Abstract
Objective
To determine the feasibility of high-fidelity simulation for studying variation in ICU admission decision making for critically ill elders with end-stage cancer.
Design
Mixed qualitative and quantitative analysis of physician subjects participating in a simulation scenario using a hospital set, actors, medical chart, and vital signs tracings. The simulation depicted a 78 year-old man with metastatic gastric cancer, life-threatening hypoxia most likely attributable to cancer progression, and stable preferences to avoid ICU admission and intubation. Two independent raters assesed the simulations and subjects completed a post-simulation web-based survey and debriefing interview.
Setting
Peter M. Winter Institute for Simulation Education and Research at the University of Pittsburgh.
Subjects
27 hospital-based attending physicians, including 6 emergency physicians, 13 hospitalists, and 8 intensivists.
Measurements and Main Results
Outcomes included qualitative report of clinical verisimilitude during the debriefing interview, survey-reported diagnosis and prognosis, and observed treatment decisions. Independent variables included physician demographics, risk attitude, and reactions to uncertainty. All (100%) reported that the case and simulation were highly realistic, and their diagnostic and prognostic assessments were consistent with our intent. Eight (29.6%) physicians admitted the patient to the ICU. Among the 8 physicians who admitted the patient to the ICU, 3 (37%) initiated palliation, 2 (25%) documented the patient’s code status (DNI/DNR), and 1 intubated the patient. Among the 19 physicians not admitting to the ICU, 13 (68%) initiated palliation and 5 (42%) documented code status. Intensivists and emergency physicians (P=0.048) were more likely to admit the patient to the ICU. Years since medical school graduation were inversely associated with the initiation of palliative care (P=0.043).
Conclusions
Simulation can reproduce the decision context of ICU triage for a critically ill patient with terminal illness. When faced with an identical patient, hospital-based physicians from the same institution vary significantly in their treatment decisions.
Keywords: terminal care, intensive care, physician decision making, cancer, simulation
One third of patients with metastatic cancer who die in the hospital are admitted to the ICU [1]. Such admission may occur despite very low expected clinical benefit, a promulgated criterion for admission refusal [2–4]. ICU admission decisions are influenced by resource availability [5–7] and physician prognostication [8], values [9, 10], and communication processes with respect to discussing patient prognosis and eliciting treatment goals [11, 12]. Increasingly, ICU admission decisions for critically ill patients are made by emergency physicians, hospitalists, and intensivists who do not have established relationships with the patient, adding levels of complexity to communication and decision making. Effective decision making now requires the rapid establishment of rapport and assessment of patient/family understanding of prognosis and goals of care.
Although there have been observational studies of physician communication [13] and decision making [14] focused on withholding or withdrawing life-sustaining treatments once in the ICU, there have been few studies of ICU admission decisions. Those that have been published focus on patient-specific clinical factors and outcomes associated with denial of ICU admission [7, 15–17]. Most of these studies were performed in Europe where limited ICU bed availability demands tight triage. None of them focused on the communication and decision making processes that impact treatment decisions and directly affect patient, family, and clinician outcomes [18].
The purpose of the current study was to assess whether simulation is a feasible way of studying variation in ICU admission decision making for a critically and terminally ill patient. Simulation offers several strengths over naturalistic observation of “real-world” decision making, including efficiency and automated data capture; avoiding patient privacy violations and complexities of consent during critical illness; and standardizing the clinical and psychosocial aspects of a case in order to isolate physician-specific determinants. The use of standardized patients and clinical simulation for education and evaluation are well-established methods for the assessment of simple communication tasks, like taking a history or breaking bad news, and time-pressured technical skills, such as running a code or intubating a difficult airway [19, 20]. However, simulation has not previously been used to study the time-pressured communication and decision scenario of ICU triage for a critically and terminally ill patient. To simulate this context, we augmented simulation technology with trained actors. This study reports qualitative assessments of the verisimilitude of a simulation to depict that decision context and a quantitative summary of physician treatment decisions and their correlates.
