Abstract
To better define the pattern of hippocampal deformity early in the course of Alzheimer’s disease, we compared the pattern of hippocampal surface variation in subjects with very mild dementia of the Alzheimer type (DAT) and nondemented subjects. The surface of the hippocampus was divided a priori on a neuroanatomical template into three zones approximating the locations of underlying subfields [Csernansky, J.G., Wang, L., Swank, J., Miller, J.P., Gado, M., McKeel, D., Miller, M.I., Morris, J.C., 2005. Preclinical detection of Alzheimer’s disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage 25, 783–792]; i.e., a lateral zone (LZ) approximating the CA1 subfield, a superior zone (SZ) approximating the combined CA2, CA3, CA4 subfields and the gyrus dentatus (GD), and an inferior-medial zone (IMZ) approximating the subiculum. Large-deformation high-dimensional brain mapping (HDBM-LD) was used to generate the hippocampal surfaces of all subjects and to register the surface zones across subjects. Average variations within each zone were calculated for the subjects with very mild DAT as compared to the average of the nondemented subjects. After correcting for multiple comparisons, the very mild DAT subjects showed significant inward variation in the left and right LZ, the left and right IMZ, but not in the left and right SZ as compared to nondemented subjects. In logistic regression analyses, inward variation of the left and right LZ or IMZ by 0.1 mm relative to the average of the nondemented subjects increased the odds of the subject being a very mild DAT subject (range—1.18 to 1.57) rather than being a nondemented subject. The odds ratios for the left and right SZ were not significant. These results represent a replication of our previous findings [Csernansky, J.G., Wang, L., Joshi, S., Miller, J.P., Gado, M., Kido, D., McKeel, D., Morris, J.C., Miller, M.I., 2000. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology 55, 1636–1643.] and suggest that inward deformities of the hippocampal surface in proximity to the CA1 subfield and subiculum can be used to distinguish subjects with very mild DAT from nondemented subjects.
Introduction
The neuropathology of Alzheimer’s disease (AD) is characterized by the accumulation of neurofibrillary tangles (NFT) and amyloid plaques (AP) in the cerebral cortex and structures of the medial temporal lobe, including the hippocampus (Bobinski et al., 1998, 1997; Mizukami et al., 1998; Muramori et al., 1998; Price and Morris, 1999; Sheng et al., 1998). Early in the course of AD, NFTs as well as threads of neurofilaments within dendrites that display cytoskeletal anomalies similar to NFTs appear in the hippocampus, especially within the hippocampal CA1 subfield and subiculum, and gradually increase in density with increasing severity of AD as defined by neuropathological criteria (Braak and Braak, 1991; Corder et al., 2000). There is also a correlation between the density of NFTs in the hippocampus and the severity of dementia as defined by the Clinical Dementia Rating (CDR) scale (Thal et al., 2000).
In vivo magnetic resonance (MR) imaging studies have reported substantial volume loss in the hippocampus in subjects with very mild or mild dementia of the Alzheimer type (DAT) (Dickerson et al., 2001; Du et al., 2001; Fox et al., 1996; Jack et al., 1997; Killiany et al., 2002). However, structural deformation of the substructures of the hippocampus in early DAT has not been adequately examined. In one recent MR imaging study, atrophy of the CA1 subfield and subiculum was revealed by measuring the width of these substructures (in the coronal plane) in subjects with mild or moderate AD (Adachi et al., 2003).
In previous MR imaging studies, we reported that subjects with very mild DAT could be discriminated from nondemented controls by examining the pattern of hippocampal surface variation. However, in those studies, we did not specifically hypothesize the relationship between DAT and specific hippocampal subfields, even though inward variation of the head and lateral surface of the body of the hippocampus, a pattern that is consistent with degeneration of the CA1 subfield and subiculum, was observed in the subjects with very mild DAT (Csernansky et al., 2000; Wang et al., 2003). Also, in a study of elderly nondemented subjects who later progressed to having very mild DAT, we predefined surface zones approximating the subfields of the hippocampus on a neuroanatomical template and found that time-to-conversion was predicted by an inward variation of the zone approximating the CA1 subfield of the left hippocampus (Csernansky et al., 2005).
In the current study, we again used a hippocampal template with predefined zones approximating the location of the underlying cellular subfields on its surface (Csernansky et al., 2005) to derive the normative pattern of hippocampal surface variation in a healthy population of individuals ranging in age from 50 to 90 years. Variations away from the average normative surface were then computed for a population of subjects with very mild DAT. We specifically tested the hypothesis that inward variation of the hippocampal surface zones approximating the CA1 subfield and subiculum would discriminate the subjects with very mild DAT from nondemented subjects of the same age range.
