Abstract
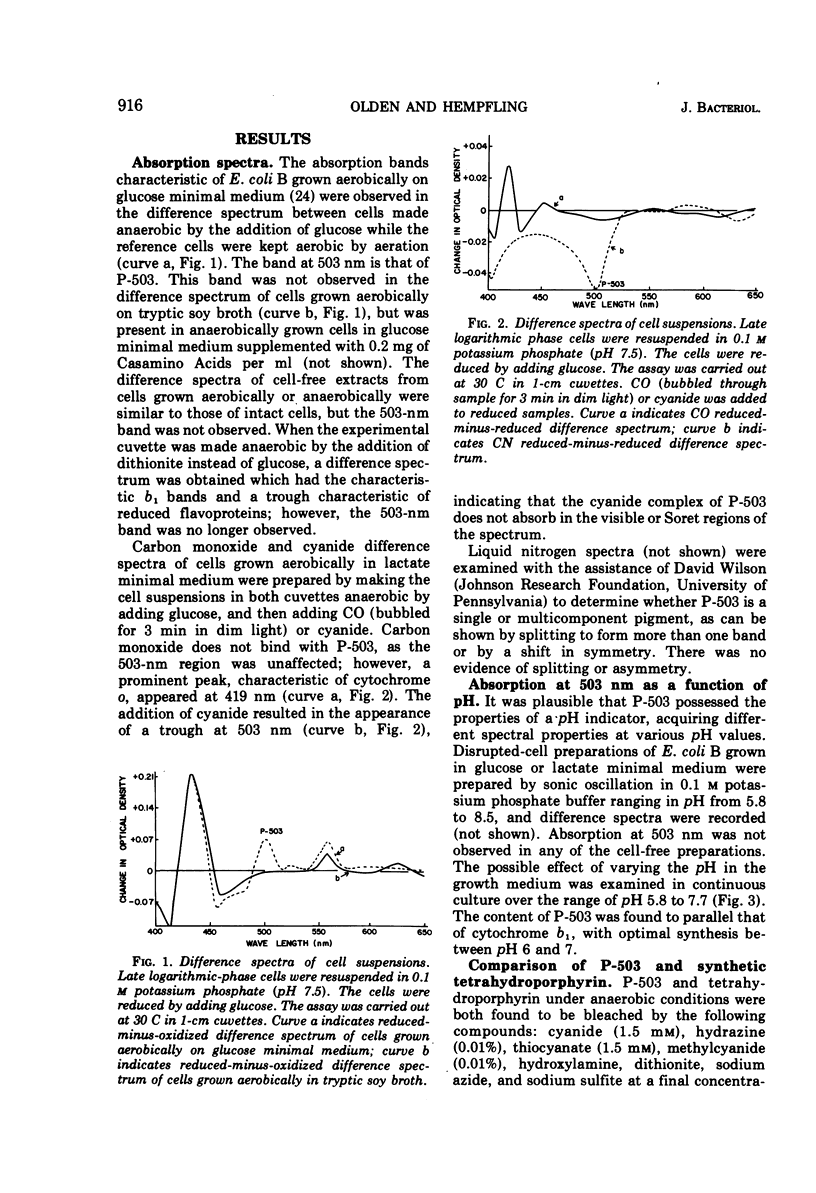
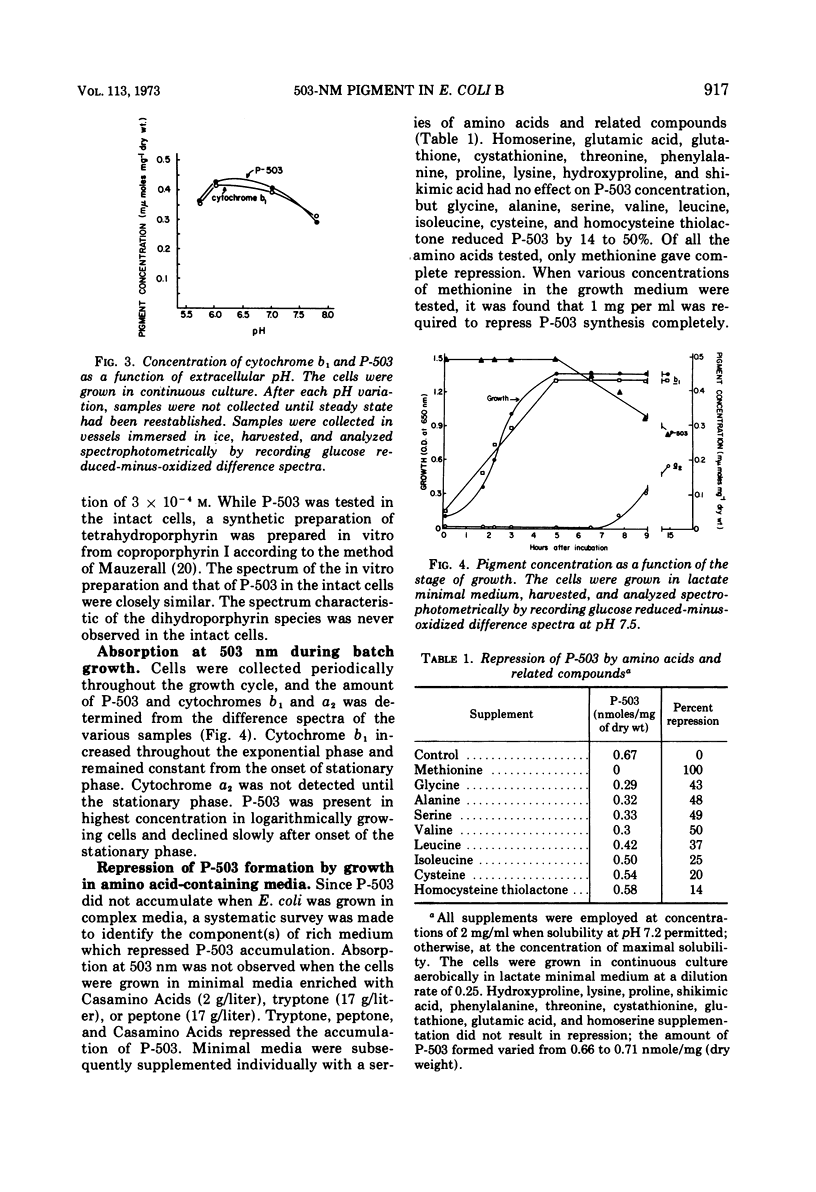
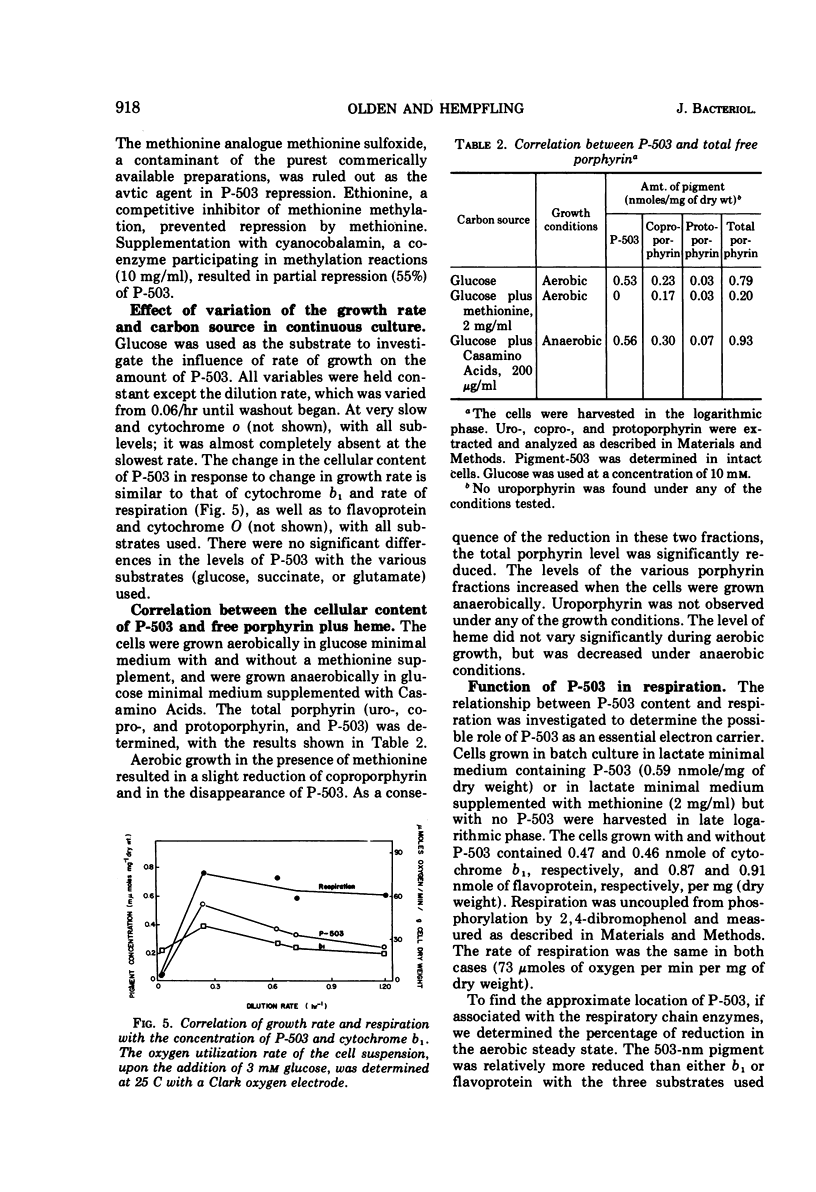
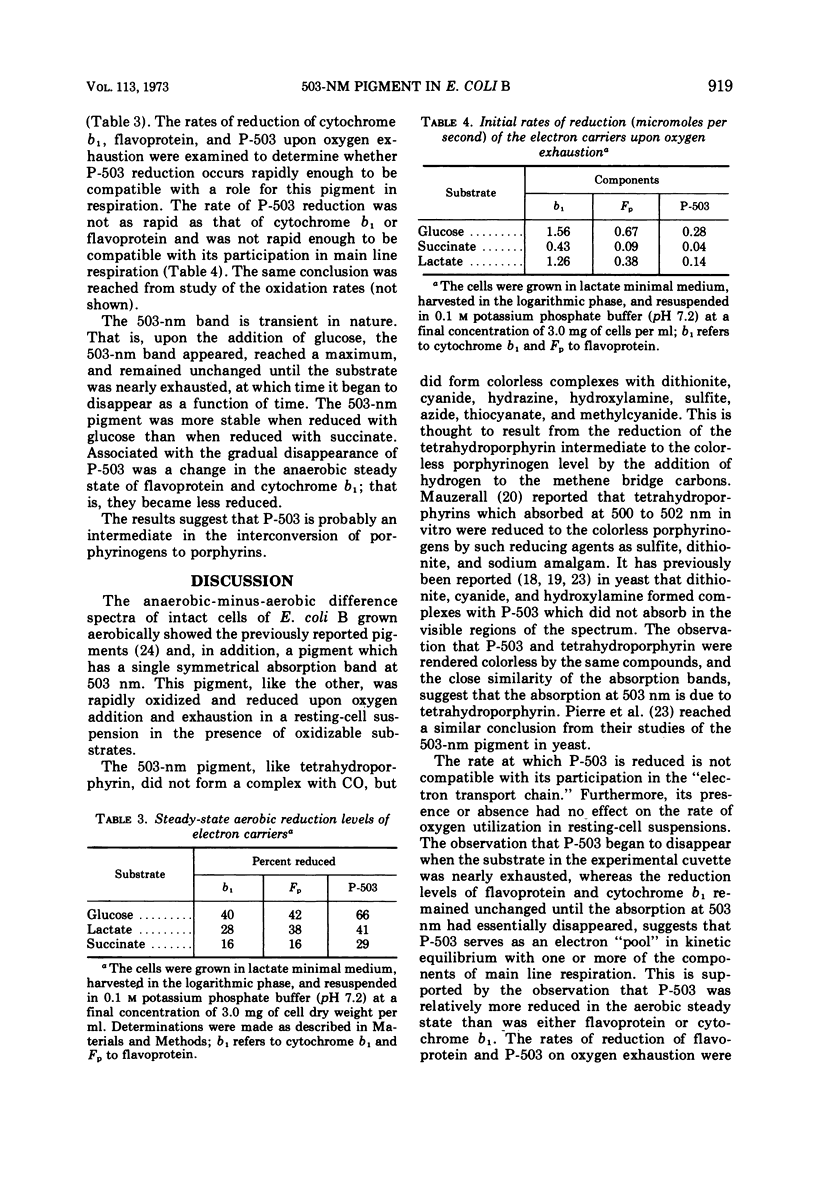
Escherichia coli B was shown to contain a pigment with a single symmetrical absorption band at 503 nm, which cannot be attributed to a cytochrome. The absorption of tetrahydroporphyrin in vitro closely resembled that of P-503 in intact cells. The compounds which rendered P-503 colorless, such as cyanide, azide, hydrazine, thiocyanate, hydroxylamine, dithionite, sulfite, and methylcyanide, also rendered tetrahydroporphyrin colorless. The pigment was present when the cells were grown aerobically or anaerobically in glucose minimal medium, or aerobically in either lactate or succinate minimal medium, but the pigment was not found in cells grown in complex media or in minimal media supplemented with methionine. A model is presented to suggest the involvement of methionine in the conversion of coproporphyrinogen to protoporphyrin. A variety of evidence suggesting that the 503-nm chromophore is in kinetic equilibrium with flavoprotein is