Summary
Objective
To investigate the effects of a matrix metalloproteinase (MMP) inhibitor on joint pathology and pain behavior in the rat meniscal transection (MNX) model of osteoarthritis (OA) and evaluate which aspects of structural disease modification contribute to symptom improvement.
Methods
OA pathology was induced in male Lewis rats, by transecting the medial collateral ligament with (MNX) or without (SHAM) a full thickness cut through the meniscus. MNX animals were orally administered an equipotent MMP 2, 8, 9, 12, 13 inhibitor (0.25, 1 and 5 mg/kg/day) or vehicle from day 1. Chondropathy, osteophytosis, osteochondral vascularity were assessed from toluidine blue stained coronal sections of the total knee joint and weight-bearing asymmetry by incapacitance. Group differences were evaluated using 1-way analysis of variance (ANOVA) and associations as Spearman's correlation coefficients.
Results
Treatment with the MMP inhibitor reduced weight-bearing asymmetry from day 14 onwards, and attenuated chondropathy (both P < 0.05). Osteochondral vascularity was elevated in MNX compared with SHAM-operated animals (P < 0.001) and reduced, dose-dependently, by MMP inhibitor treatment (r = −0.89, P < 0.05). Reduced osteochondral vascularity and chondropathy were associated with the amelioration of weight-bearing asymmetry (both P < 0.05).
Conclusion
Here we show that treatment with a MMP inhibitor reduces joint damage, osteochondral angiogenesis and behavioral evidence of pain. The association between osteochondral angiogenesis and pain behavior may be explained by perivascular nerve growth or stimulation of subchondral nerves following loss of osteochondral integrity. Our data suggest that targeting angiogenesis may have utility in the treatment of pain associated with structural damage in OA.
Keywords: Osteoarthritis, Rat model, Pain behavior, Chondropathy, Osteochondral angiogenesis
Introduction
Osteoarthritis (OA) is a chronic and progressive disorder of the joints that is widely thought of as primarily affecting the cartilage with some bone changes. However, some authors have hypothesized that the primary disorder is in the subchondral vasculature and that the changes in the cartilage and osteophyte generation are secondary phenomena1. Normal adult human cartilage is both avascular2 and insensate3. Angiogenesis occurs at the osteochondral junction in OA4, where vascular channels breach the tidemark5,6 and contain sympathetic and sensory nerves7,8. These findings lead to the hypothesis that angiogenesis facilitates innervation of articular cartilage, and therefore may be an important structural change that leads to pain. Matrix metalloproteinases (MMPs) are known to degrade the cartilage9 and therefore they may have a role in the ingrowth of blood vessels and nerves during the initiation of OA. We hypothesized that inhibition of MMPs would reduce pain behavior in OA by inhibiting osteochondral angiogenesis.
The meniscal transection (MNX)10 model of OA has been well characterized in terms of chondropathy and osteophytosis10. Recently, we have shown in this model that blood vessels also cross the osteochondral junction11, comparable with observations in the anterior cruciate ligament transaction model of OA12 and in human OA7. The MNX model is sensitive to inhibitors of MMPs10 which have a protective effect on overall cartilage damage as well as reducing osteophyte formation. However, neither vascular invasion of the cartilage nor pain behavior were reported in these studies. Some protective effects are seen for MMP inhibitors in other animal models of arthritis such as monosodium iodoacetate-induced13, adjuvant14 and canine surgically-induced arthritides15. Hind paw weight-bearing assymetry is a measure of pain behavior. The MNX model induces changes in hind paw weight distribution, which were attenuated by a COX -2 inhibitor and gabapentin16.
Associations between OA structural change and pain behavior are often weak, and are incompletely understood, both in animal models and in human disease17–19. In order to further explore possible mechanisms linking structural change and pain, we examined whether the oral administration of a MMP inhibitor would have an effect on joint pathology, osteochondral vascularity, and pain behavior, and explored possible interactions between any such effects.