Subjects and methods
Subject selection and assessment
Forty-nine subjects with very mild DAT and 86 nondemented comparison subjects participated in this study. Sixty-six of the 86 healthy, nondemented subjects were community-dwelling and enrolled in longitudinal studies of healthy aging and dementia at the Alzheimer Disease Research Center (ADRC) at Washington University School of Medicine. An additional 20 healthy, non-demented subjects were recruited from the same geographical community as the ADRC subjects as part of a study of schizophrenia at the Conte Center for the Neuroscience of Mental Disorders at Washington University School of Medicine. Members of families with genetic mutations related to AD were excluded. Also, subjects were excluded if they had other neuropsychiatric disorders that could have confounded the diagnosis of DAT.
All subjects were assessed at the time of MR scanning using a standard protocol that included semi-structured interviews with the subject and a collateral source (generally the spouse or an adult child) who was knowledgeable about the subject. A neurological examination of the subject was also performed. The diagnosis of DAT was based on information derived from the assessment indicating that the subject had experienced the gradual onset and progression of memory and other cognitive deficits. These deficits must have represented a decline from the subject’s prior abilities and have interfered with usual activities in the community and at home (American Psychiatric Association, 1994). The diagnosis and staging of DAT was made without reference to psychometric test results or neuroimaging data. The diagnosis of very mild DAT as used in our sample has been previously validated by neuropathologic findings characteristic of AD in 93% of similar cases (Berg et al., 1998).
The Clinical Dementia Rating scale (CDR) (Morris, 1993) was performed in the ADRC participants to assess the severity of dementia symptoms. The CDR rates the presence or absence of cognitive impairment on a 5 point scale (0, 0.5, 1, 2, 3—from none to severe) in 6 domains or “boxes”: (1) memory, (2) orientation, (3) judgment and problem solving, and (4) function in the community, (5) function at home and hobbies, and (6) personal care. A global CDR score is then derived using the individual ratings in the 6 domains using scoring rules (Morris, 1993), such that a global CDR score of 0 indicates no dementia and scores of 0.5, 1, 2, and 3 indicate very mild, mild, moderate and severe dementia, respectively. The individual box scores can also be totaled to yield a continuous measure of dementia severity (i.e., sum-of-the-boxes score) that ranges from 0 (0 × 6 when there is no impairment in any domain) to 18 (3 × 6 when there is maximal impairment in all domains). CDR assessments have been shown to have high interrater reliability when used at our ADRC (Berg et al., 1998) and in multicenter studies of DAT (Morris et al., 1997).
All DAT subjects were rated as having very mild dementia (i.e., CDR 0.5). Individuals meeting ADRC criteria for very mild dementia at the CDR 0.5 stage predictably progress to greater dementia severity with time and at autopsy are highly likely to have histopathological AD (Morris et al., 2001). Investigators elsewhere may characterize at least some of these very mildly demented individuals as having mild cognitive impairment rather than DAT. Conversely, the 66 healthy, nondemented subjects recruited from the ADRC were rated as having no dementia (i.e., CDR 0). The remaining healthy, nondemented subjects recruited as comparison subjects for the schizophrenia study were not rated for dementia severity using the CDR, but no dementia symptoms were identified on a structured diagnostic interview. The mean (SD) age of all healthy, nondemented subjects was 73.4 (11) years and the mean age of DAT subjects was 74.9 (7.8). Gender distribution (M/F) of the subjects was 29/57 for the nondemented subjects and 23/26 for the very mild DAT subjects (see Table 1).
Table 1.
Subject information
| Group | N | M/F ratio | Age (years) |
|---|---|---|---|
| Control (CDR 0) | 86 | 29/57 | 73.4 (11) [50.0–90.8] |
| DAT (CDR 0.5) | 49 | 23/26 | 74.9 (7.8) [50.2–89.6] |
Magnetic resonance imaging and image pre-processing
An MR scan was obtained in each subject using a Magnetom SP-4000 1.5 T imaging system, a standard head coil, and a 3D turbo-FLASH sequence (TR = 20 ms, TE = 5.4 ms, flip angle = 30°, 1-mm section thickness, 180 slices, 256-mm field of view, matrix = 256 × 256, number of acquisitions = 1, scanning time = 13.5 min), which produced 3D data with 1 mm3 isotropic resolution across the entire cranium (Venkatesan and Haacke, 1997). Signed 16-bit MR datasets were compressed to unsigned 8-bit MR datasets by linearly rescaling the voxel intensities such that the voxels with intensity levels at two standard deviations above the mean of white matter (corpus callosum) were mapped to 255, and the voxels with intensity levels at two standard deviations below the mean of CSF (lateral ventricle) were mapped to 0. The white matter and CSF means and standard deviations were obtained from sampling of voxels from the respective regions. The regions of interest for both the corpus callosum and the lateral ventricle were drawn well within their anatomical boundaries to avoid partial-volume effect of the MR scans.
