Abstract
Purpose
To examine the cost effectiveness of using a pharmacogenetic test for UGT1A1*28 variant homozygosity before administering irinotecan in metastatic colorectal cancer patients. Patients and Methods: Decision-analytic model from Medicare payer perspective followed hypothetical patients treated with FOLFIRI (5-fluorouracil/leucovorin with irinotecan). Under usual care, patients received a full dose of irinotecan. With genetic testing, irinotecan dosage was reduced 25% in homozygotes with the UGT1A1*28 variant allele. Test performance, chemotherapy toxicity, and quality-of-life weights were from clinical literature and product labels. Costs were from 2007 Medicare fee schedules. Chemotherapy efficacy after dose reduction, adverse event risk, and other parameters were varied in one-way and probabilistic sensitivity analyses. We calculated the value of investing in further studies of chemotherapy efficacy following homozygote dose reductions.
Results
Pre-treatment genetic testing costs less ($272 savings/patient tested) and yields slightly improved quality-adjusted life expectancy (0.1 quality-adjusted day/patient tested, approximately 2 quality-adjusted hours). Results depend on treatment efficacy but not adverse event risk assumptions. Testing would avoid 84 cases of severe neutropenia including 4.4 deaths. At a threshold of $100,000 per quality-adjusted life year, therapeutic efficacy of irinotecan in homozygotes after dose reduction must be ≥98.4% of full-dose efficacy for genetic testing to remain preferred. Future studies to determine whether this efficacy level can be achieved have an economic value of $22 million.
Conclusion
Pharmacogenetic testing for UGT1A1*28 variant homozygosity may be cost effective, but only if irinotecan dose reduction in homozygotes does not reduce efficacy. Future studies to evaluate reduced-dose efficacy in homozygotes should be considered.
Introduction
In 2008, colorectal cancer was diagnosed in over 150,000 men and women in the United States.1 Despite advances in screening and early detection, nearly 20% of patients present with metastatic disease.1 Many others will develop recurrent and/or unresectable colorectal cancer after completing adjuvant therapy and will be eligible for palliative chemotherapy to control their disease. While oxaliplatin-based combination chemotherapy regimens (e.g., FOLFOX+/− bevacizumab) are currently favored for first-line treatment, regimens containing irinotectan (Camptosar©) are accepted and widely used in the first-, second-, and third-line metastatic setting.2, 3
Neutropenia is a serious side effect of irinotecan treatment, particularly in patients with decreased uridine diphosphate (UDP)-glucunorosyltransferase activity.4 Administered as a prodrug, irinotecan undergoes enzymatic conversion by carboxyesterase-2 to yield the clinically active metabolite, SN-38. This active form interferes with tumor cell division by inhibiting the nuclear enzyme topoisomerase I.5 SN-38 is eliminated from the body through the biliary system, after a process of glucuronidation (conjugation to glucuronic acid) via the uridine diphosphate glucuronosyltransferase (UGT)1A1 enzyme. Several retrospective studies have demonstrated that individuals who are homozygous for UGT1A1*28 are at increased risk of irinotecan-related neutropenia and diarrhea, particularly when irinotecan is administered at higher doses.4, 6–9 This likely is due to markedly reduced mRNA transcription leading to decreased overall enzyme production, slower SN-38 glucuronidation, and a greater SN-38 plasma concentration over time, resulting in prolonged exposure to the unconjugated form of SN-38.10 As many as 11% of North Americans may be homozygous for this allele, although the prevalence is known to be lower among Asian-Americans and African-Americans compared to Caucasians.5
In 2005 the FDA approved a UGT1A1 genotype test “as an aid in the identification of patients with greater risk for decreased UDP-glucunorosyltransferase activity” 11 and therefore with an increased risk of irinotecan-induced neutropenia. The manufacturer’s label for irinotecan now recommends that physicians “consider” a dose-reduction of “at least one level” (equivalent to approximately a 17–34% dose reduction) when administering the drug to patients who are known to be homozygous for UGT1A1*28, but notes that “the precise dose reduction in this patient population is not known.”12
Data regarding the effects of irinotecan dose reduction on the incidence of side effects or overall survival in colorectal cancer patients are limited. A recent meta-analysis indicated that lower doses (<150 mg/m 2) versus medium (150 – 250 mg/m 2) and high (>250 mg/m 2) doses were associated with a lower probability of severe neutropenia in homozygotes.9 In addition, little is known about the costs associated with implementing UGT1A1 testing. Indeed, few cost-effectiveness studies have been conducted of pharmacogenetic tests for inherited mutations of any kind.13 The purpose of this study was to perform an economic evaluation of the use of pharmacogenetic testing to guide dosing of irinotecan versus irinotecan administration according to usual care in metastatic colorectal cancer patients.
