Abstract
Background:
Participants are showing great interest these days in obtaining the results of clinical trials. The aim of this study was to assess patients’ uptake and understanding of the results of the trial in which they have participated and the impact of a letter offering patients the possibility of consulting the trial results on a specific website.
Methods:
Breast cancer patients participating in a trial on the efficacy of Trastuzumab were randomly subdivided into an Internet group (who received the letter of invitation) and a control group (who did not receive it). Among 115 HER2-positive women from 21 centres, 107 (93%) answered a self-administered questionnaire.
Results:
Most of the patients in both groups had access to the Internet (72.0%). The majority (97.2%) stated that receiving information about the trial results would be useful, and the oncologist was the most frequently preferred information provider. The Internet group's declared uptake of the trial results was only slightly higher (47.1% vs 33.9% P=0.166); however, they understood the results significantly more accurately (18.8% vs 5.6% P=0.039).
Interpretation:
Although Internet was not the respondents’ preferred source of information, the possibility of using this source slightly increased the uptake and understanding of the results.
Keywords: breast cancer, randomised controlled trials, informing patients
Consistent evidence has become available that patients involved in randomised clinical trials (RCTs) want to know about the results despite their potentially negative emotional impact (Shalowitz and Miller, 2008), but previous authors have emphasised the organisational difficulties involved in this information process. It requires considerable resources, and contacting and informing the patients is time consuming for physicians and nurses, especially as a long gap often elapses after trials before the results are available. Participants may therefore be rarely informed (Fernandez et al, 2003, 2004; Partridge et al, 2004).
Few studies have focused so far on participants’ preferred mode of dissemination of trial results, or on the best methods of diffusing this information while respecting patients’ autonomy. The authors of a recent review of the literature (Shalowitz and Miller, 2008) mentioned that participants often prefer to receive the results in writing, but specified that participants in a trial on the treatment of breast cancer preferred to be given the results by their physicians (Partridge et al, 2003). In France, investigators are legally obliged to inform participants about trial results, and the French drug authority (AFSSAPS) plans to publish these results on their website (https://icrepec.afssaps.fr/Public/apropos.php).
Making RCT results available on websites seems to be one of the best solutions: it is an inexpensive method, which is easier to organise than face-to-face consultations and gives patients the opportunity to decline, as it requires their active participation. The latter point is crucial, because some patients do not want to know about the results, and this preference should be respected (Shalowitz and Miller, 2008). However, not all cancer patients use the Internet (Mancini et al, 2006), and information obtained via this medium may be less understandable and more emotionally disturbing because patients are unable to ask questions (Partridge et al, 2009).
The aim of this study was to assess patients’ uptake and understanding of the results of the trial in which they have participated, and the impact of a letter offering patients the possibility of consulting the trial results on a specific website.
Materials and methods
This study, which was approved by the French National Committee on Personal Data and Privacy (CNIL), was carried out at 21 specialised centres all over France between June 2008 and May 2009. Adult women with HER2-positive non-metastatic breast cancer included in the FNCLCC-PACS04 clinical trial, the first results of which (about the efficacy of Trastuzumab) were issued in December 2007 (Spielmann et al, 2009), were included in the study. A patient's leaflet was then written by a medical journalist with the help of expert patients from the French Cancer League, and was made available only on a password-protected website (http://www.fnclcc.fr/doc/pdf/info_patients/prive/trait-union-no13.pdf (User=BECT; Password=PACS04)) (see Supplementary online material). At the time of the trial, participants were not told that they would be able to consult the results.
Among the 166 women contacted by mail by their oncologist, 115 (69.3%) agreed to participate and answered a two-page baseline questionnaire. These 115 women were subdivided into two groups by the ‘off-site’ coordinators (UMR912) using simple random sampling methods with a concealed allocation. The Internet group (n=55) received a letter stating that the trial results were available on the password-protected website, while the control group received no letters (n=60). At 6 months after consenting, all the women were mailed a second self-administered questionnaire; 51 (92.7%) and 56 (93.3%) women, respectively, in the Internet and control groups responded.
Medical characteristics were collected from the FNCLCC-PACS04 trial database. Sociodemographic data, ‘extent of information desired’ (Zwaenepoel et al, 2006), and perception of the usefulness of being informed about the trial results were ascertained in the baseline questionnaire.