Generation of a template hippocampus with surface zones corresponding to underlying subfields
An MR scan from a healthy, nondemented subject (male, age 69, CDR 0) was used to produce the provisory neuroanatomical template. This subject was randomly selected from the group of healthy nondemented subjects, and was not otherwise included in the data analysis. A template representation of the hippocampal surface, including three zones corresponding to various underlying cellular subfields were manually delineated as previously described (Csernansky et al., 2005). Briefly, a team of experts (LW, MG, and DM) using Analyze-AVW software (Analyze-AVW, 2004) manually outlined the hippocampus in the template MR scan. The surface of the template hippocampus was then constructed as a triangulated graph (made up of thousands of points and triangular facets) at the boundary of the manual delineation. The manual outline of the hippocampus was further segmented by the same team of experts into subvolumes corresponding to the CA1, CA2, CA3, CA4 subfields, the gyrus dentatus (GD) and the subiculum, using a neuroanatomical atlas (Duvernoy, 1988). The hippocampal surface was then partitioned by determining the intersection of these subvolume segmentations with the surface. This procedure created three zones on the surface of the template hippocampus that were proximal to different underlying subfields or grouping of subfields; i.e., a lateral zone (LZ) proximal to the CA1 subfield, a superior zone (SZ) proximal to the combined CA2, CA3, CA4 subfields and the GD (NB:CA2–4 and GD were combined because the representation on the surface for each subfield was much smaller than either the CA1 or subiculum), and an inferior-medial zone (IMZ) proximal to the subiculum (see Fig. 1 in Csernansky et al., 2005).
High dimensional diffeomorphic mapping of the hippocampus
Transformations of the MR image containing the provisory hippocampal template onto the target MR images occurred in two-steps. First, the template scans and each of the target images were coarsely aligned using landmarks that had previously been placed in the template image and in the scans of all subjects in the study. These landmarks were placed at external brain boundaries, at points where the anterior and posterior commissures intersected the mid-sagittal plane, and along the boundary of each hippocampus in accordance with its principal axis, as previously described (Haller et al., 1997). Second, the precise mapping of the template image to each of the target images was defined by high dimensional diffeomorphic (i.e., invertible, continuous and differentiable) transformations. Movements of the voxels in the template MR image during these transformations were constrained by assigning the matrix of voxels the physical properties of a viscous fluid (Christensen et al., 1996). The inter-rater reliability of landmark placements as measured by hippocampal volume differences (mean 3.5%, SD 2.4%) has been previously reported (Haller et al., 1997).
Measurement of hippocampal volume and surface variation
To quantify variation of hippocampal volume and the hippocampal surface zones, the surfaces of the left and right template hippocampus, including the three zones predefined to approximate the underlying subfields, were carried along as the template image was transformed to match each of the target images. Left and right hippocampal volumes in each subject were then calculated as the volumes enclosed by the transformed hippocampal surfaces. The reliability and validity of hippocampal volume measurements derived from these transformations have been demonstrated with respect to expert manual outlining of the hippocampus in younger subjects (Haller et al., 1997) and older subjects with and without dementia (Csernansky et al., 2000; Hsu et al., 2002). Values estimating the overlap of hippocampal surface contours produced by these transformations versus manual outlining exceed 80% in such subjects, which is comparable to the accuracy of repeated attempts at manual outlining of the hippocampal surface by the same expert (Haller et al., 1997). Further, we manually outlined the three hippocampal surface zones in the right hemisphere of ten randomly selected subjects, and compared with the surface zones as mapped from the template. The intra-class correlation coefficients of the areas of the three surface zones were high: LZ-0.97; IMZ-0.97; SZ-0.90.
Total cerebral volumes were derived using elastic-based transformations (Miller et al., 1993), and used in data analyses as a measure of variation in overall brain volume.
Because the transformations from the template to each subject were diffeomorphic, each transformation had its mathematical inverse from each subject back into the template coordinate system, and all subjects are in registration with respect to the template in this way. Thus, the different zones on the hippocampal surface (i.e., lateral, superior, and inferior-medial) could be examined in all subjects using the zones predefined on the surface of the provisory hippocampal template. Notably, the partitioning of the hippocampal surface was performed using only the best approximation of the underlying volumetric subfields as they could be discerned in the template MR scan, which had been selected because of its high quality. The validity of this partitioning in individual subjects was not tested in all subjects because of the variability in quality of the MR scans collected. Nonetheless, the partitioning of the hippocampal surface was performed consistently across all subjects.