Methods
Model Overview
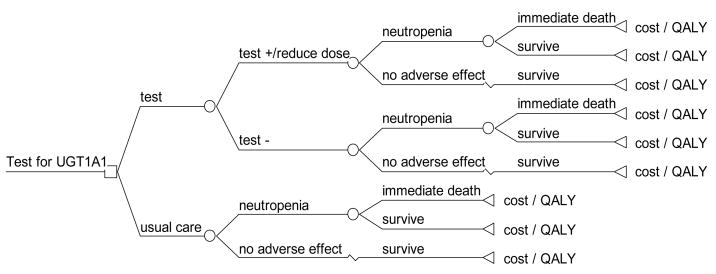
We created a decision analytic model from the Medicare payer perspective using TreeAge Pro 2008 (Williamstown, MA)14 to evaluate the cost effectiveness of testing for the UGT1A1*28 genotype prior to irinotecan administration in metastatic colorectal cancer patients compared to usual care (no testing). (Figure 1.) Hypothetical patients in the model were treated with FOLFIRI (5FU/LV with irinotecan), with a standard intermediate dose (175mg/m2) calculated for a patient with body surface area of 1.85m2 (e.g., 5′10″, 70kg person).15, 16 We chose to study the FOLFIRI regimen for the model because it is the irinotecan-containing regimen for which the most data on UGT1A1*28 testing and outcomes are currently available. In the usual care strategy, all patients received a standard intermediate dose of irinotecan. In the genetic testing strategy, patients underwent UGT1A1*28 testing prior to FOLFIRI administration. Those who were found to be homozygous for the UGT1A1*28 variant allele received a 25% dose reduction of irinotecan therapy to 131.25 mg/m2, based on the FDA’s label suggesting a dose reduction of at least one level, whereas all others received the full dose.
Figure 1.
Decision analytic model
We began by assuming that the FOLFIRI regimen had 100% efficacy in all patients and had the same efficacy at a reduced dose among homozygotes as a full dose among heterozygotes or wild types, because there is slower glucuronidation and a greater plasma concentration of SN-38 in homozygotes.6 This assumption was then varied along with other model inputs in one-way and probabilistic sensitivity analyses. A recent study indicated that response rates were not different between homozygotes, heterozygotes, or wild types, even though homozygotes experienced more frequent dose reductions due to adverse events.17
We report incremental costs and quality-adjusted life expectancy when comparing the two strategies and the economic value of additional investment to reduce uncertainties in the modeling assumptions. We also report the estimated number of severe neutropenia events and deaths avoided by testing.
Adverse Events and Life Expectancy
Severe adverse events associated with irinotecan include grade 3/4 (severe) neutropenia.18, 19 The risks of severe neutropenia were derived from randomized trials of irinotecan administration in genotyped populations (Table 1).8, 20 Among homozygotes, the probability of severe neutropenia declined from 14% to 3.7% after genetic testing and dose reduction (versus 3.7% in the rest of the population), based on a study indicating that such dose reductions decreased the risk of a severe adverse event to a risk that was statistically equivalent to that of heterozygotes and wild types (p=0.2).8 We reduced this benefit in sensitivity analyses. A fraction (23%) of patients experiencing severe neutropenia were assumed to be admitted to the hospital for management of febrile neutropenia,21 and the remainder were assumed to be treated on an outpatient basis. Because of the very small number of deaths due to adverse events reported in each trial,15, 22–26 we assumed the probability of death during a hospitalization for severe or febrile neutropenia was 0.1% (Table 1). Life expectancy following FOLFIRI treatment was assumed to be 24 months.27
Table 1.