In the second questionnaire, questions were asked (see Supplementary online material) about the participants’ expectations about the trial results, their preferences about the mode of disclosure, their declared uptake of the results and their understanding of the outcome of this trial, that is, the fact that Trastuzumab did not significantly decrease the risk of relapse 4 years after diagnosis in the FNCLCC-PACS04 trial. The question as to whether patients discussed the results with their next of kin was also addressed.
Statistical tests (SPSS 15.0) were performed between the continuous (Student's t-test) and categorical characteristics (χ2 or Fisher's exact test) of the two groups. P-values below 0.05 were taken to be statistically significant.
Results
The two randomised groups did not differ in terms of their sociodemographics, Internet access or Trastuzumab allocation. Both groups felt a priori that informing participants about the outcome of clinical trials would be useful (Table 1). Both groups stated alike (P=0.509) that access to clinical trial results should be systematic (45.7%), at either the patient's request (41.0%) or the physician's discretion (13.3%). Expectations about the results were also similar in both groups: knowing whether the trial yielded useful results (82.2% P=0.633), understanding the treatment received (20.6% P=0.468), and understanding clinical trials (9.3% P=0.745).
Table 1. Characteristics of the women studied.
|
Internet group (n=51)
|
Control group (n=56)
|
Total (n=107)
|
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P value | |
| Marital status | 0.530 | ||||||
| Married | 40 | 78.4 | 41 | 73.2 | 81 | 75.7 | |
| Education | 0.283 | ||||||
| ‘Baccalauréat’ or lower level | 33 | 67.3 | 32 | 57.1 | 65 | 61.9 | |
| Occupational activity | 0.663 | ||||||
| Yes | 24 | 47.1 | 24 | 42.9 | 48 | 44.9 | |
| Economic difficulties | 0.623 | ||||||
| Not at all | 24 | 47.1 | 28 | 51.9 | 52 | 49.5 | |
| Internet access | 0.244 | ||||||
| Yes | 34 | 66.7 | 43 | 76.8 | 77 | 72.0 | |
| Health-related Internet use | 0.400 | ||||||
| Yes | 25 | 49.0 | 32 | 57.1 | 57 | 53.3 | |
| Usefulness of clinical trial results disclosure to participants | 1.000 | ||||||
| Useful–very useful | 50 | 98.0 | 54 | 96.4 | 104 | 97.2 | |
| Trastuzumab arm in PACS04 trial | 0.585 | ||||||
| Yes | 30 | 58.8 | 30 | 53.6 | 60 | 56.1 | |
| M | SD | M | SD | M | SD | ||
| Age | 57.7 | 8.0 | 55.7 | 9.8 | 56.7 | 9.0 | 0.248 |
| Number of children | 1.9 | 1.0 | 2.0 | 1.1 | 2.0 | 1.0 | 0.568 |
| Distance from oncological centre (km) | 55.5 | 56.9 | 43.2 | 40.5 | 49.2 | 49.3 | 0.226 |
| Time since PACS04 trial inclusion (years) | 6.0 | 0.9 | 6.2 | 1.0 | 6.1 | 0.9 | 0.298 |
| ‘Extent of information desired’ score | 19.6 | 3.6 | 18.8 | 3.8 | 19.1 | 3.7 | 0.311 |
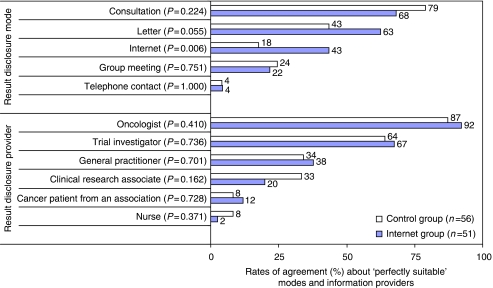
Similar preferences about how patients should be informed about clinical trial results and by whom were expressed by both groups, except that Internet was more frequently qualified as ‘perfectly suitable’ by the Internet group (43.5% vs 17.6% P=0.006; Figure 1). However, being informed via Internet was less frequently preferred than a face-to-face consultation or a mailed letter. Oncologists, followed by trial investigators, were the participants’ preferred providers of trial result information.
Figure 1.
Patients’ preferences about trial result disclosure.
