Abstract
Underwater submersion in mammals induces apnea, parasympathetically mediated bradycardia, and sympathetically mediated peripheral vasoconstriction. These effects are collectively termed the diving response, potentially the most powerful autonomic reflex known. Although these physiological responses are directed by neurons in the brain, study of neural control of the diving response has been hampered since 1) it is difficult to study the brains of animals while they are underwater, 2) feral marine mammals are usually large and have brains of variable size, and 3) there are but few references on the brains of naturally diving species. Similar responses are elicited in anesthetized rodents after stimulation of their nasal mucosa, but this nasopharyngeal reflex has not been compared directly with natural diving behavior in the rat. In the present study, we compared hemodynamic responses elicited in awake rats during volitional underwater submersion with those of rats swimming on the water's surface, rats involuntarily submerged, and rats either anesthetized or decerebrate and stimulated nasally with ammonia vapors. We show that the hemodynamic changes to voluntary diving in the rat are similar to those of naturally diving marine mammals. We also show that the responses of voluntary diving rats are 1) significantly different from those seen during swimming, 2) generally similar to those elicited in trained rats involuntarily “dunked” underwater, and 3) generally different from those seen from dunking naive rats underwater. Nasal stimulation of anesthetized rats differed most from the hemodynamic variables of rats trained to dive voluntarily. We propose that the rat trained to dive underwater is an excellent laboratory model to study neural control of the mammalian diving response, and also suggest that some investigations may be done with nasal stimulation of decerebrate preparations to decipher such control.
Keywords: nasopharyngeal reflex, blood pressure, bradycardia, pulse pressure
the mammalian diving response has been called the “master switch of life” (86); it may indeed be the most powerful autonomic reflex known. It profoundly alters normal cardiorespiratory behavior and supersedes common homeostatic reflexes active with our every breath and heartbeat. The chemoreceptor reflex, which normally adjusts respiratory rhythm or pattern when blood gases become abnormal, is inhibited during diving behavior. The baroreceptor reflex, which utilizes bulbospinal neurons in the rostral ventrolateral medulla that usually are silenced by increases in arterial blood pressure, astonishingly increases their firing with a rise in blood pressure when accompanied by stimulation of the nasal mucosa (59). The diving response in numerous species consists of several independent reflexes inducing an apnea, a dramatic bradycardia, and a peripheral vasoconstriction (6, 8, 9, 13, 14, 20, 22, 24, 41, 42). These autonomic adjustments are called the diving response because it is best seen in naturally diving marine mammals, but the response is present to some degree in all mammals tested, including the laboratory rat (49, 50, 56, 58, 67–69, 74, 98).
A long-term goal of our laboratory is to establish the neural circuits driving the reflexes comprising the diving response. Nevertheless, it is somewhat difficult to obtain data from the brains of aquatic mammals like seals diving in their natural environment (7). Some investigators have performed “forced” submersions, in which animals were “dunked” underwater in the laboratory (16, 17, 31, 35, 36, 43, 49, 50, 53, 55, 86, 95), but accessing the central nervous system is still difficult with forced underwater submersion. Moreover, it has been questioned whether the hemodynamic responses to voluntary diving are similar to those of forced submersions (16, 35, 40, 55).
Accurate targeting of brain structures is greatly enhanced by stereotaxic maps of the three-dimensional brain, but such a coordinate system is dependent on similar skulls enclosing brains of uniform size, and neither is assured in large feral mammals. However, responses similar to those seen during underwater submersion of marine mammals can be elicited in smaller laboratory mammals. For example, stimulation of the nasal mucosa of laboratory mammals with irritant vapors (15, 26, 32, 57, 60, 71, 73, 74, 76, 79, 81, 99, 100, 103) also elicits dramatic changes in cardiorespiratory function similar to those seen in aquatic mammals with submersion. This feature is advantageous since various pharmacological and physiological tests can be done on table preparations in a more controlled laboratory setting. Although the physiological responses of this nasopharyngeal reflex resemble those seen in diving mammals, it is uncertain whether the same neural circuits are utilized.
Our efforts to establish the neural circuits driving the diving response were hindered when we did experiments on the feral muskrat (16), a naturally diving rodent with little information known about its brains (15, 70). However, the laboratory rat is the most commonly used rodent for systems neuroscience research, and excellent stereotaxic atlases of its brain have been developed (80, 93). Moreover, a wealth of information is available about the control of cardiorespiratory systems of the laboratory rat. We thus hypothesized that data from voluntarily diving rats, a species with much information available concerning both their brains and cardiorespiratory functioning, would provide a valuable model for future studies of this dramatic response. Hemodynamic data are obtained from submersion of awake rats instrumented with telemetric transmitters; these data are compared with those from other experimental paradigms in the hope of establishing a table preparation that best mimics the hemodynamic changes seen in the mammalian diving response. This study compared heart rate (HR), mean arterial blood pressure (MABP), and pulse pressure (PP) of rats trained to voluntarily dive underwater to 1) those swimming on the surface of the same water maze; 2) those involuntarily submerged underwater either after being trained to dive underwater or when naive to water; and 3) those either anesthetized with urethane or decerebrated without anesthesia and then having their nasal mucosa stimulated with ammonia vapors. We also discuss the effects of anesthesia on cardiovascular activity and of stress on these responses obtained in the white laboratory rat. Part of these results have appeared previously in abstract form (74).
MATERIALS AND METHODS
Fourteen initially immature (70–90 g) and 11 adult (∼275–325 g) male Sprague-Dawley rats were obtained commercially (Harlan, Indianapolis, IN) and used in this study. All protocols were approved by the Animal Care Committee of Saint Louis University and followed the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats learned to swim and dive just after weaning through a maze constructed of Plexiglas (58) in water maintained at 30 ± 2°C to reduce thermal stress (52). The rats were first trained to negotiate the maze by swimming on the surface of the water and then trained to dive underwater through the 5-m path. When placed in the start area, the rats voluntarily initiated their own dives to reach the finish area. The rats appeared unstressed by either the training protocol or the exposure to water. No external reward was used during the training protocol.
These animals were utilized in the following manner. 1) Eight of the immature rats trained to swim and dive underwater swam on the surface of the water on 1 day (voluntary swimmer group). The next day these same rats volitionally dove underwater (voluntary diver group), after which they were immediately anesthetized and stimulated nasally with ammonia vapors (nasally stimulated, anesthetized group). 2) Another six immature rats were trained to voluntarily dive underwater, but these rats were forcibly submerged underwater (dunked diver group). 3) Five adult naive rats were involuntarily forced underwater (dunked naive group). 4) Six adult naive rats were decerebrated, and their nasal mucosa was stimulated with ammonia vapors (nasally stimulated, decerebrate group).
The immature rats were trained 5 days/wk for 5–6 wk to learn to both swim and dive underwater through a maze (58). Once these rats reached 270–290 g they, as well as the five untrained naive adult rats, were anesthetized with ketamine-xylazine (60–40 mg/kg ip), and the catheter of a biotelemetric transmitter [model PA-C40; Data Sciences International (DSI), St. Paul, MN] was inserted into their femoral artery; the transmitter itself was implanted in their abdominal cavity. These transmitters allow for the monitoring of pulsatile blood pressure in awake, spontaneously active animals. The rats were given buprenorphine (0.1 ml/100 g ip) postoperatively and healed for 5 days without training. They then continued training until the experiment.