Variable assumptions
| Variable name | Initial Value | Range | Source |
|---|---|---|---|
| Adverse Events | |||
| Probability of severe neutropenia, homozygotes with full dose | 0.14 | 0.04–0.70 | Toffoli8, Rouits18 |
| Probability of severe neutropenia, wild types and heterozygotes and homozygotes with reduced dose | 0.037 | 0.03–0.23 | Weighted average based on population prevalence; Toffoli8, Rouits18 |
| Probability of hospitalization with severe neutropenia | 0.23 | 0.23–1 | Elting21 |
| Probability of death from neutropenia | 0.001 | 0–0.03 | Trials15, 16, 22, 24 and expert opinion |
| Genetic Test Characteristics | |||
| UGT1A1 assay sensitivity | 0.9999 | 0.90–1 | Food and Drug Administration |
| UGT1A1 assay specificity | 0.9999 | 0.92–1 | Food and Drug Administration |
| Prevalence of *28 allele, homozygous | 0.11 | 0.01–0.20 | Lin30 |
| Prevalence of *28 allele, heterozygous | 0.43 | 0.40–0.46 | Lin30 |
| Quality of Life | |||
| Metastatic colorectal cancer | 0.83 | 0.8–1.0 | Brown ML31 |
| Severe neutropenia | 0.42 | 0.25–0.55 | Brown RE32 |
| Efficacy | |||
| Efficacy of reduced dose (75%) of FOLFIRI in homozygotes | 100% | 75–100% | Assumption |
| Life expectancy | 24 months | 6–24 months | Fuchs27 |
| Costs | |||
| Cost of UGT1A1 test | $102.83 | $50–750 [$75 for societal perspective] | Medicare Clinical Laboratory Fee Schedule17 |
| Cost of physician office visit | $69 | Medicare CPT 9921436 | |
| Hospitalization for severe neutropenia | $9500 | 2007 DRG weight*$7500 base rate35 (DRG 574) | |
| Chemotherapy, full dose | $12950 | Medicare Drug Acquisition Price (2007)37; multiplied by 75% for reduced dose | |
Test characteristics and allele prevalence
Genetic testing was assumed to be conducted using the Invader molecular assay developed by Third Wave Technologies (Madison, WI), an FDA-approved test for UGT1A1*28 genotyping.28 Sensitivity and specificity of the assay were reported on the FDA website as 100%.29 However, we assumed sensitivity and specificity of 99.99% in order to allow for a small chance of a laboratory error that would affect test results. In the North American population, the prevalence of homozygotes was estimated at 11%.30
Quality of Life and Costs
We used previously published quality-of-life weights (utility values) associated with metastatic colorectal cancer and severe neutropenia (Table 1). A baseline weight of 0.83 was used for the population of metastatic colorectal cancer patients.31 Severe neutropenic events were assumed to last one week, leading to an additional decrement in quality of life during that week. Febrile neutropenia alone has been reported to be associated with a quality-of-life value of 0.42 (equivalent to a utility decrement of 0.58 from perfect health).32 The quality of life of patients experiencing both metastatic colorectal cancer and a severe neutropenic event (febrile neutropenia) simultaneously was estimated at 0.42 during one week, using a lowest-value approach.33
We used the Medicare payer perspective to evaluate costs. The model included costs of UGT1A1*28 testing,34 costs of hospitalization for febrile neutropenia,35 cost of a physician office visit,36 and costs of FOLFIRI chemotherapy for a full dose in wild types and heterozygotes and for a reduced dose in homozygotes.37 The model did not include costs of subsequent chemotherapy, because the wide variety of possible treatments available impaired our ability to calculate this cost with an acceptable degree of accuracy. We wanted to estimate the maximum amount of future medical costs that might be incurred as a result of patients living longer due to the use of the test while still having the test be cost effective. We therefore estimated the threshold value at which the additional lifetime medical costs due to improved survival as a result of genetic testing would be offset by the benefits of testing. All costs were reported in 2007 U.S. dollars. Quality-adjusted life expectancy and costs were discounted at an annual rate of 3% per year, in accordance with the recommendation of the Panel on Cost-effectiveness in Health and Medicine.38
Sensitivity analyses
We performed one-way sensitivity analyses on the efficacy of FOLFIRI dose reduction, incidence of and mortality due to severe neutropenic events, genetic test performance, cost, and quality of life model inputs for a specified range of values (Table 1). Of note, we varied the efficacy of a dose reduction from 75–100% of full dose efficacy; in the model, a reduction in efficacy resulted in a proportional reduction in life expectancy after FOLFIRI treatment (e.g., a 75% reduction in efficacy resulted in a reduction in life expectancy from 24 months to 18 months). We also considered different methods for combining quality-of-life weights for severe neutropenia and metastatic colorectal cancer.33
We evaluated the impact of substituting a societal perspective for the Medicare payer perspective by adding $900 to reflect the value of patient time associated with hospitalization39 and reducing the cost of the UGT1A1*28 test from $103 (the Medicare reimbursement rate) to $75, which represented the estimated cost to conduct the test in a hospital laboratory.
Value of Information
We conducted a probabilistic sensitivity analysis by running 1000 Monte Carlo simulations that varied all non-cost variables simultaneously with a willingness-to-pay (WTP) threshold of $100,000/quality-adjusted life year (QALY).40 We applied triangular distributions41 for all parameters, where the peak was equal to the initial value and the lower and upper bounds were equal to the lowest or highest values found in the literature. The exceptions to triangular distributions were those for which data were very limited: chemotherapy efficacy, quality-of-life weights, and probability of hospitalization for neutropenia. We selected uniform distributions for these parameters, because they reflect the highest possible degree of uncertainty and are the least “informative” as they represent the distribution of possible values as a straight line within a range.
