Abstract
Exercise-induced oxidative stress is important for the muscular adaptation to training but may also cause muscle damage. We hypothesized that prolonged exercise would increase mitochondrial production of reactive oxygen species (ROS) measured in vitro and that this correlates with oxidative damage. Eight male athletes (24–32 yr) performed ultraendurance exercise (kayaking/running/cycling) with an average work intensity of 55% V̇o2peak for 24 h. Muscle biopsies were taken from vastus lateralis before exercise, immediately after exercise, and after 28 h of recovery. The production of H2O2 was measured fluorometrically in isolated mitochondria with the Amplex red and peroxidase system. Succinate-supported mitochondrial H2O2 production was significantly increased after exercise (73% higher, P = 0.025) but restored to the initial level at recovery. Plasma level of free fatty acids (FFA) increased fourfold and exceeded 1.2 mmol/l during the last 6 h of exercise. Plasma FFA at the end of exercise was significantly correlated to mitochondrial ROS production (r = 0.74, P < 0.05). Mitochondrial content of 4-hydroxy-nonenal-adducts (a marker of oxidative damage) was increased only after recovery and was not correlated with mitochondrial ROS production. Total thiol group level and glutathione peroxidase activity were elevated after recovery. In conclusion, ultraendurance exercise increases ROS production in isolated mitochondria, but this is reversed after 28 h recovery. Mitochondrial ROS production was not correlated with oxidative damage of mitochondrial proteins, which was increased at recovery but not immediately after exercise.
Keywords: antioxidative defense, fatty acids, oxidative stress
the role of exercise-induced oxidative stress in the physiological response to exercise is presently under debate. There is evidence that part of the acute response to exercise and the training-induced muscular adaptation is dependent on the presence of reactive oxygen species (ROS). This relates to mitochondrial biogenesis (40), muscular glucose uptake (20), muscle contractility (34), and insulin sensitivity (35). The oxidative stress associated with exercise may therefore be part of a normal physiological adaptation. However, excessive ROS production during a long period of time may be detrimental for exercise performance and could also have long-term health consequences. Particularly the possibility has been discussed that a vicious cycle exists where oxidatively damaged mitochondria increase their production of ROS, leading to further damage and thus to further increased production of ROS and even further damage. This cycle has been proposed to account for the ageing processes in general (3, 47) and more acutely for deteriorated function in ischemic heart (7, 24) and brain (11, 22). Furthermore, mitochondrial ROS production may also be related to insulin resistance (1). Despite these potential health risks, there are, to our knowledge, no studies examining the relationship between ROS production and oxidative damage in muscle mitochondria from humans after exercise.
It is well established that strenuous exercise causes oxidative stress in the contracting skeletal muscles (9, 18, 48). Increased free radical formation in the exercising muscles has been directly demonstrated using electron spin resonance assessment of ROS (2, 8) and biomarkers (mainly derived from lipid peroxidation) indicative of postexercise oxidative damage (2, 4, 23, 28). Intensive exercise could activate a number of intracellular sources for ROS, including myeloperoxidase, xanthine oxidase, NADH oxidase, peroxisomal oxidative enzymes (2, 17, 48), and the mitochondrial electron transport chain (ETC). The mechanism of the increased ROS production during exercise is under dispute. On the basis of the results from in vitro studies, one would expect that mitochondrial ROS production would decrease during exercise because of reduced membrane potential (Em) (17) and reduced oxygen tension (32). However, in contrast it has been shown that hypoxia increases oxidative stress in rodent muscle (25), possibly attributable to a reduction of NAD+ to NADH, which is known to be a factor that increases ROS generation (32). In a recent study, it was also shown that oxidation of free fatty acids (FFA) augments mitochondrial ROS production through a mechanism independent of membrane potential (38). These findings together with experimental evidences of oxidative modification of mitochondrial components during exercise (as reviewed in Ref. 9) suggest that mitochondria are a major source of ROS during exercise. Being a major source of ROS, muscle mitochondria are exposed to a high degree of oxidative stress, principally making them potential victims of a high degree of oxidative damage. When the antioxidant N-acetylcysteine was administered during prolonged exercise, the performance was increased in elite endurance-trained subjects but not in less trained subjects (29). The difference may relate to a higher degree of oxidative stress or a higher vulnerability to oxidative stress in athletes.
Despite the fact that mitochondria are assumed to be the most important source of ROS in exercise, ROS production in mitochondria in relation to exercise has not been well examined. To our knowledge, the effect of acute prolonged exercise on mitochondrial ROS production in human skeletal muscle has never been investigated. We hypothesized that prolonged exercise would increase mitochondrial ROS production measured in vitro and that this correlates with oxidative damage and with the plasma level of FFA. We have here used a unique experimental model to investigate this issue, namely measurements of ROS production and oxidative damage in mitochondria isolated from skeletal muscle of well-trained athletes exercising for 24 h and thus exposed to a high degree of oxidative stress for a prolonged period of time.
MATERIALS AND METHODS
The data presented here were obtained as a part of a broad study designed to study the physiological and metabolic effects of ultraendurance exercise. From this study, reports on mitochondrial respiratory function (10) and cardiorespiratory parameters (27) have already been published. These reports include detailed description of the experimental layout and mitochondrial preparation and the results from nine subjects. For clarity, the experimental conditions are briefly summarized here. Mitochondrial ROS production could not be measured in one subject because of technical problems, and we therefore report the results from the remaining eight subjects. Some of the values on plasma FFA and blood glucose (rest, postexercise, and recovery) have previously been reported for nine subjects (10). In this report, we present the whole time course of FFA and glucose for the eight subjects included in this study.
