Abstract
When the vehicle-treated, sham-operated mice underwent heat stress, the fraction survival and core temperature at +4 h of body heating were found to be 5 of 15 and 34.4°C ± 0.3°C, respectively. Castration 2 weeks before the start of heat stress decreased the plasma levels of testosterone almost to zero, protected the mice from heat-induced death (fraction survival, 13/15) and reduced the hypothermia (core temperature, 37.3°C). The beneficial effects of castration in ameliorating lethality and hypothermia can be significantly reduced by testosterone replacement. Heat-induced apoptosis, as indicated by terminal deoxynucleotidyl- transferase- mediatedαUDP-biotin nick end-labeling staining, were significantly prevented by castration. In addition, heat-induced neuronal damage, as indicated by cell shrinkage and pyknosis of nucleus, to the hypothalamus was also castration-prevented. Again, the beneficial effects of castration in reducing neuronal damage to the hypothalamus as well as apoptosis in multiple organs during heatstroke, were significantly reversed by testosterone replacement. The data indicate that testosterone depletion by castration may protect mice from heatstroke-induced multiple organ damage and lethality.
1. Introduction
Heatstroke is characterized by hyperpyrexia, multiorgan damage and dysfunction, and predominant central nervous system dysfunction (such as delirium, convulsion, or coma) [1–3]. The full spectrum of the signs and symptoms occurring during heatstroke in humans can be mimicked by the rodent heatstroke model [4]. When mice were subjected to acute heat stress, the stress response indicators such as mortality, hypothermia, and multiple organ apoptosis, were observed [5, 6]. Other line of evidence has accumulated to show that testosterone increases the susceptibility toward a wide variety of infectious diseases including human sepsis [7], shock [8], and severe injury [9]. In addition, depletion of testosterone by castration prior to soft-tissue trauma results in better maintained immune and myocardial function in male mice [10, 11]. Flutamide, an androgen receptor antagonist, has been shown to restore the depressed cell-mediated immunity [12], and cardiac and hepatic function following soft-tissue trauma and hemorrhagic shock [13]. It has also been promoted that testosterone plays a role in the regulation of heat balance in male rats [14]. This raises the possibility that testosterone depletion by castration may protect male mice from heatstroke-induced multiple organ damage and lethality.
To deal with the hypothesis, the effects of surgical castration with or without testosterone replacement on the heatstroke-induced thermoregulatory deficits (in particular, the hypothalamic neuronal damage and apoptosis and hypothermia), multiple organs dysfunction or damage and lethality were assessed in mice [5, 6].
2. Materials and Methods
2.1. Mice
All the experiments were carried out in accordance with the ethical guidelines laid down by the committee for the purpose of control and supervision of experiments on animals, Chi Mei Medical Center (Tainan, Taiwan). ICR inbred male mice, 8 weeks old, were given food and water ad libitum and acclimatized to room temperature at 24°C, relative humidity (RH) of 50 ± 8%, and a 12 h dark/light cycle for 1 week before the start of the experiment at least.
2.2. Murine Model of Heatstroke
Animals were exposed to heat stress treatment (41.2°C, RH-50–55%, 1 h) in an environment-controlled chamber [5]. The time at which mice were removed from the environmental chamber was called 0 hour. The heat-stressed mice were returned to the normal room temperature (25°C) after the end of the heat exposure. Mice that survived on day 4 of heat treatment were considered survivors, and the data were used for analysis of the results. Core temperatures were measured every 5 minutes with a copper constantan thermocouple inserted into the rectum and connected to a thermometer (HR1300, Yokogawa, Tokyo, Japan). After the 1 h heating period, animals were properly fed and hydrated. Heatstroke resembles sepsis in many aspects [15, 16]. Like many sepsis studies, we use death as an endpoint in conscious mice in this study
2.3. Castration Procedures
Fourteen days before the experiment, mice were castrated or sham-operated. Briefly, after the initiation of general anesthesia with ketamine and Xylazine (8.7 and 1.3 mg/100 g BW, IM) and application of 75% alcohol to disinfect the scrotum, a small midline incision was made and the testes were exteriorized. The spermatic vessels were tied with 4.0 silk sutures, and the testes were removed. The incision was then closed with 4.0 silk sutures. In sham-operated mice, the skin of the scrotum was incised to draw out and back the testes and closed with sutures only. An interval of 14 days after castration was chosen for subcutaneously implanting testosterone or heat stress experiments because previous studies have demonstrated the absence of any detectable plasma testosterone levels at this interval [11].