Methods
MMP inhibitor characterization
Compound potency and Matrixin family selectivity of M503902 was determined in vitro using Fluorescence Resonance Emission Transfer (FRET) assays, based on the methodology previously described20. Briefly, compounds were solubilised in dimethyl sulphoxide (DMSO, Sigma Aldrich UK) to 10 mM and serially diluted in assay buffer, (1% (v/v) DMSO, 0.1 M Tris-HCL, 0.1 M NaCl, 10 mM CaCl2, 005% (v/v) Brij 35) and 50 μl added to 96 well Black assay plates (Cliniplates, Labsystems UK) at final concentrations 0.001 nM–100μM. 25 μl of active enzyme (final concentration 0.1–0.7 mg/ml) in Zinc Calcium buffer, (0.1 M Tris-HCL pH 7.5, 0.1 M NaCl, 0.08 M ZnCl2, 0.05% (w/v) Brij 35, 0.05 M CaCl2) was added to the compound and incubated for 15 min on a plate shaker at room temperature prior to addition of substrate at Km, (see Table I for specific assay substrate). Plates were incubated in a humidity chamber at 35°C for up to 2 h.
Table I.
The ability of M503902 to inhibit purified full-length recombinant human MMPs (expressed in bacculo virus) was determined against synthetic peptide substrates, (substrate concentration at Km)
| Matrixin | Peptide substrate | M503902 |
|
|---|---|---|---|
| IC50 mean ± S.E.M (n=) | Ratio (MMPx/MMP13) | ||
| MMP-13 | MCA-Pro-Cha-Gly-Nva-Dpa-Ala-Arg-NH2 | 0.35 ± 0.05 nM3 | 1 |
| MMP-1 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 119 ± 14.5 nM3 | 340 |
| MMP-2 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 0.1 nM1 | 0.29 |
| MMP-3 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 1.9 nM1 | 5.4 |
| MMP-7 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 325 ± 28.9 nM3 | 929 |
| MMP-8 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 0.6 nM1 | 1.71 |
| MMP-9 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 0.26 ± 0.06 nM5 | 0.74 |
| MMP-12 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 0.45 nM1 | 1.26 |
| MMP-14 | MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 | 3.05 ± 0.67 nM5 | 8.7 |
| MMP-19 | MCA-Pro-Leu-Ala-Nva-Dpa-Ala-Arg-NH2 | 7.2 ± 0.24 nM11 | 20.6 |
MMPx = X is the number of matrixin in column 1.
Fluorescence was read at time 0 and every 30 min (Spectramax, Molecular Devices UK) at wavelengths of excitation 328 nm, emission 393 nm until a suitable window was reached. IC50s were calculated using an in house automated software package.
Induction of OA
All in vivo procedures were carried out in accordance to the UK Animals (Scientific Procedures) Act 1986 and approved by AstraZeneca local ethical review. OA was induced by medial MNX in male Lewis rats (270 g, Harlan, Bicester, UK), as previously described14. Briefly, pathology was induced by transecting the medial collateral ligament and making a full thickness cut through the meniscus (day 0) of the left knee. For controls, medial collateral ligament transection without MNX (SHAM) surgery was performed on the left knee of separate animals.
Treatments
Animals (10 rats per group) were dosed twice daily by oral gavage with either vehicle (hydroxyprolyl methyl cellulose/tween) or vehicle containing the MMP inhibitor, AstraZeneca M503902 (0.125, 0.5, 2.5 mg/kg from the day prior to surgery, until sacrifice at day 35). The inhibitory profile of M503902 against eleven MMPs is given in Table I.
Pain behavior
Pain behavior was measured as weight-bearing asymmetry between operated and contralateral knees. Changes in weight-bearing asymmetry were assessed at pre surgery (−2 days) and at 7, 14, 21, 28 and 35 days post surgery, using an Incapacitance meter (Linton Instruments, Diss, Norfolk, UK)21,22. This technique measures the difference in weight bearing between the ipsilateral operated limb and the contralateral control limb. Rats were placed in a Perspex container such that each paw rested on a separate transducer pad that recorded the animals weight distribution over a period of 3 sec. Each data point is the average of three readings. The hind paw weight distribution is expressed as the difference in weight between ipsilateral and contralateral limbs. Weight-bearing asymmetry was analysed as area under the curve, and possible associations with histological parameters were determined using measurements obtained prior to sacrifice 35 days after surgery.
