ABSTRACT
We evaluated the health-related quality of life (QOL) of patients undergoing anterior skull base tumor resection. The Anterior Skull Base Surgery QOL questionnaire, a disease-specific multidimensional instrument dedicated to this population, was used to collect and prospectively analyze demographic, medical, and QOL data on 48 patients. Thirty-nine patients completed the questionnaire preoperatively and at 6 and 12 months postoperatively. Seventeen patients (44%) had malignant histology and 22 (56%) had benign tumors. The overall QOL score decreased significantly at 6 months postoperatively (p < 0.05) and improved significantly at 12 months postoperatively (p < 0.04). The emotional domain improved significantly at 12 months postoperatively compared with the preoperative scores (p < 0.03). Patients with malignant tumors had lower scores at 6 months postoperatively compared with patients with benign lesions (p < 0.002), although the scores for both groups at 12 months postoperatively were similar. Adjuvant radiation therapy was associated with a poor QOL (p < 0.005). The results of this prospective study show that the overall deteriorated QOL of patients after anterior skull base tumor resection returns to baseline by 1 year after surgery. Histology and radiotherapy are significant predictors of health-related QOL in this population.
Keywords: Craniofacial resection, cranial base, subcranial approach, cancer
Previous reports have established craniofacial and subcranial approaches to the anterior skull base as being reliable surgical procedures for the removal of anterior skull base tumors.1,2 There have been great strides in the technical development of the surgical and radiological treatment of these tumors, but the physical and psychological consequences of surgery on the patient's quality of life (QOL) have not been clarified. Several prospective studies have assessed QOL issues in patients undergoing anterior skull base tumor resection.3,4,5,6,7 The main limitation of those studies is that they were mostly retrospective analyses of patients who had been operated in the past. A more accurate evaluation of the impact of treatment on the patients' physical, emotional, social, and economic functioning would require that a disease-specific questionnaire be completed shortly before and after the operation. Moreover, greater awareness of the various aspects of QOL may help surgeons improve the postoperative assessment and management of their patients, recognize the specific difficulties they experience during the postoperative period as early as possible, and target specific medical or other interventions for patients at high risk for a poor QOL.
The aim of this study was to prospectively assess the impact of surgery for extirpation of anterior skull base tumors on the patients' QOL. The secondary goal of the study was to identify predictors of postoperative functional outcome that can affect QOL.
MATERIALS AND METHODS
Patients who underwent extirpation of anterior skull base tumors at our institution between 2002 and 2007 were enrolled in this study. All operations were performed by the same interdisciplinary team using the subcranial approach to the anterior skull base.
The psychological, social, and physical well-being of the patients was assessed with the Anterior Skull Base Questionnaire (ASBQ), a disease-specific multidimensional questionnaire dedicated to individuals with tumors involving the anterior base of skull. The questionnaires were completed by the patient at three time points: 1 week before and 6 and 12 months after surgery. The study was approved by the local ethics committee. Only patients >16 years of age were enrolled in the study. All the patients gave a full medical history and underwent a physical examination on the same day. The histology of each of the lesions was recorded.
Extirpation of anterior skull base tumors was performed via the subcranial approach in all patients.8,9,10 The surgical technique, complications, and outcome have been described in detail elsewhere.11,12 The patients were followed every 3 months in the outpatient clinic.
Questionnaire
All patients completed the ASBQ. The questionnaire is a patient-based measurement designed for self administration. It consists of six domains: the role of performance (eight items), physical function (seven items), vitality (six items), pain (three items), specific symptoms (seven items), and emotion (five items), all together yielding a total of 36 questions with a nominal scale of five steps for each question.4 All questions have an identical level of importance. The domain of specific symptoms includes seven questions on several aspects that are most relevant to this patient population, such as altered taste, smell, and appearance, as well as epiphora, nasal secretions, and visual disturbances.
To identify patients who were likely to have a poor QOL after surgery, the study population was divided into three subgroups according to demographic and clinical characteristics.