The transmitter's broadcast was received with a radio receiver (model RLA3000; DSI), relayed to a Calibrated Pressure Analog Adaptor (model R11CPA; DSI), transferred through an analog-to-digital (A/D) interface [1401 plus; Cambridge Electronic Design (CED), Cambridge, UK], stored in the computer, and analyzed with Spike 2 software (CED). Systolic, diastolic, and mean arterial blood pressures were calculated, and HR was determined by counting peaks of systolic pressure. Also, we assumed that the rats did not attempt to breathe while underwater since they readily dove underwater multiple times and showed no difficulty in breathing after their experience and none drowned during submergence.
Underwater submergence and swimming.
Five to seven days after implantation of the transmitters, arterial blood pressure was recorded from awake rats either trained to dive/swim or naive to water. Eight of the trained rats swam voluntarily through the water maze, and data from these were placed in the swimming group. The next day, these same rats voluntarily dove underwater, and their data were placed in the diving group. Six other trained rats very familiar with a water environment and five untrained rats unfamiliar with water were forcibly submerged underwater for ∼20 s, and data from these were placed in the dunked diver or dunked naive groups, respectively. Hemodynamic data were recorded from all of these rats for five trials, with 5 min between trials, whether they voluntarily dove/swam or were involuntarily dunked underwater.
Nasal stimulation.
The eight rats utilized for voluntary diving were anesthetized with urethane (1 g/kg ip); data from these made up the nasally stimulated anesthetized rat group. Another six naive adult rats were anesthetized with 4.0% isoflurane and placed in a stereotaxic apparatus. After the isoflurane was reduced to 2.0%, the rats’ skulls were opened dorsally and their brain stems were transected between the superior and inferior colliculi by lowering a cutting device attached to a micromanipulator through the brain to the base of the skull. Our cutting device was crafted to be blunt and generally cut the brain with pressure. More importantly, however, vessels on the ventral surface of the brain maintained their integrity since they were compressed rather than cut. Thus bleeding generally was minimized; substantial bleeding was noted in only one rat, and this stopped within minutes. After the transection, the isoflurane anesthesia was terminated and the rat was removed from the stereotaxic device. Nasal stimulations with ammonia vapors commenced within 15 min after transection. Data from these rats made up the nasally stimulated decerebrate rat group.
For rats in either category receiving nasal stimulation, a femoral artery was cannulated for measurement of arterial blood pressure. The trachea was exposed and transected just caudal to the larynx. A cannula was placed in the distal trachea for spontaneous ventilation, while another was passed through the larynx and nasopharynx to the choanae and attached to suction. A gentle suction was generated by a vacuum pump (Gast Manufacturing) adjusted below the threshold at which normal respirations are altered. A cotton ball soaked in a 50% ammonia solution was placed near the nares, and vapors from it were drawn over the nasal mucosa from suction applied to the nasopharyngeal tube for five trials of 5 s each. Five minutes separated trials. Arterial blood pressure and respirations were sensed and impulses were transduced (Gould P23 and PT5, respectively; Grass Instruments, West Warwick, RI) and amplified (Grass 7P122). Signals for arterial blood pressure and respiration were transmitted to a computer through an A/D interface (1401 plus; CED), stored, and analyzed with Spike 2 software (CED). HR was determined by counting systolic peaks on the blood pressure trace.
Data analysis.
Data for MABP, HR, and PP were calculated for a 5-s period during stimulation and compared with similar parameters taken immediately before stimulation. Although intervals of bradycardia at its nadir and blood pressure at its peak were sought, this was not feasible in instances where these parameters were uncoordinated in time or if there was stimulus artifact due to interference of signals from their underwater source. The hemodynamic data from the rats were placed into six groups: 1) voluntary diving rats (n = 8; 40 trials); 2) voluntary swimming rats (n = 8; 40 trials); 3) awake rats trained to dive but dunked underwater (n = 6; 25 trials); 4) awake naive rats that were dunked underwater (n = 5; 20 trials); 5) anesthetized rats with nasal mucosa stimulation (n = 8; 40 trials); and 6) decerebrate rats with nasal mucosa stimulation (n = 6; 25 trials). The hemodynamic parameters of voluntarily diving rats were compared with data from the other groups of rats. Thus diving rats were compared with 1) the voluntary swimmers (see Fig. 3), 2) both groups of awake rats that were forcibly submerged underwater (see Fig. 4), and 3) both groups of anesthetized and decerebrate rats (see Fig. 5).
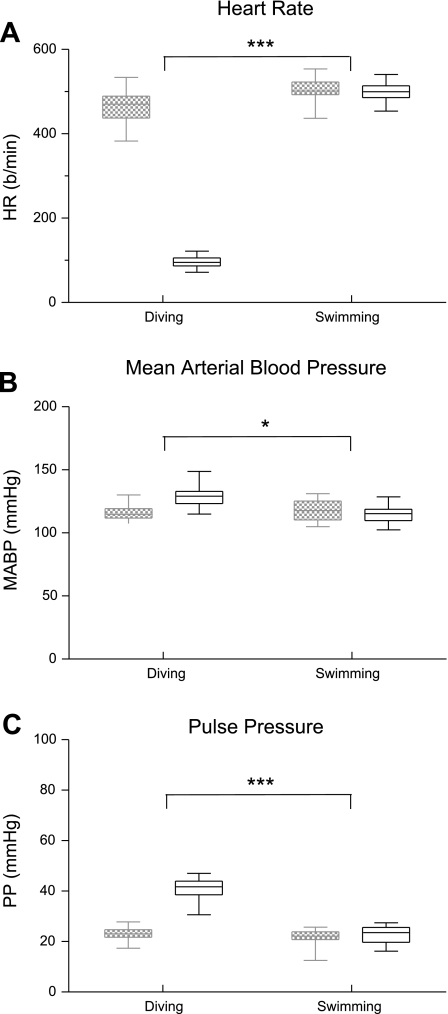
Fig. 3.
Box plots illustrating the changes in HR (A), MABP (B), and PP (C) of rats either diving or swimming. Hemodynamic parameters taken just before the exercise are in hatched boxes, while those taken during the exercise are in open boxes. Note that the hemodynamic parameters were significantly different between rats voluntarily diving underwater and those swimming on the water's surface. *P < 0.05, ***P < 0.001.
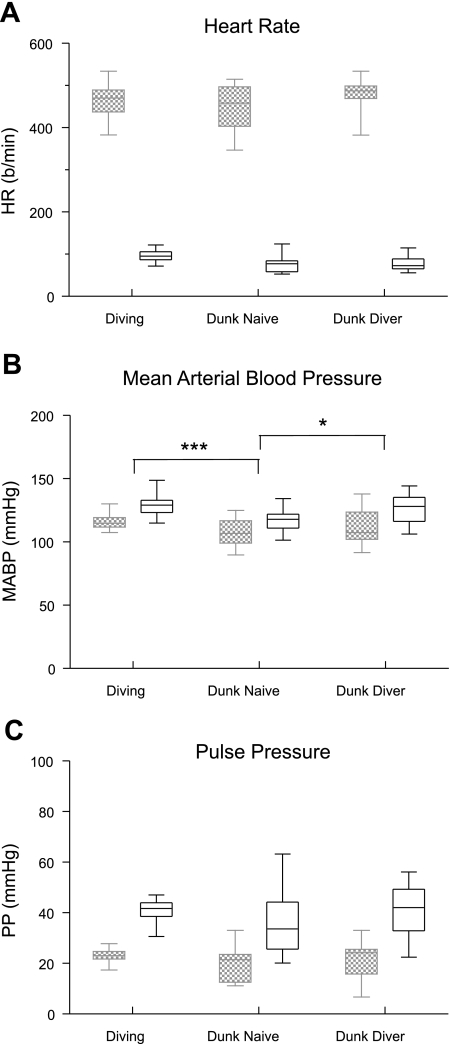
Fig. 4.