MATERIALS AND METHODS
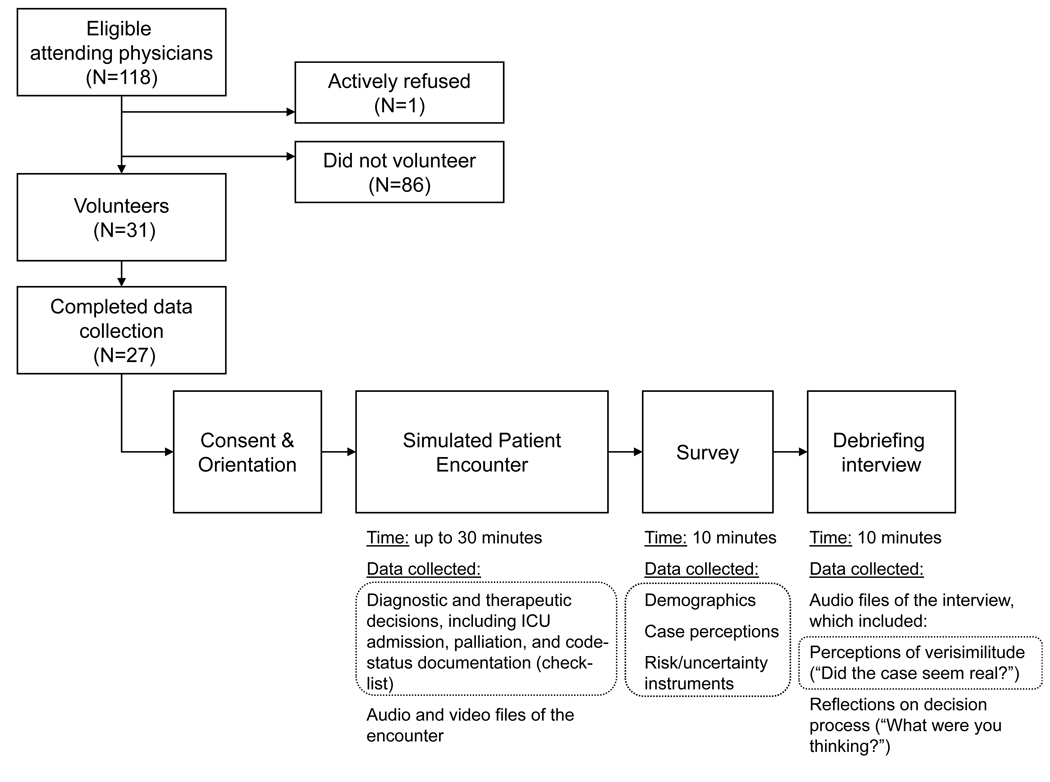
This is a mixed qualitative-quantitative study of hospital-based physician subjects’ simulation encounter at the University of Pittsburgh Peter M. Winter Institute for Simulation Education and Research (WISER). The scenario was depicted on a hospital set (emergency department set for emergency physicians, ward set for hospitalists and intensivists) by trained patient simulators (actors) playing the patient and his wife, with clinical data provided by an electronic medical record-based chart and bedside vital signs tracings on a monitor. We summarize recruitment and data collection procedures in the Figure.
Figure. Figure, Recruitment and study design.
Among 118 eligible attending physicians at one institution, 31 volunteered and 27 completed study data collection procedures. Study procedures included “role playing” a simulated encounter with a critically ill elderly man (accompanied by his wife) and completion of a post-simulation web-based survey and audio-recorded debriefing interview. Grey dashed rectangles encircle the specific data elements analyzed and reported in the current manuscript.
Simulation
Case Development
A multi-disciplinary team of physicians, including specialists in general internal medicine, palliative care, oncology, critical care, emergency medicine, and pathology contributed to case development. The case depicted a 78 year-old man with metastatic gastric cancer transferred to the hospital from a local nursing facility for shortness of breath. The clinical components of the case, including vital signs tracings, were refined in a series of pre-tests with 3 physician subjects using Sim-Man® technology alone. (SimMan® is a portable patient simulator with realistic anatomy and clinical functionality produced by Laerdal.) We then trained experienced patient simulators (actors) to play the roles of the patient and his wife based on a detailed symptom and psychosocial profile, and developed an electronic medical record-based chart containing all inpatient summaries, laboratory, pathology, and radiography reports over the last 6 months, inclusive of the patient’s full cancer course. We again pre-tested the simulation scenario with 2 physician subjects, using the actors accompanied by the chart and bedside vital sign tracings on a monitor (controlled by the SimMan® computer).
Clinical Scenario
We asked each physician to imagine that he or she had been called to the bedside by a nurse who was concerned about a gradually increasing oxygen requirement, increased respiratory rate, tachycardia, hypotension, and decreased oxygen saturation. Based upon University of Pittsburgh Medical Center policy, the patient would meet clinical criteria for a “condition C” (crisis intervention to summon a medical emergency team) and ICU admission. The chart revealed widely metastatic gastric cancer, including lymphangitic spread in the lungs, a spiral CT negative for pulmonary embolism, the assessment of the admitting physician that the shortness of breath and hypoxia were most likely due to cancer progression, though a possible pneumonia was being treated empirically, and a discharge summary 3 weeks prior from a 3-month hospital stay complicated by prolonged mechanical ventilation. The chart contained no advance care plan; however, if probed, the patient and wife would reveal that their underlying goal for care is comfort and that they have a preference for avoiding re-admission to the ICU, mechanical ventilation, and cardiopulmonary resuscitation. Responses to common questions were scripted; otherwise, the simulators were trained to follow response principles. We provide a screen shot of the video file depicting the room, vital signs tracing, and data capture and more detailed information about the simulation in an Appendix.
Subjects
We recruited emergency physicians from the Department of Emergency Medicine, hospitalists from the Division of General Internal Medicine, and intensivists from the Department of Critical Care Medicine at the University of Pittsburgh through in-person presentations at respective faculty meetings, e-mails to Department distribution lists, and calls or visits to physicians’ offices. At our institution, these 3 classes of physicians make decisions to admit patients to the ICU; only infrequently are decisions made by emergency physicians or hospitalists blocked by the intensivist on duty, which may occur if the patient does not meet clinical criteria for illness severity (i.e., not “sick enough”).
