Sir,
The issue of suitability of therapeutic drug monitoring (TDM) for imatinib continues to fuel controversies. Unlike two previous studies in gastrointestinal stromal tumours (GIST) patients (Judson et al, 2005; Delbaldo et al, 2006), a recent clinical pharmacokinetic (PK) substudy carried out on the basis of the B2222 trial, evaluating imatinib in patients with unresectable or metastatic CD117-positive GIST, found a correlation between imatinib total exposure and clinical response. Trough levels over 1100 ng ml−1 predicted a better overall benefit rate (composite clinical outcome; Demetri et al, 2009). These results are thus in line with observations made in CML patients, showing trough levels over 1000 ng ml−1 to predict a better molecular response rate (Picard et al, 2007; Larson et al, 2008).
These concentration–effect relationships confirm and strengthen our results obtained in a population of GIST patients, albeit smaller-sized (38 patients), which we had previously reported in the Journal (Widmer et al, 2008). We have indeed observed that higher free imatinib exposure predicts a higher probability of therapeutic response, when taking into account tumour KIT genotype. The strongest association was observed in patients harbouring exon 9 mutation or wild-type (wt) KIT, which is known to decrease tumour sensitivity towards imatinib (Heinrich et al, 2003). In fact, we found that, in our population of patients, free plasma concentration (the pharmacologically active species in plasma; i.e., imatinib fraction not bound to α1-acid glycoprotein (AGP); Widmer et al, 2006) was a better predictor of the clinical response rather than total concentration. This free exposure was derived from the total exposure using a mathematical model taking into account the AGP plasma level (Widmer et al, 2006). Moreover, we found a significant relationship between this free exposure and clinical response only in patients with exon 9 mutation and wt KIT. Of importance, we also observed significant correlations between total, as well as free, imatinib exposure and the occurrence of side effects (Widmer et al, 2008).
To better compare our results with those of the B2222 PK substudy, we recomputed maximum a posteriori extrapolations for both total Cmin and free Cmin in our patient samples, rather than considering the global imatinib exposure (area under the plasma concentration–time curve) that was previously analysed (Widmer et al, 2008). Among the 38 GIST patients of the previous analysis, AGP plasma levels – required to calculate free Cmin – were available for 36 patients. All these patients were included in an observational study approved by the Ethics Committee of the Lausanne Faculty of Medicine. Informed written consent was obtained from all the participants. A specific population PK model (Widmer et al, 2006) was used for this extrapolation (using NONMEM, version VI 2.0, NONMEM Project Group, University of California at San Francisco, San Francisco, CA, USA). We investigated their correlation with clinical benefit, defined as response evaluation criteria in solid tumours (RECIST) complete response, partial response or stable disease, by logistic regression analysis (using Stata version 10.1, Stata Co., College Station, TX, USA).
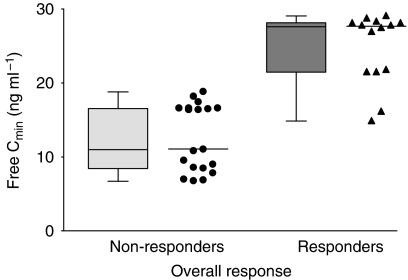
We found no significant overall association between total Cmin and response in our GIST population. Conversely, imatinib free Cmin was correlated with a clinical benefit, with responders having higher free levels than non-responders (RECIST progressive disease). This relationship did not reach significance over the whole patient sample series (i.e., irrespective of the KIT genetic profile; area under the ROC curve=0.594 and P=0.26 using logistic regression analysis on log2 values of free Cmin). However, focusing on exon 9 mutated and wt KIT cases allowed the identification of a clear relationship (area under the ROC curve=0.932 and P=0.013). The cutoff value of 20 ng ml−1 free imatinib plasma trough level corresponded to the best sensitivity (86%) and specificity (100%). The geometric average estimate of imatinib free fraction across our study samples was 1.0% (CV 45%). The mean daily doses of imatinib only tended to be slightly higher in exon 9 or wt KIT patients compared with exon 11 KIT patients (649 mg vs 590 mg daily, respectively; P=0.07 using t-test). Table 1 describes our GIST patient samples and Figure 1 shows the striking difference between the free Cmin values of responder and non-responder exon 9 or wt KIT patients (per-sample analysis).
Table 1. Left part: description of free trough plasma concentrations (free Cmin) deduced from imatinib and AGP levels available in samples from 36 GIST patients; right part: description of the samples from seven patients with exon 9 or wt KIT identified among 20 with known tumour KIT genotype.
|
All patients (wt, exon 9 and exon 11 KIT)
|
Exon 9 and wt KIT patients
|
|||
|---|---|---|---|---|
| RECIST response | n (blood samples) | Median free Cmin and range (ng ml−1) | n (blood samples) | Median free Cmin and range (ng ml−1) |
| Progressive disease | 50 | 13.4 (3.8–22.9) | 19 | 10.1 (6.1–17.4) |
| Stable disease | 63 | 15.8 (4.5–39.3) | 4 | 19.9 (13.7–20.2) |
| Partial response | 72 | 13.3 (2.8–33.0) | 2 | 20.5 (14.9–26.1) |
| Complete response | 8 | 26.0 (25.0–27.0) | 8 | 26.0 (25.0–27.0) |
Abbreviations: AGP=α1-acid glycoprotein; GIST=gastrointestinal stromal tumour; RECIST, response evaluation criteria in solid tumours.