Histology
For each animal, skin was removed and the tibiofemoral joints were isolated by cutting mid-femur and tibia. The joints including articular surfaces, joint capsule and intra-articular structures were preserved in 10% neutral buffered formalin for 48–72 h and subsequently decalcified for 24–36 h in rapid decalcification fluid (Surgipath decalcifier II – Surgipath Europe Ltd, Peterborough, UK). Trimmed joint tissues were processed by standard histological techniques and mounted in wax blocks for sectioning. Coronal sections (5 μm), through the midpoint of the joint, identified by the presence of cruciate ligament insertions, were stained with toluidine blue.
Chondropathy and osteophytosis were evaluated using the system of Janusz et al.10. In this method cartilage damage was scored on a scale of 0–5 as follows: 0. Cartilage of normal appearance; 1. Minimal fibrillation, superficial zone only; 2. Mild, extends to the upper middle zone; 3. Moderate, well into the middle zone; 4. Marked, into the deep zone but not to the tidemark; and 5. Severe, full thickness degeneration to tidemark. The amount of cartilage damage was estimated as the proportion of the section of the medial tibial plateaux involved, 1/3, 2/3 or 3/3 and the cartilage score multiplied by one, two or three respectively to give a total chondropathy score. Osteophytosis was scored on a scale of 0–3, using an eyepiece graticule as follows: 0. No osteophyte present; 1. Mild, <50 μm; 2. Moderate, 50–150 μm; and 3. Severe, >150 μm.
Osteochondral vascular density was determined by counting the number of blood vessels crossing the osteochondral junction in the entire medial tibial plateau of the mid-coronal sections. An arithmetic mean was determined for at least three replicate sections from each knee. A geometric mean (+/−95% confidence intervals) was then calculated for each group of 10 animals.
Measurements were made blinded to diagnostic group or surgical procedure, and with sections in random order.
Analysis
Data were graphically presented using Prism v 4 (GraphPad, San Diego CA). Statistical analyses used Statistical Package for the Social Sciences v.14 (SPSS inc., Chicago, Illinois). Associations are reported as Spearmann's rank correlation coefficients. Independence of associations was determined as partial correlation coefficients. Differences between groups were sought using 1-way ANOVA followed by post hoc t-tests with Bonferroni's corrections on arithmetic or geometric data, as appropriate. Data are presented in the text as mean (95% confidence interval) except where stated, and graphically as mean (S.E.M.). A two- tailed P < 0.05 was taken to indicate statistical significance.
Results
Effect of an MMP inhibitor on chondropathy, osteophytosis and blood vessels crossing the osteochondral junction
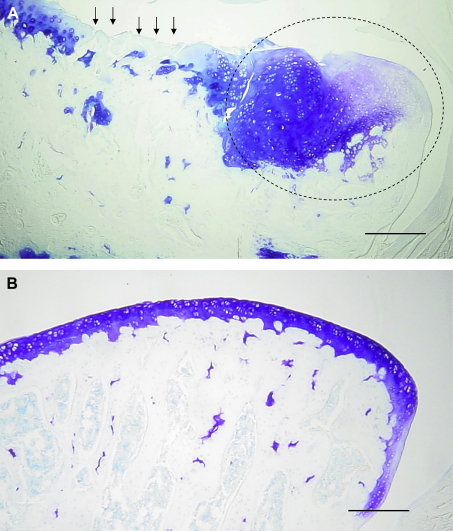
MNX was associated with the expected OA changes in the medial tibial plateaux at day 35. These included a loss of surface integrity, loss of chondrocytes and developing osteophytes [Fig. 1(A)] SHAM-operated animals did not display evidence of chondropathy or osteophytosis. [Fig. 1(B)].
Fig. 1.
