Abstract
Background
There are many unresolved issues in the diagnosis and treatment of persons with traumatic brain injury (TBI) in its post-acute and chronic phases. This article deals with two problems of clinical importance: (i) the interrelationships between structural brain damage, brain function, and clinical outcome, and (ii) post-traumatic epilepsy.
Methods
Exploratory, retrospective analysis of clinical, neuroradiological (MRI), and neuropsychological data of all patients with TBI who were treated in a cognitive neurology outpatient clinic of a German university hospital over a period of 12 years (n=320).
Results
156 patients (48.8%) had brain contusions, 83 of them (25.9%) as the sole neuroradiological abnormality. Traumatic micro-hemorrhages were seen in 148 patients (46.2%) and were the sole neuroradiological abnormality in 79 of them (24.7%). 49 patients (15.3%) had no structural brain lesion. There was no obvious correlation between the neuroradiological findings and the clinical outcome, as measured either by a general outcome parameter such as the extended Glasgow Outcome Scale (GOSE) or by neuropsychological testing. 47 patients (14.7%) had post-traumatic epilepsy; its occurrence was positively correlated with the presence of brain contusions, but not with an isolated diagnosis of diffuse axonal injury (DAI).
Conclusion
A comparison of the findings of neuroradiological studies and neuropsychological tests among patients in the chronic phase of traumatic brain injury does not reveal any simple relationship between structural and functional brain abnormalities. Diffuse axonal injury is often present in combination with other findings, and it may well be the only structural abnormality in many cases; therefore, all symptomatic patients should undergo MRI of the brain. Patients with isolated DAI seem to be less prone to post-traumatic epilepsy than those with brain contusions.
Traumatic brain injury (TBI) is one of the commonest disorders within neuropsychiatry in its widest sense. The incidence of TBI in Germany is approximately 332 per 100 000, in comparison to 182 per 100 000 for strokes (1, 2). Its annual direct and indirect costs amount to roughly 2.5 billion euros (2). It is generally accepted that its overall burden for health economics is equal to the total costs of other well-known neurological diseases such as Parkinson’s disease, multiple sclerosis, Guillain-Barré syndrome, amyotrophic lateral sclerosis, and myasthenia combined (3). However, despite an overabundance of literature—the PubMed database has more than 50 000 hits for the search term “traumatic brain injury”—the complex area of TBI is actually somewhat overlooked in both medical training and subsequent general medical and neuropsychiatric practice.
There are many questions regarding the diagnosis, prognosis and best possible treatment of traumatic brain injury in its post-acute and chronic phases that cannot yet be satisfactorily answered. This article focuses mainly on the clinically significant aspects of potential interrelationships between structural brain damage, brain function, and clinical outcome, and on the frequency and conditions for onset of post-traumatic epilepsy. It is based on an exploratory, retrospective analysis of the clinical data of all TBI patients of the Cognitive Neurology Outpatient Clinic at the University of Leipzig, Germany from 1996 to 2007. The overwhelming majority (>85%) of the neuroradiological information provided is derived from MRI data. Until now this type of evaluation, based on MRI data alone, has not been standard for this patient population in either clinical practice or larger clinical studies. It therefore deserves particular mention.
The descriptive findings are discussed in the context of searches of the literature. The research makes a contribution to the diagnosis and interpretation of morphological/structural and functional consequences of traumatic brain lesions. Ideally, this should also stimulate a process at the end of which this potentially chronic and serious brain damage is given the professional attention which it deserves on the strength of epidemiological data.
Methods
Database and data collection
Between January 1, 1996 and December 31, 2007 (the last day covered by this evaluation), 320 patients (18%) with a primary diagnosis of TBI (the inclusion criterion) were treated in the Cognitive Neurology Outpatient Clinic at the University of Leipzig. For this retrospective analysis, the following data on these patients were taken from electronic and original medical records:
Sex
Age on the dates of the injury and magnetic resonance imaging (MRI)
Closed or open TBI
Glasgow Coma Scale (GCS) score (according to ER records or, if these do not exist or are not available, calculated retrospectively for the patient’s condition at the site of the accident when attended by a professional paramedic) (e1, e2)
Previous illnesses
Medications taken at the time of the injury
Cause of TBI
Presence of polytrauma, skull fracture, generalized brain edema, or hypoxic encephalopathy.
