Abstract
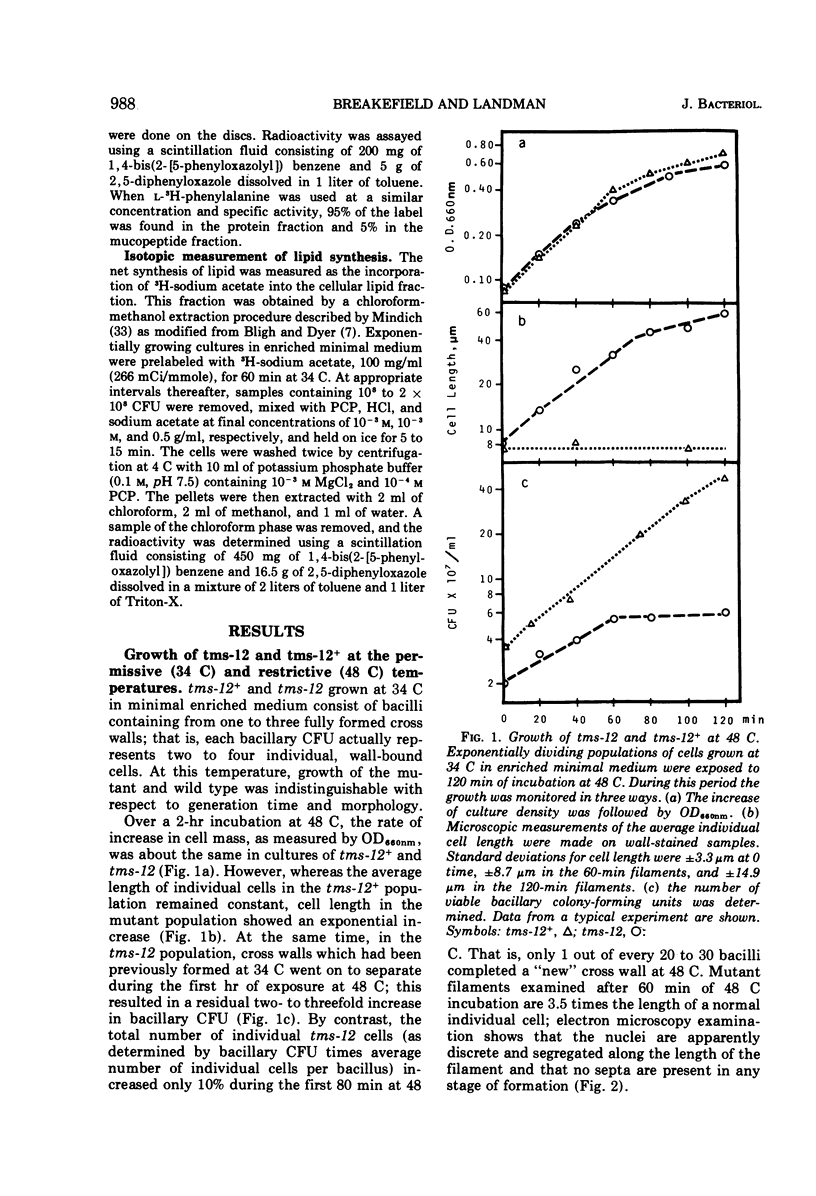
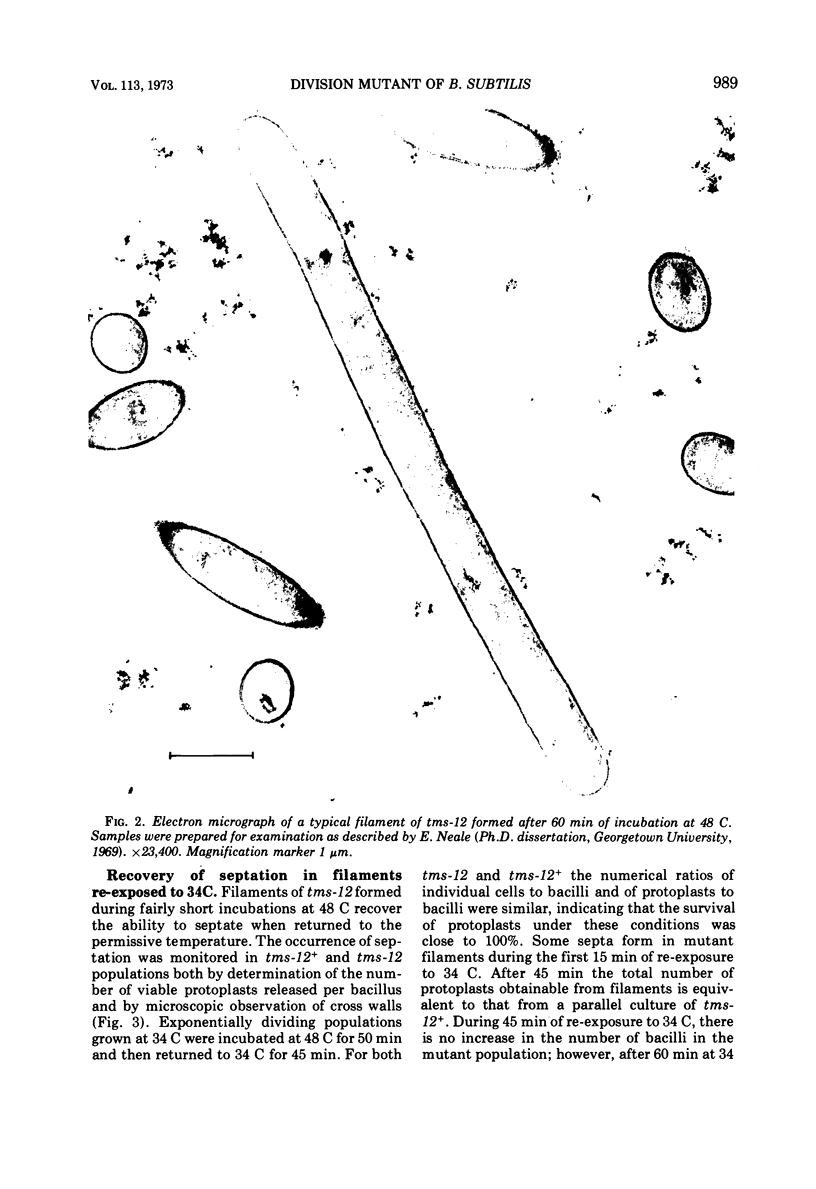
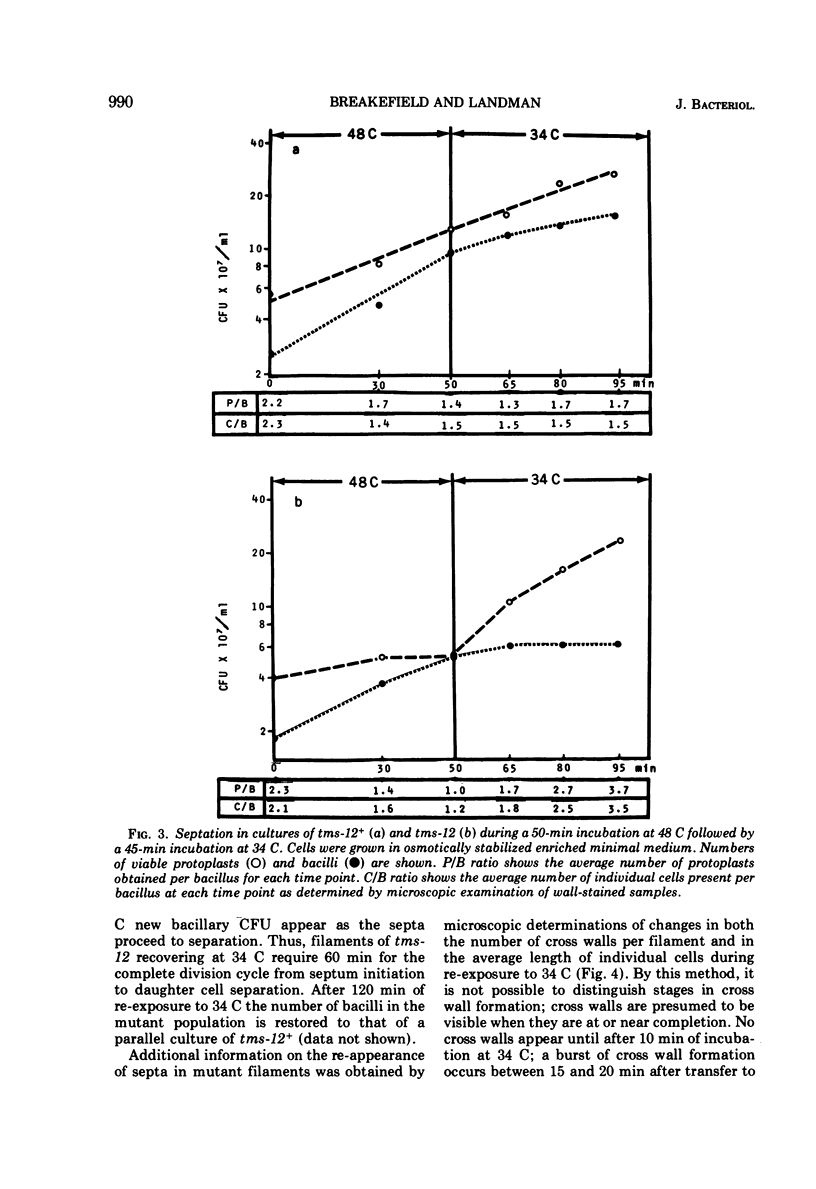
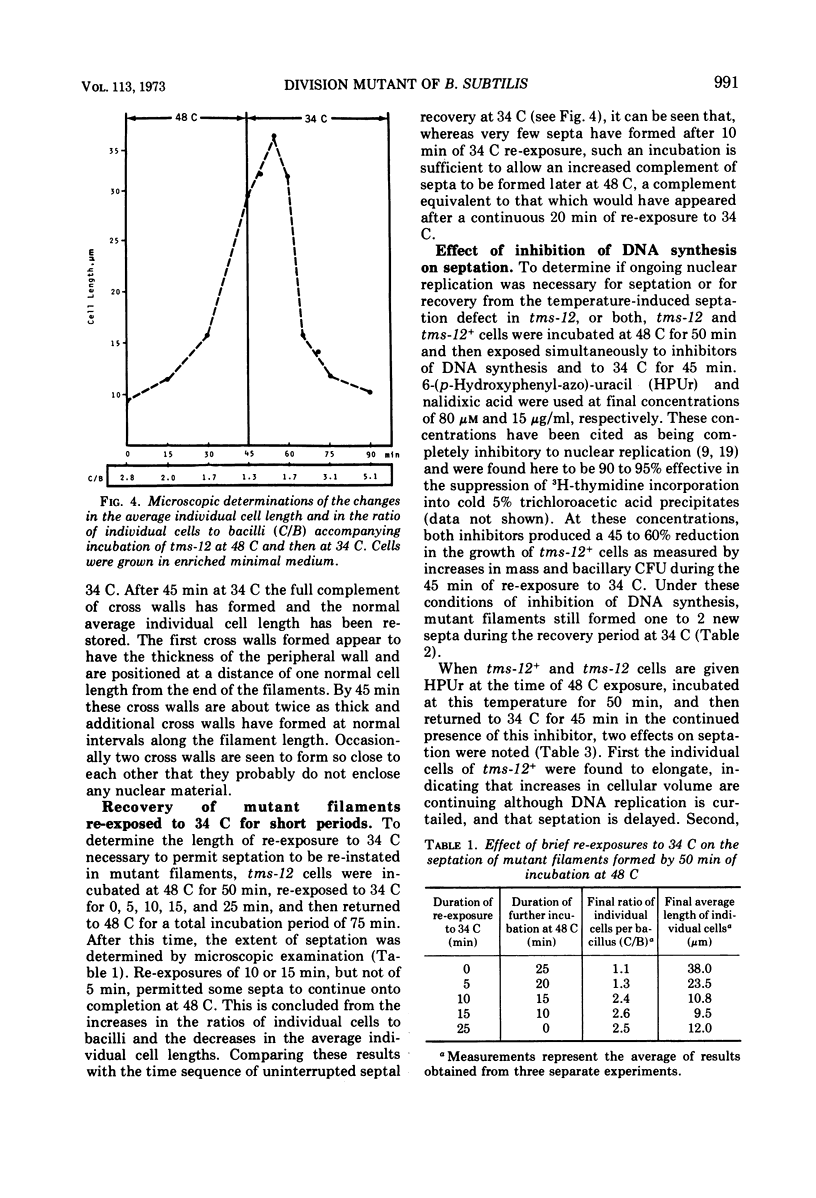
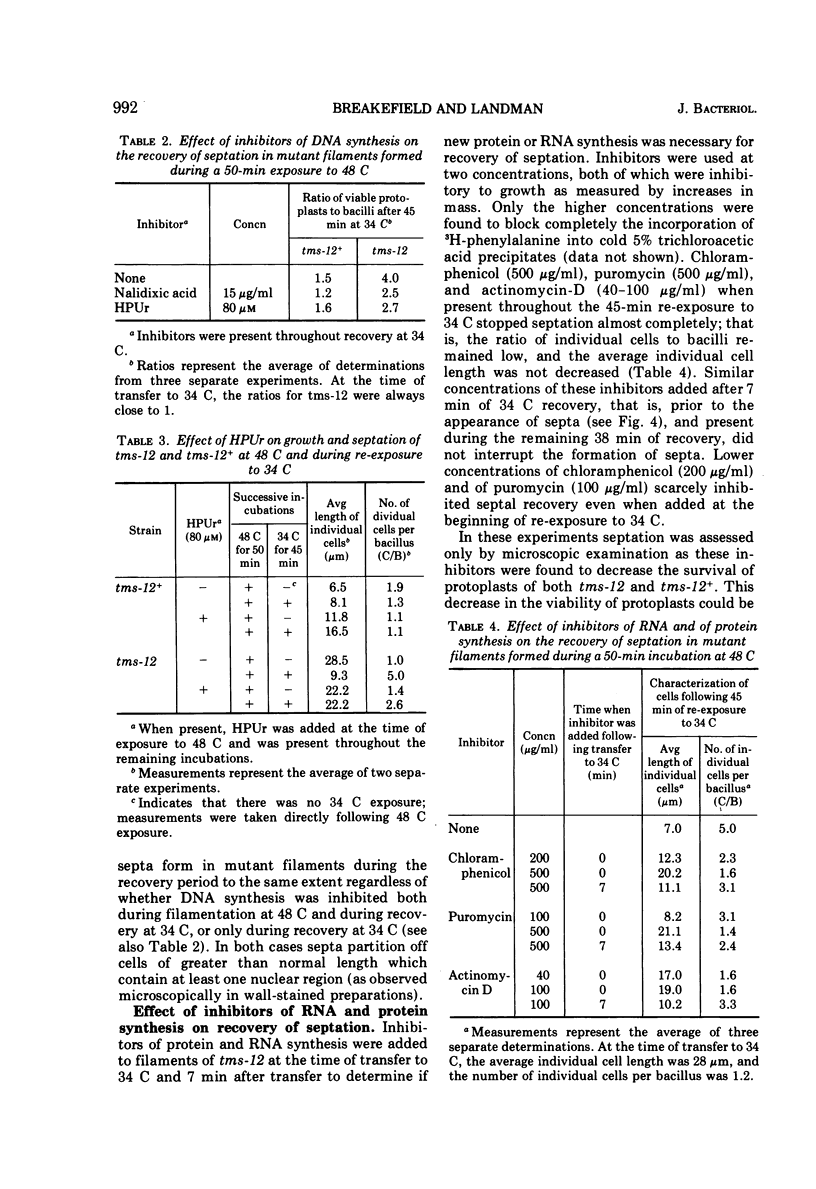
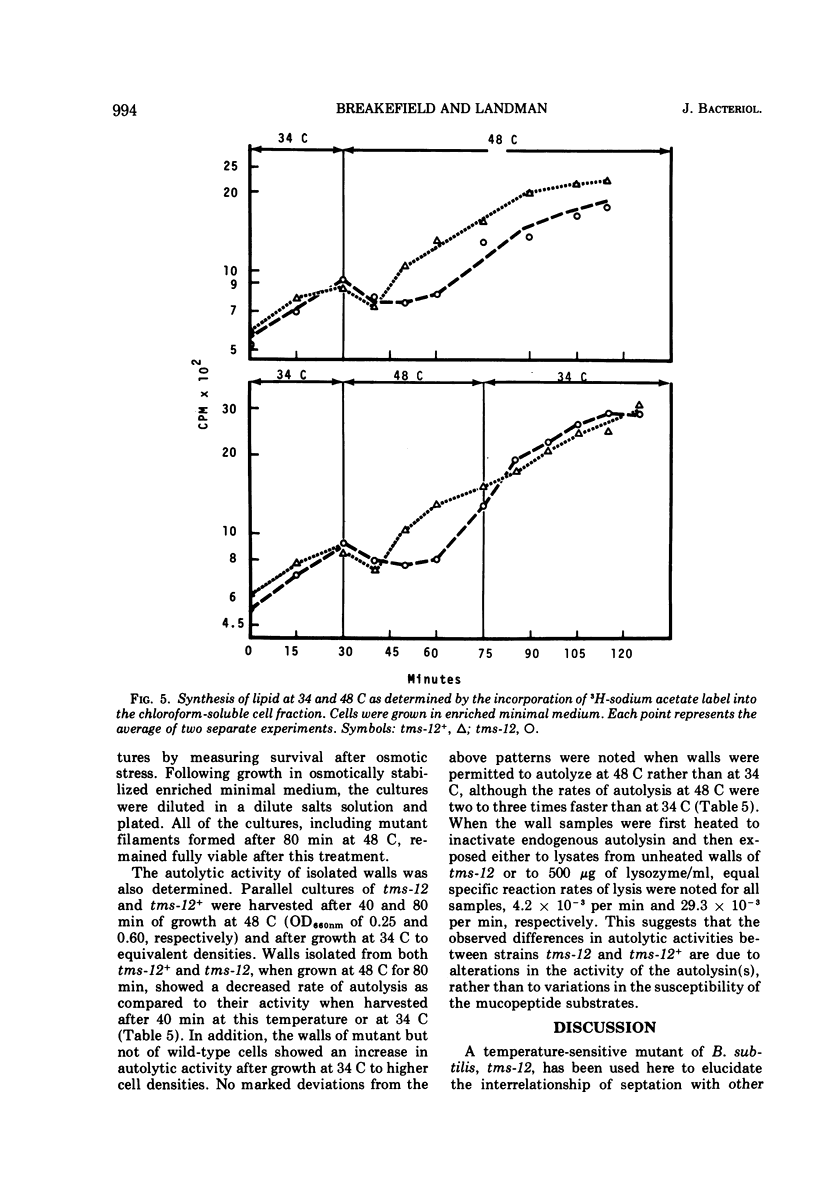
A temperature-sensitive divisionless mutant of Bacillus subtilis 168, tms-12, is shown to be defective in an early step in septum formation at the restrictive temperature. The nature of this defect has been studied by comparing the growth and composition of mutant and wild-type (tms-12+) cells at the restrictive (48 C) and permissive (34 C) temperatures. At 48 C, tms-12 cells grow as nonseptate, multinucleate filaments. Filamentation does not appear to be a result of alterations in properties of the cell wall, since the