Each patient provided between 1 and 12 samples over 3 years (median: 4 samples per patient), along with current RECIST response status.
Figure 1.
Box plots and individual values of extrapolated free trough concentrations (free Cmin) in exon 9 mutated or wt KIT patients, contrasting responders’ samples (complete response, partial response, or stable disease; n=14; median=25.7 ng ml−1) vs non-responders’ (progressive disease; n=19; median=10.1 ng ml−1).
This per-sample analysis was performed because imatinib doses administered to each patient during the course of this 3-year-long observational study could be increased or decreased. The concentration, and possibly the response or adverse events related to treatment, may therefore vary at some point for a given patient. Interestingly, a similar analysis carried out on a per-patient basis (i.e., expressing only a single mean free Cmin and one median response for each individual patient) provided a similar relationship and cut-off, without, however, reaching statistical significance because of the limited number of patients with exon 9 or wt KIT. The results from our observational study should therefore still be considered cautiously and will have to be confirmed in a larger patient population. In fact, extrapolation of free concentrations still remains to be formally confirmed by direct measurement of free plasma levels of imatinib in patients’ blood, an aspect that is currently being addressed in ongoing studies initiated at our Institution with GIST and CML patients.
Altogether, the results from the B2222 substudy and our results call for attention to imatinib concentration–effect relationships in the management of GIST patients, which deserve further in-depth exploration. In our opinion, future investigations should take into account not only total plasma concentrations but also free plasma concentrations (either measured or computed with regard to AGP and albumin levels), as well as the tumour genotype. We are currently expanding our set of observations in a larger GIST population in that endeavour. We also agree with the authors of the B2222 PK substudy regarding the urgent need for prospective controlled trials to assess whether a TDM programme would optimise the treatment outcomes in GIST patients, as it has been called for in CML patients (Blasdel et al, 2007). Similar considerations might apply as well to other new tyrosine kinase inhibitors currently fleshing out our armament of targeted anticancer agents. Convenient analytical methods already exist for their measurement in patients’ plasma (Haouala et al, 2009), but, as for imatinib, further clinical evaluation of their concentration–response relationships and of the benefit of optimising their concentration exposure is warranted in cancer patients.
Footnotes
Dr Decosterd, Dr Leyvraz and Dr Buclin have received grant-in-aid supports from Novartis for research projects other than the clinical study mentioned in this letter.
References
- Blasdel C, Egorin MJ, Lagattuta TF, Druker BJ, Deininger MW (2007) Therapeutic drug monitoring in CML patients on imatinib. Blood 110(5): 1699–1701; author reply 1701 [DOI] [PubMed] [Google Scholar]
- Delbaldo C, Chatelut E, Re M, Deroussent A, Seronie-Vivien S, Jambu A, Berthaud P, Le Cesne A, Blay JY, Vassal G (2006) Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res 12(20): 6073–6078 [DOI] [PubMed] [Google Scholar]
- Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M (2009) Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 27(19): 3141–3147 [DOI] [PubMed] [Google Scholar]
- Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, Ris HB, Leyvraz S, Widmer N, Decosterd LA (2009) Therapeutic drug monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877(22): 1982–1996 [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21(23): 4342–4349 [DOI] [PubMed] [Google Scholar]
- Judson I, Peiming M, Peng B, Verweij J, Racine A, di Paola ED, van Glabbeke M, Dimitrijevic S, Scurr M, Dumez H, van Oosterom A (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55(4): 379–386 [DOI] [PubMed] [Google Scholar]
- Larson RA, Druker BJ, Guilhot FA, O’Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111(8): 4022–4028 [DOI] [PubMed] [Google Scholar]
- Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, Lassalle R, Marit G, Reiffers J, Begaud B, Moore N, Molimard M, Mahon FX (2007) Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109(8): 3496–3499 [DOI] [PubMed] [Google Scholar]
- Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, Rochat B, Eap CB, Henry H, Biollaz J, Buclin T (2006) Population pharmacokinetics of imatinib and role of alpha-1-acid glycoprotein. Br J Clin Pharmacol 62(1): 97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer N, Decosterd LA, Leyvraz S, Duchosal MA, Rosselet A, Debiec-Rychter M, Csajka C, Biollaz J, Buclin T (2008) Relationship of imatinib free plasma levels and target genotype with efficacy and tolerability. Br J Cancer 98(10): 1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]