The following injury patterns were recorded:
Subarachnoid hemorrhages (SAHs)
Subdural hemorrhages (SDHs)
Epidural hemorrhages (EDHs)
Hygromata
Contusions and their locations
Traumatic microbleeds (TMBs) and their locations as a marker of diffuse axonal injury (DAI)
Diffuse vascular injury (DVI) or traumatic intracerebral hematomas (9)
Midbrain and/or brainstem injuries
Injuries to blood vessels that supply the brain.
Patient subgroups had already been included in several individual studies, particularly regarding potential structural and functional correlates of DAI (4 8).
The following were recorded as the functional and clinical outcome parameters at the time of patients’ semi-inpatient hospital stays (median 12 months post-trauma):
Results of standardized neuropsychological testing
Extended Glasgow Outcome Scale (GOSE) score (e3)
Post-traumatic epilepsy
Psychiatric morbidity (according to ICD-10 criteria wherever possible)
Ongoing prescription of psychoactive drugs.
MRI
274 patients (86%) underwent at least one brain MRI scan after having the procedure explained to them and after consenting to it. There were contraindications for MRI in 46 patients. The scans were performed using two 3-Tesla whole-body scanners (for information on machine specifications and scan protocols, see [4, 5]).
Neuropsychological examinations
All patients underwent neuropsychological examination of attention and psychomotor speed (e4), executive functions (e5, e6), learning/memory (e7, e8), and intelligence (e9). Where there were results for more than one point in time, those from the patients’ first stay at the clinic were used for this evaluation.
Statistical calculations
The data gathered were statistically tested for any significant correlations between clinical variables (Fisher’s exact test, Mann-Whitney U test, Spearman’s rank correlation). Unless explicitly stated otherwise, the enquiry in each case was exploratory, and significance levels are therefore not given. One exception to this is the hypothesis-led enquiry into statistically significant correlations between structural findings and the results of neuropsychological tests (Mann-Whitney U test, Spearman’s rank correlation). Specifically, the patients’ results in the following neuropsychological tests were used as parameters:
TAP (Test for Attentional Performance) test battery (e4)
Behavioral Assessment of the Dysexecutive Syndrome (BADS) (e5)
Stroop test (e6)
California Verbal Learning Test (CVLT) (e7)
Wechsler Memory Scale—Revised (WMS-R) (e8)
Multiple-Choice Vocabulary Intelligence Test (MWT A/B) (e9).
Altogether, confirmation of 22 hypotheses was sought (multiple significance level = 0.05; local significance level following correction for multiple testing [Bonferroni correction] = 0.0023). All statistical calculations were carried out using SPSS software, version 15.
Results
Clinical histories
Table 1 provides an overview of patient demographics. Road traffic accidents (RTAs) were the leading cause of TBI, accounting for 69% of cases (221 patients). Next came falls, with 25.6% (82 patients); and blows to the head, with 5% (16 patients). In one patient (0.3%), brain injury was caused by a blow and a fall combined. Within the RTA group, car accidents (43.4%, 139 patients) were the leading cause, followed by bicycle/motorcycle accidents (20.6%, 66 patients) and pedestrians involved in RTAs (5%, 16 patients).