To compare variation in the location of the three zones of the hippocampal surface in the very mild DAT subjects with the nondemented subjects recruited for this study, composite (i.e., average) left and right hippocampal surfaces were generated for the two subject groups. For the nondemented group, the average transformation was computed and then applied to the template hippocampal surface (Miller et al., 1997), thus generating the average hippocampal surface for the nondemented group. The average hippocampal surface for the very mild DAT group was similarly obtained. For the very mild DAT group, at each point on its average hippocampal surface, we then calculated the perpendicular displacement from the corresponding point on the average surface obtained using the population of the nondemented subjects. Because the surface of the template hippocampus had been partitioned into three anatomical zones (i.e., LZ, SZ, and IMZ), we were also able to calculate the mean variation of the surface points within each of these three zones for the very mild DAT subjects with respect to the nondemented subjects. Negative values of the surface measures represented inward variation of the surface while positive values represented outward variation of the surface.
Data analysis
In our previous study of very mild DAT subjects, inward deformities that discriminated DAT subjects from nondemented comparison subjects were localized to the lateral and inferior portions, but not the superior portion, of the hippocampal surface (see Fig. 4 in Csernansky et al., 2000 and Fig. 6 in Wang et al., 2003). We therefore hypothesized that for the current study, group differences would be found in the LZ and IMZ, but not in the SZ, and tested this hypothesis using t tests with Bonferroni adjustment for multiple comparisons. Effect sizes (Cohen’s d) were also calculated for each zone per hemisphere.
To investigate whether an overall difference between DAT and healthy comparison subjects could be detected, a repeated-measures mixed model was used, with diagnostic group as main effect, hemisphere and surface zones as two different repeated measurements, and age as a covariate.
Finally, to determine post hoc whether hippocampal volume and surface measures could be used for discriminant purposes, logistic regression procedures were used to determine odds ratios, significance (95% confidence limits) and the C-statistics (SAS Institute Inc., 2000) for each variable (i.e., volume and zone variation). The odds ratio is like regression coefficients, whereas the C-statistic is like area under the ROC curve which can be interpreted as probability of correct classification. Spearman correlations (partialing out total cerebral brain volumes) were also estimated to examine the relationships between measures of hippocampal volume and surface variation and age at the time of MR scanning. An alpha of .05 was maintained for all analyses.
Results
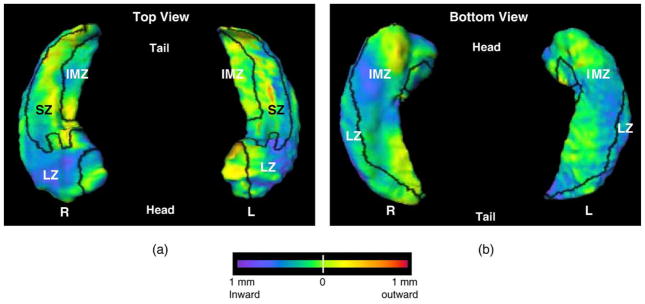
The average surface in the healthy, nondemented group of subjects was used to represent the “normal” hippocampal surface across both sexes and the range of ages represented in the healthy, nondemented population. The variation of the DAT group with respect to the nondemented average is depicted in Fig. 1, with the boundaries between the three zones of the hippocampal surface (i.e., lateral, superior, and inferior-medial) drawn in black.
Fig. 1.
Visualization of the pattern of hippocampal surface deformities in subjects with very mild DAT compared with nondemented subjects. Panel a shows the left and right hippocampi from the top (dorsal surface), while panel b shows the left and right hippocampi from the bottom (ventral surface). Boundaries between the three zones of the hippocampal surface (i.e., lateral, superior and inferior-medial) are drawn in black (see text for the method of defining the three zones) and all three zones are labeled. The flame coloring represents the difference between the mean surface of the subjects with very mild DAT and the mean surface of nondemented subjects. Inward variation of the hippocampal surface is represented by cooler colors (i.e., blue to purple), while outward variation is represented by warmer colors (i.e., orange to red).