A). Representative image of the medial tibial plateau of a menisectomised (MNX) animal at 35 days post surgery showing full thickness cartilage fibrillation and proteoglycan loss (arrows). A large osteophyte (circled) has formed at the joint margin. B). Representative image of the medial tibial plateau of a sham (SHAM) operated animal at 35 days. There is no evidence of proteoglycan loss or fibrillation of the cartilage surface. The joint margin appears normal. Scale bars 100 μm.
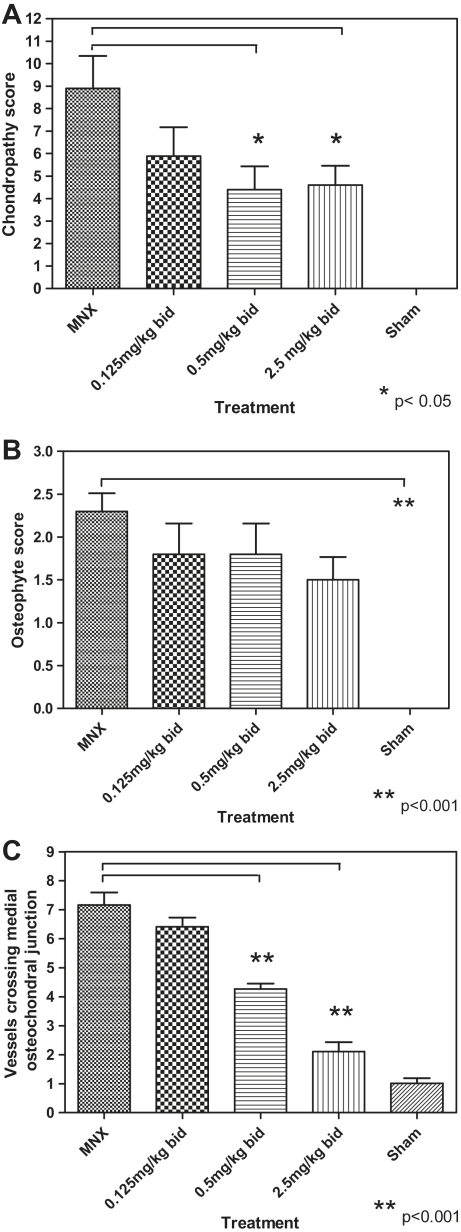
The MMP inhibtor used was shown to be an equipotent inhibitor of MMPs 2, 8, 9,12 and 13 (Table I). Administration of the MMP inhibitor was associated with a dose-dependent reduction in chondropathy score [r = − 0.37, P < 0.01, Fig. 3(A)] with significant reduction of chondropathy at 5 mg/kg/day (4.6, C.I. 2.6–6.5) and at 1 mg/kg/day (4.4, C.I. 2.0–6.7), compared to MNX untreated controls (8.9, C.I. 5.6–12.1), each P < 0.05.
Fig. 3.
The effect of increasing doses of a MMP inhibitor, in the rat MNX model of OA, on chondropathy (Panel A), osteophytosis (Panel B) and the number of vessels crossing the osteochondral junction (Panel C). Treatment with 0.5 and 2.5 mg bid resulted in significant decreases in the chondropathy scores (P < 0.05) and in the number of blood vessels crossing the osteochondral junction (P < 0.001).There was no significant effect on osteophytosis.
Osteophytes were not seen in SHAM-operated animals. The mean osteophyte score for MNX animals was 2.3 (C.I. 1.8–2.8) compared to SHAM scores of 0.0 (P < 0.0001) [Fig. 3(B)]. Increasing dose of the MMP inhibitor was not significantly associated with decreasing osteophyte scores (r = −0.29, P > 0.05, Table II).
Table II.