Data Collection
Two independent raters (AEB, HH) observed each simulation live and used a checklist to record physician decisions, represented by statements to the patient (“I’m going to turn up your oxygen now”) or to the investigator controlling the simulation (“I’d like to request a non-rebreather with 100% FiO2”). The primary outcome assessed by the raters was the ICU triage decision; secondary outcomes included diagnostic, therapeutic, and consultation requests during the simulation, and the final treatment plan. If subjects did not tell the patient the treatment plan explicitly before leaving the room, an investigator (AEB) asked the subject upon exit what his/her treatment plan would be. Disagreements between raters were resolved through discussion.
At the completion of the simulation experience, each subject completed a web-based survey to collect demographic information (age, sex, year of medical school graduation, specialty, year of residency and/or fellowship completion, years at University of Pittsburgh), perceptions of the simulated case (diagnosis underlying current decompensation, probability of surviving the current hospitalization, probability of 3-month survival, perception of the patient’s goals of care), risk attitude (modified 2-item Nightingale instrument [21, 22]), and responses to uncertainty (15-item Gerrity instrument) [23, 24].
After completion of the survey, one investigator (AEB) conducted a debriefing interview, asking each subject to provide a narrative report of their perceptions of the case verisimilitude. We assessed feasibility by the observed willingness of each physician to “suspend disbelief” and engage fully in the role-play, subjective reports of their experience during the debriefing interview, and survey items assessing the physicians’ diagnosis and prognosis for the case.
Analyses
One investigator (AEB) qualitatively assessed each physician’s report of case verisimilitude during the debriefing interview. A second investigator (HH) confirmed these qualitative assessments by listening to an audio recording of the interview. Both investigators also qualitatively assessed each physician’s open-ended response to the survey question “What is the cause of the patient’s current clinical deterioration?” to assess whether or not they identified cancer and the most likely culprit. There were no disagreements between investigators in their qualitative assessments of verisimilitude or diagnosis. We summarized subject characteristics, physician perceptions of prognosis, and simulation outcomes descriptively, using means and percentages. Because of the small sample size, we used non-parametric Mann-Whitney sign rank tests and Fisher’s exact tests, as appropriate, and explored only univariable, not multivariable, relationships between predictor variables and the primary outcome: ICU admission, and two secondary outcomes: palliation of dyspnea and documentation of code status. The variables role, residency, and fellowship were highly overlapping, as were age and years since medical school graduation, so we selected role and years since graduation as predictor variables. All statistical analyses were performed using STATA 9.0 (College Station, TX).
Human Subjects
The protocol, which required deliberate omission of the specific study outcome (ICU triage) from the consent form, was approved by the University of Pittsburgh Institutional Review Board. Subjects completed written informed consent with the understanding that they were participating in a study of treatment decisions for critically ill patients made by hospital based physicians who do not have an established relationship with the patient. After completing the simulation, each subject was debriefed with full details of the study aims and invited to withdraw participation after this disclosure.
RESULTS
Subjects
From among 118 eligible physicians, 31 volunteered and 27 completed the simulation. All physicians who were approached personally by the principal investigator (AEB) agreed to participate; those who did not volunteer did so passively (by ignoring “blast” e-mails; only 1 physician actively refused to participate by saying “no” to an e-mail); and 4 volunteers could not be scheduled due to time constraints.
We describe the sample of 27 subject physicians in Table 1. All hospitalists were general internists; one had sub-specialty training in palliative care. The intensivists were heterogeneous, including 3 internal medicine-, 2 general surgery- , 2 anesthesiology-, and 1 emergency medicine-trained physician(s). Overall, their mean age was 41 years, with an average of 15 years since medical school graduation and 10 years at the University of Pittsburgh. Two-thirds were men and over 80% were white. Most were risk averse over gains and losses.
Table 1.
Characteristics of the Study Physicians (N=27)
| Characteristic | Measure N=27 |
|---|---|
| Role, n (%) | |
| Emergency physician | 6 (22.2 %) |
| Hospitalist | 13 (48.1 %) |
| Intensivist | 8 (29.6 %) |
| Residency training, n (%) | |
| Emergency medicine | 7 (25.9 %) |
| General surgery | 2 (7.4 %) |
| Internal medicine | 16 (59.3 %) |
| Anesthesiology | 2 (7.4 %) |
| Fellowship type, n (%) | |
| Critical care/pulmonary critical care | 8/ (29.6 %) |
| No fellowship | 7 (25.9 %) |
| General medicine | 7 (25.9 %) |
| Emergency medicine research | 3 (11.1 %) |
| Palliative care | 1 (3.7 %) |
| Age, mean ± SD (range) | 41.3 ± 9.5 (30–67) |
| Years since medical school graduation, mean ± SD (range) | 15.3 ± 9.0 (4–41) |
| Years at the University of Pittsburgh, mean ± SD (range) | 10.2 ± 8.9 (1–37) |
| Female, n (%) | 9 (33.3 %) |
| Race, n (%) | |
| Non-Hispanic white | 22 (81.5 %) |
| Asian | 5 (18.5 %) |
| Risk Attitude, n (%) | |
| Risk averse, certain gain (vs. risk seeking) | 21 (77.8 %) |
| Risk averse, certain loss (vs. risk seeking) | 18 (66.7 %) |
| Physicians’ Reactions to Uncertainty, mean ±SD | |
| Anxiety due to uncertainty subscale (possible range: 5–30) | 15.1 ±5.1 |
| Concern about bad outcomes subscale (possible range: 3–18) | 8.5 ±3.6 |
| Reluctance to disclose uncertainty to patients subscale (possible range: 5–30) | 10.8 ±3.9 |
| Reluctance to disclose mistakes to physicians subscale (possible range: 2–12) | 4.2 ±1.7 |
Qualitative Assessment of Verisimilitude
All (100%) reported that the case and simulation were highly realistic, as described by one subject during the debriefing interview:
It was very realistic, uh, it definitely, uh, emotionally evoked the same response as I would have in a clinical scenario and it did not feel the least bit contrived. It was good. Good simulation. I thought it was realistic. It had a lot of face validity, um, I didn’t think it was far-fetched…I have seen – been in situations like this and I found myself, uh, feeling the same emotions that I do clinically – um, fear to say death, fear of saying you’re going to die, alluding to it in general terms, um, probing gently to see how far the patient understands his or her condition. Um, I found myself doing all those same automatic things I do in real practice.