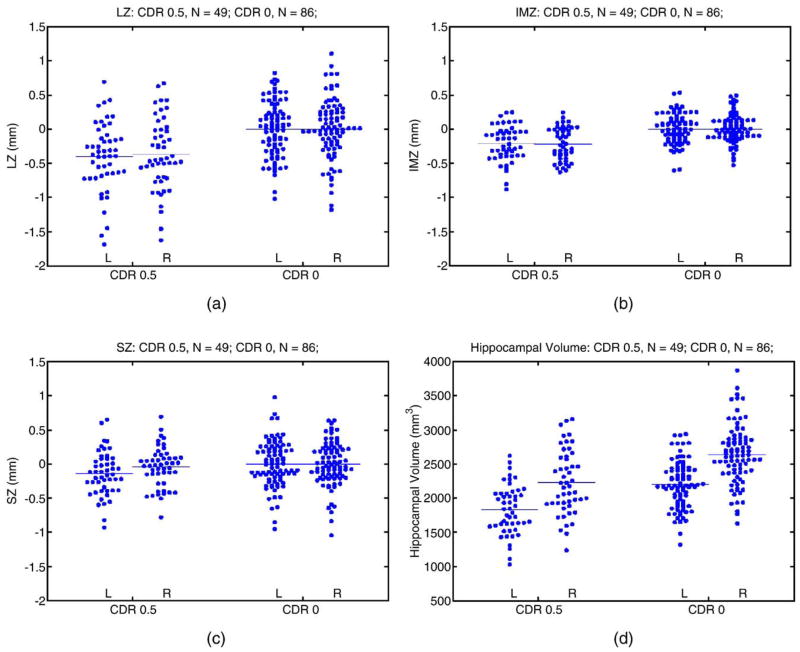
The scatter plots of mean hippocampal surface displacements in each zone for the DAT and control groups were displayed in Fig. 2. In the DAT group, the mean hippocampal surface displacements as well as effect sizes were negative for each zone when compared with the healthy group (see Table 2). Adjusting for multiple comparisons, the hypothesis that the mean hippocampal displacements in the DAT and healthy subjects were the same was rejected in the left and right LZ, the left and right IMZ, but not in the left and right SZ. When the displacements across all zones and hemispheres were modeled as repeated measurements, there was a significant group effect (F = 83.5, df = 1,133, P < 0.0001); similar results were obtained when age was used as a covariate (F = 78.5, df = 1,132, P < 0.0001). Further, surface displacements for the three zones on the left and right sides (except right SZ) were inversely correlated with age in the healthy comparison subjects (Spearman’s rho ranging −0.27 to −0.51, right SZ 0.34, all P values <0.05), but not in the DAT subjects (Spearman’s rho ranging from −0.06 to −0.18, except for the right SZ, which was 0.39, all P values >0.05). However, there were no statistical differences between the slopes for the two subject groups when each measure of surface variation was regressed against age.
Fig. 2.
Scatter plots of hippocampal surface deformation and volumes in DAT subjects and controls. Between-group comparison statistics are shown in Table 2.
Table 2.
Hippocampal surface deformation in DAT subjects compared with controls
| Lateral zone |
Inferior-medial zone |
Superior zone |
||||
|---|---|---|---|---|---|---|
| L | R | L | R | L | R | |
| Mean (SD) surface Displacement (mm) | −0.40a (0.50) | −0.37a (0.52) | −0.21a (0.26) | −0.22a (0.24) | −0.14 (0.32) | −0.04 (0.29) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.018b | 0.42 |
| Effect sizec | −0.92 | −0.78 | −0.92 | −1.0 | −0.43 | −0.14 |
Lateral zone: proximal to the CA1 subfield; inferior-medial zone: proximal to the subiculum subfield; superior zone: proximal to the combined CA2, CA3, CA4 and GD subfields.
Significant difference when compared with Controls (P < 0.0001) -Null hypothesis was rejected after Bonferroni correction for multiple comparisons.
After Bonferroni correction for multiple comparisons (six) this difference became nonsignificant.
Effect size (Cohen’s d): comparison between Control and DAT groups. Negative effect sizes indicate DAT smaller than Control.
Correlations among the hippocampal structural variables were listed in Table 3. As expected, there were direct correlations between surface variation measures and between surface variation measures and hippocampal volumes. Within the group of DAT subjects, hippocampal volume and surface variation measures were not correlated with sum-of-boxes scores for either group. This would not be unexpected since the DAT subjects had been selected to have restricted range of sum of boxes scores—they were all CDR 0.5.
Table 3.
Correlations among hippocampal morphometric measures
| Morphometric measure | Lateral zone displacement |
Inferior-medial zone displacement |
Superior zone displacement |
Volume |
|||||
|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | ||
| Control | Lateral zone displacement | L | 0.72 | 0.77 | 0.47 | (−0.14) | −0.30 | 0.93 | 0.68 |
| R | 0.61 | 0.58 | (0.11) | −0.43 | 0.75 | 0.91 | |||
| Inferior-medial zone displacement | L | 0.55 | (−0.04) | (−0.04) | 0.90 | 0.69 | |||
| R | (0.14) | (0.07) | 0.60 | 0.79 | |||||
| Superior zone displacement | L | (−0.03) | (0.03) | (0.15) | |||||
| R | (−0.21) | (−0.16) | |||||||
| Volume | L | 0.78 | |||||||
| R | |||||||||
| DAT | Lateral zone displacement | L | 0.80 | 0.91 | 0.60 | −0.54 | −0.34 | 0.95 | 0.75 |
| R | 0.75 | 0.80 | −0.43 | (−0.27) | 0.77 | 0.95 | |||
| Inferior-medial zone displacement | R | 0.64 | −0.37 | (−0.23) | 0.96 | 0.73 | |||
| R | (−0.10) | (0.11) | 0.66 | 0.92 | |||||
| Superior zone displacement | L | 0.64 | −0.36 | −0.32 | |||||
| R | (−0.23) | (−0.08) | |||||||
| Volume | L | 0.76 | |||||||
| R | |||||||||
Non-significant correlations (Spearman’s rho) are in parentheses.