Table 1. Patients’ demographic data.
| Number/Range | Mean/Median | Percentage | |
| n | 320 | ||
| Sex: | |||
| M | 242 | 76 | |
| F | 78 | 24 | |
| Age at time of injury (years) | 3 to 72 | 32, 3/30 | |
| Age at time of head MRI (years) | 15 to 77 | 34, 2/31, 5 | |
| Latency of TBI until MRI (months) | 1 to 360 | 27, 6/12 | |
| GCS score | 244/3 to 15 | 6, 4/3 | |
| GOSE score | 313/4 to 8 | 6/6 |
n: number; M: male; F: femaleMRI: magnetic resonance imaging; TBI: traumatic brain injury; GCS: Glasgow Coma Scale; GOSE: extended Glasgow Outcome Scale
Structural findings
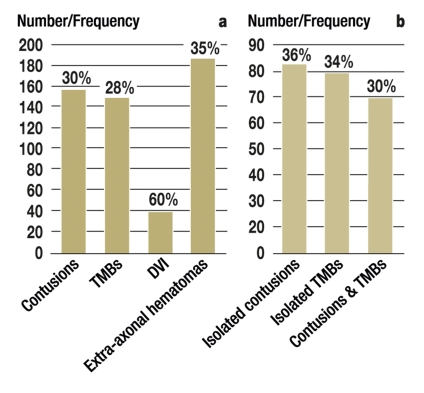
156 patients (48.8%) had brain contusions, 83 of them (25.9%) as the sole neuroradiological abnormality, i.e. there were no other traumatic parenchymatous changes (Figure and Table 2). Traumatic microbleeds were seen in 148 patients (46.2%) and were the sole neuroradiological abnormality in 79 of them (24.7%) (Figure and Table 2). Significant generalized brain atrophy according to visual criteria was observed in 13 patients (4%). As no quantitative analyses were carried out regarding this, no statistical calculations were performed. However, 12 of these patients showed clear signs of traumatic microbleeds. 27 patients (8.4%) had isolated extra-axial injuries. In 49 patients (15.3%), no structural consequences of trauma were detectable on imaging at any time. Thus, examination of patients with isolated extra-axial injury included, imaging revealed no chronic morphological parenchymatous traumatic signal changes in a total of 76 patients (23.8%).
Figure.
Frequencies and relative distribution of different types of primary traumatic brain damage
Absolute and relative frequencies of different categories of primary focal traumatic brain damage in the patient group with radiologically detectable trauma sequelae
Absolute and relative frequencies for the presence of contusions and traumatic microbleeds in isolation or combination (DVI: diffuse vascular injury; TMBs: traumatic microbleeds)
Table 2. List of the most common locations of contusions and traumatic microbleeds*.
| Location | Contusions | Location | Traumatic micro-‧hemorrhages | ||
| n | % (rel.) | n | % (rel.) | ||
| Frontal | 117 | 36.6 (75) 2.2 | Frontal | 124 | 38.8 (83.8) |
| – Isolated polar | 7 | 5.3 | – Isolated | 38 | 11.9 (25.7) |
| – Isolated basal | 17 | – Isolated F1 | 29 | 9.1 (19.6) | |
| Temporal | 91 12 | 28.4 (58) 3.8 | Corpus callosum | 52 | 16.2 (35.1) |
| – Isolated polar | – Corpus | 20 | 6.2 (38.5) | ||
| - Splenium | 11 | 3.4 (21.1) | |||
| – Genu | 4 | 1.2 (7.6) | |||
| – Corpus + splenium | 11 | 3.4 (21.1) | |||
| Frontal + temporal | 28 | 8.8 | Frontal + corpus callosum | 11 | 3.4 (7.4) |
| Parietal | 12 | 3.8 (7.7) | |||
| Occipital | 9 | 2.8 (5.8) | |||
| Cerebellar | 3 | 0.9 (1.9) | |||
| Multiple | |||||
| – Frontal pole/front base | 10 | 3.1 | |||
| – Frontal pole/temporal pole | 7 | 2.2 | |||
| – Frontal pole/front base/temporal pole | 7 | 2.2 | |||
| – Front base/temporal pole | 14 | 4.4 | |||
*Absolute and relative, in relation to each specific injury type and location; F1: Gyrus frontalis superiorN.B.: Pathologies of the same kind in multiple locations were counted each time; for the locations of traumatic microbleeds, see also (4) rel.: relative; n: number
Clinical condition
On the GOSE, 18 patients (5.6%) achieved a score of 4, 74 patients (23.1%) a score of 5, 124 patients (38.8%) a score of 6, 88 patients (27.5%) a score of 7, and 9 patients (2.8%) the highest score, 8; the median of the sample was 6. For 7 patients (2.2%), there were not enough data for sufficiently accurate calculation. There was a weak correlation between severity of initial TBI according to the GCS and outcome according to the GOSE (Spearman’s rank correlation [rs] = 0.335, p<0.001).