Another subject explained: “Very quickly [after entering the room] it became real…I was thinking that, um, this patient was critically ill and either that he had to go to the ICU or we had to decide that we were going make him comfort measures.” Additionally, subjects’ diagnostic and prognostic assessments were consistent with our intent. Specifically, the majority (78%) of physicians explicitly identified cancer as the probable underlying cause of the patient’s dyspnea; 6 (22%) did not mention cancer in their differential, instead implicating acute derangements such as pneumonia, sepsis, and pulmonary embolism. In response to the closed-ended survey question about prognosis with probabilities provided in 10% increments, 12 (44%) of the physicians estimated that the patient’s probability of surviving the hospitalization was 0–9%, 11 (41%) estimated it as 10–19%, 2 (7%) as 20–29%, 1 (4%) as 30–39% and 1 (4%) as 50–59%. Twenty-three (85%) estimated that the patient’s probability of surviving beyond 3 months was 0–9%, 4 (15%) estimated it as 10–19%.
Descriptive Summary of Treatment Decisions
During the simulation, 8 (30%) of the physicians initiated non-invasive positive pressure ventilation. For their final treatment plan, 8 (30%) admitted the patient to the ICU. Among the 8 physicians who admitted the patient to the ICU, 3 (37%) initiated palliation for dyspnea, 2 (25%) documented the patient’s code status (DNI/DNR), and 1 intubated the patient. Among the 19 physicians who did not admit the patient to the ICU, 13 (68%) initiated palliation for dyspnea and 5 (42%) documented code status.
Predictors of Treatment Decisions
In univariable, non-parametric tests of association, intensivists and emergency physicians were more likely to admit the patient to the ICU (p=0.048), and years since medical school graduation were inversely associated with the initiation of palliation (p=0.043); there were no predictors of code status documentation (Table 2). Risk attitude and reactions to uncertainty were not correlated with any of the decision outcomes. We did not explore the relationship between prognostic estimate and treatment plan because these estimates were obtained after the simulation and were thus contingent upon the chosen treatment plan.
Table 2.
Univariable Relationship between Physician Characteristics and Treatment Plan (N=27)
| Variable | ICU | P-value* | Palliation | P-value* | Code status | P-value* |
|---|---|---|---|---|---|---|
| Sex, n/N (%) | 0.676 | 0.244 | 1.00 | |||
| Male | 6/18 (33%) | 12/18 (67%) | 5/18 (28%) | |||
| Female | 2/9 (22%) | 4/9 (44%) | 2/9 (22%) | |||
| Role, n/N (%) | 0.048 | 1.00 | 1.00 | |||
| Emergency physician | 3/6 (50%) | 3/6 (50%) | 2/6 (33%) | |||
| Hospitalist | 1/15 (8%) | 8/13 (62%) | 3/13 (23%) | |||
| Intensivist | 4/8 (50%) | 5/8 (63%) | 2/ 8 (25%) | |||
| Years since graduation, difference | 1.6 years | 0.613 | −8.0 years | 0.043 | 1.9 years | 0.421 |
| Risk Attitude – certain gain, n/N (%) | 1.00 | 0.160 | 0.633 | |||
| Risk averse | 6/21 (29%) | 14/21 (67%) | 5/21 (24%) | |||
| Risk seeking | 2/6 (33%) | 2/6 (33%) | 2/6 (33%) | |||
| Risk Attitude – certain loss, n/N (%) | 0.201 | 0.551 | 0.363 | |||
| Risk averse | 7/18 (39%) | 11/18 (61%) | 6/18 (33%) | |||
| Risk seeking | 1/9 (11%) | 5/9 (56%) | 1/9 (11%) | |||
| PRU score, difference | ||||||
| Anxiety due to uncertainty | 1.8 | 0.241 | 1.7 | 0.488 | −0.6 | 0.934 |
| Concern about bad outcomes | 0.4 | 0.915 | 0.6 | 0.942 | −0.7 | 0.656 |
| Reluctance to disclose uncertainty to patients | 0.5 | 0.852 | −1.7 | 0.244 | 1.6 | 0.331 |
| Reluctance to disclose mistakes to physicians | −0.6 | 0.645 | 0.2 | 0.810 | 1.1 | 0.099 |
p-values reflect Fisher exact tests for dichotomous predictors and Mann-Whitney rank tests for continuous predictors
PRU – Physicians’ Reactions to Uncertainty.