Box plots illustrating the changes in HR (A), MABP (B), and PP (C) in rats either voluntarily submerged underwater (Diving) or involuntarily “dunked” underwater. Hemodynamic parameters taken just before submersion are in hatched boxes, while those taken during submersion are in open boxes. Note that there were no differences in the changes of HR or PP to underwater submersion, but the increases in MABP of involuntarily submerged rats were significantly different from those of voluntarily diving rats. *P < 0.05, ***P < 0.001.
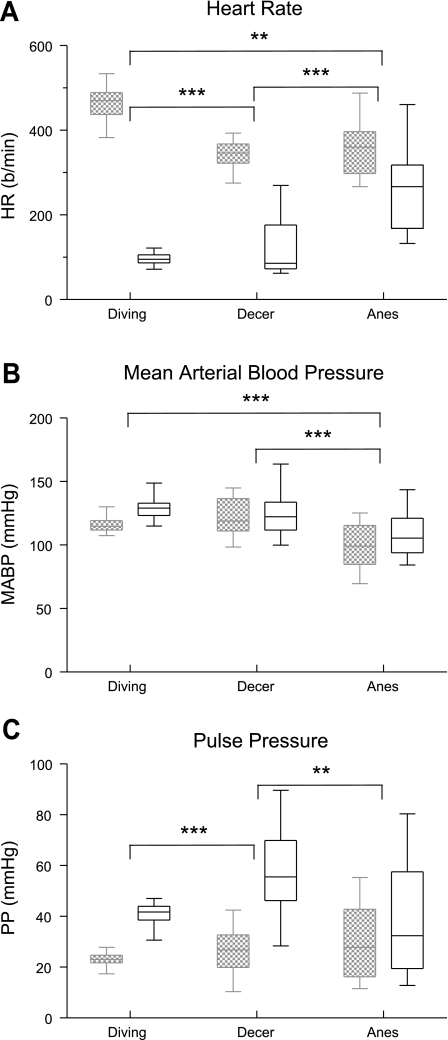
Fig. 5.
Box plots illustrating the changes in HR (A), MABP (B), and PP (C) in rats during voluntary underwater submersion and those with an intercollicular decerebration and stimulated nasally with ammonia vapors or those anesthetized with urethane and stimulated nasally. Hemodynamic parameters taken just before submersion or stimulation are in hatched boxes, while those taken during submersion or stimulation are in open boxes. Note that the responses became more variable when the rats were either anesthetized or decerebrated. **P < 0.01, ***P < 0.001.
One-way ANOVA using the Bonferroni post hoc test determined significance between the means of the variables of control and experimental data for each group with SPSS software (v. 13). Data generated from multiple trials from each rat were summed with those from the other rats in each group, and significance from these data from all trials was calculated. Data are presented as means ± SE, and significance was calculated as P < 0.05. Groups themselves were then compared with ANOVA using the post hoc Bonferroni test and multiple comparisons. Comparisons of groups were plotted as box plots, which are very useful when two or more data sets are compared. Box plots present a vertical view of the data and show the shape of its distribution, its central value, and its spread. The box itself represents 50% of the data, the 75th percentile marks the top of the box, the 25th percentile marks the bottom, while the median (50th percentile) is shown as a line through the box. Whiskers show the most extreme (maximum and minimum) values in the data set and extend a maximum of 1.5 times the range in the box. Data outside these parameters are considered outliers. Outliers for the present study were few (not illustrated), but for Figs. 3, 4, and 5 were MABP at 147 and 148 mmHg as well as PP of 30 mmHg for experimental data in the diving group, HR at 437 and 449 beats per minute (bpm) as well as PP of 13 and 15 mmHg for control data in the swimming group, and HR of 134 bpm for experimental data in the dunked naive group.
RESULTS
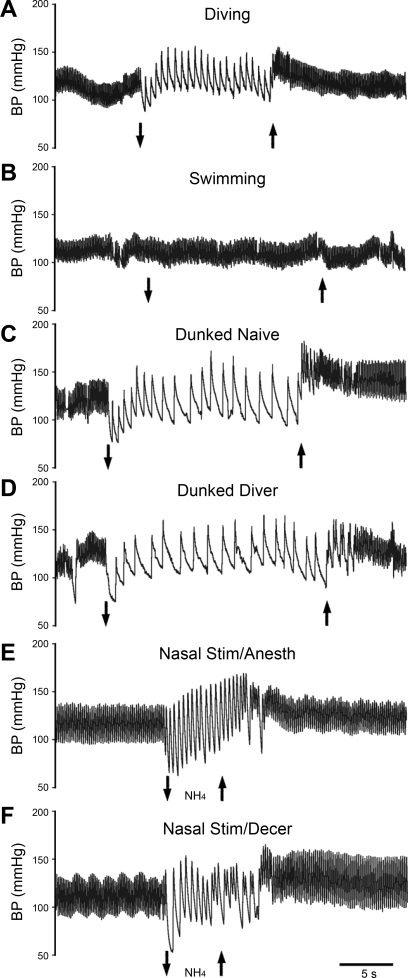
This study compared the multiple hemodynamic variables of HR, MABP, and PP from rats voluntarily diving underwater with those swimming on the water's surface, with both trained and untrained awake rats involuntarily dunked underwater, and with those anesthetized or decerebrate and stimulated nasally with ammonia vapors (Table 1). All stimuli induced marked changes in hemodynamic parameters (Fig. 1). The parasympathetically mediated bradycardia almost always appeared simultaneously in millisecond scales with submersion of the snout in voluntarily diving rats, while the sympathetically mediated increase in MABP usually peaked within 2–3 s after submergence in these animals. The bradycardia often was delayed for a second in the naive dunked rats, however, while MABP often increased either just before or immediately after submergence, peaking within 3–4 s after submergence. Interestingly, the dunked diving rats showed both behaviors depending on the rat. Passing ammonia vapors over the nasal mucosa usually showed immediate increases in MABP and a bradycardia, but since the drop in HR (and thus cardiac output) was less pronounced after nasal stimulation, the increase in MABP began within 0–2 s. Also, MABP usually peaked at the end of the stimulation period. The bradycardia in the decerebrate rats was immediate and very pronounced in most animals, but the sympathetic activation was muted in most of these rats.
Table 1.
Hemodynamic variables induced in different experimental paradigms
| Swimming |
Diving |
Dunk Naive |
Dunk Dive |
Decerebrate |
Anesthetized |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | |
| HR, bpm | 505 ± 4 | 501 ± 3 | 466 ± 6 | 96 ± 2 | 451 ± 12 | 76 ± 4 | 488 ± 4 | 77 ± 3 | 343 ± 6 | 117 ± 11 | 358 ± 9 | 256 ± 14 |
| MABP, mmHg | 118 ± 1 | 115 ± 1 | 116 ± 1 | 129 ± 1 | 107 ± 2 | 117 ± 2 | 113 ± 3 | 126 ± 2 | 121 ± 3 | 124 ± 3 | 100 ± 2 | 109 ± 3 |
| PP, mmHg | 22 ± 0.5 | 23 ± 0.5 | 23 ± 0.4 | 41 ± 0.6 | 19 ± 2 | 37 ± 3 | 22 ± 0 | 41 ± 1 | 27 ± 2 | 56 ± 3 | 30 ± 2 | 38 ± 3 |
Data are means ± SE of hemodynamic variables induced in rats after swimming, voluntary diving underwater, forced submergence underwater (Dunk), or nasal stimulation with ammonia vapors (Decerebrate and Anesthetized). Ctrl, control; Exp, experimental; HR, heart rate; bpm, beats/min; MABP, mean arterial blood pressure; PP, pulse pressure.
Fig. 1.