There were also statistical correlations both between GCS/GOSE and evidence of a structural brain lesion in general (p<0.001/ p = 0.006, Mann-Whitney U test), and between GCS/GOSE and evidence of substantial cranial trauma (patients with extra-axial injury patterns but no parenchymatous lesions) (p<0.001/p = 0.007, Mann-Whitney U test). There was also evidence of statistical correlations between GCS/GOSE and history of brain edema during the acute phase (p<0.001/p = 0.008, Mann-Whitney U test). Finally, there were correlations between the GCS and primary presence of contusions/traumatic microbleeds (p = 0.004/p = 0.005, Mann-Whitney U test) but not between evidence of contusions/traumatic microbleeds as the primary or sole neuroradiological abnormality and GOSE scores (contusions: p = 0.051 and p = 0.228; traumatic microbleeds: p = 0.244 and p = 0.467, Mann-Whitney U test).
Correlations between imaging, clinical, and neuropsychological findings
Contrary to the hypothetical assumption, there was no correlation between GCS/GOSE and neuropsychological test results (Spearman’s rank correlation, multiple significance level p = 0.05, local significance level p = 0.0023), nor was any statistically significant correlation found between the latter and the following parameters (Mann-Whitney U test, p>0.0023 in each case):
Traumatic microbleeds (general or isolated)
Contusions (general or isolated)
Other traumatic parenchymatous brain lesions
Corpus callosum, midbrain, or brainstem lesions
Generalized brain edema during the acute phase.
Post-traumatic epilepsy
47 patients (14.7%) suffered post-traumatic epilepsy. Onset was not correlated with TBI severity according to the GCS (p = 0.739, Mann-Whitney U test), but it was negatively correlated with outcome according to the GOSE (p = 0.048, Mann-Whitney U test). Post-traumatic epilepsy occurred with detectable contusions in 20% of patients. In contrast, only 10% of patients with traumatic microbleeds were affected. There was a statistical correlation between post-traumatic epilepsy and isolated contusions (p<0.001, Fisher’s exact test), but not between the former and isolated traumatic microbleeds (p = 0.713, Fisher’s exact test).
Discussion
Types and causes of traumatic brain lesions
The findings on the type, frequency, pattern, and mechanism of traumatic brain damage partly confirm the results stated in the literature (2, 9– 11). This is particularly true, for example, for the distribution of contusional injuries, which tend to affect the structures of the frontal pole/front base and temporal pole (9, 10). Discrepancies from the conclusions of current large epidemiological studies (2), such as those on the cause and severity of TBI, may be dependent on various different factors. Worthy of particular mention are the retrospective nature of data collection and the fact that all the information on the patient population was obtained from a single clinic which focuses mainly on cognitive rehabilitation. This latter fact undoubtedly results in selection bias. Despite this limitation, however, the evaluation does yield well-founded information on the range of structural and functional findings from a sample of patients suffering from chronic problems as a result of TBI. It is therefore suitable for use as a source of data, including for comparative investigations of other establishments.
The results of the research show that traumatic microbleeds are present in around half of cases, either alone or with contusions. If we consider these changes to be neuroradiological markers of DAI (4), this means that “pure DAI” is present in around half of cases. This has direct consequences for imaging diagnostics: all symptomatic TBI patients should undergo MRI examination (figure). Particularly suitable sequences in this context are currently T2*-weighted gradient-echo imaging (T2*GRE), susceptibility-weighted imaging (SWI), and diffusion tensor imaging (DTI) (4, 14, 15).