The declared uptake of the clinical trial results was non-significantly higher in the Internet group (47.1%) than in the control group (33.9% P=0.166). Multiple sources of uptake were declared by 23.5% of the Internet group, as compared with 10.7% of the control group (P=0.077). These sources were a website (33.3% vs 8.9% in the Internet vs control group; P=0.002), the oncologist (29.4% vs 28.6% P=0.924), and other sources (11.8% vs 8.9% P=0.629). Among the 43 patients who declared having had access to the results, 22 (51.2%) had discussed these results with a close relative and 5 others (11.6%) planned to do so. Open commentaries showed that the results were often discussed with the spouse and other relatives, the oncologist, the general practitioner, and other patients. Talking about the results was said to be easier when they were perceived as positive. Negative results were also discussed, however, in order to obtain reassurance about their personal significance. One patient's next of kin were surprised by the randomisation procedure. Two patients reported a lack of communication because the cancer was ‘over’ and was no longer a topic of discussion in the family.
The trial results were better understood by the Internet group (18.8%) than by the control group (5.6% P=0.039). Among the patients who declared they had obtained the results via a website, 37.5% of the Internet group understood the results fairly well, which was not the case in the control group (0% P=0.262).
Discussion
This is the first randomised study in which participants’ uptake of the results of a RCT has been measured.
First, a fairly high proportion of women declared that they had consulted the results of the trial in comparison with what health professionals’ statements might suggest (Partridge et al, 2004). As Trastuzumab was already being used to treat metastatic breast cancer, a lot of information about this drug was circulating on Internet, and some confusion about the specific results of the FNCLCC-PACS04 trial may therefore have occurred. As uptake of results was self-declared, it was probably over-estimated by the patients, but presumably to the same extent in both groups. This would be consistent with some patients’ poor understanding of the results, which may have been due to confusion between information about the effectiveness of Trastuzumab in general and the specific results of the FNCLCC-PACS04 trial. It can be difficult for health practitioners and patients to find out exactly what they want to know about drugs on Internet when these drugs are already being used for other indications.
Second, it was previously reported that 90% of the participants in a phase II trial giving negative results chose to receive the results in response to a letter describing the early closure of the study and proposing to divulge the results (Partridge et al, 2005). The comparatively low uptake observed in the Internet group could not be entirely attributable to the lack of availability of Internet or the fact that little use of Internet was made for health-related purposes. Some patients declared spontaneously in their open comments that if they had known that the trial results had become available, they would have asked for an appointment with their oncologist. This would be consistent with our findings showing that the preferred mode of disclosure on the whole was consultation with the oncologist. However, the Internet method of disclosure was preferred more frequently by those who had actually used this method. Some performance bias may have occurred here, but these results also possibly suggest that these participants were favourably impressed by this method. This is consistent with studies showing that most patients were comfortable with receiving written results by impersonal means, especially if a two-step procedure is used for this purpose (Partridge et al, 2005, 2009; Fernandez et al, 2009). Although it was reported in a previous study (Fernandez et al, 2009) that websites are not patients’ favourite way of receiving the results, this method was acceptable to some patients. Therefore, this could be one of the modes of disclosure proposed to patients, as suggested by Partridge and Winer (2009), as it enables each patient to choose the most appropriate mode.
Third, to assess the effects of website information with greater power (80%), four times more patients would have to be included. Recruiting patients for this study was particularly difficult: we had to find an RCT of which the results had not been issued at the time of the study; the patients with HER2-positive receptors included here amounted to less than 20% of the total number of patients participating at 44 centres in the FNCLCC-PACS04 trial. In addition, about 20% of the participants could no longer be reached 6 years after the RCT because they were not in good health or were no longer alive. Despite this main limitation, the strengths of this study were the nation-wide recruitment and the fact that this is the first time, to our knowledge, that a standard situation has been compared with an experiment designed to enhance the uptake of results. In addition, the second point that was objectively assessed showed that the use of Internet significantly improved patients’ understanding of the trial results despite the low statistical power. In another study, 69% of the participants who were mailed the results interpreted them correctly (Partridge et al, 2009). The poor understanding observed here may have been due to the existence of discrepancies between the present results (showing the lack of effectiveness of Trastuzumab) and those previously presented in the literature and could have arisen from the need to specify more clearly the question addressed here (the effect of 18-month Herceptin treatment after chemotherapy on the 4-year risk of cancer progression). The poor understanding shown by some participants might also be attributable to their low levels of literacy and their lack of familiarity with medical language.