2.4. Testosterone Supplement
For testosterone replacement study, the castrated groups were subcutaneously implanted with testosterone 2 weeks after the castration. Briefly, after the initiation of general anesthesia with ketamine and Xylazine (8.7 and 1.3 mg/100 g BW, IM), a small incision was made in the skin of the back, a pellet of testosterone (0.5 mg/pellet, 21-day release; Innovative Research) was subcutaneously implanted. For the other sham-implanted animals, the skin of the back was incised and then closed with 3.0 silk suture only. Animals were subjected to thermal experiments 2 weeks after implanted surgery.
2.5. Experimental Groups
Four major groups of animals were designated for the experiment. In the normothermic control (NC) groups, the animals were exposed to room temperature (26°C) throughout the entire experiments. The sham-operated heatstroke (SOH) mice, the castrated, vehicle-treated heatstroke (CVH) mice, and the castrated, testosterone-treated heatstroke (CTH) mice were treated with heat regimen of 41.2°C for 1 h. Before the start of experiments, their core temperature was within the normal body temperature range of 37.0 ~ 37.6.
2.6. Blood Sampling and Plasma Testosterone Assays
Peripheral blood samples were harvested from urethane-anesthetized mice (1.4 g/kg BW, IP) by heart puncture via a syringe containing 3.8% sodium citrate (9 : 1 vol/vol). The blood was centrifuged to isolate upper layer plasma. Plasma concentration of testosterone was measured by enzyme immunoassay (EIA) as described in the instructions provided by manufacturer's kits (Cayman Chemical USA, Catalog No.582701), which presents 6 pg/mL of sensitivity, and 100% of specificity.
2.7. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assays
The heat-treated mice were sacrificed at “heat off 2.5 h” under the heat stress model. Perfusion and prefixed procedure with the PBS and 10% formaldehyde were performed after the general anesthesia with urethane (1.4 g/kg BW, IP). The brain, liver, spleen, and kidney were excised and postfixed in a solution containing 30% sucrose and 10% formaldehyde for at least 24 hours. After fixation, the organs were embedded separately in Tissue Tek OCT embedding medium (Miles). Snap frozen samples were cryostat sectioned (8 μm thick) and placed on slides coated with poly-L-lysine for TUNEL assays. TUNEL staining was done using a BD ApoAlert DNA Fragmentation Assay kit (BD Biosciences-Clontech), according to detailed protocol provided by manufacture. In brief, tissue slides were pretreated with 20 μg/mL proteinase K solution for 5 minutes and were incubated with the reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and fluorescein-conjugated deoxyuridine triphosphate (dUTP) for 1 hour at 37°C. Afterwards, sections were washed with PBS, their nuclei were costained with 4,6-diamidino-2-phenylindole (DAPI) using DAPI-containing VectashieldR Mounting Medium (VECTOR Laboratories, Burlingame, CA), and subsequently analyzed using Olympus E800 fluorescent microscope equipped with Olympus Coolpix 995 digital camera (both from Olympus, Japan). Apoptosis induction efficacy was calculated as percentage of fluorescein-positive to DAPI-stained nuclei.
2.8. Neuronal Damage Score
At the end of experiment, the brain was removed, fixed in 10% neutral buffered formalin, and embedded in paraffin blocks. Serial (5 μm) sections through the hypothalamus were stained with hematoxylin and eosin for microscopic evaluation. The extent of hypothalamic neuronal damage was scored on a scale of 0–3, modified from the grading system of Pulsinelli et al. [17], in which 0 is normal 1 indicates that approximately 30% of the neurons were damaged, 2 indicates that approximately 60% of the neurons were damaged, and 3 indicates that approximately 100% of the neurons are damaged. Each hemisphere was evaluated independently without the examiner knowing the experimental conditions. Degenerative damage was considered to have occurred in any neurons showing pyknosis of the nucleus and cell shrinkage.