Associations between MMP inhibitor treatment, OA structural severity, osteochondral vascularity and pain behavior
| MMP inhibitor dose | Chondropathy | Osteophytosis | Osteochondral vascularity | |
|---|---|---|---|---|
| Chondropathy | −0.38* | |||
| Osteophytosis | −0.29 | 0.64** | ||
| Osteochondral vascularity | −0.89** | 0.39* | 0.32* | |
| Pain behavior | 0.46** | −0.38* | −0.20 | −0.33* |
Values are Spearman's correlation coefficients. *P < 0.05, **P < 0.01
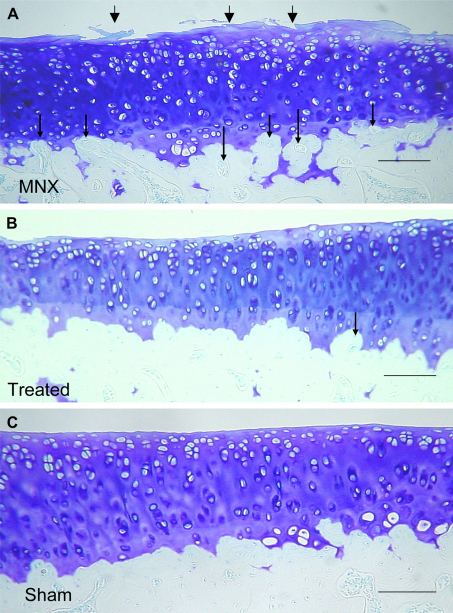
Blood vessels crossing the osteochondral junction [Figs. 2(A) and 3(C)] were more numerous in the medial tibial plateaux 35 days after MNX (7.1, C.I 6.1–8.2) than in SHAM-operated animals (1.0, CI 0.6–1.4) (P < 0.001). The osteochondral vascularity observed in the MNX animals was reduced by treatment with the MMP inhibitor at 5 mg/kg/day (2.1, CI 1.3–2.8; mean difference 5.0, CI 3.8–6.2) and 1 mg/kg/day (4.2, CI 3.8–4.6; mean difference 2.9, CI 1.6–4.1, both P < 0.001). At the highest dose, osteochondral vascularity was similar to the SHAM-operated controls (mean difference 1.0, CI −0.1–2.3, P > 0.05). Administration of the inhibitor was associated with a dose-dependent decrease in the number of vessels crossing the osteochondral junction (r = −0.89, P < 0.01, Table II).
Fig. 2.
A). Representative image of the medial tibial plateaux of MNX animal showing loss of proteoglycan and cartilage fibrillation (large arrows) vascular channels arising from the subchondral bone are evident at the osteochondral junction (small arrows). B). Medial tibial plateu from an MNX animal treated with 5.0 mg/kg/day M503092. The cartilage surface shows no fibrillation but there is some proteoglycan loss as evidence by diminished toluidine blue staining. Fewer vascular channels (small arrow) are seen in treated animals. C). SHAM-operated animal showing intact cartilage and no vascular channels in this section. Sections were stained with toluidene blue and imaged with a small diameter substage diaphragm to demonstrate blood vessels as refractile structures within channels. Scale bars 100 μm.
Effect of MMP inhibitor on pain behavior
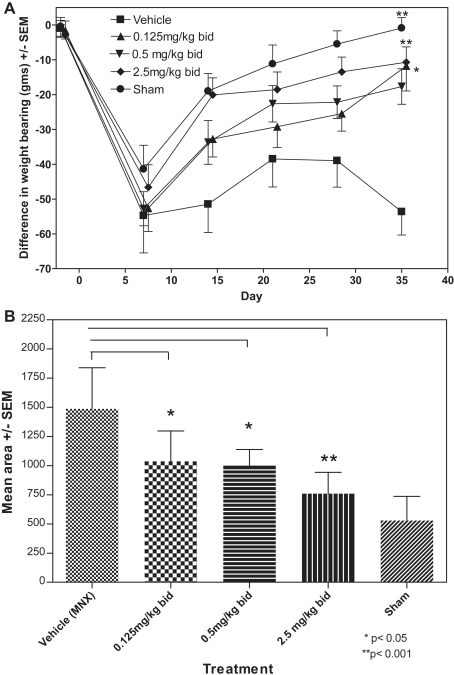
Weight-bearing asymmetry was similar in both MNX and SHAM-operated animals at day 7, persisted in MNX operated, and normalized in SHAM-operated animals by day 35 (Fig. 4). Area under the curve (AUC) for weight-bearing asymmetry in SHAM-operated animals was 530, CI 322–733, lower than in MNX operated animals 1488, CI 1137–1839 (P < 0.001). Administration of the MMP inhibitor was associated with a dose-dependent reduction in AUC for weight-bearing asymmetry compared with vehicle-treated MNX controls of 1037, CI 777–1297 (P < 0.05) for 0.25 mg/kg/day, 999, CI 860–1138 (P < 0.05) for 1 mg/kg/day and 760, 576–943 (P < 0.001) for 5 mg/kg/day.