This is the first attempt to assess an Internet-based method of informing patients. The results obtained show that this method only slightly improved the patients’ understanding and non-significantly improved the declared rate of consultation of the RCT results. The usefulness of obtaining RCT results declared by the majority of the patients contrasted with the rather low rate of uptake declared by those in the Internet group. This question needs to be investigated more closely in larger samples, as well as using qualitative methods to determine the best way of meeting participants’ need for information about the results of trials.
Acknowledgments
This survey was funded by the French National Cancer Institute (INCa ‘SEHS et recherche clinique’ 2006 R080I IIAA). We are indebted to the members on the Patients’ Committee for Clinical Trials of the Ligue Nationale Contre le Cancer who participated actively in defining the aims of the present study. We thank the members on the Patients’ Committee of the Paoli-Calmettes Institute for reviewing the survey questionnaires. We also thank Dr Mayer (Centre Georges-François Leclerc, Dijon), Dr Coeffic (Institut privé de cancérologie, Grenoble), Dr Monnier (Centre André Boulloche, Montbélliard), Dr Dohollou (Polyclinique Bordeaux Nord), Dr Audhuy (Hopital Pasteur, Colmar), Dr Brain (René Huguenin, St Cloud), Dr Cany (Polyclinique Francheville, Périgeux), Dr Couderc (Clinique de l’Ormeau, Tarbes), Dr Mousseau (CHU Grenoble), Dr Eymard (Institut Jean Godinot, Reims), Dr Deniaud (Centre hospitalier de Bayonne), Dr Jaubert (Clinique Tivoli, Bordeaux) and Dr Houyau (Clin Claude Bernard, Albi), who participated in the inclusion of patients.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Fernandez CV, Gao J, Strahlendorf C, Moghrabi A, Pentz RD, Barfield RC, Baker JN, Santor D, Weijer C, Kodish E (2009) Providing research results to participants: attitudes and needs of adolescents and parents of children with cancer. J Clin Oncol 27: 878–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CV, Kodish E, Shurin S, Weijer C (2003) Offering to return results to research participants: attitudes and needs of principal investigators in the Children's Oncology Group. J Pediatr Hematol Oncol 25: 704–708 [DOI] [PubMed] [Google Scholar]
- Fernandez CV, Skedgel C, Weijer C (2004) Considerations and costs of disclosing study findings to research participants. CMAJ 170: 1417–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J, Nogues C, Adenis C, Berthet P, Bonadona V, Chompret A, Coupier I, Eisinger F, Fricker JP, Gauthier-Villars M, Lasset C, Lortholary A, N’Guyen TD, Vennin P, Sobol H, Lyonnet DS, Julian-Reynier C (2006) Patients’ characteristics and rate of Internet use to obtain cancer information. J Public Health (Oxf) 28: 235–237 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Burstein HJ, Gelman RS, Marcom PK, Winer EP (2003) Do patients participating in clinical trials want to know study results? J Natl Cancer Inst 95: 491–492 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Hackett N, Blood E, Gelman R, Joffe S, Bauer-Wu S, Knudsen K, Emmons K, Collyar D, Schilsky RL, Winer EP (2004) Oncology physician and nurse practices and attitudes regarding offering clinical trial results to study participants. J Natl Cancer Inst 96: 629–632 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Winer EP (2009) Sharing study results with trial participants: time for action. J Clin Oncol 27: 838–839 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP (2009) The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Res Treat 115: 123–129 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Wong JS, Knudsen K, Gelman R, Sampson E, Gadd M, Bishop KL, Harris JR, Winer EP (2005) Offering participants results of a clinical trial: sharing results of a negative study. Lancet 365: 963–964 [DOI] [PubMed] [Google Scholar]
- Shalowitz DI, Miller FG (2008) Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med 5: 714–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann M, Roche H, Delozier T, Canon JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels JP, Lortholary A, Orfeuvre H, Campone M, Hardy-Bessard AC, Coudert B, Maerevoet M, Piot G, Kramar A, Martin AL, Penault-Llorca F (2009) Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 36: 6129–6134 [DOI] [PubMed] [Google Scholar]
- Zwaenepoel L, Hoorens V, Peuskens J, Laekeman G (2006) The ‘extent of information desired’-scale in psychiatric in-patients: a behavioural approach. Patient Educ Couns 62: 72–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.