Compound symmetry and autoregressive covariance model tests were applied for comparing different subgroups of patients at various time points. The SAS™ system for Windows (SAS Institute Inc., Cary, NC) was used for statistical analysis. Differences were considered significant at p < 0.05. All error bars and (±) signs are standard deviation.
RESULTS
A total of 63 patients underwent open surgery for extirpation of tumors involving the anterior skull base in our institution during the study period. Ten of them were not adequately fluent in Hebrew or English and could not complete the ASBQ questionnaire. Five patients were <16 years old. Of the 48 remaining patients who were enrolled in the study, seven did not complete the three required questionnaires, and two died before the 12-month end point. The response rate for completing the questionnaire, after excluding the patients who died, was 85%. Thus, a total of 39 patients fulfilled study entry criteria. They all gave a full medical history and underwent a physical examination on the same day. Their mean age was 39 ± 15 years (range 16 to 76, median 38.5) and 21 (54%) were males. Their demographic data are shown in Table 1. Seventeen patients (44%) had malignant lesions and 22 (56%) had benign tumors (Table 2): there was no significant difference in age or sex between the two histological groups.
Table 1.
Demographic Characterization of the Patients
| Demographic Characteristic | No. (%) |
|---|---|
| Gender | |
| Male | 21 (54) |
| Female | 18 (46) |
| Age (y) | |
| <60 | 34 (87) |
| >60 | 5 (13) |
| Mean age | 39 |
| Malignant tumor | |
| Yes | 17 (44) |
| No | 22 (56) |
| Radiotherapy | |
| Yes | 16 (41) |
| No | 23 (59) |
| Major complication | |
| Yes | 10 (26) |
| No | 29 (74) |
| Recurrent disease | |
| Yes | 14 (36) |
| No | 25 (64) |
Table 2.
Tumor Histology
| Histology Type | n | % |
|---|---|---|
| Malignant tumors | ||
| Squamous cell carcinoma | 5 | 13 |
| Sarcoma | 4 | 10 |
| Esthesioneuroblastoma | 4 | 10 |
| Chordoma | 1 | 2.5 |
| Hemangiopericytoma | 1 | 2.5 |
| Adenocarcinoma | 1 | 2.5 |
| Sinonasal undifferentiated carcinoma | 1 | 2.5 |
| Benign tumors | ||
| Osteoma | 8 | 20.5 |
| Inverted papilloma | 4 | 10 |
| Meningoencephalocele | 3 | 8 |
| Fibrous dysplasia | 2 | 5 |
| Meningioma | 2 | 5 |
| Juvenile angiofibroma | 2 | 5 |
| Mucocele | 1 | 2.5 |
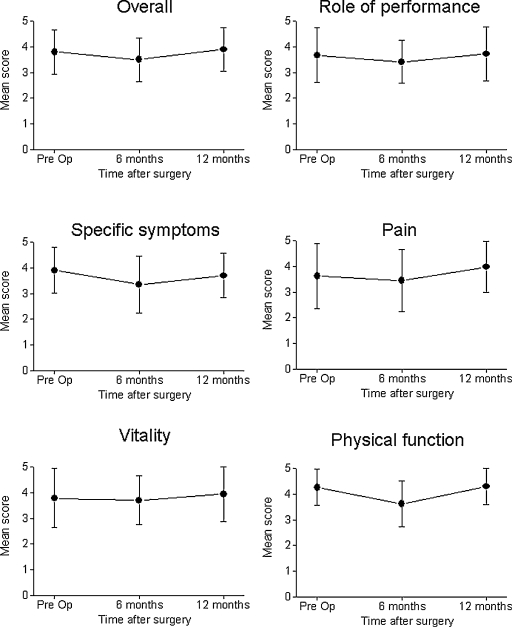
The patients were followed every 3 months for an average of 26 months (median 28, range 6 to 66). The results of the impact of surgery on the selected aspects of QOL are summarized in Table 3. There was significant decrease in the overall QOL score at 6 months after surgery (from 3.8 ± 0.87 to 3.5 ± 0.85, p < 0.05) and gradual improvement in the overall QOL score (to 3.9 ± 0.85, p < 0.04) at 12 month after surgery (Fig. 1). There was a significant decrease in the scores of the domains of the role of performance (p < 0.004), physical function (p < 0.004), and specific symptom (p < 0.004). The scores of the vitality and pain domains before and 6 month after the surgery were not statistically different. Interestingly, the QOL scores in the emotional domain improved at 6 and 12 months after surgery (Fig. 2A). Although we found significant improvement in the specific symptom domain at 12 months after surgery, these scores were still lower than the scores reported before the operation (3.7 ± 1.0 and 3.9 ± 0.9, respectively, p < 0.05). Despite the significant decline in the overall QOL that was apparent at 6 months after surgery, the QOL scores were similar to those prior to the operation at 12 months postoperatively.
