Abstract
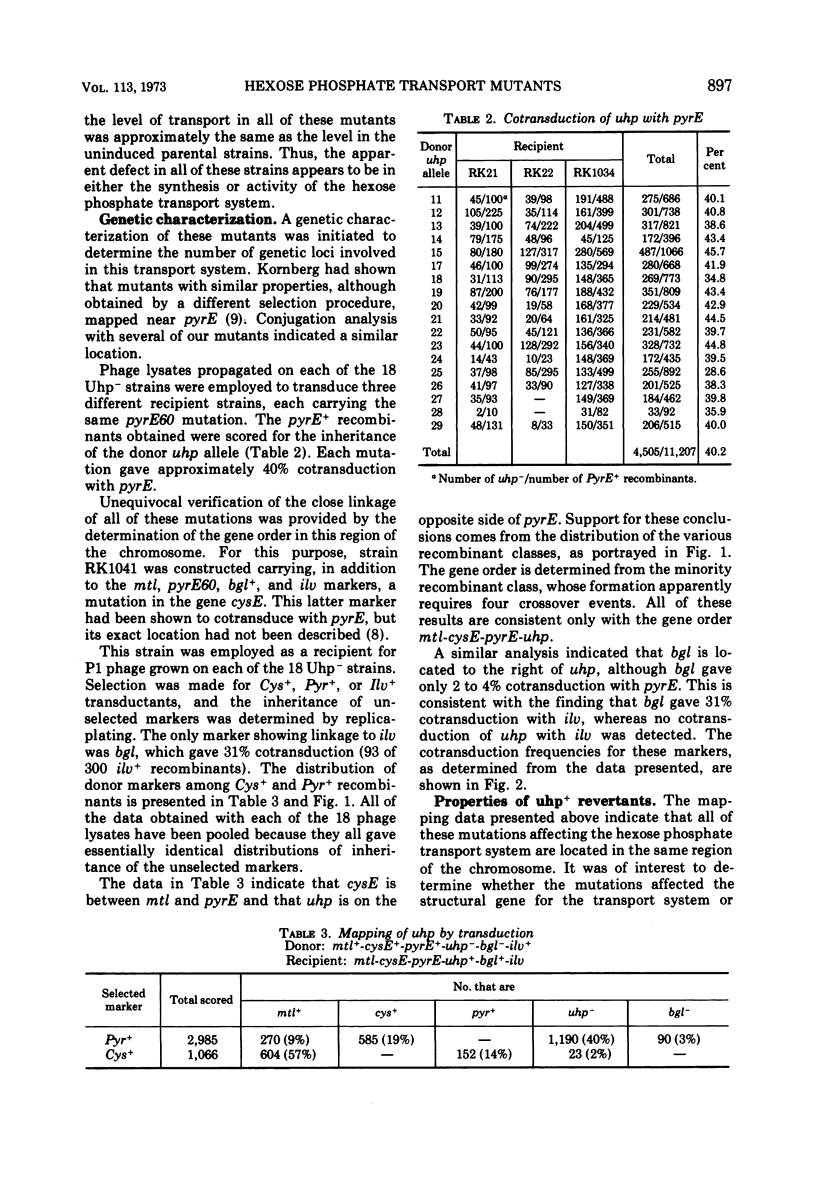
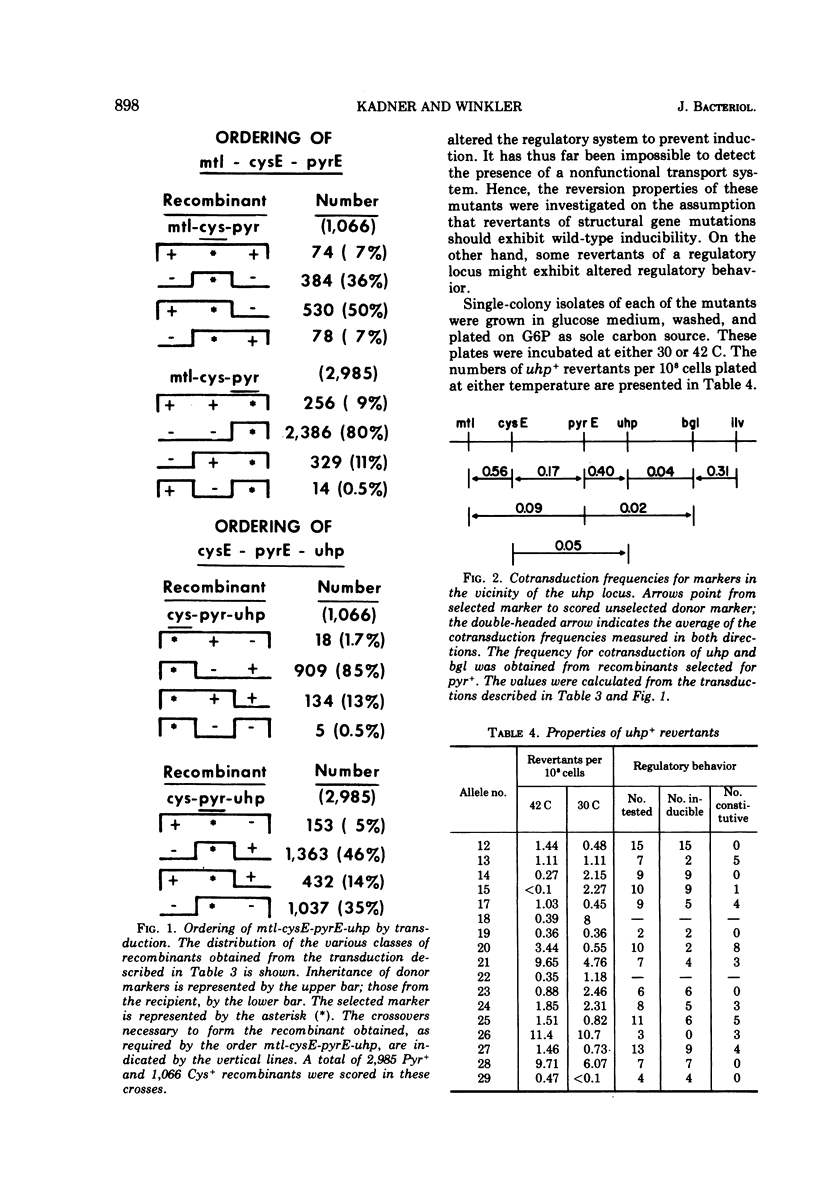
Mutants of Escherichia coli defective in the hexose phosphate transport system were isolated. Negative selection by penicillin treatment or positive selection with phosphonomycin was employed. These mutants grew normally on all carbon sources other than hexose phosphates. The map location of the mutations in 18 independently isolated mutant strains was investigated by transduction crosses. All of the mutations were found to lie in the same region of the chromosome, in the region represented by min 72 on the Taylor map. The order of the genes in this region was found to be mtl-cysE-pyrE-uhp-bgl-ilv. Revertants of some of the mutants exhibited altered regulatory control of this transport system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen B. G., Leanza W. J., Beattie T. R., Patchett A. A., Arison B. H., Ormond R. E., Kuehl F. A., Jr, Albers-Schonberg G., Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969 Oct 3;166(3901):123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W. Dehydrogenase activity involved in the uptake of glucose 6-phosphate by a bacterial membrane system. J Biol Chem. 1972 Jul 25;247(14):4561–4565. [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L., Smith Janet. Isolation and properties of a regulatory mutant in the hexose phosphate transport system of Escherichia coli. FEBS Lett. 1971 Mar 5;13(3):133–136. doi: 10.1016/0014-5793(71)80218-1. [DOI] [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of hexose phosphate uptake by Escherichia coli. Nature. 1969 Dec 27;224(5226):1261–1262. doi: 10.1038/2241261a0. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Pogell B. M., Maity B. R., Frumkin S., Shapiro S. Induction of an active transport system for glucose 6-phosphate in Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):406–415. doi: 10.1016/0003-9861(66)90047-6. [DOI] [PubMed] [Google Scholar]
- SERCARZ E. E., GORINI L. DIFFERENT CONTRIBUTION OF EXOGENOUS AND ENDOGENOUS ARGININE TO REPRESSOR FORMATION. J Mol Biol. 1964 Feb;8:254–262. doi: 10.1016/s0022-2836(64)80135-2. [DOI] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Compartmentation in the induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1970 Feb;101(2):470–475. doi: 10.1128/jb.101.2.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Kinetics of exogenous induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1971 Jul;107(1):74–78. doi: 10.1128/jb.107.1.74-78.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