Tracings of arterial blood pressure (BP) in rats during a trial of voluntary diving underwater (case 1967; A), swimming on the water's surface (case 1967; B), forced submersion (“dunking”) of a rat naive to water (case 2045; C), forced submersion of a rat trained to dive underwater (case 2079; D), nasal stimulation with ammonia vapors in a rat anesthetized with urethane (case 1968; E), and nasal stimulation with ammonia vapors in a decerebrate rat (case R2372; F). Down arrow indicates the start of swimming, underwater submersion, or stimulation of the nasal mucosa with ammonia vapors, while up arrow indicates the end of stimulation. All traces are at the same time scale.
Comparison of control and experimental data.
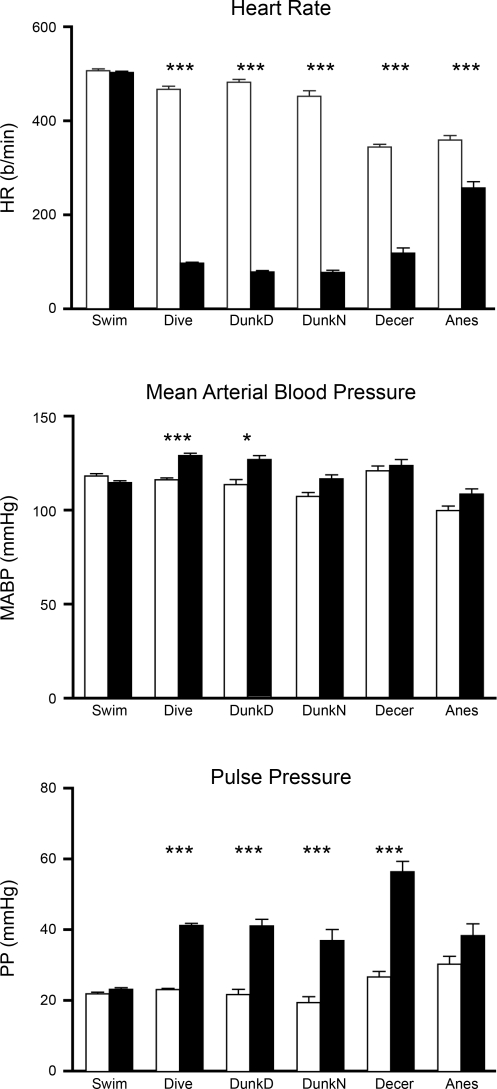
Comparison of the hemodynamic data between the control and the experimental variables for swimming rats showed no significant change in HR, MABP, or PP (Fig. 2). However, comparisons of data from the voluntary diving rats showed significant differences in HR (P < 0.001), MABP (P < 0.001), and PP (P < 0.001) (Fig. 2). Similar tests comparing data from trained rats involuntarily submerged (dunked divers) showed highly significant (P < 0.001) bradycardia and rise in PP, while the increase in MABP was also significant (P < 0.05) (Fig. 2). After naive rats were dunked involuntarily, HR was significantly reduced (P < 0.001) and PP significantly increased (P < 0.001), but MABP rose only slightly (Fig. 2). Comparison of the hemodynamic data between control and experimental variables for nasally stimulated decerebrate rats showed significant differences (P < 0.001) for HR and PP, but the change in MABP was not significant (Fig. 2). HR was significantly different (P < 0.001) in anesthetized rats stimulated nasally with ammonia vapors, but neither MABP nor PP was significant (Fig. 2). Thus the hemodynamic responses obtained from voluntary diving laboratory rats compared favorably with those seen in diving marine mammals. HR and PP responses from decerebrate rats stimulated nasally with ammonia vapors also were comparable to those from voluntary submersion of trained rats.
Fig. 2.
Changes in the hemodynamic parameters of heart rate (HR), mean arterial blood pressure (MABP), and pulse pressure (PP) from control values obtained before stimulation in rats swimming (Swim), voluntarily or involuntarily submerged [Dive, dunked diver (DunkD) dunked naive (DunkN)], or stimulated nasally with ammonia vapors [decerebrate (Decer), anesthetized (Anes)]. Open bars, control values obtained before stimulation; filled bars, data obtained during swimming, submersion, or nasal stimulation. b/min, Beats per minute. *P < 0.05, ***P < 0.001.
Comparing experimental data between groups.
A purpose of this study was to determine an effective and efficient way to induce the diving response experimentally and thus allow for easier study of its neural control. Since the cardiorespiratory behavior we recorded during the voluntary submergence of the laboratory rat mimics that of marine mammals underwater, we consider these responses the typical diving response for the rat. Thus the hemodynamic data of voluntarily diving rats were compared with those swimming on the water's surface, with those involuntarily dunked underwater, and with those either anesthetized or decerebrated and stimulated nasally with ammonia vapors to determine whether these other paradigms could be used to augment studies of the neural control of the diving response.
Voluntary diving versus voluntary swimming.
The hemodynamic changes induced in diving rats versus those swimming appeared markedly different (Fig. 1, A and B). Multiple comparisons showed that HR and PP (P < 0.001) and MABP (P < 0.05) were significantly different when these variables were compared between rats voluntarily diving underwater and those swimming on the water's surface (Fig. 3).
Voluntary diving versus forced submersion.
The underwater submersion of awake rats either voluntarily or involuntarily always induced dramatic hemodynamic changes (Fig. 1, A, C, and D). Multiple comparisons between the voluntary divers, the involuntarily dunked diver group, and the involuntarily dunked naive rats showed that the drop in HR to underwater submersion was similar in all three groups (Fig. 4A). The difference in the rise in MABP during underwater submersion in voluntarily diving rats was significant (P < 0.001) only compared with dunked naive rats, while the changes in MABP of dunked naive rats also were significantly (P < 0.05) different from that of dunked divers (Fig. 4B). The increase in PP between the three groups was not significantly different (Fig. 4C).
Voluntary diving versus nasal stimulation.
Nasal stimulation also produced significant hemodynamic changes, but these generally were different from those seen in voluntary diving rats (Fig. 1, A, E, and F). Multiple comparisons between the voluntary diving rats and the nasally stimulated decerebrate or nasally stimulated anesthetized rats showed that the drop in HR was significantly different between the voluntary diving group and both the decerebrate (P < 0.001) and anesthetized (P < 0.01) rats (Fig. 5A). The median HR of rats diving underwater and nasally stimulated decerebrate preparations, however, were similar. The bradycardia was also significantly different (P < 0.001) between the decerebrate and anesthetized rats. The rise in MABP in nasally stimulated anesthetized rats was significantly different (P < 0.001) compared with both the voluntary diving rats and the decerebrate rats (Fig. 5B). Comparing the increase in PP between these same groups showed that its rise in nasally stimulated decerebrate rats was significantly different from both the voluntary diving rats (P < 0.001) and the anesthetized rats (P < 0.01) (Fig. 5C).
DISCUSSION
Hemodynamic effects of underwater submersion.
All cells in a mammal need oxygen to survive; the source of oxygen usually is ambient air, respired through the lungs and transported to all cells via the vascular system using the heart as a pump. However, this source of oxygen is lost to mammals when underwater. The diving response is a very powerful collection of reflexes that allow an organism to preserve intrinsic oxygen, contained especially within its blood, and thus prolong aerobic metabolism in its two most important organs, the heart and the brain. The mammalian diving response consists of a respiratory apnea, a parasympathetically mediated bradycardia, and a sympathetically mediated peripheral vasoconstriction. All these cardiorespiratory parameters are controlled by neurons in the central nervous system.