Table 3.
The Impact of Surgery on Selected Aspects of Quality of Life
| Variable | Time after Surgery | Overall | Role Emotional | Specific Symptoms | Pain | Vitality | Physical Function | Role of Performance |
|---|---|---|---|---|---|---|---|---|
| Preop, preoperatively. | ||||||||
| All patients | Before (n = 39) | 3.8* | 3.32 | 3.9 | 3.62 | 3.78 | 4.26 | 3.67 |
| 6 mo (n = 31) | 3.5* | 3.52 | 3.35 | 3.45 | 3.7 | 3.62 | 3.41 | |
| 12 mo (n = 21) | 3.9* | 3.79 | 3.7 | 3.98 | 3.94 | 4.3 | 3.72 | |
| Pathology | ||||||||
| Benign | Before (n = 22) | 3.91 | 3.33‡ | 4.03 | 3.69 | 3.88 | 4.43 | 3.81 |
| 6 mo (n = 19) | 3.91 | 3.85‡ | 3.77 | 3.88 | 4.17 | 4.1 | 3.71 | |
| 12 mo (n = 11) | 3.96 | 3.91‡ | 3.83 | 4.07 | 3.92 | 4.3 | 3.73 | |
| Malignant | Before (n = 17) | 3.66‡ | 3.32* | 3.76* | 3.51* | 3.65† | 4.04‡ | 3.48 |
| 6 mo (n = 13) | 2.91‡ | 3.06* | 2.74* | 2.82* | 3.02† | 2.91‡ | 2.98 | |
| 12 mo (n = 9) | 3.83‡ | 3.63* | 3.52* | 3.85* | 3.98† | 4.3‡ | 3.69 | |
| Complication | ||||||||
| No | Before (n = 29) | 3.92 | 3.32 | 4.09 | 3.74 | 3.93 | 4.34* | 3.81 |
| 6 mo (n = 25) | 3.6 | 3.71 | 3.57 | 3.49 | 3.73 | 3.67* | 3.46 | |
| 12 mo (n = 17) | 3.91 | 3.82 | 3.75 | 3.93 | 3.93 | 4.32* | 3.72 | |
| Yes | Before (n = 10) | 3.47 | 3.33 | 3.9 | 3.27 | 3.43 | 4.04* | 3.26 |
| 6 mo (n = 7) | 3.14 | 2.84 | 2.57 | 3.29 | 3.58 | 3.42* | 3.23 | |
| 12 mo (n = 4) | 3.85 | 3.66 | 3.5 | 4.17 | 4.02 | 4.21* | 3.71 | |
| Recurrent surgery | ||||||||
| No | Preop (n = 25) | 3.67 | 3.32* | 3.88 | 3.39 | 3.65 | 4.03† | 3.48 |
| Before (n = 21) | 3.5 | 3.56* | 3.41 | 3.43 | 3.66 | 3.51† | 3.46 | |
| 12 mo (n = 14) | 3.83 | 3.92* | 3.72 | 4.01 | 3.79 | 4.01† | 3.66 | |
| Yes | Before (n = 14) | 4.03 | 3.32 | 3.96 | 4.02 | 4.01 | 4.67† | 4 |
| 6 mo (n = 11) | 3.52 | 3.44 | 3.23 | 3.48 | 3.79 | 3.83† | 3.31 | |
| 12 mo (n = 7) | 4.04 | 3.52 | 3.65 | 3.9 | 4.26 | 4.9† | 3.82 | |
| Radiotherapy | ||||||||
| No | Before (n = 23) | 3.90 | 3.37† | 4.04 | 3.61 | 3.87 | 4.44 | 3.77 |
| 6 mo (n = 19) | 3.91 | 3.79† | 3.90 | 3.70 | 4.10 | 4.05 | 3.72 | |
| 12 mo (n = 14) | 4.03 | 4.02† | 3.85 | 4.21 | 4.01 | 4.32 | 3.90 | |
| Yes | Before (n = 16) | 3.97‡ | 3.24 | 3.67‡ | 3.62* | 3.67‡ | 4.10‡ | 3.56* |
| 6 mo (n = 12) | 2.84‡ | 2.94 | 2.44‡ | 2.91* | 3.05‡ | 2.90‡ | 2.92* | |
| 12 mo (n = 7) | 3.72‡ | 3.32 | 3.53‡ | 3.57* | 3.80‡ | 4.45‡ | 3.44* | |
p < 0.05.