Fig. 4.
Time course showing the effect of increasing doses of a MMP inhibitor on pain behavior, as measured by a difference in weight bearing, in the MNX and SHAM animals. Vehicle-treated animals continued to show pain behavior but this behavior was dose-dependently decreased by increasing doses of M503092. SHAM animals returned to normal weight bearing over the course of the experiment. Values for 0.125 mg/kg/bid and 2.5 mg/kg/bid have been offset by 0.5 days to increase the clarity of presentation by preventing overlap of error bars. Areas under the curve were calculated for the 10 individual animals in each treatment group and a mean with 95% CI's computed. Differences between groups were sought with 1-way ANOVA with Bonferroni corrections.
Relationship between pathology and pain behavior
Associations between the measured parameters are given in Table II. Weight-bearing asymmetry was associated with osteochondral vascularity, chondropathy.and although not signifcant osteophytosis. The reduction of osteochondral vascularity with increasing dosage of the MMP inhibitor, was independent of the reduction in chondropathy (partial correlation coefficient −0.88, P < 0.0001).
Discusssion
OA is a major cause of pain and disability, and is traditionally characterized as a degenerative condition associated with damage to articular cartilage and subchondral bone. However, the precise link between structural changes and pain in OA has been difficult to establish, with often only weak associations found between chondropathy or osteophytosis, and symptom severity23,24. Observational data in human OA have indicated that subchondral bone or bone marrow lesions25 may be a source of pain26 and we hypothesise that invasion of the articular cartilage by subchondral blood vessels may be a key structural change leading to OA pain. In support of this hypothesis we report here that in the MNX animal model of OA treated with a MMP inhibitor, that osteochondral vascularity, as well as chondropathy, was associated with pain behavior.
MMPs, particularly MMP13, have received attention in OA research, because of their ability to degrade cartilage matrix proteins such as type II collagen. Janusz et al10 demonstrated a reduction in chondropathy following MMP inhibition with an Matrix Metalloproteinase inhibitor (MMPi) inhibitor, structurally related to hydroxamic acid (Proctor and Gamble) in a meniscal injury model of OA. Our result with the AstraZeneca MMP inhibitor, M503902, supports the importance of MMPs in the development of chondropathy in this animal model of OA.
Adult articular cartilage is normally an avascular tissue, although vascular growth occurs at the osteochondral junction in OA, both in man4,7, and in rats after MNX11. Degradation of the vascular basement membrane and surrounding extracellular matrix by MMPs are necessary in order to allow endothelial cells to migrate and invade surrounding tissue during angiogenesis27. Reduction of osteochondral vascular density in MNX rats by MMP inhibitor treatment suggests that MMPs are also necessary for osteochondral angiogenesis in OA. Inhibition of osteochondral angiogenesis was observed at a dose of MMP inhibitor which did not significantly affect chondropathy and osteophytosis, and was, at least in part, independent of these other aspects of structural change. MMP inhibition therefore may directly reduce osteochondral angiogenesis. Angiogenesis at the osteochondral junction requires bone remodelling as well as degradation of cartilage matrix, as blood vessels are normally separated from the articular cartilage by the subchondral bone plate. The MMP inhibitor studied here also inhibited the metallo-gelatinases MMP2 and 9, which are know to be involved in bone remodelling28. Our data also lead us to suggest that osteochondral angiogenesis may be dependent on a restricted range of factors, and therefore may be more susceptible to inhibition by specific MMP inhibitors, than are chondropathy or osteophytosis.
