ABSTRACT
Gunshot wounds (GSWs) to the head are frequently fatal. Rarely, the bullet may lodge in the skull base and not cause significant brain injury. Typically, the bullet fragments are felt to be inert and do not require operative extirpation if they are within the bony confines of the skull base. We report the case of a bullet in the jugular foramen causing recurrent syncope that resolved after surgical removal of the bullet. The medical records from a patient who suffered a GSW to the head were retrospectively reviewed and the treatment and outcome documented. In 2000, a 20-year-old man suffered a GSW to the head. Immediate evaluation revealed the bullet in the right skull base at the jugular foramen, but no parenchymal brain injury. One year after the GSW, he began to experience stereotypical spells resulting in loss of consciousness. Extensive cardiovascular workup was normal. In 2002, the patient underwent removal of the bullet. He has been syncope-free since the operation and returned to his career in the military. We believe the retained bullet in this patient was irritating the IX–X cranial nerves, resulting in syncope, similar to the mechanism in vagoglossopharyngeal neuralgia. Removing the bullet relieved the irritation and stopped the syncopal spells.
Keywords: Gunshot wounds, jugular foramen, Syncope, vagoglossopharyngeal neuralgia
There has been a dramatic increase in violence, and particularly gunshot wounds (GSWs) to the head, during the last two decades. This has become a worsening public health problem, with a significant impact on the U.S. population.1,2 Victims of GSWs to the head usually have high morbidity and mortality with survival rates from 34 to 93% in multiple series, with a very dismal prognosis for return to independent living3,4,5,6 However, a very small, fortunate minority of patients, who arrive in good neurological condition, can go on to a good functional recovery.7
Occasionally the bullet trajectory from a GSW to the head may be completely outside of the intracranial cavity, with the bullet taking a course through the sinonasal cavities and/or soft tissues and bone of the skull base. In this situation, a conservative surgical approach can often be taken, with retained fragments left in place. With rare exceptions, retained bullet fragments do not appear to pose sufficient risk for infection or other complications to warrant aggressive removal.8,9,10,11
We report the case of a bullet embedded in the jugular foramen causing recurrent, disabling syncope that resolved after surgical extirpation through a transcervical, transjugular approach. Several cases of vagoglossopharyngeal neuralgia associated with syncope and cardiac arrest have been reported in the literature,12 with only one case reported in 1974 of paroxysmal glossopharyngeal neuralgia and cardiac arrest caused by a GSW to the mandible with retained bullet fragments in the tongue.13 To our knowledge, this is the first case report of likely vagoglossopharyngeal-related syncope caused by a bullet retained in the jugular foramen.
CASE REPORT
A 20-year-old military police officer on duty was involved in an altercation in which he suffered a GSW to the head from a 0.38-caliber pistol. The bullet initially entered through the left face at the nasolabial fold and lodged without fragmenting in the right jugular foramen. He did not immediately lose consciousness but suffered profuse bleeding from the nose and mouth and through the entry wound. Upon arrival to an outside emergency department, he was intubated, hemodynamically stable, and was noted to have a normal neurological exam although his lower cranial nerves could not be assessed.
A computed tomographic scan of the head confirmed the bullet in the right jugular foramen, without any evidence of parenchymal brain injury or subarachnoid hemorrhage (Figs. 1 and 2). A formal cerebral angiogram revealed dissection and occlusion of the right internal carotid artery (ICA; Fig. 3) several centimeters from its origin from the common carotid artery. There was good collateral flow to the right anterior circulation via the anterior and posterior communicating arteries. The right internal jugular vein (IJV) was patent but small.
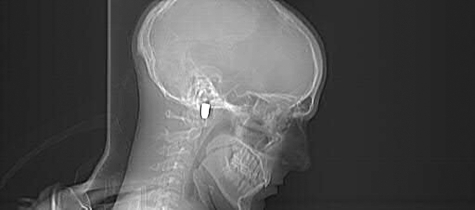
Figure 1.
Lateral radiograph reveals a large-caliber bullet in the region of the right skull base/inferior jugular foramen.
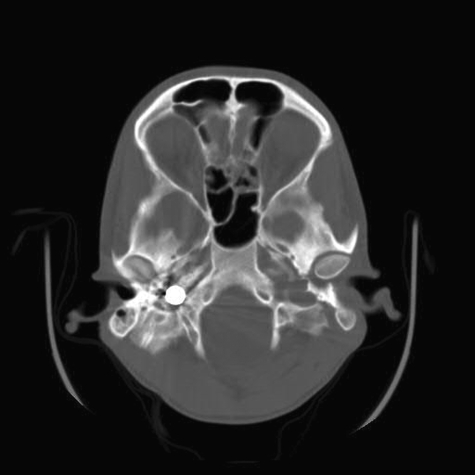
Figure 2.