Lack of evidence of traumatic brain damage
Patients in whom no structural brain damage is evident and patients with no definite evidence of substantial TBI (i.e. also with no isolated extra-axial injury) combined represent the category “minor head injury” (12, e10, e11). There were significant correlations to both the GCS and the GOSE for both groups. The relatively high proportion, almost a quarter (23.8%), of patients who fell into these two categories is emphatic evidence of the significance of this problem. It also makes it clear that some patients suffer from chronic health disorders (general, neurological, cognitive, or psychological) comprising “post-concussion syndrome” even after mild TBI (12, 13, e10). After all, these sequelae were sufficiently pronounced for an outpatient clinic to be attended and/or for the attention of primary care physicians, neurologists/neuropsychiatrists, or inpatient rehabilitation establishments to be considered necessary. The causes and conditions for onset of such disorders are hotly disputed (e10, e12). Conventional neuroradiological procedures have not proved helpful to date (14, e13, e14). Further efforts regarding objective evidence of potential underlying neuronal damage (15) are therefore necessary and worthwhile, with respect to both diagnostic and therapeutic issues and medical law.
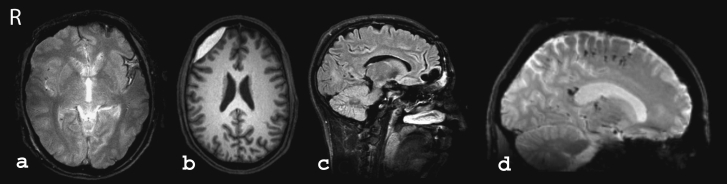
Examples of classical magnetic resonance imaging (MRI) findings following traumatic brain injury
Residue of traumatic subarachnoid hemorrhage in the left insula in T2*-weighted GRE imaging. The hypointense image of the cortical gyri, resulting from a buildup of blood or blood waste products on the brain surface, is clearly visible.
Right frontolateral extra-axial hematoma in the subacute phase in T1-weighted MRI. The hematoma appears significantly hyperintense. It rests on the brain surface and compresses it slightly. Note the exceptionally biconvex shape of the subdural hematoma shown here.
Frontal pole contusion in the chronic phase (FLAIR weighting). The lesion is filled with fluid and appears hypointense. The typical (in this case double-pointed) hollow or dish shape is clearly visible.
Multiple small traumatic hematomas at the corticomedullary boundary of the frontal lobe and in the corpus callosum (splenium) in T2*-weighted GRE MRI, sagittal view. This finding points very much towards a diagnosis of DAI/TAI.
T2*-GRE: T2*-weighted gradient-echo imaging; DAI/TAI: diffuse axonal injury/traumatic axonal injury (from: Scheid R. Bildgebende Diagnostik bei leichtgradigen Hirntraumen im Verlauf. Der medizinische Sachverständige. 2009; 105:216–21. With the kind permission of Gentner publishing house, Stuttgart, Germany
Neuropsychological data and magnetic resonance imaging
No conclusive relationship to structural injury patterns has been found using either the GOSE or neuropsychological tests. This comes in addition to equivalent results of a separate study involving patients with isolated traumatic microbleeds (5). It is true that varying profiles of patients with focal and diffuse damage patterns were described by Wallesch et al. according to the relevant clinical scales (Neurobehavioral Rating Scale, Frontal Lobe Score) (16, e15) and that these point to varying disorders, mainly in the frontal-subcortical neuronal circuits in the context of contusional and DAI-compatible injuries as functional neuroanatomical causes. However, in general, focal and diffuse injuries combined probably contribute to general and neuropsychological outcomes, and in this case neuropsychological tests are of only limited use in distinguishing between these different injury patterns (17).
Many studies of TBI involving neuropsychological data yield inconsistent findings on structural or functional correlations (5, 16– 19, e14, e16). Regarding etiology, it must be borne in mind that TBI is heterogeneous and consists of different dynamic processes at different times of assessment, and that these processes are also affected by often multiple neuropsychiatric (co-)morbidities (20). There are also multiple suspected adaptive and neuroplastic processes about whose conditions, progress, and efficacy current knowledge is still rudimentary (e17). Interestingly, our results are also in line with the evaluation of a current long-term psychiatric observation. This long-term observation concludes that most post-traumatic psychiatric disorders on Axes I and II according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) have only a very limited relationship to outcome and the specific location of cortical contusions (Axis I: Major clinical disorders including developmental and learning disabilities; Axis II: personality disorders, mental retardation) (21).