2.9. Statistical Analysis
All values, except those on Table 3, are expressed as the mean ± SEM and were analyzed by one-way analysis of variance followed by the Fisher's least significance test as a post hoc test for multiple comparisons among means. For the data in Table 3, the Wilcoxon signed-rank test was used. The Wilcoxon tests convert the scores or values of a variable to ranks, require calculation of the sum of the ranks, and provide critical values for the sum necessary to test the null hypothesis at a given level of significance. These data are presented as the “median,” followed by first and third quartiles. All results were considered statistically significant at P < .05.
Table 3.
Mean (±SEM) of neuronal damage score values in hypothalamus for different groups of mice.
| Treatment groups | Neuronal damage score |
|---|---|
| (1) Normothermic controls (NCs) | 0 (0, 0) |
| (2) Sham-operated, vehicle-treated heatstroke (SOH) mice | 2 (2, 2)* |
| (3) Castrated, vehicle-treated heatstroke (CVH) mice | 0 (0, 0.25)** |
| (4) Castrated, testosterone-treated heatstroke (CTH) mice | 1 (1, 1)*** |
*P < .05 in comparison to group 1; **P < .05 in comparison to group 2;
***P < .05 in comparison to group 3. Vehicle or testosterone was administered 14 days before whole body heating (WBH), and the tissue section was obtained for neuronal damage score assay 2.5 h post-WBH. Data are means ± SEM of 7 mice per group.
3. Results
3.1. Castration Attenuates Heat-Induced Lethality and Thermoregulatory Deficit
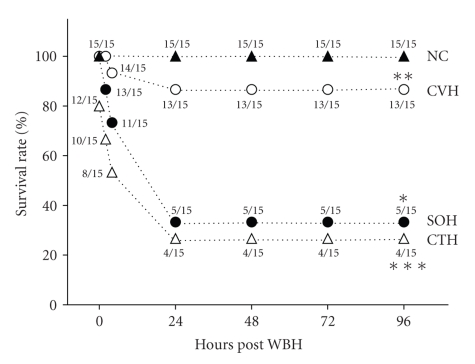
WBH treatment was used to induce heatstroke and thermoregulatory deficit (e.g., hypothermia) in mice as described in Section 2. Both Figures 1 and 2 indicate that 1 h of WBH resulted in 33% mortality and hypothermia (~27°C core temperature) monitored at 4 h of WBH in SOH mice. However, in CVH mice, the values of both percentage of survival and core temperature were significantly reached to new levels of 87% and 34.5°C, respectively. When the CTH groups were exposed to the same heat treatment, the values of both percentage of survival and core temperature were significantly returned to 27% and 28°C, respectively.
Figure 1.
Percentage of survival for normothermic controls (NCs), castrated, vehicle-treated heatstroke (CVH) mice, sham-operated, vehicle-treated heatstroke (SOH) mice, and castrated, testosterone-treated heatstroke (CTH) mice. *P < .05 compared with NC group. **P < .05 compared with SOH group. ***P < .05 compared with CVH group.
Figure 2.
The rectal temperature changes 1–4 hours post-whole body heating (WBH) for normothermic controls (NC), castrated, vehicle-treated heatstroke (CVH) mice, castrated, testosterone-treated heatstroke (CTH) mice, and sham-operated, heatstroke (SOH) mice. Data are means ± SEM (n = 6~9). *P < .05 compared with NC group. **P < .05 compared with SOH group. ***P < .05 compared with CVH group.
3.2. Castration Decreases Plasma Levels of Testosterone
Table 1 summarizes the plasma levels of testosterone for different groups of mice monitored at 4 h of WBH in SOH, CVH, and CTH groups or the equivalent time in NC group. As compared to those of NC or SOH groups, the CVH mice displayed significantly lower levels of plasma testosterone (4 ± 2 pg/mL versus 403 ± 91 or 525 ± 115 pg/mL). However, CTH mice showed significantly higher levels of plasma testosterone (260 ± 24 pg/mL versus 4 ± 2 pg/mL) as compared to those of CVH (Table 1).
Table 1.