DISCUSSION
In this pilot study of 27 experienced hospital-based physicians from one academic medical center, we established that simulation technology augmented by simulated patients is a feasible method for studying physician decision making for critically ill patients in time-pressured situations. Subjects uniformly endorsed the realism of this particular clinical scenario and reported the experience of “suspension of disbelief” that enabled them to engage with the simulated patients and treat the encounter as if it were real. This highly promising technology allowed us to standardize clinical and psychosocial aspects of the case in order to measure physician-specific attributions of decision making; to circumvent the ethical and logistical barriers to studying decision making in the “real” world; and to capture high quality data for analyses.
Although the patient and his wife had stable and informed preference for avoiding ICU admission, mechanical ventilation and cardiopulmonary resuscitation, when study physicians were confronted with dyspnea, hypoxia, and hypotension, they varied substantially in their decision to either admit the patient to the ICU for respiratory intubation or retain the patient on the floor, usually in anticipation of death. Only 13 (48%) physicians fully treated patient and in accordance with his goals and preferences (not admitting him to the ICU and initiating palliation for his dyspnea). The only significant predictors of behavior in this small sample were that intensivists and emergency physicians were more likely than hospitalists to admit to the ICU and those who had been more recently trained were more likely to initiate palliation for dyspnea.
It is important to note that physicians who admitted the patient to the ICU did not simply fail to ascertain the patient’s preference for avoiding ICU admission; indeed 75% admitted the patient to the ICU despite ascertainment of his preference not to be admitted. Such overruling or disavowal usually focused on the goal of “stabilizing” the patient; it mostly involved the physician’s stipulation that a trial of aggressive treatment was warranted, but sometimes the stipulation that the ICU was better suited for providing attentive palliative care. Our detailed qualitative analyses of the simulation encounters and the physician debriefing interviews to elucidate physician rationalizations are beyond the scope of the current manuscript.
The findings from the current study add physician specialty (and likely other unmeasured idiosyncrasies) to the known list of influences on ICU admission decisions, including ICU bed availability [5–7], inaccuracy in prognostication [8], and patient age and comorbidity [7, 15–17]. Our study raises additional concerns about variations in palliation and code status documentation. Although the promise of evidence-based medicine has increased our expectations of uniform care delivery, most clinical decisions still fall outside of the purview of nationally-promulgated guidelines. Decisions that reflect patient values, sometimes called preference-sensitive care, need to be discovered through negotiation between doctors and patients. However, our study demonstrates that doctors faced with a “life and death” preference-based decision do not reliably engage in this negotiation and thus come to different treatment decisions. This underscores the importance of better understanding and targeting physician communication and decision making for intervention in such situations – a goal best achieved in the simulation setting. Simulation-based training is a promising new approach for improving complex cognitive skills in high-stress situations [25–29].
Our findings are consistent with many prior studies implicating physicians’ role in observed variations in treatment. Many vignette-based studies have documented variation in decision making across heterogenous samples of physicians to understand such phenomena as regional and cross-national variations in aggregate spending and procedure use [22, 30, 31], racial variations in health care utilization [32], and variations in willingness to withhold or withdraw life-sustaining treatments [33–37]. Observational studies, such as Cook and colleagues’ multi-center study of 15 ICUs across the US and Canada found that the physician's perception that the patient preferred not to use life support, the physician's predictions of a low likelihood of survival in the intensive care unit and a high likelihood of poor cognitive function were among the strongest determinants of the withdrawal of ventilation in critically ill patients, not age or the severity of the illness and organ dysfunction [14]. This finding was disturbing because the 5-center Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT) trial and many other studies raise strong doubts about accuracy of physicians’ beliefs about patient preferences [38]. Another observational study of 9 intensivists in a single center by Garland and colleagues demonstrated that individual physicians had more influence on costs than all other variables except the severity and type of acute illness, with average daily discretionary costs varying 43% across physicians [39].
Our power to draw conclusions about the mechanisms underlying physician-specific variation in decision making is limited by our small pilot sample and the abbreviated collection of data about study subjects. Nonetheless, the fact that decision making was influenced by specialty and years since graduation from medical school suggests that training, experience or social norms may play a role. Further study of these factors is clearly warranted. Our primary aim, however, was to demonstrate feasibility, and in this effort we have been successful. Although it may be considered a limitation that our subjects were recruited from a single academic medical center, impairing generalizability, this design feature could also be seen as a strength, given that we found substantial variation despite physicians’ practice within a single facility governed by the same formal norms. It is possible that physician behavior in the simulation was not identical to the way they might have behaved in a “real” clinical scenario, particularly if they were aware of the investigators’ interests in end-of-life ICU use and patient-doctor communication. If anything, this would bias our findings towards more patient-centered care. Indeed, two participants wondered during their debriefing interview whether in a real case they might have reflexively admitted to the ICU rather than exploring treatment preferences and offering palliation in a ward bed. Despite these limitations, ours is the first study to our knowledge to use simulation to explore physician variation in ICU admission decision making.