Post-traumatic epilepsy
TBI is a major cause of epileptic fits (22). Approximately 15% of patients suffered from post-traumatic epilepsy. This figure is higher than generally assumed (4% to 7%) (e18, e19) but roughly corresponds to the observed incidence of approximately 17% in TBI patients with non-penetrating injuries in rehabilitation establishments (e20).
Contusions have been established as a risk factor (e21, e22). A corresponding correlation was also found in our patients. Worthy of note is the low prevalence of post-traumatic seizures in patients with isolated traumatic microbleeds. It could be deduced from this that patients with “pure DAI” are at lower risk. There are no systematic findings on this in the literature. This is not a trivial observation, as intra-cerebral hemorrhages are generally associated with the opposite: a higher risk of seizures (23, e20). A possible explanation for this may be the extracortical location of the majority of the associated changes. However, in most cases of epilepsy with “subclinical cerebrovascular disorders,” for example, the cerebellar white matter is also primarily affected (24, 25, e23), and such disorders are also associated with the onset of cerebral micro-hemorrhages (e24). Regardless of the possible pathogenesis, the finding should nevertheless be verified by further research, as it may be prognostically relevant.
The literature provides no completely consistent information on the relationship between post-traumatic seizures and clinical outcome in the literature (e20). The evidence found here of an inverse correlation with the GOSE supports the assumption that post-traumatic epilepsy, unlike post-traumatic early seizures, has a negative effect on general functional outcome as a result of the other health-related, psychological, and potentially work-related consequences associated with it.
Key Messages.
In most cases, no simplistic structural–functional relationships can be identified in the chronic phase following traumatic brain injury (TBI).
Due to the relative frequency of isolated traumatic microbleeds, in one quarter of the patients examined, all symptomatic TBI patients should be examined using suitable MRI sequences.
Similar to contusions, traumatic microbleeds occur mainly in specific locations. However, the lack of a correlation with neuropsychological data supports the assumption that only the tip of the iceberg of the underlying pathologies associated with diffuse axonal injury (DAI) can be portrayed using currently available imaging methods.
Up to 15% of patients suffer post-traumatic epilepsy with probable long-term negative effects on clinical or functional outcome. Patients with isolated DAI seem to be less prone to post-traumatic epilepsy than those with brain contusions.
The possibility of a neuronal basis for potentially chronic health problems following TBI with no detectable structural brain damage requires further intensive research.
Acknowledgments
The authors would like to thank the patients and in particular all the staff at the Cognitive Neurology Outpatient Clinic at the University of Leipzig, Germany, without whose help and ongoing dedication this article could not have been written.
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Gesundheitsbericht für Deutschland 2006. www.gbe-bund.de.