Finally, each measure of volume and surface variation was entered into a separate logistic regression procedure to examine its ability to discriminate the two groups, with the healthy nondemented subjects as the reference group; these results are summarized in Table 4. A significant odds ratio of 1.23 for 0.1 mm increment of the left LZ can be interpreted as follows: for a given subject, when the LZ is displaced inwardly by 0.1 mm (i.e., negative when compared to the mean of the healthy comparison subjects; inward displacement of 0.1 mm is equivalent to volume reduction of 68.8 mm3), the odds of this subject being a DATsubject is 1.23 times the odds of it being a healthy comparison subject1. When the confidence limits for a particular variable do not include 1, the odds ratio is significant. When either LZ or IMZ surface displacement was increased by 0.1 mm, a significant odds ratio was obtained (range 1.18 to 1.57). However, odds ratios for the SZ were non-significant. Finally, odds ratios for both left and right hippocampal volumes were also significant. Because correlations among these variables were high, individual surface variables were not combined for further discriminant purposes.
Table 4.
Odds ratios and C-statistics for each morphometric variable when used in a logistic regression procedure
| Morphometric measure | Unit of increase | Odds ratio | 95% Confidence limits | C-statistics | |
|---|---|---|---|---|---|
| Lateral zone displacement | L | 0.1 mm | 1.23 | 1.12–1.36 | 0.73 |
| R | 0.1 mm | 1.18 | 1.09–1.29 | 0.71 | |
| Inferior-medial zone displacement | L | 0.1 mm | 1.48 | 1.25–1.80 | 0.73 |
| R | 0.1 mm | 1.57 | 1.31–1.92 | 0.74 | |
| Superior zone displacement | L | 0.1 mm | 1.14 | 1.02–1.28 | 0.63 |
| R | 0.1 mm | 1.05 | 0.94–1.18 | 0.42 | |
| Volume | L | 68 mm3 | 1.23 | 1.13–1.35 | 0.77 |
| R | 68 mm3 | 1.15 | 1.09–1.24 | 0.73 |
When the confidence limits for a particular variable do not include 1, the odds ratio is significant. Significant odds ratios were found for the left and right lateral zone displacements and inferior-medial zone displacements. Other variables had 95% confidence limits too close to 1 to be significant. Interpretation: e.g., left lateral zone: for a decrease of 0.1 mm (i.e., inward displacement when compared to the mean of healthy comparison subjects), the odds of being a DAT subject vs. the odds of being a healthy comparison subject is 1.23. Inward displacement of 0.1 mm is equivalent to volume reduction of 68.8 mm3. The C-statistic is like area under the ROC curve which can be interpreted as probability of correct classification.
Discussion
In this study, we tested the hypothesis that inward variation of the lateral hippocampal surface zone (approximating the CA1 subfield) and inferior-medial hippocampal surface zone (approximating the subiculum) would discriminate the subjects with very mild DAT from nondemented subjects of the same age range. The results suggest that irregularities of the hippocampal surface can be used to distinguish subjects with an early form of DAT from nondemented subjects. This result represents a replication of our previous findings2 (Csernansky et al., 2000). However, in our previous study, we used exploratory statistical methods to identify the pattern of hippocampal surface deformation that was associated with early DAT (Csernansky et al., 2000). The observation of such patterns formed the hypothesis that was tested in the present study: we predefined zones on the surface of the hippocampus that were proximal to the underlying subfields, and then tested the specific hypothesis that inward variation of the hippocampal surface in proximity to the CA1 subfield (i.e., LZ) and subiculum (IMZ), but not the remaining subfields (SZ), could be used to characterize subjects with very mild DAT. We speculate that inward variation of these two surface zones represents localized loss of volume within the proximal hippocampal substructures. However, it should be kept in mind that changes deep within the internal structure of the hippocampus could have had distant effects on the conformation of the hippocampal surface.
Given that AD is a progressive disease, one might have expected that the rate of decrease in hippocampal volume or inward variation of the hippocampal surface zones as a function of age would have been greater in the DAT subjects than in the healthy nondemented subjects. However, our results showed no group differences in the slopes representing the relationship between each of the structural measures and age. This could be due to the fact that the nondemented group showed no statistically significant correlations at all (see Table 5 and Table 6), and it should be kept in mind that all of the DAT subjects were highly similar with regard to the severity of their dementia regardless of their age. Thus, our data support the interpretation that the degree of deformity in hippocampal structure is similar within a group of subjects with very mild DAT, regardless of their age. Taking this interpretation a step further, it would suggest the effect of having DAT on hippocampal structure has masked any normative relationship between hippocampal structure and age. This interpretation is consistent with the results of our previous comparison of DAT and healthy nondemented subjects (Csernansky et al., 2000), where we included an additional comparison group of healthy younger subjects and found that the effect of age on hippocampal structure was separable from the effect of having very mild DAT. To determine whether the rate of hippocampal degeneration is more rapid in DAT subjects than healthy nondemented subjects, longitudinal studies are needed, and a preliminary study from our group (Wang et al., 2003), as well as other groups (Du et al., 2004), suggests that this does occur.