ratio of mucopeptide to teichoic acid, the autolytic activity, and the ability of the walls to protect cells against osmotic shock are comparable in tms-12 filaments and tms-12+ bacilli grown at 48 C. Synthesis of deoxyribonucleic acid and the segregation of nucleoids also proceed normally during filamentation. The synthesis of membrane, however, is delayed during filamentation of tms-12. No gross alterations were observed in the protein or lipid composition of membranes isolated from mutant filaments. Septum formation resumes when filaments are returned to 34 C and appears to be associated with an increased synthesis of membrane. The occurrence of septa was monitored both by microscopic observation of cross walls and by assays of the number of viable protoplasts released from bacillary filaments upon removal of the cell wall. Septation recovery can be blocked by inhibitors of ribonucleic acid and protein synthesis added during, but not after, the first 7 min of recovery at 34 C. By contrast, inhibition of deoxyribonucleic synthesis does not block recovery.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N., Rowbury R. J. Temperature-sensitive cell division component in a mutant of Salmonella typhimurium. J Gen Microbiol. 1971 Jul;67(1):107–115. doi: 10.1099/00221287-67-1-107. [DOI] [PubMed] [Google Scholar]
- Anraku N., Landman O. E. Control of the synthesis of macromolecules during amino acid and thymine starvation in Bacillus subtilis. J Bacteriol. 1968 May;95(5):1813–1827. doi: 10.1128/jb.95.5.1813-1827.