Axial computed tomographic scans confirm the bullet is located in the inferior right jugular foramen.
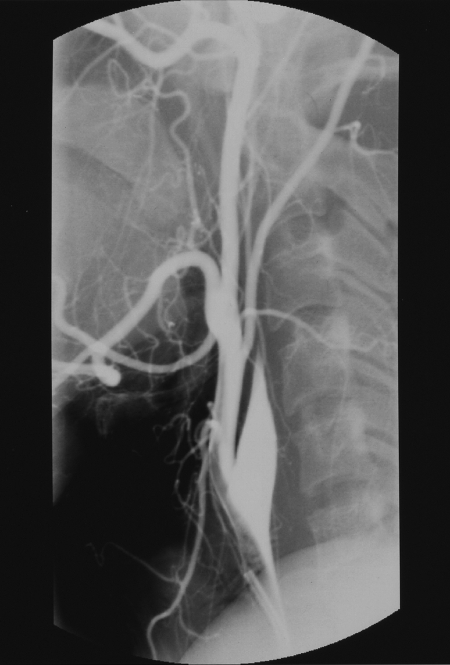
Figure 3.
Right common carotid artery injection demonstrates occlusion of the right internal carotid artery known to be secondary to gunshot wound.
The patient recovered well without requiring any acute surgical intervention. He returned to his occupation with the military, performing administrative work. Approximately 1 year after the incident, he began to have stereotypical spells of a sudden, poorly localized pain in the right side of his head, nausea, and diaphoresis followed by 30 to 60 seconds of loss of consciousness. He had fallen on one occasion and sustained a minor head injury secondary to the syncope. There was no identified trigger to the spells including head or neck movement, swallowing, chewing, talking, exercising, or neck compression. There was no associated tonic or clonic movements or bowel or bladder incontinence or aura. He had no associated headache and would recover rather quickly after a spell with normal mental status. The spells would occur from one or two times per month initially but were becoming more frequent, occurring almost once a week by the time he was referred to our institution for evaluation.
Two years following his injury, he was referred to our institution for evaluation of his spells. The spells were threatening his military career, such that he would be unable to resume his prior duties without restriction, as well as presenting a risk of further injury with repeated syncope. His neurological exam was normal except his right vocal fold was very weak but still mobile and he had pooling of secretions in the right piriform sinus. Extensive cardiac workup including echocardiography was normal.
A computed tomographic scan of the head confirmed the bullet was still in the right jugular foramen (Fig. 2). A formal cerebral angiogram continued to show the right ICA was occluded with no suggestion of pseudoaneurysm formation. It was our hypothesis that the patient was experiencing recurrent syncope secondary to vagal and glossopharyngeal irritation from the retained bullet and subsequent vagal overactivity.
Operation
A transcervical transmastoid approach to the jugular foramen was performed. Through a standard postauricular C-shaped incision, the right mastoid and occiput were exposed. A wide cortical mastoidectomy was performed, and the sigmoid sinus was decompressed down to the jugular bulb. The descending VII nerve was identified down to the stylomastoid foramen but not mobilized from its canal. The tip of the mastoid and the styloid process were removed. The digastric, stylohyoid, and stylopharyngeus muscles were mobilized anteroinferiorly. The incision was extended into the upper neck, and the IJV was identified and protected. The fibrous occluded ICA and X, XI, and XII cranial nerves were identified and followed toward the skull base. The bullet was discovered at the skull base posteromedial to the lower cranial nerves and anteromedial to the IJV and removed (Fig. 4).
Figure 4.
The intact bullet after removal.
There were no postoperative complications and the patient left the hospital on postoperative day 3. He had no new neurological deficits including no change in his moderately weak right vocal fold. At 6 weeks postoperatively, he was able to resume all activities without restrictions. He has had no further syncopal spells now 6 years following surgery. He is currently deployed in Iraq with the U.S. military serving full duty as a military police officer.