Using the results of the probabilistic sensitivity analysis with a WTP threshold of $100,000/QALY, we estimated the value of obtaining better quality data to reduce uncertainties in our model, and therefore the value that represents the maximum amount that should be spent to conduct a study of dose reductions in homozygotes. First we calculated the expected value of perfect information (EVPI). EVPI can be interpreted as the value of completely removing the possibility of making an incorrect decision based on the current analysis, due to its inherent uncertainty.42 We then calculated the expected value of partial perfect information (partial EVPI) for resolving just the uncertainty about the efficacy of a reduced dose of irinotecan in homozygotes. The partial EVPI represents the maximum value society should be willing to pay to eliminate the uncertainty about the efficacy assumption alone. We assumed that the test would continue to be useful for 5 years before being replaced by new technologies, and that the size of the population to which the test can be applied is 29, 260 metastatic colorectal cancer patients annually,21 63% of whom we estimate are age 65 and older based on SEER data from 1973–2002.43 Finally we compared the partial EVPI to how much a clinical study might cost to improve decision making concerning dose reductions.
Results
Adverse events and Cost-effectiveness
For every 10,000 patients tested, 1,100 (11%) would receive a 25% dose reduction as a result of being identified as UGT1A1*28 homozygous. On average, this treatment strategy would avoid 84.5 cases of severe neutropenia (including 4.5 deaths due to neutropenia) and would save $2.7 million in treatment costs. Taking into account the cost of testing and assuming no reduction in treatment efficacy, the average cost savings and quality-adjusted life expectancy savings per patient tested was $272.34 and 0.073 quality-adjusted days (Table 2), not even 2 quality-adjusted hours. If all 29,000 metastatic colorectal cancer patients diagnosed each year in the U.S. were treated with chemotherapy that included irinotecan, the estimated net annual savings could be as high as $7.96 million and 6 quality-adjusted life years, as a result of avoiding 245 severe neutropenic events (including 13 fatal events).
Table 2.
Cost-effectiveness Results Depending on Efficacy of Dose Reduction in Homozygotes.
| Strategy | Cost | Incremental Cost | Effectiveness (QALYs) | Incremental Effectiveness | Incremental Cost- effectiveness ratio (ICER) |
|---|---|---|---|---|---|
| Test 100% efficacy | $12,786 | 1.6349 | |||
| No test | $13,058 | $272 | 1.6347 | −0.0002 QALYs = −0.073 days | Dominated |
| Test 99% efficacy | $12,786 | 1.6331 | |||
| No Test | $13,058 | $272 | 1.6347 | 0.0016 QALYs = 0.584 days | $170,569 |
| Test 98.4% efficacy | $12,786 | 1.6319 | |||
| No test | $13,058 | $272 | 1.6347 | 0.0027 QALYs = 0.99 days | $100,073* |
| Test 95% efficacy | $12,786 | 1.6259 | |||
| No test | $13,058 | $272 | 1.6437 | 0.0088 QALYs =3.21 days | $30,965 |
| Test 85% efficacy | $12,786 | 1.6079 | |||
| No test | $13,058 | $272 | 1.6347 | 0.0268 QALYs =9.78 days | $10,165 |
| Test 75% efficacy | $12,786 | 1.5899 | |||
| No test | $13,058 | $272 | 1.6347 | 0.0448QALYs =16.35 days | $6,081 |
Note: When efficacy of a reduced dose in homozygotes falls below 98.4%, the no-testing strategy is below the willingness-to-pay threshold of $100,000/QALY.
The results were sensitive to the efficacy of a reduced dose and somewhat sensitive to the cost of the genetic test but not to adverse event risk. In one-way sensitivity analyses, the estimated efficacy of a reduced dose of irinotecan administered to homozygous carriers following testing must be at least 98.4% of full-dose efficacy for testing to remain the preferred strategy at a willingness-to-pay threshold of $100,000/QALY (Table 2), meaning that efficacy in homozygotes must hold nearly constant following a dose reduction. The cost of the genetic test must be less than $395 (versus the current Medicare cost of $103) for testing to remain the preferred strategy at a WTP threshold of $100,000/QALY; at less than $375, testing both cost less and increased QALYs. Results were not sensitive to the range of values tested for adverse events (incidence of and mortality due to severe neutropenia); for example, if the probability of severe neutropenia after dose reduction in homozygotes was 10.5% (25% reduction) versus 3.7%, the threshold for treatment efficacy of a reduced dose was 98.3%. Results were also not sensitive to life expectancy, genetic test performance (i.e., sensitivity and specificity), and quality-of-life weights (data not shown). Adopting a societal perspective by incorporating patient time costs and a lower test cost did not change the optimal strategy or trends in the sensitivity analyses.