Plasma levels of testosterone for different groups of mice.
| Treatment groups | Plasma testosterone (pg/mL) |
|---|---|
| (1) Normothermic controls (NCs) | 403 ± 91 |
| (2) Sham-operated heatstroke (SOH) mice | 525 ± 115 |
| (3) Castrated, vehicle-treated heatstroke (CVH) mice | 4 ± 2* |
| (4) Castrated, testosterone-treated heatstroke (CTH) mice | 260 ± 24*,** |
*P < .01 in comparison with group 1 or group 2;
**P < .05 in comparison with group 3. The blood sampling was obtained for testosterone assay immediately before the start of thermal experiments (or 14 days after vehicle or testosterone treatment). Data are means ± SEM of 7 mice per group.
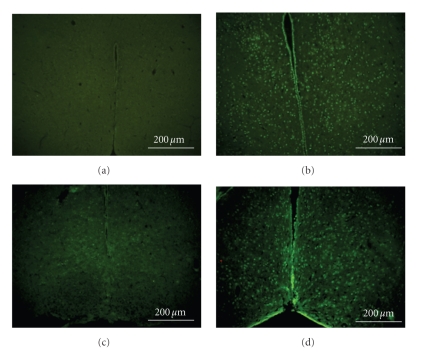
3.3. Castration Attenuates Heat-Induced Increased Numbers of TUNEL-Positive Cells in Hypothalamus
As summarized in Table 2, the numbers of TUNEL-positive cells in the hypothalamus evaluated at 2.5 h postWBH were 0, 344 ± 107, 70 ± 17, and 312 ± 95 per hypothalamic section, respectively, for NC (n = 7), SOH mice (n = 7), CVH (n = 7), and CTH (n = 7). Photomicrographs of TUNEL-positive cells in the hypothalamus of an NC, a SOH mouse, a CVH mouse, and a CTH mouse were shown in Figures 3(a)to 3(d). As compared to those of NC group, SOH mice had higher numbers of TUNEL-positive cells in their hypothalami (Figure 3(b)). The heat-induced increased numbers of hypothalamic TUNEL-positive cells could be significantly reduced by castration (Figure 3(c)). However, the beneficial effects of castration were significantly reversed by testosterone replacement (Figure 3(d)).
Table 2.
Mean (±SEM) number of TUNEL-positive cells per tissue section for different groups of mice.
| Treatment groups | Hypothalamus spleen kidney |
|---|---|
| (1) Normothermic controls (NCs) | 0 |
| (2) Sham-operated, vehicle-treated heatstroke (SOH) mice | 344 ± 107* |
| (3) Castrated, vehicle-treated heatstroke (CVH) mice | 70 ± 17** |
| (4) Castrated, testosterone-treated heatstroke (CTH) mice | 312 ± 95*** |
*P < .05 in comparison to group 1; **P < .05 in comparison to group 2;
***P < .05 in comparison to group 3. Vehicle or testosterone was administered 14 days before whole body heating, and the tissue section was obtained for TUNEL assay 2.5 h post-WBH. Data are means ± SEM of 7 mice per group.
Figure 3.
Photomicrographs of TUNEL, staining of the hypothalamus for a normothermic control (NC) (a), a sham-operated, heatstroke (SOH) mouse (b), a castrated, vehicle-treated heatstroke (CVH) mouse (c), and a castrated, testosterone-treated (CTH) mouse (d).
3.4. Castration Reduces Heat-Induced Neuronal Damage Cell Shrinkage and Nucleus Pyknosis in Hypothalamus
Table 3 summarizes the effects of heat exposure on the neuronal damage scores of the hypothalamus from NC mice, SOH mice, CVH mice, or CTH mice. The scores for hypothalamic neuronal damage in SOH mice significantly (P < .05) exceeded those of the respective NC mice. However, the hypothalamic neuronal damage scores in CVH mice were significantly (P < .05) lower than those of SOH mice. Furthermore, it was found that the hypothalamic neuronal damage score in CTH mice were significantly (P < .05) higher than those of CVH mice. Two and half hours after termination of heat stress, SOH mice exhibited cell shrinkage and pyknosis of the nucleus in the hypothalamus (Figure 4(b)). The heatstroke-induced neuronal damage in the hypothalamus was markedly less in CVH mice (Figure 4(c)) or CTH mice (Figure 4(d)). Both TUNEL and HE stainings revealed that apoptosis, cell shrinkage and pyknosis that occurred in the hypothalamus of a SOH (Figure 5(b)) mouse could be reduced by castration as shown in a CVH mouse (Figure 5(c)), which could be reversed by testosterone replacement in a CTH mouse (Figure 5(d)).