Many consider seals and whales as premier diving mammals, but the diving response exists in some form in all mammals tested (6, 8, 9, 14, 20, 41, 42), including the laboratory rat (49, 50, 56, 67–69, 74, 98). Although many regard rats as strictly terrestrial, feral rats often live in sewers, rivers, and marshes and are excellent swimmers (30). Moreover, the laboratory rat is descended from the semiaquatic wild rat.
The diving response appears very early in development and may be a safeguard against the hypoxia developed during birth in mammals (88). The intensity of the diving response decreases after birth in humans (27) but has been strongly implicated as the cause of neonatal death in cases of sudden infant death syndrome (48, 51). The constant utilization of the diving response by marine mammals during their ontogeny, however, reinforces the bradycardia (28, 65) as well as secondary metabolic and behavioral adaptations (37, 66) to hypoxia induced by underwater submersion. Thus we trained recently weaned rats to reinforce this behavior optimally before inserting the telemetric transmitters. Early training also allowed the rats to become very familiar with a water environment, but inducing new environments to naive adult rats often promotes stress responses (vide infra).
Diving underwater initiates an abrupt bradycardia in marine mammals, while MABP remains relatively unaltered (6, 9, 20, 31, 39, 41, 86, 104). We show a dramatic bradycardia, a slight increase in MABP, and an increase in PP in laboratory rats during voluntary submergence. The 79% drop in HR, 11% increase of MABP, and 81% increase in PP in voluntarily diving rats during underwater submersion here is comparable to that seen by others (49, 56, 98). Cardiac output decreases in proportion to the bradycardia, and MABP is maintained near predive values because of the large increase in peripheral resistance throughout the dive (49, 56). The large increase in PP partially may be maintained by the massive release of epinephrine from the adrenal gland, since this organ is quickly activated during underwater submersion in both mammals (10, 33) and birds (46, 47), but epinephrine would have little influence on the PP of initial heartbeats. Since these responses in the laboratory rat during underwater submersion are similar to those seen in diving marine mammals, we consider them the typical mammalian diving response of the rat.
Voluntary diving versus voluntary swimming.
We compared the cardiorespiratory responses of rats voluntarily diving underwater to those voluntarily swimming on the water's surface. Although the swimming rats experienced sensations similar to those of the rats diving underwater (e.g., body immersion and thermal sensation from the water as well as proprioception from exercising muscles), these activities produced markedly different hemodynamic results. Comparisons for HR, MABP, and PP between diving and swimming groups of rats showed that all were significantly different (P < 0.001). Our rats swam in water at temperatures (29–32°C) that were warmer than ambient air, but far from the extremes known to induce changes in heart rate (1).
Placing an untrained rodent into a pool of water usually induces swimming behavior and is commonly done experimentally either to promote exercise or to induce stress. However, both exercise (38) and stress (34, 87) usually induce an increase in MABP and a tachycardia, and this usually is seen when placing naive rats in water. Swimming on the water's surface for a short time in the present study apparently was not enough exercise for our trained rats to induce significant changes in HR or PP. However, there was an insignificant drop in MABP here that generally confirmed the results of others (1). Psychological studies also use swimming to induce stress levels in rodents naive to a water environment (102). However, there were no increases in HR or blood pressure in our swimming rats, suggesting that their training (58) since juveniles minimized their stress response. Indeed, it was our impression that our rats actually “looked forward” to their aquatic exercises.
The initial stimulus inducing the dramatic hemodynamic changes seen during diving versus those in swimming is unknown. However, these profound changes always were initiated with immersion of the rats’ nares in the water. The importance of immersing an innervated snout in water to induce the diving response has been shown similarly by others (16, 18, 43, 85). We previously have investigated (54, 72, 75, 77) the anterior ethmoidal nerve, a small branch of the ophthalmic division of the trigeminal nerve that innervates mucosa surrounding the opening of the nares. Indeed, stimulating this nerve electrically induces responses similar to those seen in diving mammals (54, 83), while numbing the nasal mucosa with local anesthetics inhibits the response (103). We have termed this nerve the “gatekeeper” (75), since it is the first to sense substances entering the upper respiratory tract and eventually the lungs. We suggest that respiration is terminated if these small-diameter fibers sense substances incompatible with life.
Since the cardiovascular consequences of swimming markedly differ from those of underwater submergence, swimming behavior cannot be utilized as a substitute for studies investigating the voluntary diving of rats underwater.
Voluntary diving versus forced submersion.
Numerous investigators have chosen to submerge mammals underwater forcibly in the laboratory to better control their experiments (16, 36, 43, 49, 50, 53, 86, 89, 92, 95). However, many have speculated that the cardiorespiratory behavior of forced dives in aquatic mammals is similar, but not equal to, that seen during natural diving (35, 40, 55). The changes in HR and PP of awake rats voluntarily diving underwater and either group dunked underwater involuntarily were statistically similar in the present study, contrasting that seen during forced submersion of untrained harbor seals versus those familiar with involuntary submergence (35). However, our rats were trained to “dive” underwater voluntarily, while the seals in the study of Jobsis et al. (35) were trained by repeatedly “dunking” them under water. Nevertheless, the fact that bradycardia was unchanged either with or without dive training here promotes the reflex nature of the bradycardia to underwater submersion in the rat.
PP was also not different between the groups, but the data (Fig. 4) were much more scattered in groups dunked underwater involuntarily. Moreover, the MABP responses of naive rats dunked underwater involuntarily were statistically different from those of either group previously trained to dive. This fact suggests that perhaps a psychological component may influence hemodynamics when the diving response is forcibly induced in mammals. Perhaps emotional factors like fear induced variable increases in sympathetic nerves in these cases.
These responses of naive rats make the model of involuntary submersion questionable for studies of the diving response. We thus agree with the speculation of others that voluntary and forced dives are different, and data from forced submersion experiments on naive animals especially should be interpreted cautiously. Nevertheless, if hemodynamic behavior is studied in rats during forced submersion, we suggest it is best studied in rats trained to dive underwater since the responses of dunked divers were similar to those of the voluntary diver group.
Voluntary diving versus nasal stimulation in anesthetized rats.
Many investigations of the brain are best done on anesthetized animals secured in a stereotaxic apparatus, so that coordinates may be used to localize nuclei in the brain. Although this procedure limits the utility of underwater submersion for inducing the diving response, similar responses can be induced by stimulating the nasal mucosa of rodents with noxious vapors. In this regard we stimulated the nasal mucosa of anesthetized muskrats with ammonia vapors in the past to help us decipher the neural control of the diving response (15, 54, 57, 71, 73, 79, 103). We thus compared the cardiovascular responses to underwater submersion in an awake rat voluntarily diving underwater to those elicited in the same animal by nasal stimulation with ammonia vapors under urethane anesthesia.
It has been recognized for considerable time, however, that general anesthetics effect cardiovascular reflex responses to stimuli (3–5, 21, 29, 44, 45, 90, 105), including those in the diving response to underwater submersion (15, 19, 95, 101). Unmyelinated and small myelinated fibers innervate the nasal mucosa and provide most of the fibers in the anterior ethmoidal nerve (2, 12, 23, 54, 96, 97). These small fibers also are probably important for the hemodynamic responses elicited by nasal stimulation with ammonia vapors (84). General anesthetics influence the firing behavior of both peripheral afferent fibers as well as neurons in the central nervous system. For example, Aδ and C fibers found in somatosensory nerves often increase the frequency of their firing in mammals under volatile anesthetics (11, 61–63, 94), but the potentials initiated in similar-sized fibers in the carotid sinus nerve are unaffected by injected urethane (90). Moreover, neurons in the rostral ventrolateral medulla, which mediates sympathetic activation during nasal stimulation (59), are inhibited by urethane (90), while pentobarbitone may actually enhance reflex activity relayed through this region (91). The hemodynamic responses elicited in the anesthetized rats here also were more erratic than those of voluntary divers, as judged by the large spread of data (Fig. 5). We speculate that the wider variability of bradycardia and changes in MABP elicited by nasal stimulation of the anesthetized rats was due to the urethane anesthetic, presumably affecting neurons in the central nervous system.