The MNX model of OA is associated with a biphasic pattern of pain behavior. We observed that early weight-bearing asymmetry at day 7 affects equally both MNX and SHAM-operated animals. Pain behavior at this stage is most likely attributable to capsular damage and synovitis, which also are similar 7 days after either MNX or SHAM surgery11. Synovitis resolves in SHAM-operated animals by 28 days after surgery, indicating capsular repair11, whereas MNX animals develop chondropathy, osteophytosis and increased osteochondral vascularity. Persistent weight-bearing asymmetry 35 days after MNX, but not after SHAM surgery, indicates that OA structural change rather than capsular damage leads to pain behavior. Inhibition of weight-bearing asymmetry by the MMP inhibitor at day 35 in MNX animals, but not 7 days after either MNX or SHAM operation, leads us to believe that the MMP inhibitor reduces pain behavior by inhibiting OA structural change, rather than by reducing early tissue damage or inflammation.
Effects on chondropathy scores at day 35 were relatively modest, with chondropathy scores reduced by the highest dose to approximately half the values observed in vehicle-treated MNX animals. This contrasts with a near abolition of pain behavior at day 35. Residual chondropathy and osteophytosis therefore do not necessarily prevent pain relief by structural disease modification. These findings corroborate clinical data indicating only weak associations between chondropathy or osteophytosis and reported pain, and the clinical observation that patients with extensive structural change on radiographs may report few symptoms18. The reduction in chondropathy scores is likely due to a direct effect of the MMPi on proteinases, such as collagenase 3 or MMP9, that can degrade cartilage matrix proteins. However, an indirect effect mediated through inhibition of angiogenesis is also possible.
Sensory nerves grow along blood vessels following angiogenesis in subcutaneous tissues29 and during callus formation30, and nerves are localised within vascular channels at the osteochondral junction in OA7. Furthermore, increased osteochondral vascularity is associated with abnormally high hydraulic conductance through the articular cartilage31 such that osteochondral angiogenesis may expose subchondral nerves to painful stimuli in OA. We show here that reduced osteochondral angiogenesis was associated with a marked reduction in pain behavior. We postulate therefore that inhibition of osteochondral angiogenesis may be a key component of structural disease modification that may improve pain in OA.
In conclusion, here we show that there is vascular penetration of articular cartilage in a rat surgical model of OA and that treatment with an MMP inhibitor is associated with reduction in osteochondral angiogenesis and pain behavior. Osteochondral angiogenesis may lead to key structural changes that contribute to pain in OA, raising the possibility that anti-angiogenic agents may reduce OA pain.
Conflict of interest
The authors have no conflicts of interest to declare in regard to the manuscript entitled: Effects of the metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis.
Acknowledgements
PIM. was supported by a grant 17064 from The Arthritis Research Campaign.The authors would like to thank Chris Stannard, Clare Day MSc and Ruth Webster BSc for their technical assistance.