Figure 4.
Photomicrographs of neuronal damage of the hypothalamus of a normothermic control (NC) (a), a sham-operated vehicle-treated heatstroke (SOH) mouse (b), a castrated, vehicle-treated heatstroke (CVH) mouse (c), and a castrated, testosterone-treated heatstroke (CTH) mouse (d). Two and half hours post-whole body heating, the hypothalamus of a SOH mouse or a CTH mouse showed cell shrinkage, pyknosis of the nucleus, and loss of Nissl substance. However, following castration, neuroprotection was induced (as shown in a CVH mouse) ×200.
Figure 5.
Photomicrographs of TUNEL, HE, and merged (TUNEL+HE) staining of the hypothalamus of an NC mouse (a), a SOH mouse (b), a CVH mouse (c), and a CTH mouse (d). Apoptosis (green fluorescence; TUNEL staining) broadly colocalized with HE staining (purple color) of neurons to yield the blue color. Apoptosis is associated with pyknosis of the nucleus (b and d) ×400.
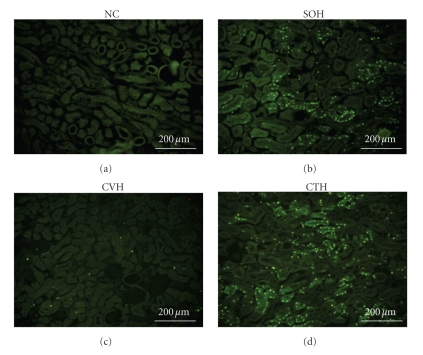
3.5. Castration Downregulates the Heat Stress-Induced Apoptosis in Splenocytes, Hepatocytes, and Kidney Cells
TUNEL assays of splenocytes, hepatocytes, and kidney cells were done 2.5 h after the termination of heat stress for different groups of mice. The numbers of TUNEL-positive cells of spleen, and kidney from SVH mice were significantly higher than those of NC mice (Table 2). As compared to SOH mice, CVH mice had significantly lower numbers of TUNEL-positive cells in multiple organs (Table 2). In addition, the CTH mice showed significantly higher numbers of TUNEL-positive in all these 3 organs as compared to those of CVH mice. Photomicrographs showing examples of TUNEL-positive cells of spleen and kidney for different groups of mice were depicted in both Figures 6 and 7, respectively.
Figure 6.
Photomicrographs of TUNEL staining of the spleen for an NC mouse, a SOH mouse, a CVH mouse, and a CTH mouse.
Figure 7.
Photomicrographs of TUNEL staining of the kidney for an NC mouse, a SOH mouse, a CVH mouse, and a CTH mouse.
4. Discussion
It has been demonstrated that plasma levels of estradiol are related to heat tolerance in anesthetized rats [18]. For example, the heat tolerance of estrus female rats was superior to the heat tolerance of ovariectomized or leuprolide-treated female rats. Induction of high levels of plasma estradiol caused by intravenous delivery of a high dose (1 mL/kg) of Premarin in estrus female, ovariectomized, or leuprolide-treated female rats conferred protection after heatstroke occurrence, as reflected by prolonged survival time. Evidence has also been provided to suggest that Premarin can act via estrogen receptors to rescue the unanesthetized, unrestrained mice from heatstroke-induced lethality [6]. In the present study, we have further evidence to promote that testosterone depletion by castration is able to protect the unanesthetized, unrestrained mice from heatstroke-induced lethality. It appears that low testosterone and/or high estradiol are able to protect mice from heatstroke-induced lethality. The contention is consistent with several clinical and experimental studies which demonstrate that gender dimorphism in immune and organ responsiveness and in the susceptibility and morbidity from shock, trauma, and sepsis [19]. In view of these findings, clinically relevant therapeutic strategies should be performed using estrogen or Premarin, and/or the androgen receptor antagonist, flutamide in heatstroke victims since heatstroke resembles sepsis in many aspects [15, 16].
Testosterone produced by Leydig cells of the testes is the major androgen in the circulation of men and adult males of most mammalian species. Androgen may proceed to amplify the action of testosterone through its conversion to dihydroxatone or its aromatization to estradiol [20]. This raises the possibility that the increased levels of both dihydrosterone and estradiol may be induced following castration-induced testosterone depletion in the current model. However, our findings reveal that castration does not affect the plasma levels of both dihydrosterone and estradiol in our mice and indicate that low testosterone, rather than high estradiol, is the main cause for the beneficial effect of castration in preventing heat-induced lethality (the data are not shown here).