Although the bradycardia induced in our nasally stimulated rats anesthetized with urethane was significantly different from control levels, similar to a previous report (84), it also was significantly different from that induced in voluntary underwater diving. Moreover, the change in MABP of the anesthetized rats was significantly different from that of both voluntary diving and nasal stimulation of decerebrate rats without anesthesia. Our data suggest that the responses of the nasally stimulated anesthetized rat are the least similar to those of the voluntary diving rat.
Voluntary diving versus nasal stimulation in decerebrate rats.
The diving response has been shown to be present in decerebrate animals previously (25, 53, 78), suggesting that the neural circuits for the diving response are intrinsic to the brain stem. We tested the effects of the ammonia stimulus on the nasal mucosa of rats whose brain stems had been transected after initial sedation with the volatile anesthetic isoflurane. While volatile anesthetics themselves also can affect cardiovascular reflex activity (29, 62, 64, 82, 105), they are quickly eliminated from the body (see Nagasaki et al., 2001). The median HRs seen after submersion in awake voluntary diving rats in the present study were similar to those seen in rats whose brain stems were transected (present study; Ref. 78). This emphasizes the reflex nature of this response and again negates an emotional component during diving in rats. The MABP responses of decerebrate and voluntary diving rats were similar here, although PP was significantly more robust and variable in the decerebrate rats than in voluntary divers. Electrical stimulation of the anterior ethmoidal nerves of the rat in working heart-brain stem preparations (83) also induces dramatic bradycardia and apnea, and these preparations are not anesthetized, but arterial blood pressure is not measured. We suggest that decerebrate preparations may prove valuable for deciphering the neural circuit for the diving response once the proper level of transection is formulated.
Summary.
The brain drives behavior, including the cardiorespiratory changes seen during underwater submersion. However, investigations elucidating the neural control of the diving response in marine mammals are difficult because of their native aquatic environment, their relatively large brains, and the paucity of information about them. We provide data here on some hemodynamic variables induced during diving behavior of the laboratory rat, which is commonly used in neuroscience research. We show that the hemodynamic changes recorded telemetrically in rats to underwater submergence are remarkably similar to those seen in naturally diving species. We show that the typical cardiorespiratory responses noted in a voluntarily diving rat are not mimicked by a rat swimming on the water's surface. Moreover, the cardiorespiratory responses to underwater submersion in the laboratory rat have been almost invariable in the over one thousand trials we have recorded. Involuntary dunking of animals also induced hemodynamic responses similar to those seen in voluntary diving. The invariability of bradycardia during voluntary or involuntary submergence suggests that this parameter may be reflex in origin. Our data suggest, however, that variables such as stress may be involved when the rats are involuntarily submerged. We document that responses from anesthetized rats stimulated nasally with ammonia vapors were the least similar to those of awake rats diving voluntarily, while some responses from decerebrate rats stimulated nasally were similar to those seen during diving. We thus suggest that investigations of this nasopharyngeal reflex may be best if decerebrate preparations are used to eliminate the effects of injected anesthetics. Although respirations were not recorded in the awake rats submerged in this study, they apparently were inhibited during submergence, since rats neither had difficulty breathing after emergence from the water nor drowned despite diving repeatedly. We thus promote the common laboratory rat as a logical model for studies detailing the complex neural components driving the mammalian diving response.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-64772 and monies from the Saint Louis University School of Medicine.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Baker MA, Horvath SM. Influence of water temperature on heart rate and rectal temperature of swimming rats. Am J Physiol 207: 1073–1076, 1964 [DOI] [PubMed] [Google Scholar]
- 2. Beidenbach MA, Beuerman RW, Brown AC. Graphic-digitizer analysis of axon spectra in ethmoidal and lingual branches of the trigeminal nerve. Cell Tissue Res 157: 341–352, 1975 [DOI] [PubMed] [Google Scholar]
- 3. Blake DW, Blombery PA, Korner PI. Effects of ketamine, althesin, and thiopentone on the Valsalva-contrictor and heart rate reflexes of the rabbit. J Auton Nerv Syst 5: 291–301, 1982 [DOI] [PubMed] [Google Scholar]
- 4. Blake DW, Korner PI. Role of baroreceptor reflexes in the hemodynamic and heart rate responses to althesin, ketamine, and thiopentone anesthesia. J Auton Nerv Syst 3: 55–70, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Blake DW, Korner PI. Effects of ketamine and althesin anesthesia on baroreceptor-heart rate reflex and hemodynamics of intact and pontine rabbits. J Auton Nerv Syst 5: 145–154, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Blix AS, Folkow B. Cardiovascular adjustments to diving in mammals and birds. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc, 1983, sect. 2, vol. III, p. 917–945 [Google Scholar]
- 7. Butler PJ. Diving beyond the limits. News Physiol Sci 16: 222–227, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Butler PJ, Jones DR. The comparative physiology of diving in vertebrates. Adv Comp Physiol Biochem 8: 179–364, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev 77: 837–899, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Byku M, Gan Q, Panneton WM. A physiological and neuroanatomical comparison of rats after either voluntary or forced dives (Abstract). FASEB J 18: A1102, 2004 [Google Scholar]
- 11. Campbell JN, Raja SN, Meyer RA. Halothane sensitizes cutaneous nociceptors in monkeys. J Neurophysiol 52: 762–770, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Cauna N, Hinderer KH, Wentges RT. Sensory receptor organs of the human nasal respiratory mucosa. Am J Anat 124: 187–210, 1969 [DOI] [PubMed] [Google Scholar]
- 13. Davis RW, Polasek L, Watson R, Fuson A, Williams TM, Kanatous SB. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp Biochem Physiol A Mol Integr Physiol 138: 263–268, 2004 [DOI] [PubMed] [Google Scholar]
- 14. de Burgh Daly M. Breath-hold diving: mechanisms of cardiovascular adjustments in the mammal. Recent Adv Physiol 10: 201–245, 1984 [Google Scholar]
- 15. Doyle RE, Panneton WM, Vogler GA, Romeo JP, Watson BJ, Higgins B. The muskrat in biomedical research. Lab Anim Sci 38: 667–674, 1988 [PubMed] [Google Scholar]
- 16. Drummond PC, Jones DR. The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J Physiol 290: 253–271, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dykes RW. Factors related to the dive reflex in harbor seals: respiration, immersion, bradycardia, and lability of the heart rate. Can J Physiol Pharmacol 52: 248–257, 1974 [DOI] [PubMed] [Google Scholar]
- 18. Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can J Physiol Pharmacol 52: 259–265, 1974 [DOI] [PubMed] [Google Scholar]
- 19. Elsner R, Franklin DL, Van Citters RL, Kenney DW. Cardiovascular defense against asphyxia. Science 153: 941–949, 1966 [DOI] [PubMed] [Google Scholar]