References
- 1.Imhof H., Breitenseher M., Kainberger F., Trattnig S. Degenerative joint disease: cartilage or vascular disease? Skeletal Radiol. 1997;26(7):398–403. doi: 10.1007/s002560050254. [DOI] [PubMed] [Google Scholar]
- 2.Moses M.A., Sudhalter J., Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990;248(4961):1408–1410. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- 3.Dye S.F., Vaupel G.L., Dye C.C. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773–777. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]
- 4.Walsh D.A., Bonnet C.S., Turner E.L., Wilson D., Situ M., McWilliams D.F. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15(7):743–751. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Clark J.M. The structure of vascular channels in the subchondral plate. J Anat. 1990;171:105–115. [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan H., Jundt J., Riddle J.M., Pitchford W., Christopherson T. The tibial subchondral plate. A scanning electron microscopic study. J Bone Joint Surg Am. 1987;69(8):1212–1220. [PubMed] [Google Scholar]
- 7.Suri S., Gill S.E., Massena de Camin S., Wilson D., McWilliams D.F., Walsh D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortier L.A., Nixon A.J. Distributional changes in substance P nociceptive fiber patterns in naturally osteoarthritic articulations. J Rheumatol. 1997;24(3):524–530. [PubMed] [Google Scholar]
- 9.Billinghurst R.C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janusz M.J., Bendele A.M., Brown K.K., Taiwo Y.O., Hsieh L., Heitmeyer S.A. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10(10):785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 11.Mapp P.I., Avery P.S., McWilliams D.F., Bowyer J., Day C., Moores S. Angiogenesis in two animal models of osteoarthritis. Osteoarthritis Cartilage. 2008;16(1):61–69. doi: 10.1016/j.joca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Hayami T., Pickarski M., Zhuo Y., Wesolowski G.A., Rodan G.A., Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Janusz M.J., Hookfin E.B., Heitmeyer S.A., Woessner J.F., Freemont A.J., Hoyland J.A. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001;9(8):751–760. doi: 10.1053/joca.2001.0472. [DOI] [PubMed] [Google Scholar]
- 14.Conway J.G., Wakefield J.A., Brown R.H., Marron B.E., Sekut L., Stimpson S.A. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med. 1995;182(2):449–457. doi: 10.1084/jem.182.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L.P., Jr., Smith G.N., Jr., Brandt K.D., Myers S.L., O'Connor B.L., Brandt D.A. Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis Rheum. 1992;35(10):1150–1159. doi: 10.1002/art.1780351007. [DOI] [PubMed] [Google Scholar]
- 16.Bove S.E., Laemont K.D., Brooker R.M., Osborn M.N., Sanchez B.M., Guzman R.E. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14(10):1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Lanyon P., Muir K., Doherty S., Doherty M. Influence of radiographic phenotype on risk of hip osteoarthritis within families. Ann Rheum Dis. 2004;63(3):259–263. doi: 10.1136/ard.2002.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart D.J., Spector T.D. The classification and assessment of osteoarthritis. Baillieres Clin Rheumatol. 1995;9(2):407–432. doi: 10.1016/s0950-3579(05)80198-0. [DOI] [PubMed] [Google Scholar]
- 19.Conaghan P.G., Felson D.T. Structural associations of osteoarthritis pain: lessons from magnetic resonance imaging. Novartis Found Symp. 2004;260:191–201. discussion 201–5, 277–9. [PubMed] [Google Scholar]
- 20.Knight C.G., Willenbrock F., Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296(3):263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- 21.Mihara M., Higo S., Uchiyama Y., Tanabe K., Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthritis Cartilage. 2007;15(5):543–549. doi: 10.1016/j.joca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Bove S.E., Calcaterra S.L., Brooker R.M., Huber C.M., Guzman R.E., Juneau P.L. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11(11):821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 23.Hannan M.T., Felson D.T., Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 24.Lethbridge-Cejku M., Scott W.W., Jr., Reichle R., Ettinger W.H., Zonderman A., Costa P. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore longitudinal study of aging. Arthritis Care Res. 1995;8(3):182–188. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 25.Hunter D.J., Zhang Y., Niu J., Goggins J., Amin S., LaValley M.P. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 26.Felson D.T., Niu J., Guermazi A., Roemer F., Aliabadi P., Clancy M. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 27.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krane S.M., Inada M. Matrix metalloproteinases and bone. Bone. 2008;43(1):7–18. doi: 10.1016/j.bone.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Walsh D.A., Hu D.E., Mapp P.I., Polak J.M., Blake D.R., Fan T.P. Innervation and neurokinin receptors during angiogenesis in the rat sponge granuloma. Histochem J. 1996;28(11):759–769. doi: 10.1007/BF02272149. [DOI] [PubMed] [Google Scholar]
- 30.Aoki M., Tamai K., Saotome K. Substance P- and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. Int Orthop. 1994;18(5):317–324. doi: 10.1007/BF00180235. [DOI] [PubMed] [Google Scholar]
- 31.Hwang J., Bae W.C., Shieu W., Lewis C.W., Bugbee W.D., Sah R.L. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 2008;58(12):3831–3842. doi: 10.1002/art.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]