Our previous results have shown that mice display increased production of cellular ischemia (e.g., glutamate and lactate-to-pyruvate ratio) and injury (e.g., glycerol) markers in the hypothalamus following heatstroke [6]. The current findings further show that both apoptosis (as indicated by TUNEL staining) and neuronal damage (as indicated by both cell shrinkage and pyknosis by H and E staining) in the hypothalamus occur during heatstroke. Apparently, the hypothermia that occurred after heatstroke in mice [5, 6] may have resulted from neuronal apoptosis and degeneration in the hypothalamus. The heat-induced thermoregulatory deficits as well as neuronal degeneration and apoptosis in the hypothalamus can be significantly prevented by testosterone depletion caused by surgical castration (as shown in the present results). Furthermore, the beneficial effects of testosterone in preventing neuronal damage and apoptosis in the hypothalamus and thermoregulatory deficit (e.g., hypothermia occurs during room temperature exposure) can be reversed after testosterone replacement. In addition to ischemic damage to the hypothalamus, severe heat causes apoptosis of spleen and renal cells, which can be ameliorated by testosterone depletion.
As mentioned in the Introduction section, multiorgan dysfunctions ensued from severe heatstroke include cardiac depression, cerebral ischemia and neuronal damage, systemic inflammation, hepatic and renal failure, systemic inflammation, and hypercoagulable state. The increased proinflammatory cytokine release by kupffer cells normally observed in intact inflammatory male mice following trauma-hemorrhage can be prevented by castration [12, 21, 22]. Castration of male rats 2 weeks prior to the onset of trauma-hemorrhage prevented the depression of myocardial function [10]. Flutamide (an androgen receptor antagonist) has also been shown to prevent the depression of cardiovascular responses following trauma and severe blood loss in male rats [14]. In addition, evidence has accumulated to indicate that testosterone is able to enhance both platelet aggregation [23, 24] and vasoconstriction [25, 26]. These observations prompted us to think that testosterone depletion may improve heat tolerance during heatstroke by reducing multiple organ dysfunction.
In summary, the current results demonstrate that testosterone depletion by castration may rescue mice from heat-induced multiple organ damage and lethality. In order to transfer those effects into clinical usage, studies mimicking castration by the use of an androgen receptor antagonist such as Flutamide should be conducted following the onset of heatstroke in future studies.
Acknowledgment
This study was supported by grants from the National Science Council, Taipei, Taiwan and the Chi Mei Medical Center (Tainan, Taiwan).
References
- 1.Bouchama A, Knochel JP. Heat stroke. The New England Journal of Medicine. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 2.Knochel JP, Reed G. Disorders of heat regulation. In: Maxwell MH, Kleeman CR, Narins RG, editors. Clinical Disorders of Fluid and Electrolyte Mechanism. 5th edition. New York, NY, USA: McGraw-Hill; 1994. pp. 1549–1590. [Google Scholar]
- 3.O’Donnell TF, Jr., Clowes GH., Jr. The circulatory abnormalities of heat stroke. The New England Journal of Medicine. 1972;287(15):734–737. doi: 10.1056/NEJM197210122871502. [DOI] [PubMed] [Google Scholar]
- 4.Chang C-K, Chang C-P, Chiu W-T, Lin M-T. Prevention and repair of circulatory shock and cerebral ischemia/injury by various agents in experimental heatstroke. Current Medicinal Chemistry. 2006;13(26):3145–3154. doi: 10.2174/092986706778742945. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Premachandran S, Sharma D, Bagewadikar RS, Poduval TB. Therapeutic treatment with L-arginine rescues mice from heat stroke-induced death: physiological and molecular mechanisms. Shock. 2005;24(4):341–347. doi: 10.1097/01.shk.0000180983.55623.2b. [DOI] [PubMed] [Google Scholar]
- 6.Shen K-H, Lin C-H, Chang H-K, Chen W-C, Chen S-H. Premarin can act via estrogen receptors to rescue mice from heatstroke-induced lethality. Shock. 2008;30(6):668–674. doi: 10.1097/SHK.0b013e31817538cb. [DOI] [PubMed] [Google Scholar]