The threshold analysis indicated that in order for genetic testing to remain the dominant strategy, the average cost for the 13 patients who would avoid death due to a severe neutropenic event cannot exceed $618,955 per patient during their remaining life expectancy after completion of the FOLFIRI regimen.
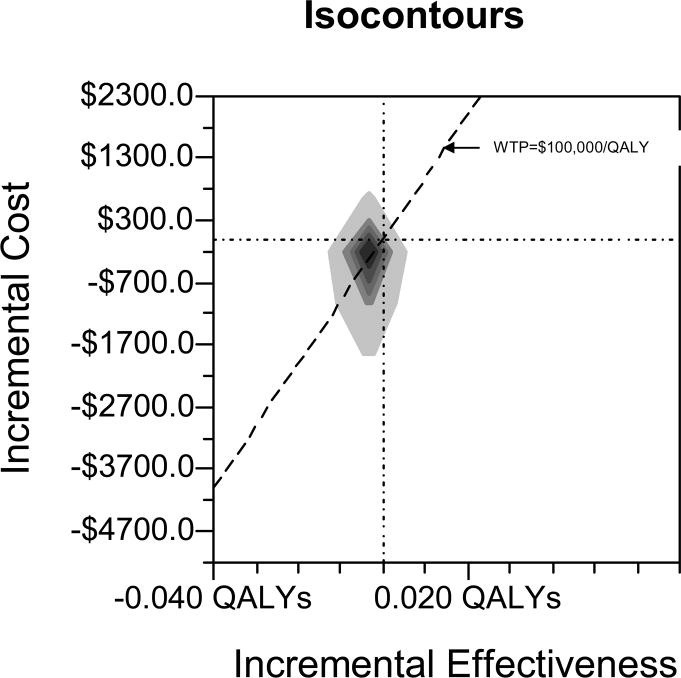
In probabilistic sensitivity analysis, testing dominated usual care in only 9.1% of simulations. Usual care was preferred in 72% of simulations, with an additional 7.5% where usual care resulted in an increase in QALYs but was above the WTP threshold of $100,000/QALY (Figure 2).
Figure 2. Probabilistic Sensitivity Analysis with Willingness-to-Pay (WTP) Threshold of $100,000/QALY.
Note: All non-cost variables were varied according to triangular or uniform distributions (see text). Testing costs less and is more effective in 9.1% of simulations. Usual care was preferred in 72.0% of simulations. X and Y axes are indicated by horizontal and vertical lines, respectively, where they cross zero.
Value of Studies to Reduce Uncertainties
The partial EVPI for the efficacy variable was $22 million over the next 5 years. In other words, a research investment of up to $22 million to evaluate the efficacy of irinotecan dose reduction in homozygotes would be justified based on a WTP threshold of $100,000/QALY if irinotecan remained an accepted and widely-used treatment alternative for metastatic colorectal cancer patients in the United States for the next 5 years. Given that Medicare primarily provides health insurance coverage for patients age 65 and older (including approximately 18,000 metastatic colorectal cancer patients in this age group diagnosed each year), the partial EVPI just from the Medicare perspective was $13.8 million. The total EVPI was $21.4 million, or $13.4 million from the Medicare perspective, which was slightly lower than the partial EVPI. The higher partial EVPI is likely due to the large and important effect of the efficacy variable in the model, which is not fully reflected in the total EVPI because uncertainty in other model parameters is correlated with uncertainty in the efficacy parameter and works in the opposite direction.