Table 5.
Correlation of hippocampal surface deformation with age, partialing out total cerebral brain volumes
| Lateral zone |
Inferior-medial zone |
Superior zone |
||||
|---|---|---|---|---|---|---|
| L | R | L | R | L | R | |
| Control (N = 86) | −0.40 (P < 0.0001) | −0.32 (P = 0.0029) | −0.20 (P = 0.069) | −0.11 (P = 0.31) | −0.43 (P < 0.0001) | 0.32 (P = 0.0033) |
| DAT (N = 49) | −0.19 (P = 0.19) | −0.18 (P = 0.23) | −0.17 (P−0.26) | −0.19 (P = 0.19) | −0.06 (P = 0.67) | 0.12 (P = 0.41) |
Table 6.
Correlation of hippocampal surface deformation with age
| Lateral zone |
Inferior-medial zone |
Superior zone |
||||
|---|---|---|---|---|---|---|
| L | R | L | R | L | R | |
| Control (N = 86) | −0.51 (P < 0.0001) | −0.48 (P < 0.0001) | −0.35 (P = 0.001) | −0.27 (P = 0.011) | −0.49 (P < 0.0001) | 0.34 (P = 0.0013) |
| DAT (N = 49) | −0.18 (P = 0.21) | −0.16 (P = 0.26) | −0.15 (P−0.31) | −0.18 (P = 0.21) | −0.06 (P = 0.70) | 0.12 (P = 0.40) |
Not surprisingly, correlations between shape measurements and volumes were very high (Spearman’s rho ranging from 0.60 to 0.95 except for one, see Table 3), and we decided not to include these variables together in the logistic regression model we developed, to avoid the numerical instability that might be associated with variable colinearity. Nonetheless, our results suggest that measures of hippocampal structure can be used to classify the group membership of individual DAT subjects. Ultimately, similar methods might be used in clinical populations to improve the diagnosis of DAT early in its course. However, the subjects in this study were relatively free of other neurological and medical disorders that could have complicated group discrimination, and in clinical populations of elderly subjects such disorders are common.
The pattern of surface deformation found to discriminate the DAT subjects from the healthy nondemented subjects is consistent with the pattern of neuropathological findings found within the hippocampus (Bobinski et al., 1998, 1997; Mizukami et al., 1998; Muramori et al., 1998; Price and Morris, 1999; Sheng et al., 1998). More specifically, hippocampal degeneration within the CA1 subfield and subiculum appears to be disproportionate to other components of the hippocampal formation early in the course of AD. This may reflect a hierarchy of AD-mediated neuronal vulnerability (Mizukami et al., 1998). We recently completed a correlative of antemortem hippocampal volume and postmortem neuropathology in AD subjects with a wide range of severity (Csernansky et al., 2004). In this study, a correlation was found between hippocampal volume and NFT but not amyloid plaque density within the hippocampal formation. Unfortunately, because of the small number of subjects available and the way in which the tissue had been blocked, it was not possible to determine whether there was a similar correlation between change in the volume of specific subfields and the NFT density within those subfields.
The manual partitioning of the hippocampal surface was performed using only the best approximation of the underlying 3D volumetric subfields as they could be discerned in the template MR scan, which had been selected because of its high quality. The validity of this partitioning in individual subjects was not tested in all subjects because of the variability in quality of the MR scans collected. Nonetheless, the automated partitioning of the hippocampal surface was performed consistently across all subjects because the diffeomorphic transformations from the template ensured reliable registration across subjects, thus allowing for the examination of the different zones on the hippocampal surface in all subjects based the zones predefined on the surface of the provisory hippocampal template. Similar strategies have been employed by others to examine functions within hippocampal subfields in high-resolution MR scans (Zeineh et al., 2003).
The mean hippocampal surface generated in the healthy nondemented subjects in this study might be used in future studies of the effects of neuropsychiatric disorders on hippocampal volume and shape to create a more definitive template, against which the structure of individual subjects or groups of subjects would be assessed. In the present study, we used a provisory template developed from an individual subject that we presumed was representative of the populations being compared. However, use of a neuroanatomical template based on the mean of a large number of healthy subjects across a range of ages relevant to a particular group of neurological diseases would be advantageous in that details of neuroanatomical structure that might be peculiar to any single subject would not influence the results. One of the major goals of antemortem neuroimaging studies of AD is to be able to apply methods such as those used in the present study to detect the presence and severity of the disease process of AD in individual subjects as early as possible. Precise quantitation of normative neuroanatomical structures, as well as the discovery of specific structural deviations that discriminate early forms of AD from the process of healthy aging, will be necessary to achieve that goal.