discussed. However, it is not a participant in main line respiration, as its rate of reduction upon exhaustion of oxygen was too slow, and the rate of respiration in resting-cell suspensions was independent of P-503 concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRETT J. The prosthetic group of cytochrome a2. Biochem J. 1956 Dec;64(4):626–639. doi: 10.1042/bj0640626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER R. THE BIOSYNTHESIS OF COPROPORPHYRINOGEN, MAGNESIUM PROTOPORPHYRIN MONOMETHYL ESTER AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS CAPSULATA. Biochem J. 1963 Oct;89:100–108. doi: 10.1042/bj0890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEEB S. S., HAGER L. P. CRYSTALLINE CYTOCHROME B1 FROM ESCHERICHIA COLI. J Biol Chem. 1964 Apr;239:1024–1031. [PubMed] [Google Scholar]
- Demain A. L., White R. F. Porphyrin overproduction by Pseudomonas denitrificans: essentiality of betaine and stimulation by ethionine. J Bacteriol. 1971 Aug;107(2):456–460. doi: 10.1128/jb.107.2.456-460.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELKIND M. M., SUTTON H. Lethal effect of visible light on a mutant strain of haploid yeast. II. Absorption and action spectra. Arch Biochem Biophys. 1957 Nov;72(1):96–111. doi: 10.1016/0003-9861(57)90177-7. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 1. The effect of growth conditions. Biochem J. 1962 Jun;83:539–549. doi: 10.1042/bj0830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 2. The effects of ethionine and threonine. Biochem J. 1962 Jun;83:550–559. doi: 10.1042/bj0830550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUCHER C. R., KOCHOLATY W. A comparison of the cytochrome structure and radiation effects in Azotobacter. Arch Biochem Biophys. 1957 May;68(1):30–38. doi: 10.1016/0003-9861(57)90323-5. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Formation of protoporphyrin from coproporphyrinogen in extracts of various bacteria. J Bacteriol. 1970 May;102(2):398–403. doi: 10.1128/jb.102.2.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitakahara J. R., Polglase W. J. The 503nm pigment of Escherichia coli. Biochem J. 1970 Dec;120(4):771–775. doi: 10.1042/bj1200771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENMAYER A. Oxidative metabolism and absorption spectra of anaerobically grown yeast; manometric data and absolute absorption spectra. J Cell Comp Physiol. 1959 Feb;53:93–117. doi: 10.1002/jcp.1030530110. [DOI] [PubMed] [Google Scholar]
- LINDENMAYER A., SMITH L. CYTOCHROMES AND OTHER PIGMENTS OF BAKER'S YEAST GROWN AEROBICALLY AND ANAEROBICALLY. Biochim Biophys Acta. 1964 Dec 9;93:445–461. doi: 10.1016/0304-4165(64)90329-0. [DOI] [PubMed] [Google Scholar]
- Labbe P., Volland C., Chaix P. A propos de la nature et du rôle d'un pigment absorbant à 503 millimicrons chez certaines levures. Biochim Biophys Acta. 1967 Jul 5;143(1):70–78. doi: 10.1016/0005-2728(67)90110-7. [DOI] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- NOSOH Y. ABSORPTION SPECTRUM OF ACTIVELY RESPIRING YEAST CELLS. Arch Biochem Biophys. 1964 May;105:439–445. doi: 10.1016/0003-9861(64)90028-1. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activity in extracts from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Sep 24;37(1):116–122. doi: 10.1016/0006-291x(69)90888-2. [DOI] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