Discussion
Pharmacogenetic testing has the potential to fundamentally alter risk-benefit tradeoffs associated with pharmacotherapy, introducing “personalized medicine” concepts into clinical practice. Cost-effectiveness analyses of pharmacogenetic tests can provide important insights into the relative economic value of this new management paradigm, but few of these cost-effectiveness studies have been published.13, 44 This is the first cost-effectiveness study of UGT1A1*28 testing to guide chemotherapy dosing of irinotecan. Testing itself would cost up to $3 million per year, given the test cost and eligible population. We found that incorporating UGT1A1*28 testing in the clinical management of metastatic colorectal cancer treated with irinotecan may result in lower overall medical costs and higher quality-adjusted life expectancy, but our findings were sensitive to several uncertainties in the model’s parameter estimates as indicated by the sensitivity analyses. In particular, the maintenance of clinical efficacy of irinotecan after dose reduction in UGT1A1*28 homozygotes was a key determinant of cost-effectiveness results. That is, if treatment efficacy is not fully maintained after the FDA-recommended dose reduction of irinotecan, testing will not be a cost-effective alternative. The small reduction in treatment-related risks (severe neutropenia and/or death) gained by testing is outweighed by the risk of shortened survival due to under-treatment. However, limited data in this area do suggest that homozygotes experience slower glucuronidation and a greater plasma concentration of SN-38,6 and that response rates are not different between homozygotes, heterozygotes, or wild types, even with differential dose reductions across groups.17
Our analysis indicates that the federal government should be willing to invest in further research to reduce the uncertainty associated with UGT1A1 genotype testing and irinotecan dose-reduction. Specifically, at a WTP threshold of $100,000/QALY and with a 5-year time horizon for the genetic testing technology, the federal government should consider investing at least $13.8 million specifically to evaluate the efficacy of a reduced irinotecan dose in UGTA1*28 homozygous colorectal cancer patients ages 65 and older. In comparison, the estimated expected cost to conduct an average Phase III clinical trial is $27.1 million (in 2000 US$),45 although some analysts consider this to be a 2-4-fold overestimate.46, 47 The cost to conduct a clinical trial in homozygotes may differ substantially from this amount, however, because irinotecan is not a novel therapeutic entity and already has been proven effective in metastatic colorectal cancer patients. Alternatively, an observational clinical study could be considered that might provide valuable information more quickly.
This analysis is subject to limitations. First, the probabilities of severe neutropenia and death from neutropenia in colorectal cancer patients were difficult to estimate, because data were drawn from randomized controlled trials using different regimens, patients were more closely monitored in clinical trials than in usual care (with dose reductions for patients with adverse events), and the number of events or deaths in any one trial was small or nil. Because we used estimates drawn from clinical trials, our results may under-estimate the value of genetic testing to avoid these events. On the other hand, dose reductions reported in some studies were greater than the dose reduction we modeled based on the FDA label. Results were consistent, however, when we varied the amount of dose reduction benefit from avoiding adverse events. Second, there were only two published clinical trials reporting results in a relatively small number of genotyped patients. So, while genetic test sensitivity and specificity are fairly well established, estimates of clinical sensitivity and specificity – or the probabilities that a positive test predicts an adverse event or death – have a wide range. Although we accounted for the fact that a patient with severe neutropenia could be hospitalized versus treated on an outpatient basis, this is likely to depend on local care patterns and patient ability to self-manage at home. Varying this parameter, however, did not affect the model results.
We also did not consider alternative dosing approaches. For instance, the results of a study by Toffoli and colleagues8 indicated that successfully administering full-dose irinotecan in UGT1A1*28 homozygotes can produce superior response rates compared to non-homozygotes (OR 0.19, 95%CI: 0.04–0.89), albeit with higher rates of hematologic and non-hematologic toxicities (OR 4.9, 95%CI: 1.36–17.9). This suggests that the magnitude of the recommended dose reduction in homozygotes should be carefully considered, as a potential effectiveness advantage (i.e., improved response rate and/or survival) may be significantly compromised. In addition, alternative dosing regimens of irinotecan may have different safety profiles, with the importance of UGT1A1*28 status enhanced or reduced depending on pharmacodynamic parameters associated with the dosing schedule. Finally, prophylactic use of agents such as pegfilgrastim or filgrastim could help reduce the incidence of neutropenia in homozygotes,48 thereby allowing a full dose of irinotecan to be used in the first cycle, but also contributing to the cost of this treatment strategy.
Our analysis focused on the use of irinotecan for the treatment of metastatic colorectal cancer. The broad therapeutic application of irinotecan (e.g. tumors of the upper gastrointestinal tract and central nervous system49–51) should also be taken into account when considering both the clinical impact of dosing recommendations and the potential economic value of pharmacogenetic testing. The proportion of metastatic colorectal cancer patients who will eventually receive irinotecan is not known; however, if fewer than 29,214 cancer patients were expected to receive irinotecan in a year, the value of future research would decrease.
Cost-effectiveness analysis can help policy makers evaluate the clinical utility of new medical technologies such as pharmacogenetic tests, make decisions about reimbursement, and identify priorities and investment levels for further research. In the case of UGT1A1*28 testing, we found that routine testing may slightly improve clinical outcomes and quality of life, but is only cost saving if clinical efficacy (survival) is maintained following an irinotecan dose reduction in homozygotes. Further studies to evaluate the impact of irinotecan dose reduction on clinical efficacy would be worth an investment of up to $13.8 million dollars, based on the impact of such testing on costs, life expectancy, and quality of life for Medicare beneficiaries alone, and even more if all use of irinotecan were considered.
Acknowledgments
This work was supported by each author’s institution and in part by the American Cancer Society [Grant Numbers MRSGT-4-002-01-CPHPS to HTG and MRSG-07-232-01-CPHPS to MJH].