Acknowledgments
This research was supported by PHS grants P01 AG03991, P50 AG05681, R01 MH60883, P50 MH71616, P41 RR15241 and by a grant from the American Health Assistance Foundation. The authors would like to thank the Clinical and Psychometric Cores of the Washington University Alzheimer’s Disease Research Center (ADRC) for subject assessments.
Footnotes
In the logistic regression procedure, the healthy comparison group is the reference group, the stricter interpretation of the statistical output is that for a given subject, when the LZ is displaced outward by 0.1 mm (positive increase), the odds of this subject being a healthy comparison subject is 1.23 times the odds of it being a DAT subject. Since diagnosing healthy subjects is not intuitive, we turned the interpretation around because the reciprocity property of the odds ratio allows one to do that.
Ten subjects were in both the current and the previous studies and were rated as CDR 0 in both studies. However, more recent MR scans were used for the current study.
References
- Adachi M, Kawakatsu S, Hosoya T, Otani K, Honma T, Shibata A, Sugai Y. Morphology of the inner structure of the hippocampal formation in Alzheimer disease. AJNR Am J Neuroradiol. 2003;24:1575–1581. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington, DC: 1994. p. xxvi.p. 886. [Google Scholar]
- Analyze-AVW. Analyze-AVW. Mayo Medical Foundation; Rochester, MN: 2004. [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bobinski M, Wegiel J, Tarnawski M, Bobinski M, Reisberg B, de Leon MJ, Miller DC, Wisniewski HM. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Tarnawski M, Wegiel J, Reisberg B, Miller DC, Wisniewski HM. Neuronal and volume loss in CA1 of the hippocampal formation uniquely predicts duration and severity of Alzheimer disease. Brain Res. 1998;805:267–269. doi: 10.1016/s0006-8993(98)00759-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Christensen GE, Rabbitt RD, Miller MI. Deformable templates using large deformation kinematics. IEEE Trans Image Process. 1996;5:1–13. doi: 10.1109/83.536892. [DOI] [PubMed] [Google Scholar]
- Corder EH, Woodbury MA, Volkmann I, Madsen DK, Bogdanovic N, Winblad B. Density profiles of Alzheimer disease regional brain pathology for the huddinge brain bank: pattern recognition emulates and expands upon Braak staging. Exp Gerontol. 2000;35:851–864. doi: 10.1016/s0531-5565(00)00147-9. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Hamstra J, Wang L, McKeel D, Price JL, Gado M, Morris JC. Correlations between antemortem hippocampal volume and postmortem neuropathology in AD subjects. Alzheimer Dis Assoc Disord. 2004;18:190–195. [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris J. Preclinical detection of Alzheimer’s disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Ganzer S, Zhu XP, Jagust WJ, Miller BL, Reed BR, Mungas D, Yaffe K, Chui HC, Weiner MW. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62:422–427. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. An Atlas of Applied Anatomy. J.F. Bergmann Verlag; Munich: 1988. The human hippocampus. [Google Scholar]
- Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer’s disease (see comments) Lancet. 1996;348:94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease (see comments) Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs. hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Miller MI, Christensen GE, Amit Y, Grenander U. Mathematical textbook of deformable neuroanatomies. Proc Natl Acad Sci U S A. 1993;90:11944–11948. doi: 10.1073/pnas.90.24.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Banerjee A, Christensen GE, Joshi SC, Khaneja N, Grenander U, Matejic L. Statistical methods in computational anatomy. Stat Methods Med Res. 1997;6:267–299. doi: 10.1177/096228029700600305. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ikonomovic MD, Grayson DR, Sheffield R, Armstrong DM. Immunohistochemical study of GABAA receptor alpha1 subunit in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Brain Res. 1998;799:148–155. doi: 10.1016/s0006-8993(98)00437-5. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): current version and scoring rules (see comments) Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s disease cooperative study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Muramori F, Kobayashi K, Nakamura I. A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci. 1998;52:593–599. doi: 10.1111/j.1440-1819.1998.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS System for Windows, V8. SAS Institute Inc; Cary, North Carolina: 2000. [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Progressive neuronal DNA damage associated with neurofibrillary tangle formation in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:323–328. doi: 10.1097/00005072-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Thal DR, Holzer M, Rub U, Waldmann G, Gunzel S, Zedlick D, Schober R. Alzheimer-related tau-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp Neurol. 2000;163:98–110. doi: 10.1006/exnr.2000.7380. [DOI] [PubMed] [Google Scholar]
- Venkatesan R, Haacke E. Role of high resolution in magnetic resonance (MR) imaging: applications for MR angiography, intracranial T1-weighted imaging, and image interpolation. Int J Imaging Syst Technol. 1997;8:529–543. [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. NeuroImage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face–name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]