CONCLUSIONS
Simulation can reproduce the decision context of ICU triage for a critically ill patient with terminal illness. When faced with an identical patient, hospital-based physicians from the same institution vary significantly in their treatment decisions. Skills-based training using simulation may offer an opportunity for physicians to practice and improve time-pressured communication and decision making tasks to reduce variation and better align with patient treatment preferences.
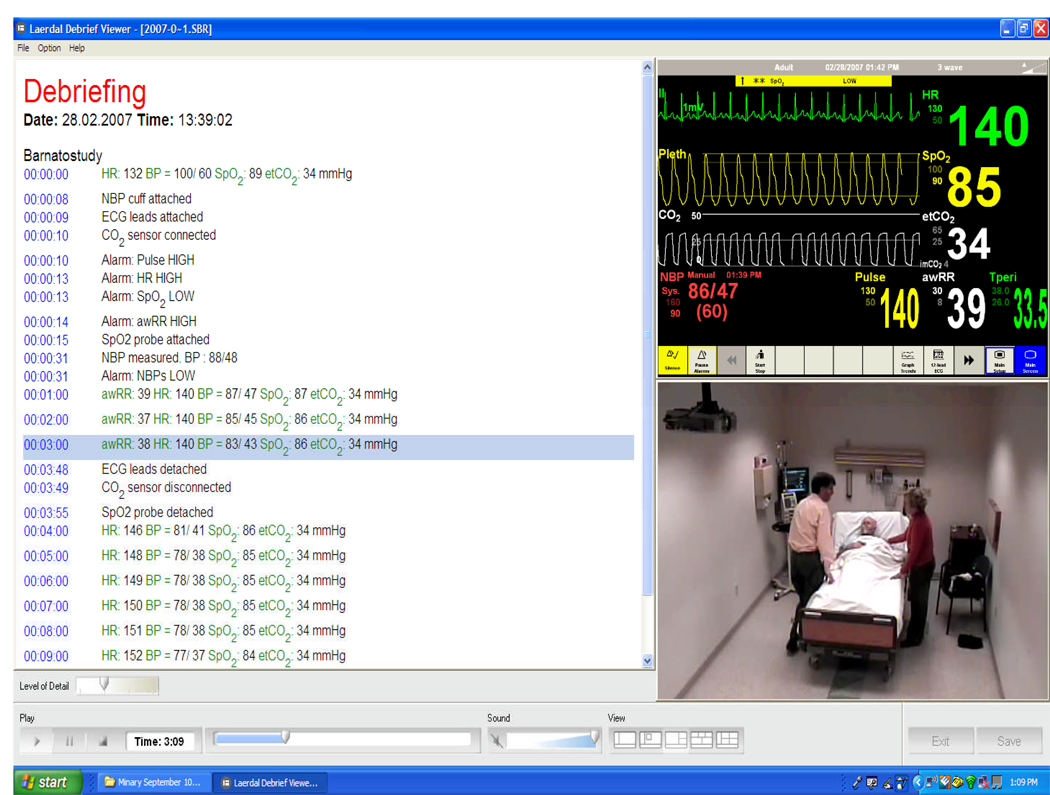
Appendix Figure. Appendix Figure, Screen shot of the recorded simulation.
This image represents a screen shot of the data captured during each subject’s simulation encounter (the physician in the image is a co-investigator, not a study subject). In the left panel is a time-based recording of events, the upper right panel is the vital sign tracing on the monitor behind the patient’s bed, and the lower right panel is a video image of the room, with the physician and the actors playing the patient and his wife.
ACKNOWLEDGMENTS
The authors thank many University of Pittsburgh faculty and staff for their contributions: Kenneth Smith, Mark Roberts, and Eric Milbrandt for pre-testing the simulated cases; Paul Phrampus, Thomas Dongilli, Jon Mazur, and Christine Barton at WISER for technical support and simulation resources; Melissa Saul for the de-identified electronic medical records upon which the case materials were based; Terri Martin for de-identified radiographs; Steven Raab for the fictitious pathology report; and the 27 attendings who volunteered their time as study subjects.
Source of support: Dr. Barnato was supported by a career development award from the National Institute on Aging (K08 AG21921), this research project was supported by a pilot grant from the University of Pittsburgh Institute to Enhance Palliative Care.
APPENDIX – SIMULATION DETAILS
The case was presented to each subject as a “bullet,” supported by printed, typed components of an electronic medical record (EMR), including admission history and physical (H&P for inpatient physicians; ED evaluation for emergency physicians) including assessment and plan; laboratory and radiology reports for the current admission and the prior admission; discharge and operative summary from the discharge 3 weeks earlier, and a pathology report. Radiology reports for chest radiographs, chest, abdomen, and pelvis computed tomography for the current and prior admission were drawn from de-identified reports from a patient in the University of Pittsburgh Medical Archival System (MARS); other materials were fictional, but modeled upon the EMR reports of one investigator’s (LE) patients.
We instructed the subjects to treat the interaction as they would a real encounter. The only exception was that the subjects were allowed to ask the investigator controlling the simulation (AEB) for information that could not be obtained from the patient or wife (e.g., physical examination findings), or for treatments (e.g., increased oxygen, fluid bolus). The only new information provided in response to subject query was physical examination findings; all other requests, such as for new laboratory or radiographic tests, were met with the response “the test results are unchanged from the most recent information available in the chart.”