- 2.Rickels E, von Wild K, Wenzlaff P, Bock WJ. Epidemiologie und Versorgung - Ergebnisse einer prospektiven Studie. München: W. Zuckschwerdt Verlag; 2006. Schädel-Hirn-Verletzung. [Google Scholar]
- 3.Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review—traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Sci World J. 2007;12:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid R, Walther K, Guthke T, Preul C, von Cramon DY. Cognitive sequelae of diffuse axonal injury. Arch Neurol. 2006;63:418–424. doi: 10.1001/archneur.63.3.418. [DOI] [PubMed] [Google Scholar]
- 6.Scheid R, Zimmer C, Schroeter ML, Ballaschke O, von Cramon DY. The clinical spectrum of blunt cerebrovascular injury. Neurologist. 2006;12:255–262. doi: 10.1097/01.nrl.0000243977.17242.ab. [DOI] [PubMed] [Google Scholar]
- 7.Scheid R, Ott DV, Roth H, Schroeter ML, von Cramon DY. Comparative MR-imaging at 1. 5 T and 3 T for the evaluation of traumatic microbleeds. J Neurotrauma. 2007;24:1811–1816. doi: 10.1089/neu.2007.0382. [DOI] [PubMed] [Google Scholar]
- 8.Schroeter ML, Ettrich B, Schwier BS, Scheid R, Guthke T, von Cramon DY. Diffuse axonal injury due to traumatic brain injury alters inhibition of imitative response tendencies. Neuropsychologia. 2007;45:3149–3156. doi: 10.1016/j.neuropsychologia.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Graham DI, Gennarelli TA, McIntosh TA. Trauma. Greenfield’s neuropathology. In: Graham DI, Lantos PI, editors. 7th ed. London, New York: Arnold; 2002. pp. 823–898. [Google Scholar]
- 10.Bigler ED. Neuroimaging correlates of functional outcome. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: principles and practice. New York: Demos Medical Publishing; 2007. pp. 201–224. [Google Scholar]
- 11.Brown AW, Elovic EP, Kothari S, Flanagan SR, Kwasnica C. Congenital and acquired brain injury. 1. Epidemiology, pathophysiology, prognostication, innovative treatments, and prevention. Arch Phys Med Rehabil. 2008;89(Suppl 1):3–8. doi: 10.1016/j.apmr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Ropper AH, Gorson KC. Concussion. N Engl J Med. 2007;356:166–172. doi: 10.1056/NEJMcp064645. [DOI] [PubMed] [Google Scholar]
- 13.Deb S, Lyons I, Koutzoukis C. Neurosychiatric sequelae one year after minor head injury. J Neurol Neurosurg Psychiatry. 1998;65:899–902. doi: 10.1136/jnnp.65.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metting Z, Rödiger LA, De Keyser J, van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet Neurol. 2007;6:699–710. doi: 10.1016/S1474-4422(07)70191-6. [DOI] [PubMed] [Google Scholar]
- 15.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallesch CW, Curio N, Kutz S, Jost S, Bartels C, Synowitz H. Out-come after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Injury. 2001;15:401–412. doi: 10.1080/02699050010005959. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JT, Hadley DM, Wiedmann KD, Teasdale GM. Neuropsychological consequences of two patterns of brain damage shown by MRI in survivors of severe head injury. J Neurol Neurosurg Psychiatry. 1995;59:328–331. doi: 10.1136/jnnp.59.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felmingham KL, Baguley IJ, Green AM. Effects of diffuse axonal injury on speed of information processing following severe traumatic brain injury. Neuropsychol. 2004;18:564–571. doi: 10.1037/0894-4105.18.3.564. [DOI] [PubMed] [Google Scholar]
- 19.Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch CW. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Injury. 2005;19:101–108. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- 20.McAllister TW. Neuropsychiatric aspects of TBI. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: principles and practice. New York: Demos Medical Publishing; 2007. pp. 835–861. [Google Scholar]
- 21.Koponen S, Taiminen T, Kurki T, et al. MRI findings and Axis I and II psychiatric disorders after traumatic brain injury: a 30-year retrospective follow-up study. Psychiatry Res. 2006;146:263–270. doi: 10.1016/j.pscychresns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 23.Temkin NR. Risk factors for post-traumatic seizures. Epilepsia. 2003;44(Suppl 10):18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- 24.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 25.Werhan KJ. Epilepsy in the Elderly [Altersepilepsie] Dtsch Arztebl Int. 2009;106(9):135–142. doi: 10.3238/arztebl.2009.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Teasdale G, Jennett B. Assessment of coma and impaired con-sciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- e2.Katz DI, Alexander MP. Traumatic brain injury, Predicting course of recovery and outcome for patients admitted to rehabilitation. Arch Neurol. 1994;51:661–670. doi: 10.1001/archneur.1994.00540190041013. [DOI] [PubMed] [Google Scholar]