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Boylen C. W., Ensign J. C. Ratio of teichoic acid and peptidoglycan in cell walls of Bacillus subtilis following spire germination and during vegetative growth. J Bacteriol. 1968 Aug;96(2):421–427. doi: 10.1128/jb.96.2.421-427.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. C., Marmur J. Identification of conserved genetic functions in Bacillus by use of temperature-sensitive mutants. Bacteriol Rev. 1968 Dec;32(4 Pt 1):302–312. [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Martin D. T., Begg K. J. Independence of cell division and DNA replication in Bacillus subtilis. Nat New Biol. 1971 Jun 30;231(26):274–276. doi: 10.1038/newbio231274a0. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi M., Sicard N. Induction of filament formation and thymineless death in Escherichia coli K-12. J Bacteriol. 1970 Apr;102(1):293–295. doi: 10.1128/jb.102.1.293-295.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L., SCHAECHTER M. A model for statistics of the cell division process. J Gen Microbiol. 1962 Nov;29:435–454. doi: 10.1099/00221287-29-3-435. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton P. M., Donachie W. D. Deoxyribonucleic acid synthesis and cell division in a lon- mutant of Escherichia coli. J Bacteriol. 1970 Jun;102(3):810–814. doi: 10.1128/jb.102.3.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kaneko H., Tamura G. Thermosensitive mutant of Escherichia coli requiring new protein synthesis to recover cellular division ability. Biochem Biophys Res Commun. 1971 Feb 19;42(4):669–675. doi: 10.1016/0006-291x(71)90540-7. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Patch C. T., Landman O. E. Comparison of the biochemistry and rates of synthesis of mesosomal and peripheral membranes in Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):345–357. doi: 10.1128/jb.107.1.345-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulton R. J. Analysis of the multiseptate potential of Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):762–767. doi: 10.1128/jb.104.2.762-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc E. P. Biochemical determination of bacterial morphology and the geometry of cell division. J Theor Biol. 1970 Jun;27(3):471–497. doi: 10.1016/s0022-5193(70)80010-8. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]




