [PubMed] [Google Scholar]
- Laverty A, Jaffé A, Cunningham S. Establishment of a Web-based registry for rare (orphan) pediatric lung diseases in the United Kingdom: the BPOLD registry. Pediatr Pulmonol. 2008;43:451–456. doi: 10.1002/ppul.20783. [DOI] [PubMed] [Google Scholar]
- Reincke M, Petersenn S, Buchfelder M, et al. The German Acromegaly Registry: description of the database and initial results. Exp Clin Endocrinol Diabetes. 2006;114:498–505. doi: 10.1055/s-2006-948313. [DOI] [PubMed] [Google Scholar]
- Patel S G, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98:1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- Niland J C. NCCN Internet-based data system for the conduct of outcomes research. Oncology (Williston Park) 1998;12:142–146. [PubMed] [Google Scholar]
- Marshall W W, Haley R W. Use of a secure Internet Web site for collaborative medical research. JAMA. 2000;284:1843–1849. doi: 10.1001/jama.284.14.1843. [DOI] [PubMed] [Google Scholar]
- Khaitan L, Apelgren K, Hunter J, Traverso L W, Society of American Gastrointestinal Endoscopic Surgeons (SAGES) Outcomes Initiative A report on the Society of American Gastrointestinal Endoscopic Surgeons (SAGES) Outcomes Initiative: what have we learned and what is its potential? Surg Endosc. 2003;17:365–370. doi: 10.1007/s00464-002-8844-4. [DOI] [PubMed] [Google Scholar]
- Lallas C D, Preminger G M, Pearle M S, et al. Internet based multi-institutional clinical research: a convenient and secure option. J Urol. 2004;171:1880–1885. doi: 10.1097/01.ju.0000120221.39184.3c. [DOI] [PubMed] [Google Scholar]
- Proctor S J, Wilkinson J. A Web-based study concept designed to progress clinical research for “orphan” disease areas in haematological oncology in the elderly: the SHIELD programme. Crit Rev Oncol Hematol. 2007;61:79–83. doi: 10.1016/j.critrevonc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Schell S R. Creation of clinical research databases in the 21st century: a practical algorithm for HIPAA Compliance. Surg Infect (Larchmt) 2006;7:37–44. doi: 10.1089/sur.2006.7.37. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss D M. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. 2004;100:813–819. doi: 10.3171/jns.2004.100.5.0813. [DOI] [PubMed] [Google Scholar]
- Jones J B, Snyder C F, Wu A W. Issues in the design of Internet-based systems for collecting patient-reported outcomes. Qual Life Res. 2007;16:1407–1417. doi: 10.1007/s11136-007-9235-z. [DOI] [PubMed] [Google Scholar]
- Lum F, Schachat A P, Jampel H D. The development and demise of a cataract surgery database. Jt Comm J Qual Improv. 2002;28:108–114. doi: 10.1016/s1070-3241(02)28010-4. [DOI] [PubMed] [Google Scholar]
- Berry D L, Trigg L J, Lober W B, et al. Computerized symptom and quality-of-life assessment for patients with cancer part I: development and pilot testing. Oncol Nurs Forum. 2004;31:E75–E83. doi: 10.1188/04.ONF.E75-E83. [DOI] [PubMed] [Google Scholar]
- Taenzer P, Bultz B D, Carlson L E, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology. 2000;9:203–213. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mullen K H, Berry D L, Zierler B K. Computerized symptom and quality-of-life assessment for patients with cancer part II: acceptability and usability. Oncol Nurs Forum. 2004;31:E84–E89. doi: 10.1188/04.ONF.E84-E89. [DOI] [PubMed] [Google Scholar]