DISCUSSION
Vagoglossopharyngeal neuralgia is a well-known, but rare condition characterized by clusters of unilateral attacks of sharp, stabbing, and shooting pain localized in the throat radiating to the ear or vice versa. The pain shoots from the pharynx, tonsil, and posterior tongue base upward to the Eustachian tube and inner ear or mandibular angle and usually lasts for seconds to a few minutes. These paroxysms are usually triggered by swallowing, sneezing, chewing, coughing, talking, cleaning the throat, and even sudden movements of the head.14 This may be idiopathic but has also been described with structural abnormalities such as cerebellopontine angle tumors, intracranial vascular compression, nasopharyngeal tumors, parapharyngeal abscess, trauma, multiple sclerosis, and cranial base tumors.15,16,17,18 There is one other case of glossopharyngeal neuralgia associated with cardiac arrest secondary to a GSW.13 A report in 1974 related a soldier shot in the mandible with bullet fragments in the tongue. He developed pain typical of glossopharyngeal neuralgia and paroxysmal cardiac arrest responsive to atropine. His pain and bradycardia came under control with carbamazepine, and he did not require surgical removal of the bullet fragments. The authors suggest that scarring and fibrosis high in the neck likely resulted in compression of the IX and X nerves.13 Rarely, vagoglossopharyngeal neuralgia is felt to be secondary to an elongated styloid process that directly irritates the glossopharyngeal nerve with head turning, also known as Eagle's syndrome.19
There are many well-documented cases of syncope associated with vagoglossopharyngeal neuralgia.20,21,22,23 The pathophysiology is explained by the close connections that exist between the glossopharyngeal nerve and the vagus nerve. The general visceral afferent component of cranial nerve IX (glossopharyngeal) receives viscerosensory input from the carotid sinus baroreceptors as well as tactile sensation from the posterior third of tongue, pharynx, middle ear, and Eustachian tube. The carotid sinus is a baroreceptor that detects increases in blood pressure. The general visceral sensory information then travels via the inferior ganglion of the glossopharyngeal nerve and synapses in the caudal portion of the nucleus solitarius. From here, reflex connections are made with the dorsal motor nucleus of X (vagus) and also the ventrolateral medulla. The dorsal motor nucleus of X sends out preganglionic parasympathetic fibers to the heart to serve as a “vasodepressor center,” reducing heart rate. The ventrolateral medulla serves as a “vasopressor center”—that is, neurons in this area receive solitary input and project to the cord where they influence the activity of preganglionic sympathetic motor neurons.24,25
An increase in systemic blood pressure results in an increased firing rate of the carotid and aortic baroreceptors. Impulses are transmitted to the caudal portion of the nucleus solitarius via IX (carotid baroreceptor) and X (aortic baroreceptor). From here, impulses pass to excite the dorsal motor nucleus of X, resulting in bradycardia, and to inhibit the ventral lateral medulla, resulting in reduced blood pressure (inhibiting sympathetics to blood vessels)24,25 (Fig. 5). An irritation of the general visceral afferent component of the glossopharyngeal nerve could thus result in a similar reflexive pathway resulting in bradycardia, hypotension, and syncope.
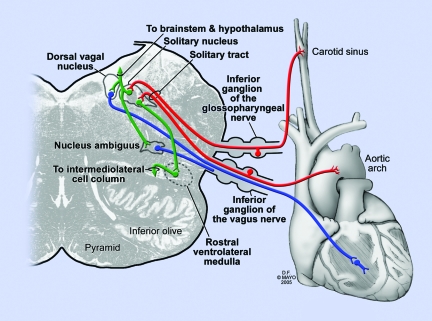
Figure 5.
Red: General visceral afferents from IX and X. Blue: Preganglionic parasympathetic efferents. Green: The solitary nucleus sends excitatory input to the dorsal vagal nucleus that increases parasympathetic outflow to the heart, resulting in bradycardia. It also sends inhibitory input to the rostral ventrolateral medulla, reducing firing in the intermediolateral cell column of the spinal cord, reducing sympathetic outflow to the blood vessels and causing associated vasodilatation.
The mainstay of treatment for vagoglossopharyngeal neuralgia is surgical division of the IX nerve root and upper rootlets of X in the cerebellopontine angle, or potentially microvascular decompression if an offending vessel is discovered at the time of surgical exploration.12,14,15 Traditional neuromodulatory medications such as carbamazepine, phenytoin, and gabapentin have also been tried but with less success compared with trigeminal neuralgia.22,23,26 Additionally, radiofrequency rhizotomy and stereotactic radiosurgery have been tried with limited success.12,26,27 We were reluctant to sacrifice further IX and X nerve function in this patient, who already had clinical evidence of vocal fold weakness but was swallowing well. We did not feel microvascular decompression would have a role in the treatment of this mechanism. We felt the best option would be to remove the potential irritant to the nerves by removing the foreign body—in this case, a bullet. Temporary cardiac pacing has been useful in some cases during surgical exploration for microvascular decompression or section of IX and upper rootlets of X, but because the patient's prolonged electrocardiographic monitoring was normal preoperatively, we did not feel this was necessary. There was no alteration of cardiac rhythm during the operation.