p < 0.01.
p < 0.001.
Figure 1.
Estimates of quality of life scores before and after anterior skull base tumor resections. There was a significant decrease in the overall scores at 6 months after surgery (p < 0.05) and a recovery to baseline at 1 year after surgery. Pre Op, preoperatively.
Figure 2.
Quality of life (QOL) score in the emotional domain. (A) Overall QOL scores. (B) QOL scores in patients with benign tumors showing significant improvement between 6 and 12 months after surgery (p < 0.05). (C) QOL scores in patients with malignant tumors showing a decline in scores at 6 months after surgery and improvement at 12 months postoperatively (p < 0.005). Pre Op, preoperatively.
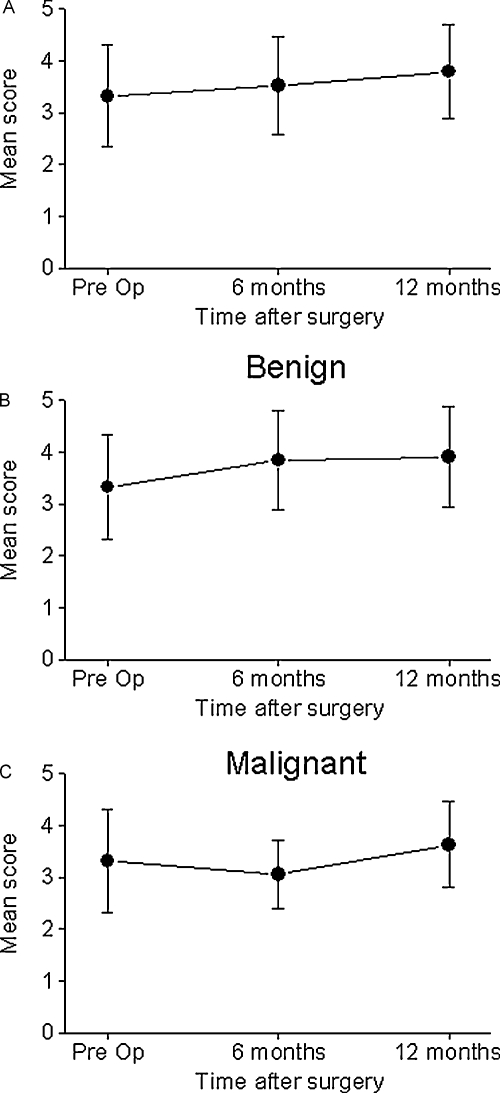
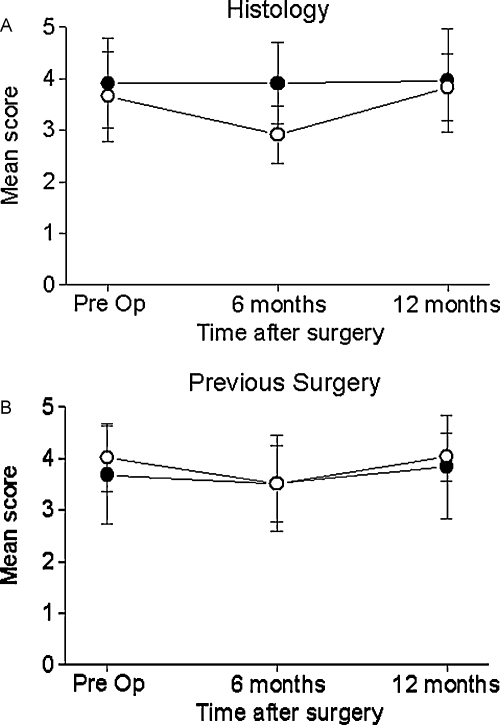
The patients were divided into three subgroups according to demographic and clinical characteristics to identify those who were likely to have a poor QOL after surgery. Figure 3 shows that patients with malignant tumors had significantly decreased QOL scores at 6 months after surgery (p < 0.004). There was, however, a significant improvement in their QOL at 12 months after surgery (p < 0.002). In contrast, the QOL scores of the patients with benign tumors did not change throughout the whole postoperative period. There was significant difference in the overall QOL between the two histologically defined groups (p < 0.002). Adjuvant radiation therapy was also associated with significantly poor QOL scores in the domains of physical functioning (p < 0.004), role of performance (p < 0.03), vitality (p < 0.006), and specific symptoms (p < 0.004).
Figure 3.
Impact of histology and prior surgery on quality of life (QOL) scores. (A) Malignant histology (open circles) was associated with lower overall QOL scores at 6 months after surgery, whereas patients with benign tumors (closed circles) had stable QOL scores (p < 0.005). (B) Prior skull base surgery had no significant impact on QOL scores (previous surgery, open circles). Pre Op, preoperatively.
Ten patients had a major complication, including osteoradionecrosis of the frontal bone (n = 5), abducens nerve palsy (n = 2), deep vein thrombosis (n = 2), and nasal obstruction due to adhesions (n = 1). The QOL scores of the patients with and without complications were similar (Table 3).
DISCUSSION
Surgery for extirpation of anterior skull base tumors may be associated with significant morbidity.2 Some of the functional deficits experienced by these patients are visual dysfunction, anosmia, chronic nasal discharge, epiphora, dysgeusia, breathing difficulties, and cosmetic deformations.12 The short- and long-term complications associated with craniofacial surgery have been described in detail.1,13 We previously studied the