We would like to thank Elisabeth Fenwick, PhD, University of Glasgow, United Kingdom, for methodological expertise and assistance; Debra Leonard, MD, PhD, and Hanna Rennert, PhD, Weill Cornell Medical College for sharing insights about pharmacy testing and billing; and Scott Cantor, PhD, of MD Anderson Cancer Center and participants of the “Using Modeling to Inform Public Health” seminar at Yale University School of Medicine.
Footnotes
Presented in part at the Society for Medical Decision Making 29th Annual Meeting, October 21-24 2007, Pittsburgh, PA.
References
- 1. [Accessed July 9, 2008];American Cancer Society, Cancer Facts and Figures. http://www.cancer.org/docroot/stt/stt_0.asp.
- 2.NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Clinical guidelines. 2008 July 9;1 www.nccn.org.
- 3.Food and Drug Administration. [Accessed July 29, 2007];Talk Paper, FDA approves Campostar for first line therapy in treatment of advanced colorectal cancer. http://www.fda.gov/bbs/topics/ANSWERS/ANS01011.html.
- 4.Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics Journal. 2002;2(1):43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 5.Humerickhouse R, Lohrbach K, Li L, Bosron WF, Dolan ME. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Research. 2000 Mar 1;60(5):1189–1192. [PubMed] [Google Scholar]
- 6.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. Journal of Clinical Oncology. 2004 Apr 15;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 7.Marcuello E, Altes A, Menoyo A, Del Rio E, Gomez-Pardo M, Baiget M. UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. British Journal of Cancer. 2004 Aug 16;91(4):678–682. doi: 10.1038/sj.bjc.6602042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toffoli G, Cecchin E, Corona G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. Journal of Clinical Oncology. 2006 Jul 1;24(19):3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 9.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. Journal of the National Cancer Institute. 2007 Sep 5;99(17):1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 10.Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Research. 1994 Jul 15;54(14):3723–3725. [PubMed] [Google Scholar]
- 11.United States Food and Drug Administration. [Accessed September 30, 2007];Invader UGT1A1 molecular assay 510(k) summary. http://www.fda.gov/cdrh/pdf5/K051824.pdf.
- 12.CampostarR (irinotecan HCl), Hepatic dysfunction, pancreatitis, UGT1A1, July 21, 2005, Final label. http://www.fda.gov/cder/foi/label/2005/020571s024,027,028lbl.pdf.
- 13.Phillips KA, Van Bebber SL. A systematic review of cost-effectiveness analyses of pharmacogenomic interventions. Pharmacogenomics. 2004 Dec;5(8):1139–1149. doi: 10.1517/14622416.5.8.1139. [DOI] [PubMed] [Google Scholar]
- 14.TreeAge Software, Inc. [Accessed July 29, 2007]; http://www.treeage.com/products/index.html.
- 15.Andre T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. European Journal of Cancer. 1999 Sep;35(9):1343–1347. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 16.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. Journal of Clinical Oncology. 2004 Jan 15;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 17.Kweekel DMGH, Van der Straaten T, Antonini NF, Punt CJA, Guchelaar H-J. UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group study. British Journal of Cancer. 2008 Jul 22;99(2):275–282. doi: 10.1038/sj.bjc.6604461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth AD, Yan P, Dietrich D, Fiocca R, Bodoky G, Labianca D, Cunningham D, Van Cutsem E, Bosman F, Tejpar S. Does UGT1A1*28 homozygosity predict for severe toxicity in patients treated with 5-fluorouracil (5-FU)-irinotecan (IRI)? Results of the PETACC 3-EORTC 40993-SAKK 60/00 trial comparing IRI/5-FU/folinic acid (FA) to 5-FU/FA in stage II–III colon cancer. ASCO 2008 Gastrointestinal Cancers Symposium; 2008. [Google Scholar]
- 19.Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Database. 05/01/2008;:ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed February 2008, 2008.