Surgical approaches to the jugular foramen have been well described.28,29,30 The transmastoid, infralabyrinthine route with removal of the styloid provided excellent exposure of the bullet and pertinent surrounding neural and vascular structures. Our exposure was aided by the ICA being occluded and thus not at risk. Because the bullet was mostly in the extracranial portion of the jugular foramen, we did not have to interfere with conductive hearing or reroute the facial nerve.
CONCLUSION
Although rare, direct irritation of the IX–X cranial nerve complex can result in ipsilateral throat, ear, and/or head pain associated with syncope. In our case, the irritating focus was a bullet in the extracranial jugular foramen. The transcervical transmastoid approach provided good exposure of this region of the skull base, with preservation of all pertinent neurovascular structures. Undoubtedly, the exposure was made easier because the ICA was previously occluded. Removing the irritative factor in this case resolved the recurrent syncope and allowed the patient to resume his military career.
REFERENCES
- Koop C E, Lundberg G B. Violence in America: a public health emergency. Time to bite the bullet back. JAMA. 1992;267:3075–3076. [PubMed] [Google Scholar]
- Richmond T S, Lemaire J. Years of life lost because of gunshot injury to the brain and spinal cord. Am J Phys Med Rehabil. 2008;87:609–615. quiz 615–618. doi: 10.1097/PHM.0b013e31817fb496. [DOI] [PubMed] [Google Scholar]
- Kennedy F, Gonzalez P, Dang C, Fleming A, Sterling-Scott R. The Glasgow Coma Scale and prognosis in gunshot wounds to the brain. J Trauma. 1993;35:75–77. doi: 10.1097/00005373-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Kaufman H H, Makela M E, Lee K F, Haid R W, Jr, Gildenberg P L. Gunshot wounds to the head: a perspective. Neurosurgery. 1986;18:689–695. doi: 10.1227/00006123-198606000-00002. [DOI] [PubMed] [Google Scholar]
- Part 2: Prognosis in penetrating brain injury. J Trauma. 2001;51(2 suppl):S44–S86. [PubMed] [Google Scholar]
- Rosenfeld J V. Gunshot injury to the head and spine. J Clin Neurosci. 2002;9:9–16. doi: 10.1054/jocn.2001.0949. [DOI] [PubMed] [Google Scholar]
- Levy M L. Outcome prediction following penetrating craniocerebral injury in a civilian population: aggressive surgical management in patients with admission Glasgow Coma Scale scores of 6 to 15. Neurosurg Focus. 2000;8:e2. doi: 10.3171/foc.2000.8.1.153. [DOI] [PubMed] [Google Scholar]
- Suddaby L, Weir B, Forsyth C. The management of 22 caliber gunshot wounds of the brain: a review of 49 cases. Can J Neurol Sci. 1987;14:268–272. doi: 10.1017/s0317167100026597. [DOI] [PubMed] [Google Scholar]
- Botelho R V, Romero P C, Coelho R V, Fontoura E A. Carotid artery-sygmoid sinus fistula: a rare complication of gunshot wound on the base of the cranium. Neurosurg Rev. 1999;22:121–123. doi: 10.1007/s101430050044. [DOI] [PubMed] [Google Scholar]
- Rengachary S S, Carey M, Templer J. The sinking bullet. Neurosurgery. 1992;30:291–294. discussion 294–295. doi: 10.1227/00006123-199202000-00029. [DOI] [PubMed] [Google Scholar]
- Hagan R E. Early complications following penetrating wounds of the brain. J Neurosurg. 1971;34(2 Pt 1):132–141. doi: 10.3171/jns.1971.34.2part1.0132. [DOI] [PubMed] [Google Scholar]
- Rushton J G, Stevens J C, Miller R H. Glossopharyngeal (vagoglossopharyngeal) neuralgia: a study of 217 cases. Arch Neurol. 1981;38:201–205. doi: 10.1001/archneur.1981.00510040027002. [DOI] [PubMed] [Google Scholar]
- al-Ubaidy S S, Bakeen G. Paroxysmal glossopharyngeal neuralgia associated with cardiac arrest. Br J Oral Surg. 1974;11:243–245. doi: 10.1016/0007-117x(74)90108-5. [DOI] [PubMed] [Google Scholar]