- e3.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- e4.Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP). [Test battery for the assessment of attention] Psychologische Testsysteme. Würselen. 1993 [Google Scholar]
- e5.Wilson B, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioural assessment of the dysexecutive syndrome. Thames Valley Test Company. Bury St. Edmunds. 1996 [Google Scholar]
- e6.Wolfram H, Neumann J, Wieczorek V. VEB Georg Thieme. Leipzig: 1986. Psychologische Leistungstests in der Neurologie und Psychiatrie. [Psychological performance test in neurology and psychiatry] [PubMed] [Google Scholar]
- e7.Delis DC, Kramer JH, Kaplan E, Obler BA. The Psychological Corporation. San Antonio;: 1987. The California verbal learning test: Adult version. [Google Scholar]
- e8.Härting C, Markowitsch HJ, Neufeld U, Calabrese P, Deisinger K, Kessler J. [German version of the revised version of the Wechsler memory scale] Bern: Verlag Hans Huber; 2000. Wechsler Gedächtnis Test - Revidierte Fassung (WMS-R) [Google Scholar]
- e9.Lehrl S, Merz J, Burkhard G, Fischer B. Mehrfachwahl–Wortschatz-Intelligenztest (MWT A/B). [Multiple choice vocabulary intelligence test] Erlangen: perimed Fachbuch-Verlagsgesellschaft mbH. 1991 [Google Scholar]
- e10.Iverson GL, Zasler ND, Lange RT. Post-concussive disorder. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: principles and practice. New York: Demos Medical Publishing; 2007. pp. 373–403. [Google Scholar]
- e11.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Workshop Scientific Team and Advisory Panel Members: -Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Meares S, Shores EA, Taylor AJ, et al. Mild traumatic brain injury does not predict acute postconcussion syndrome. J Neurol -Neurosurg Psychiatry. 2008;79:300–306. doi: 10.1136/jnnp.2007.126565. [DOI] [PubMed] [Google Scholar]
- e13.Hughes DG, Jackson A, Mason DL, Berry E, Hollis S, Yates DW. Abnormalities on magnetic resonance imaging seen acutely follow-ing mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550–558. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- e14.Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25:1049–1056. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- e15.Wallesch CW, Curio N, Galazky I, Jost S, Synowitz H. The neuropsychology of blunt head injury in the early postacute stage: effects of focal lesions and diffuse axonal injury. J Neurotrauma. 2001;18:11–20. doi: 10.1089/089771501750055730. [DOI] [PubMed] [Google Scholar]
- e16.Kamikubo T, Ohashi M, Hashimoto K, Miyano S. Cognitive dysfunction in 32 diffuse axonal injury cases. No To Shinkei. 2003;55:669–673. [PubMed] [Google Scholar]
- e17.Kothari S, Flanagan SR, Kwasnica C, Brown AW, Elovic EP. Congenital and acquired brain injury. 5. Emerging concepts in prognostication, evaluation, and treatment. Arch Phys Med Rehabil. 2008;89(Suppl 1):S27–S31. doi: 10.1016/j.apmr.2007.12.014. [DOI] [PubMed] [Google Scholar]
- e18.Jennett B. 2nd ed. Chicago: William Heinemann; 1975. Epilepsy after non-missile head injuries. [DOI] [PubMed] [Google Scholar]
- e19.Annegers JF, Grabow JD, Groover RV, Laws ER, Jr, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- e20.Yablon SA, Dostrow VG. Posttraumatic seizures and epilepsy. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: principles and practice. New York: Demos Medical Publishing; 2007. pp. 835–861. [Google Scholar]
- e21.Angeleri F, Majkowski J, Cacchiò G, et al. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia. 1999;40:1222–1230. doi: 10.1111/j.1528-1157.1999.tb00850.x. [DOI] [PubMed] [Google Scholar]
- e22.Asikainen I, Kaste M, Sarna S. Early and late posttraumatic sei-zures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- e23.Brodie MJ, French JA. Management of epilepsy in adolescents and adults. Lancet. 2000 356:323–329. doi: 10.1016/S0140-6736(00)02515-0. [DOI] [PubMed] [Google Scholar]
- e24.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]