impact of anterior skull base surgery on the health-related QOL of patients and found that 44% of the patients may suffer from significant sequelae that can impact their functional outcome following surgery.3 In the current study, we prospectively evaluated the QOL of patients undergoing anterior skull base tumor resection. Questionnaires were completed before surgery and throughout the postoperative period. The overall results of this study show that patients undergoing skull base surgery have reduced QOL scores in most of the domains of the ASBQ at 6 months after surgery and that this was followed by a significant improvement in their QOL 6 months later. Our current results are in accordance with previous retrospective studies showing good QOL scores within 12 months after skull base surgery,3,5 and refute other studies on patients with malignant intracranial tumors that demonstrated severely deteriorating long-term QOL measures, with marked decline in cognitive, physical, emotional, and social functioning.14
Compared with patients undergoing surgery for malignant neoplasms, the patients with benign tumors maintained their overall QOL scores throughout the entire postoperative period. Furthermore, most of them showed improvement in the emotional domain scores at 6 to 12 months after surgery, reaching higher scores than before surgery after 1 year.
The stability of QOL scores at 12 months after surgery is an important finding of this study. We had shown earlier that there was a gradual improvement in QOL measures during the first 6 postoperative months and that there was no significant change in QOL 12 months after surgery.3 The changes in QOL measures that we recorded after skull base surgery are similar to those described by De Jesús et al for meningiomas involving the cavernous sinus,15 but different from those observed by Osoba et al for patients with high-grade gliomas.14
As we had reported previously, patients undergoing radiation therapy had a worse score than the patients who did not.3,4 Radiation therapy was given to patients with malignant tumors, and the coexistence of both factors probably contributed to the deteriorating health-related QOL of these patients. A similar impact of radiation therapy on the patient's QOL was found in patients with head and neck cancer.16
The main limitation of our current study is that it involves a relatively small group of patients with different types of neoplasms. Further prospective, multicenter studies are required to more accurately assess the QOL characteristics of patients with various anterior skull base tumors. Comparative studies are essential for examining the impact of different surgical modalities that have no clear survival advantage on the patients' QOL.