- 20. Elsner R, Gooden B. Diving and Asphyxia: A Comparative Study of Animals and Man. New York: Cambridge Univ. Press, 1983, p. 1–168 [PubMed] [Google Scholar]
- 21. Faber JE. Effects of althesin and urethan-chloralose on neurohumoral cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol 256: R757–R765, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol 84: 254–271, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Finger TE, St. Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol 294: 293–305, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Foster GE, Sheel AW. The human diving response, its function, and its control. Scand J Med Sci Sports 15: 3–12, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Gabbott GRJ, Jones DR. The effect of brain transection on the response to forced submergence in ducks. J Auton Nerv Syst 36: 65–74, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Gieroba ZJ, Yu YH, Blessing WW. Vasoconstriction induced by inhalation of irritant vapour is associated with appearance of Fos protein in C1 catecholamine neurons in rabbit medulla oblongata. Brain Res 636: 157–161, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Goksör E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr 91: 307–312, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Greaves DK, Schreer JF, Hammill MO, Burns JM. Diving heart rate development in postnatal harbour seals, Phoca vitulina. Physiol Biochem Zool 78: 9–17, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Halliwill JR, Billman GE. Effect of general anesthesia on cardiac vagal tone. Am J Physiol Heart Circ Physiol 262: H1719–H1724, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Hanney PW. Rodents: Their Lives and Habits. Vancouver: David and Charles, 1975 [Google Scholar]
- 31. Hill RD, Schneider RC, Liggins GC, Schuette AH, Elliott RL, Guppy M, Hochachka PW, Qvist J, Falke KJ, Zapol WM. Heart rate and body temperature during free diving of Weddell seals. Am J Physiol Regul Integr Comp Physiol 253: R344–R351, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Ho CY, Kou YR. Protective and defensive airway reflexes evoked by nasal exposure to wood smoke in anesthetized rats. J Appl Physiol 88: 863–870, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Hochachka PW, Liggins GC, Guyton GP, Schneider RC, Stanek KS, Hurford WE, Creasy RK, Zapol DG, Zapol WM. Hormonal regulatory adjustments during voluntary diving in Weddell seals. Comp Biochem Physiol 112B: 361–375, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Inagaki H, Kuwahara M, Tsubone H. Effects of psychological stress on autonomic control of heart in rats. Exp Anim 53: 373–378, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Jobsis PD, Ponganis PJ, Kooyman GL. Effects of training on forced submersion responses in harbor seals. J Exp Biol 204: 3877–3885, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Jones DR, Furilla RA, Heieis MR, Cabbott A, Smith FM. The effect of the stress of forcible submergence on the diving response in muskrats (Ondatra zibethica). Can J Zool 60: 187–193, 1982 [Google Scholar]
- 37. Jorgensen C, Lydersen C, Brix O, Kovacs KA. Diving development in nursing harbour seal pups. J Exp Biol 204: 3993–4004, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Soc, 1996, sect. 12, p. 381–447 [Google Scholar]
- 39. Kooyman GL. Physiology without restraint in diving mammals. Mar Mamm Sci 1: 166–178, 1985. [Google Scholar]
- 40. Kooyman GL. Diverse Divers: Physiology and Behavior. Berlin: Springer, 1989 [Google Scholar]
- 41. Kooyman GL, Castellini MA, Davis RW. Physiology of diving in marine mammals. Annu Rev Physiol 43: 343–356, 1981 [DOI] [PubMed] [Google Scholar]
- 42. Kooyman GL, Ponganis PJ. The physiological basis of diving to depth: birds and mammals. Annu Rev Physiol 60: 19–32, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Koppányi T, Dooley MS. Submergence and postural apnea in the muskrat. Am J Physiol 88: 592–595, 1929 [Google Scholar]
- 44. Korner PI, Langsford G, Starr D, Uther JB, Ward W, White SW. The effects of chloralose-urethane and sodium pentobarbitone anesthesia on the local and autonomic components of the circulatory response to arterial hypoxia. J Physiol 199: 283–302, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Korner PI, Uther JB, White SW. Circulatory effects of chloralose-urethane and sodium pentobarbitone anesthesia in the rabbit. J Physiol 199: 253–265, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lacombe AMA, Jones DR. Neural and humoral effects on hindlimb vascular resistance of ducks during forced submergence. Am J Physiol Regul Integr Comp Physiol 261: R1579–R1586, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Lacombe AMA, Jones DR. Role of adrenal catecholamines during forced submergence in ducks. Am J Physiol Regul Integr Comp Physiol 261: R1364–R1372, 1991 [DOI] [PubMed] [Google Scholar]
- 48. Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome. Respir Physiol Neurobiol 159: 127–138, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Lin YC. Autonomic nervous control of cardiovascular response during diving in the rat. Am J Physiol 227: 601–605, 1974 [DOI] [PubMed] [Google Scholar]
- 50. Lin YC, Baker DG. Cardiac output and its distribution during diving in the rat. Am J Physiol 228: 733–737, 1975 [DOI] [PubMed] [Google Scholar]
- 51. Lobban CDR. The human dive reflex as a primary cause of SIDS: a review of the literature. Med J Aust 155: 561–563, 1991 [PubMed] [Google Scholar]
- 52. MacArthur RA. Aquatic thermoregulation in the muskrat (Ondatra zibethicus): energy demands of swimming and diving. Can J Zool 62: 241–248, 1984 [Google Scholar]
- 53. Martner J, Wadenvik H, Lisander B. Apnoea and bradycardia from submersion in “chronically” decerebrated cats. Acta Physiol Scand 101: 476–480, 1977 [DOI] [PubMed] [Google Scholar]
- 54. McCulloch PF, Faber KM, Panneton WM. Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res 830: 24–31, 1999 [DOI] [PubMed] [Google Scholar]
- 55. McCulloch PF, Jones DR. Cortical influences on diving bradycardia in muskrats (Ondatra zibethicus). Physiol Zool 63: 1098–1117, 1990 [Google Scholar]
- 56. McCulloch PF, Ollenberger GP, Bekar LK, West NH. Trigeminal and chemoreceptor contributions to bradycardia during voluntary dives in rats. Am J Physiol Regul Integr Comp Physiol 273: R814–R822, 1997 [DOI] [PubMed] [Google Scholar]
- 57. McCulloch PF, Panneton WM. Fos immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience 78: 913–925, 1997 [DOI] [PubMed] [Google Scholar]
- 58. McCulloch PF, Panneton WM. Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res 984: 42–53, 2003 [DOI] [PubMed] [Google Scholar]
- 59. McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the nasal mucosa. J Physiol 516: 471–484, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McRitchie RJ, White SW. Role of trigeminal olfactory, carotid sinus and aortic nerves in the respiratory and circulatory response to nasal inhalation of cigarette smoke and other irritants in the rabbit. Aust J Exp Biol Med Sci 52: 127–140, 1974 [DOI] [PubMed] [Google Scholar]
- 61. Mutoh T, Kanamaru A, Tsubone H, Nishimura R, Sasaki N. Respiratory reflexes in response to nasal administration of halothane to anesthetized, spontaneously breathing dogs. Am J Vet Res 61: 260–267, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Mutoh T, Nishimura R, Kim H, Matsunaga S, Sasaki N. Cardiopulmonary effects of sevoflurane, compared with halothane, enflurane, and isoflurane, in dogs. Am J Vet Res 58: 885–890, 1997 [PubMed] [Google Scholar]