- 20.Rouits E, Boisdron-Celle M, Dumont A, Guerin O, Morel A, Gamelin E. Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clinical Cancer Research. 2004 Aug 1;10(15):5151–5159. doi: 10.1158/1078-0432.CCR-03-0548. [DOI] [PubMed] [Google Scholar]
- 21.Elting L, Lu C, Escalante CP, Giordano SH, Trent JC, Cooksley C, Avritscher EBC, Shih YCT, Ensor J, Bekele BN, Gralla RJ, Talcott JA, Rolston K. Outcomes and Cost of Outpatient or Inpatient Management of 712 Patients With Febrile Neutropenia. Journal of Clinical Oncology. 2008 Feb 01;26(4):606–610. doi: 10.1200/JCO.2007.13.8222. [DOI] [PubMed] [Google Scholar]
- 22.Rougier P, Van Cutsem E, Bajetta E, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer.[erratum appears in Lancet 1998 Nov 14;352(9140):1634] Lancet. 1998 Oct 31;352(9138):1407–1412. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998 Oct 31;352(9138):1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. Journal of Clinical Oncology. 2003 Mar 1;21(5):807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 25.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. Journal of Clinical Oncology. 2005 Aug 1;23(22):4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 26.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) British Journal of Cancer. 2006 Mar 27;94(6):798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs CS, Marshall J, Barrueco J. Randomized, Controlled Trial of Irinotecan Plus Infusional, Bolus, or Oral Fluoropyrimidines in First-Line Treatment of Metastatic Colorectal Cancer: Updated Results From the BICC-C Study. Journal of Clinical Oncology. 2008 February 1;26(4):689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 28.Third Wave Products. [Accessed July 29, 2007];Current Products, Advancing personalized medicine: The InvaderR UGT1A1 molecular assay. http://www.twt.com/company/pressreleases/2006/fda_feb_06.htm.
- 29.United States Food and Drug Administration. Center for drug evaluation and research, Table of valid genomic biomarkers in the context of approved drug labels. http://www.fda.gov/cder/genomics/genomic_biomarkers_table.htm.
- 30.Lin J-P, O’Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006 Oct 3;114(14):1476–1481. doi: 10.1161/CIRCULATIONAHA.106.633206. [DOI] [PubMed] [Google Scholar]
- 31.Brown ML, Nayfield SG, Shibley LM. Adjuvant therapy for stage III colon cancer: economics returns to research and cost-effectiveness of treatment. Journal of the National Cancer Institute. 1994 Mar 16;86(6):424–430. doi: 10.1093/jnci/86.6.424. [DOI] [PubMed] [Google Scholar]
- 32.Brown RE, Hutton J. Cost-utility model comparing docetaxel and paclitaxel in advanced breast cancer patients. Anti-Cancer Drugs. 1998 Nov;9(10):899–907. doi: 10.1097/00001813-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Bonds DE, Freedberg KA. Combining utility measurements: exploring different approaches. Disease Management and Health Outcomes. 2001;9(9):507–516. [Google Scholar]
- 34. [Accessed February 2007];Medicare Laboratory Fee Schedule. http://www.cms.hhs.gov/
- 35. [Accessed February 2007];Medicare Diagnosis-related Group Weights. http://www.cms.hhs.gov/
- 36. [Accessed February 2007];Medicare Physician Fee Schedule. 2007 http://www.cms.hhs.gov.
- 37. [Accessed February 2007];Medicare Drug Acquisition Price. http://www.cms.hhs.gov.
- 38.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 39.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. Journal of the National Cancer Institute. 2007 Jan 3;99(1):14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 40.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Archives of Internal Medicine. 2003 Jul 28;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 41.Hunink MGP. Decision making in health and medicine: Integrating evidence and values. Cambridge, United Kingdom: Cambridge University Press; 2001. [Google Scholar]
- 42.Briggs A, Sculpher M, Claxton K. Decision Modeling for Health Economic Evaluation. Oxford, England: Oxford University Press; 2006. [Google Scholar]
- 43. [Accessed March 2007];Surveillance Epidemiology and End Results. http://seer.cancer.gov/
- 44.Phillips KA, Van Bebber SL. Measuring the value of pharmacogenomics. Nature Reviews Drug Discovery. 2005 Jun;4(6):500–509. doi: 10.1038/nrd1749. [DOI] [PubMed] [Google Scholar]
- 45.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of Health Economics. 2003 Mar;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 46.Light DW, Warburton RN. Extraordinary claims require extraordinary evidence. Journal of Health Economics. 2005 Sep;24(5):1030–1033. doi: 10.1016/j.jhealeco.2005.07.001. discussion 1034–1053. [DOI] [PubMed] [Google Scholar]
- 47.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Affairs. 2006 Mar-Apr;25(2):420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 48.Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, Siena S, Lalisang RI, Samonigg H, Clemens MR, Zani V, Liang BC, Renwick J, Piccart MJ. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Annals of Oncology. 2003 Jan;14(1):29–34. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 49.Cloughesy T, Filka E, Nelson G, Kabbinavar F, Friedman H, Miller LL, Elfring GL. Irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. American Journal of Clinical Oncology. 2002 April;25(2):204–208. doi: 10.1097/00000421-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Vredenburgh J, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. Journal of Clinical Oncology. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 51.Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K, Bugat R. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Annals of Oncology. 2008 Jun 16; doi: 10.1093/annonc/mdn166. Epub. [DOI] [PubMed] [Google Scholar]