- 7.Schröder J, Kahlke V, Staubach K-H, Zabel P, Stüber F. Gender differences in human sepsis. Archives of Surgery. 1998;133(11):1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 8.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14(2):81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 9.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. The Journal of Trauma. 2000;48(5):932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Remmers DE, Cioffi WG, Bland KI, Wang P, Angele MK, Chaudry IH. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Annals of Surgery. 1998;227(6):790–799. doi: 10.1097/00000658-199806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage: critical role of testosterone. Archives of Surgery. 1996;131(11):1186–1192. doi: 10.1001/archsurg.1996.01430230068012. [DOI] [PubMed] [Google Scholar]
- 12.Wichmann MW, Angele MK, Ayala A, Cioffi WG, Chaudry IH. Flutamide: a novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8(4):242–248. [PubMed] [Google Scholar]
- 13.Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. American Journal of Physiology. 1997;273(6):H2919–H2925. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 14.Shvareva N, Kaplanski J, Abramovich L, Sod-Moriah UA. Testosterone modifies response to chronic heat exposure in rats. Comparative Biochemistry and Physiology Part A. 1998;120(4):575–578. doi: 10.1016/s1095-6433(98)10078-8. [DOI] [PubMed] [Google Scholar]
- 15.Grogan H, Hopkins PM. Heat stroke: implications for critical care and anaesthesia. British Journal of Anaesthesia. 2002;88(5):700–707. doi: 10.1093/bja/88.5.700. [DOI] [PubMed] [Google Scholar]
- 16.Lu K-C, Wang J-Y, Lin S-H, Chu P, Lin Y-F. Role of circulating cytokines and chemokines in exertional heatstroke. Critical Care Medicine. 2004;32(2):399–403. doi: 10.1097/01.CCM.0000108884.74110.D9. [DOI] [PubMed] [Google Scholar]
- 17.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Annals of Neurology. 1982;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 18.Chen S-H, Chang F-M, Niu K-C, Lin MY-S, Lin M-T. Resuscitation from experimental heatstroke by estrogen therapy. Critical Care Medicine. 2006;34(4):1113–1118. doi: 10.1097/01.CCM.0000205756.04845.15. [DOI] [PubMed] [Google Scholar]
- 19.Angele MK, Frantz MC, Chaudry IH. Gender and sex hormones influence the response to trauma and sepsis—potential therapeutic approaches. Clinics. 2006;61(5):479–488. doi: 10.1590/s1807-59322006000500017. [DOI] [PubMed] [Google Scholar]
- 20.Bilińska B, Wiszniewska B, Kosiniak-Kamysz K, et al. Hormonal status of male reproductive system: androgens and estrogens in the testis and epididymis. In vivo and in vitro approaches. Reproductive Biology. 2006;6(supplement 1):43–58. [PubMed] [Google Scholar]
- 21.Angele MK, Knöferl MW, Schwacha MG, et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. American Journal of Physiology. 1999;277(1):C35–C42. doi: 10.1152/ajpcell.1999.277.1.C35. [DOI] [PubMed] [Google Scholar]
- 22.Ayala A, Perrin MM, Ertel W, Chaudry IH. Differential effects of hemorrhage on Kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (IL-1, IL-6 and TNF) release. Cytokine. 1992;4(1):66–75. doi: 10.1016/1043-4666(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 23.Johnson M, Ramey E, Ramwell PW. Androgen mediated sensitivity in platelet aggregation. American Journal of Physiology. 1977;232(4):H381–385. doi: 10.1152/ajpheart.1977.232.4.H381. [DOI] [PubMed] [Google Scholar]
- 24.Uzunova A, Ramey E, Ramwell PW. Effect of testosterone, sex and age on experimentally induced arterial thrombosis. Nature. 1976;261(5562):712–713. doi: 10.1038/261712a0. [DOI] [PubMed] [Google Scholar]
- 25.Ajayi AAL, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91(11):2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda K, Ruff A, Morinelli TA, Mathur RS, Halushka PV. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. American Journal of Physiology. 1994;267(3):H887–H893. doi: 10.1152/ajpheart.1994.267.3.H887. [DOI] [PubMed] [Google Scholar]