- 63. Mutoh T, Tsubone H, Nishimura R, Sasaki N. Effects of volatile anesthetics on vagal C-fiber activities and their reflexes in anesthetized dogs. Respir Physiol 112: 253–264, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Nagasaki G, Tanaka M, Nishikawa T. The recovery profile of baroreflex control of heart rate after isoflurane or sevoflurane anesthesia in humans. Anesth Analg 93: 1127–1131, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Noren SR, Cuccurullo V, Williams TM. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus). J Comp Physiol [B] 174: 139–147, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Noren SR, Williams TM, Pabst DA, McLellan WA, Dearolf JL. The development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J Comp Physiol [B] 171: 127–134, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Ollenberger GP, Matte G, Wilkinson AA, West NH. Relative distribution of blood flow in rats during surface and submerged swimming. Comp Biochem Physiol 119A: 271–277, 1998 [DOI] [PubMed] [Google Scholar]
- 68. Ollenberger GP, West NH. Contribution of hypercapnia and trigeminal stimulation to cerebrovascular dilation during simulated diving. Am J Physiol Regul Integr Comp Physiol 274: R921–R930, 1998 [DOI] [PubMed] [Google Scholar]
- 69. Ollenberger GP, West NH. Distribution of regional cerebral blood flow in voluntarily diving rats. J Exp Biol 201: 549–558, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Panneton M, Watson BJ. Stereotaxic atlas of the brainstem of the muskrat, Ondatra zibethicus. Brain Res Bull 26: 479–509, 1991 [DOI] [PubMed] [Google Scholar]
- 71. Panneton WM. Controlled bradycardia induced by nasal stimulation in the muskrat, Ondatra zibethicus. J Auton Nerv Syst 30: 253–264, 1990 [DOI] [PubMed] [Google Scholar]
- 72. Panneton WM. Primary afferent projections from the upper respiratory tract in the muskrat. J Comp Neurol 308: 51–65, 1991 [DOI] [PubMed] [Google Scholar]
- 73. Panneton WM. Trigeminal mediation of the diving response in the muskrat. Brain Res 560: 321–325, 1991 [DOI] [PubMed] [Google Scholar]
- 74. Panneton WM, Gan Q. Cardiovascular changes induced in freely diving, swimming and nasally stimulated rats (Abstract). Soc Neurosci Abstr 28: 923.2, 2003 [Google Scholar]
- 75. Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience 141: 889–906, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Panneton WM, Gan Q, Sun W. Pressor responses to nasal stimulation are unaltered after disrupting the CPA. Auton Neurosci 144: 13–21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res 874: 48–65, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Panneton WM, Sun W. Cardiorespiratory changes after nasal stimulation persist after partial pontine transection (Abstract). Soc Neurosci Abstr 27: 170. 14, 2001 [Google Scholar]
- 79. Panneton WM, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus: evidence for excitatory amino acid transmission. Brain Res 691: 37–45, 1995. [DOI] [PubMed] [Google Scholar]
- 80. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 81. Peterson DF, Coote JH, Gilbey MP, Futuro-Neto HA. Differential pattern of sympathetic outflow during upper airway stimulation with smoke. Am J Physiol Regul Integr Comp Physiol 245: R433–R437, 1983 [DOI] [PubMed] [Google Scholar]
- 82. Picker O, Schwarte LA, Roth HJ, Greve J, Scheeren TW. Comparison of the role of endothelin, vasopressin and angiotensin in arterial pressure regulation during sevoflurane anaesthesia in dogs. Br J Anaesth 92: 102–108, 2004 [DOI] [PubMed] [Google Scholar]
- 83. Rozloznik M, Paton JFR, Dutschmann M. Repetitive paired stimulation of nasotrigeminal and peripheral chemoreceptor afferents cause progressive potentiation of the diving bradycardia. Am J Physiol Regul Integr Comp Physiol 296: R80–R87, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res 1075: 122–132, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Schagatay E, Van Kampen M. Apneic snout immersion in trained pigs elicits a “diving response”. Adv Exp Med Biol 393: 73–76, 1995 [PubMed] [Google Scholar]
- 86. Scholander PF. The master switch of life. Sci Am 209: 92–106, 1963 [DOI] [PubMed] [Google Scholar]
- 87. Sgoifo A, Koolhaas JM, Musso E, De Boer SF. Different sympathovagal modulation of heart rate during social and nonsocial stress episodes in wild-type rats. Physiol Behav 67: 733–738, 1999 [DOI] [PubMed] [Google Scholar]
- 88. Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A 123: 221–234, 1999 [DOI] [PubMed] [Google Scholar]
- 89. Stephenson R, Butler PJ, Dunstone N, Woakes AJ. Heart rate and gas exchange in freely diving American mink (Mustela vison). J Exp Biol 134: 435–442, 1988 [DOI] [PubMed] [Google Scholar]
- 90. Sun MK, Reis DJ. Urethane directly inhibits chemoreflex excitation of medullary vasomotor neurons in rats. Eur J Pharmacol 293: 237–243, 1995 [DOI] [PubMed] [Google Scholar]
- 91. Sun MK, Spyer KM. Reticulospinal vasomotor neurones in decerebrate rats: effect of pentobarbitone. J Auton Nerv Syst 33: 85–90, 1991 [DOI] [PubMed] [Google Scholar]
- 92. Swain UG, Gilbert FF, Robinette JD. Heart rates in the captive, free-ranging beaver. Comp Biochem Physiol A Comp Physiol 91: 431–435, 1988. [DOI] [PubMed] [Google Scholar]
- 93. Swanson LW. Brain Maps: Structure of the Rat Brain. A Laboratory Guide with Printed and Electronic Templates for Data, Models and Schematics. Amsterdam: Elsevier, 2004 [Google Scholar]
- 94. Tanelian DL, MacIver MB. Differential excitatory and depressant anesthetic effects on mammalian A-delta and C fiber sensory afferents. Ann NY Acad Sci 625: 273–275, 1991 [DOI] [PubMed] [Google Scholar]
- 95. Tchobroutsky C, Merlet C, Rey P. The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Respir Physiol 8: 108–117, 1969 [DOI] [PubMed] [Google Scholar]
- 96. Wallois F, Gros F, Condamin M, Macron M. Postnatal development of the anterior ethmoidal nerve in cats: unmyelinated and myelinated nerve fiber analysis. Neurosci Lett 160: 221–224, 1993 [DOI] [PubMed] [Google Scholar]
- 97. Wallois F, Larnicol N, Rose D, Duron B. A comparative HRP study of the neuronal supply to the inferior and superior nasal meatus in the cat. Neurosci Lett 139: 234–238, 1992 [DOI] [PubMed] [Google Scholar]
- 98. Whishaw IQ, Schallert T. Hippocampal RSA (theta), apnea, bradycardia, and effects of atropine during underwater swimming in the rat. Electroencephalogr Clin Neurophysiol 42: 389–396, 1977 [DOI] [PubMed] [Google Scholar]
- 99. White S, McRitchie RJ, Korner PI. Central nervous system control of cardiorespiratory nasopharyngeal reflexes in the rabbit. Am J Physiol 228: 404–409, 1975 [DOI] [PubMed] [Google Scholar]
- 100. White SW, McRitchie RJ, Franklin DL. Autonomic cardiovascular effects of nasal inhalation of cigarette smoke in the rabbit. Aust J Exp Biol Med Sci 52: 111–126, 1974 [DOI] [PubMed] [Google Scholar]
- 101. Whyane TF, Smith NT, Eger EI, Stoelting RK, Whitcher CE. The effects of halothane anesthesia on reflex cardiovascular responses to simulated diving and the Valsalva maneuver. Anesthesiology 34: 262–270, 1971 [DOI] [PubMed] [Google Scholar]
- 102. Wood SK, Verhoeven RE, Savit AZ, Kenner CR, Fishbach PS, Woods JH. Facilitation of cardiac vagal activity by CRF-R1 antagonists during swim stress in rats. Neuropsychopharmacology 31: 2580–2590, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61: 195–200, 1996 [DOI] [PubMed] [Google Scholar]
- 104. Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol 47: 968–973, 1979 [DOI] [PubMed] [Google Scholar]
- 105. Zimpfer M, Sit SP, Vatner SF. Effects of anesthesia on the canine carotid chemoreceptor reflex. Circ Res 48: 400–406, 1981. [DOI] [PubMed] [Google Scholar]