The patient’s primary symptoms were progressive dyspnea, air hunger, and anxiety without pain. If probed, the wife and patient would report that they understood from the oncologist at their last visit 1 week ago that the cancer was metastatic and that there were no treatment options to slow the progress of the cancer; that their underlying goal for care is comfort; and that they have a preference for avoiding re-admission to the ICU, mechanical ventilation, and cardiopulmonary resuscitation based upon their recent experience with a complicated 3-month hospital admission requiring prolonged mechanical ventilation. Upon specific questioning, the wife would report that they had a “living will” at home and that the patient had a “do not resuscitate” order in the nursing home (neither of which were transmitted with the patient).
Responses to common questions were scripted; otherwise, the simulators were trained to follow response principles. They only answered directly posed questions; otherwise, the wife was to mirror the physicians’ statements. For example, if the doctor said: “If we admit your husband to the ICU, we can fix his low oxygen level and help his breathing,” the wife was to respond “Is that what you are suggesting?” If the physician strongly recommended a course of action, the wife was to acquiesce; if the family was given more than one treatment option, they would ask the physician to explain the options in detail and then choose the least intensive option.
The vital signs tracings, reflecting steady deterioration over the simulation, were unresponsive to all interventions ordered by physicians, with the exception of narcotics, which decreased respiratory rate. The patient’s level of consciousness waned slowly over time, and more abruptly with administration of narcotics. If the physician ordered non-invasive positive pressure ventilation (BiPAP), the investigator told the subject over the microphone “We’ve begun BiPAP. Now, imagine 3 hours have elapsed. The patient’s vital signs are as you see them now [unchanged] and he’s not tolerating BiPAP. He wants it off.” We halted the simulation when the physician articulated a plan and left the room or 30 minutes elapsed, whichever came first.
REFERENCES
- 1.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633–638. [PubMed] [Google Scholar]
- 3.Azoulay E, Pochard F, Chevret S, Vinsonneau C, Garrouste M, Cohen Y, Thuong M, Paugam C, Apperre C, De Cagny B, et al. Compliance with triage to intensive care recommendations. Crit Care Med. 2001;29(11):2132–2136. doi: 10.1097/00003246-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Afessa B. The intensive care support of patients with malignancy: do everything that can be done. Intensive Care Med. 2006;32(1):3–5. doi: 10.1007/s00134-005-2835-6. [DOI] [PubMed] [Google Scholar]
- 5.Strauss MJ, LoGerfo JP, Yeltatzie JA, Temkin N, Hudson LD. Rationing of intensive care unit services. An everyday occurrence. Jama. 1986;255(9):1143–1146. [PubMed] [Google Scholar]
- 6.Singer DE, Carr PL, Mulley AG, Thibault GE. Rationing intensive care--physician responses to a resource shortage. N Engl J Med. 1983;309(19):1155–1160. doi: 10.1056/NEJM198311103091905. [DOI] [PubMed] [Google Scholar]
- 7.Sinuff T, Kahnamoui K, Cook DJ, Luce JM, Levy MM. Rationing critical care beds: a systematic review. Crit Care Med. 2004;32(7):1588–1597. doi: 10.1097/01.ccm.0000130175.38521.9f. [DOI] [PubMed] [Google Scholar]
- 8.Smith WR, Poses RM, McClish DK, Huber EC, Clemo FL, Alexander D, Schmitt BP. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest. 2002;121(5):1610–1617. doi: 10.1378/chest.121.5.1610. [DOI] [PubMed] [Google Scholar]
- 9.Zussman R. Intensive Care: Medical Ethics and the Medical Profession. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- 10.Cassell J. Life and Death in Intensive Care. Philadelphia, PA: Temple University Press; 2005. [Google Scholar]
- 11.Kaufman SR. And a Time to Die: How American Hospitals Shape the End of Life. New York, NY: Scribner; 2005. [Google Scholar]
- 12.Christakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. Chicago, IL: The University of Chicago Press; 1999. [Google Scholar]
- 13.McDonagh JR, Elliott TB, Engelberg RA, Treece PD, Shannon SE, Rubenfeld GD, Patrick DL, Curtis JR. Family satisfaction with family conferences about end-of-life care in the intensive care unit: increased proportion of family speech is associated with increased satisfaction. Crit Care Med. 2004;32(7):1484–1488. doi: 10.1097/01.ccm.0000127262.16690.65. [DOI] [PubMed] [Google Scholar]
- 14.Cook D, Rocker G, Marshall J, Sjokvist P, Dodek P, Griffith L, Freitag A, Varon J, Bradley C, Levy M, et al. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349(12):1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 15.Sprung CL, Geber D, Eidelman LA, Baras M, Pizov R, Nimrod A, Oppenheim A, Epstein L, Cotev S. Evaluation of triage decisions for intensive care admission. Crit Care Med. 1999;27(6):1073–1079. doi: 10.1097/00003246-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27(9):1459–1465. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- 17.Garrouste-Orgeas M, Montuclard L, Timsit JF, Reignier J, Desmettre T, Karoubi P, Moreau D, Montesino L, Duguet A, Boussat S, et al. Predictors of intensive care unit refusal in French intensive care units: a multiple-center study. Crit Care Med. 2005;33(4):750–755. doi: 10.1097/01.ccm.0000157752.26180.f1. [DOI] [PubMed] [Google Scholar]