CONCLUSION
The QOL scores of most patients undergoing anterior skull base tumor resection deteriorate within 6 months after surgery and undergo significant improvement at 12 months postoperatively. Malignant histology and radiotherapy are associated with poor QOL scores at 6 months postoperatively, only to subsequently improve and reach the same level as patients with benign histology at 1 year after surgery. These findings may be used for better management of these patients with the aim of enhancing their QOL.
ACKNOWLEDGMENT
We thank Esther Eshkol for her editorial assistance. Supported in part by a grants from the Israel Science Foundation, The US-Israel Binational Science Foundation, and the Israeli Cancer Association to Ziv Gil.
REFERENCES
- Patel S G, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98:1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- Gil Z, Patel S G, Singh B, et al. International Collaborative Study Group Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: an international collaborative study. Cancer. 2007;110:1033–1041. doi: 10.1002/cncr.22882. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Spektor S, et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2003;129:1303–1309. doi: 10.1001/archotol.129.12.1303. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss D M. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. 2004;100:813–819. doi: 10.3171/jns.2004.100.5.0813. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Spektor S, Khafif A, Fliss D M. Patient, caregiver, and surgeon perceptions of quality of life following anterior skull base surgery. Arch Otolaryngol Head Neck Surg. 2004;130:1276–1281. doi: 10.1001/archotol.130.11.1276. [DOI] [PubMed] [Google Scholar]
- Woertgen C, Rothoerl R D, Hosemann W, Strutz J. Quality of life following surgery for malignancies of the anterior skull base. Skull Base. 2007;17:119–123. doi: 10.1055/s-2006-953513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Saeki N, Mine S, et al. Evaluation of outcome and QOL in patients with craniofacial resection for malignant tumors involving the anterior skull base. Neurol Res. 2000;22:545–550. doi: 10.1080/01616412.2000.11740716. [DOI] [PubMed] [Google Scholar]
- Gil Z, Cohen J T, Spektor S, Shlomi B, Fliss D M. Anterior skull base surgery without prophylactic airway diversion procedures. Otolaryngol Head Neck Surg. 2003;128:681–685. doi: 10.1016/S0194-59980223285-4. [DOI] [PubMed] [Google Scholar]
- Gil Z, Cohen J T, Spektor S, Fliss D M. The role of hair shaving in skull base surgery. Otolaryngol Head Neck Surg. 2003;128:43–47. doi: 10.1067/mhn.2003.14. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Leider-Trejo L, et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17:25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss D M, Gil Z, Spektor S, et al. Skull base reconstruction after anterior subcranial tumor resection. Neurosurg Focus. 2002;12:e10. doi: 10.3171/foc.2002.12.5.11. [DOI] [PubMed] [Google Scholar]
- Gil Z, Fliss D M. Pericranial wrapping of the frontal bone after anterior skull base tumor resection. Plast Reconstr Surg. 2005;116:395–398. discussion 399. doi: 10.1097/01.prs.0000172761.65844.d0. [DOI] [PubMed] [Google Scholar]
- Ross D A, Marentette L J, Moore C E, Switz K L. Craniofacial resection: decreased complication rate with a modified subcranial approach. Skull Base Surg. 1999;9:95–100. doi: 10.1055/s-2008-1058155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoba D, Aaronson N K, Muller M, et al. Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J Neurooncol. 1997;34:263–278. doi: 10.1023/a:1005790632126. [DOI] [PubMed] [Google Scholar]
- De Jesús O, Sekhar L N, Parikh H K, Wright D C, Wagner D P. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915–919. discussion 919–920. doi: 10.1097/00006123-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Epstein J B, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389–398. doi: 10.1002/hed.1049. [DOI] [PubMed] [Google Scholar]