- Evans R W, Torelli P, Manzoni G C. Glossopharyngeal neuralgia. Headache. 2006;46:1200–1202. doi: 10.1111/j.1526-4610.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- Ferrante L, Artico M, Nardacci B, Fraioli B, Cosentino F, Fortuna A. Glossopharyngeal neuralgia with cardiac syncope. Neurosurgery. 1995;36:58–63. discussion 63. doi: 10.1227/00006123-199501000-00007. [DOI] [PubMed] [Google Scholar]
- Phuong H L, Matsushima T, Hisada K, Matsumoto K. Glossopharyngeal neuralgia due to an epidermoid tumour in the cerebellopontine angle. J Clin Neurosci. 2004;11:758–760. doi: 10.1016/j.jocn.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Minagar A, Sheremata W A. Glossopharyngeal neuralgia and MS. Neurology. 2000;54:1368–1370. doi: 10.1212/wnl.54.6.1368. [DOI] [PubMed] [Google Scholar]
- Resnick D K, Jannetta P J, Bissonnette D, Jho H D, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–68. discussion 68–69. doi: 10.1227/00006123-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Soh K B. The glossopharyngeal nerve, glossopharyngeal neuralgia and the Eagle's syndrome—current concepts and management. Singapore Med J. 1999;40:659–665. [PubMed] [Google Scholar]
- Esaki T, Osada H, Nakao Y, et al. Surgical management for glossopharyngeal neuralgia associated with cardiac syncope: two case reports. Br J Neurosurg. 2007;21:599–602. doi: 10.1080/02688690701627138. [DOI] [PubMed] [Google Scholar]
- Elias J, Kuniyoshi R, Carloni W V, Borges M R, Peixoto C A, Pimentel D. Glossopharyngeal neuralgia associated with cardiac syncope. Arq Bras Cardiol. 2002;78:510–519. doi: 10.1590/s0066-782x2002000500008. [DOI] [PubMed] [Google Scholar]
- Savica R, Laganà A, Calabrò R S, Casella C, Musolino R. Vagoglossopharyngeal neuralgia: a rare case of sincope responding to pregabalin. Cephalalgia. 2007;27:566–567. doi: 10.1111/j.1468-2982.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- Giza E, Kyriakou P, Liasides C, Dimakopoulou A. Glossopharyngeal neuralgia with cardiac syncope: an idiopathic case treated with carbamazepine and duloxetine. Eur J Neurol. 2008;15:e38–e39. doi: 10.1111/j.1468-1331.2008.02097.x. [DOI] [PubMed] [Google Scholar]
- Ropper A H, Brown R H. In: Ropper AH, Brown RH, editor. Adams and Victor's Principles of Neurology. 8th ed. New York: McGraw-Hill; Diseases of the cranial nerves.
- Ganong W F. Review of Medical Physiology. 22nd ed. New York: Lange Medical Books/McGraw Hill Medical; 2005. pp. 605–613.
- Taha J M, Tew J M., Jr Long-term results of surgical treatment of idiopathic neuralgias of the glossopharyngeal and vagal nerves. Neurosurgery. 1995;36:926–930. discussion 930–931. doi: 10.1227/00006123-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Stieber V W, Bourland J D, Ellis T L. Glossopharyngeal neuralgia treated with gamma knife surgery: treatment outcome and failure analysis. Case report. J Neurosurg. 2005;102(suppl):155–157. doi: 10.3171/jns.2005.102.s_supplement.0155. [DOI] [PubMed] [Google Scholar]
- Oghalai J S, Leung M K, Jackler R K, McDermott M W. Transjugular craniotomy for the management of jugular foramen tumors with intracranial extension. Otol Neurotol. 2004;25:570–579. discussion 579. doi: 10.1097/00129492-200407000-00026. [DOI] [PubMed] [Google Scholar]
- Liu J K, Sameshima T, Gottfried O N, Couldwell W T, Fukushima T. The combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach for resection of glomus jugulare tumors. Neurosurgery. 2006;59(1 suppl 1):ONS115–ONS125. discussion ONS115–ONS125. doi: 10.1227/01.NEU.0000220025.81500.8D. [DOI] [PubMed] [Google Scholar]
- Sanna M, Bacciu A, Falcioni M, Taibah A, Piazza P. Surgical management of jugular foramen meningiomas: a series of 13 cases and review of the literature. Laryngoscope. 2007;117:1710–1719. doi: 10.1097/MLG.0b013e3180cc20a3. [DOI] [PubMed] [Google Scholar]