- 18.Boyle DK, Miller PA, Forbes-Thompson SA. Communication and end-of-life care in the intensive care unit: patient, family, and clinician outcomes. Crit Care Nurs Q. 2005;28(4):302–316. doi: 10.1097/00002727-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Proceedings of the AAMC'S Consensus Conference on the Use of Standardized Patients in the Teaching and Evaluation of Clinical Skills; Acad Med; December 3–4, 1992; Washington, D.C. 1993. pp. 437–483. [PubMed] [Google Scholar]
- 20.Weller JM. Simulation in undergraduate medical education: bridging the gap between theory and practice. Med Educ. 2004;38(1):32–38. doi: 10.1111/j.1365-2923.2004.01739.x. [DOI] [PubMed] [Google Scholar]
- 21.Nightingale SD, Grant M. Risk preference and decision making in critical care situations. Chest. 1988;93(4):684–687. doi: 10.1378/chest.93.4.684. [DOI] [PubMed] [Google Scholar]
- 22.Poses RM, De Saintonge DM, McClish DK, Smith WR, Huber EC, Clemo FL, Schmitt BP, Alexander-Forti D, Racht EM, Colenda CC, 3rd, et al. An international comparison of physicians' judgments of outcome rates of cardiac procedures and attitudes toward risk, uncertainty, justifiability, and regret. Med Decis Making. 1998;18(2):131–140. doi: 10.1177/0272989X9801800201. [DOI] [PubMed] [Google Scholar]
- 23.Gerrity MS, White KD, DeVellis RF, Dittus RS. Physician's reactions to uncertainty: refining the constructs and scales. Motivation and Emotion. 1995;19:175–191. [Google Scholar]
- 24.Gerrity MS, DeVellis RF, Earp JA. Physicians' reactions to uncertainty in patient care. A new measure and new insights. Med Care. 1990;28(8):724–736. doi: 10.1097/00005650-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40(3):254–262. doi: 10.1111/j.1365-2929.2006.02394.x. [DOI] [PubMed] [Google Scholar]
- 26.Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70–S81. doi: 10.1097/00001888-200410001-00022. [DOI] [PubMed] [Google Scholar]
- 27.Fritz PZ, Gray T, Flanagan B. Review of mannequin-based high-fidelity simulation in emergency medicine. Emerg Med Australas. 2007 doi: 10.1111/j.1742-6723.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 28.Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10–28. doi: 10.1080/01421590500046924. [DOI] [PubMed] [Google Scholar]
- 29.Kneebone R. Evaluating clinical simulations for learning procedural skills: a theory-based approach. Acad Med. 2005;80(6):549–553. doi: 10.1097/00001888-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Sirovich BE, Gottlieb DJ, Welch HG, Fisher ES. Variation in the tendency of primary care physicians to intervene. Arch Intern Med. 2005;165(19):2252–2256. doi: 10.1001/archinte.165.19.2252. [DOI] [PubMed] [Google Scholar]
- 31.Winslow C, Kosecoff J, Chassin M, Kanouse D, Brook R. The appropriateness of performing coronary artery bypass surgery. JAMA. 1988;260(4):505–509. [PubMed] [Google Scholar]
- 32.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, et al. The effect of race and sex on physicians' recommendations for cardiac catheterization. [see comments]. [erratum appears in N Engl J Med 1999 Apr 8;340(14):1130] New England Journal of Medicine. 1999;340(8):618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 33.Christakis NA, Asch DA. Physician characteristics associated with decisions to withdraw life support. Am J Public Health. 1995;85(3):367–372. doi: 10.2105/ajph.85.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson LC, Danis M, Garrett JM, Multran E. Who decides? Physicians' willingness to use life-sustaining treatment. Archives of Internal Medicine. 1996;156(7):785–789. doi: 10.1001/archinte.156.7.785. [DOI] [PubMed] [Google Scholar]
- 35.Kelly WF, Eliasson AH, Stocker DJ, Hnatiuk OW. Do specialists differ on do-not-resuscitate decisions? Chest. 2002;121(3):957–963. doi: 10.1378/chest.121.3.957. [DOI] [PubMed] [Google Scholar]
- 36.Swanson JW, McCrary SV. Doing all they can: physicians who deny medical futility. Journal of Law, Medicine & Ethics. 1994;22(4):318–326. doi: 10.1111/j.1748-720x.1994.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 37.Wenger NS, Carmel S. Physicians' religiosity and end-of-life care attitudes and behaviors. Mt Sinai J Med. 2004;71(5):335–343. [PubMed] [Google Scholar]
- 38.Anonymous: A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274(20):1591–1598. [PubMed] [Google Scholar]
- 39.Garland A, Shaman Z, Baron J, Connors AF., Jr Physician-attributable differences in intensive care unit costs: a single-center study. Am J Respir Crit Care Med. 2006;174(11):1206–1210. doi: 10.1164/rccm.200511-1810OC. [DOI] [PubMed] [Google Scholar]