Subjects
Eight healthy men (age 29 ± 1 yr, body mass index 24.4 ± 0.7 kg/m2, V̇o2peak cycling 62.4 ± 2.0 ml·kg−1·min−1, type I skeletal muscle fibers 55.7 ± 3.2%) participated in this study. The participants belonged to the elite of Swedish ultraendurance, multisport performance athletes. All subjects were informed about the procedure and possible risks and discomfort involved in the experiment and about their right to terminate the experiment at any time point and signed written consent forms. The design of the study and the procedures were approved by the Regional Ethical Review Board in Stockholm, Sweden.
Pretests
The athletes performed pretests on a kayak ergometer (Dansprint, Hovide, Denmark), cycle ergometer (Monark ergomedic 893E; Monark Exercise, Vansbro, Sweden), and on a treadmill (Rodby Electronics, Vansbro, Sweden). After a brief warm-up, the work rate was raised every minute above the estimated work rate to reach V̇o2peak. VO2 was measured continuously with an online system (AMIS 2001; Innovision, Odense, Denmark). All subjects also performed a test to determine the onset of blood lactate accumulation (4 mmol/l) during kayaking, cycling, and running to make sure that they worked below their individual threshold during the main study.
Test Protocol for Main Study
The athletes arrived to the test lab in the morning after three days of standardized food intake and without strenuous exercise followed by one night of fast. The subjects performed 24 h of exercise consisting of 12 blocks (4 each of kayaking, running, and cycling) on a workload corresponding to ∼60% of their individual V̇o2peak in respective activity. Each block consisted of 110 min of exercise followed by 10 min of rest. The last exercise bout was cycling. The subjects were allowed to eat standardized food (59% carbohydrate, 29% fat, and 12% protein), as we aimed to give each person 50% of the estimated energy expenditure. Subjects were allowed to drink water freely during the experiment.
Sample Collection
Blood samples for analyses of FFA, insulin, and glucose were drawn at sitting rest, before exercise (preexercise), during the periods of steady-state cycling (i.e., every sixth hour), and after 28 h of recovery. A polyethylene catheter was inserted in an anticubital vein before exercise to facilitate the repeated blood sampling. Muscle biopsy samples were taken from the vastus lateralis muscle before exercise, after exercise (within 30 min postexercise), and after 28 h of recovery. The preexercise biopsies were taken about 5–12 days before the main test to avoid complications/disturbances during the strenuous exercise period. The biopsies taken postexercise and 28 h postexercise were taken from different legs. After local anesthesia (1–2 ml Carbocain, 20 mg/ml; Astra-Zeneca, Södertälje, Sweden), an incision was made through the skin and fascia, and a biopsy was taken using a Weil Blackesly conchotome. The biopsy (∼150 mg wet weight of muscle) was divided into two portions. One portion (∼50 mg) was frozen in liquid nitrogen and stored at −80°C, freeze-dried, and used for preparation of skeletal muscle homogenate in cold buffer containing: 2 mmol/l HEPES, 1 mmol/l EDTA, 5 mmol/l EGTA, 10 mmol/l MgCl2, 50 mmol/l β-glycerophosphate, 1 mmol/l Na3VO4, 2 mmol/l DTT, 1% Triton X-100, 20 μg/ml leptin, 50 μg/ml aprotinin, and 40 μg/ml PMSF, pH 7.40. The other portion (∼100 mg) was immediately used for isolation of mitochondria as previously described (42). In short, muscle specimens were disintegrated with scissors and treated with 0.4 mg/ml protease (Sigma P-4789), followed by homogenization and differential centrifugation. The final mitochondria pellet was resuspended in a buffer (225 mM mannitol, 75 mM sucrose, 10 mM Tris·HCl, 0.1 mM EDTA, and 0.2% bovine serum albumin, pH 7.4) and kept on ice until analysis of ROS production (described below). Protein concentration was determined with Pierce Protein Assay Kit (Pierce, Rockford, IL).
Mitochondrial ROS Net Production
Mitochondrial H2O2 net production was determined fluorometrically by the use of the Amplex red reagent (Molecular Probes, Eugene, OR). Oxidation of Amplex red coupled by horseradish peroxidase (HRP) causing reduction of H2O2 produces the red fluorescent oxidation product resorufin (21). Mitochondria (0.15–0.2 mg of mitochondrial protein/ml) were incubated at 37°C in a buffer consisting of 225 mM mannitol, 75 mM sucrose, 10 mM Tris base, 10 mM K2HPO4, 0.1 mM EDTA, 0.08 mM MgCl2, and 0.2% bovine serum albumin, pH 7.1. All incubations also contained 5 μM Amplex red, 12 U/ml HRP and 45 U/ml superoxide dismutase (SOD). The reaction was initiated by addition of succinate (5 mM) followed by addition of antimycin A (3 μg/ml). The rationale for using succinate (complex II substrate) is that in pretests we found very low ROS production with pyruvate-malate (complex I substrate) and also that this is the established method to measure mitochondrial ROS production, thus allowing comparison with the literature (16, 30). The increase in fluorescence at an excitation wavelength of 560 nm (slit 2.5 nm) and emission of 590 nm (slit 5 nm) was followed in a 300-μl microcuvette for 10–15 min with a Hitachi F-2500 spectrofluorometer. Addition of 50 U/ml catalase decreased the fluorescence signal by ∼99% (not shown). The rate of H2O2 production was calculated as the change in fluorescence intensity during the linear increase, i.e., after termination of any lag period. Calibration curves were obtained by adding known amounts of freshly diluted H2O2 (concentration checked at 240 nm using a molar extinction coefficient of 43.6) to the assay medium in the absence and presence of human skeletal muscle mitochondria. The standard curve was linear at least up to 900 nM H2O2.
Immunoblotting
Protein modification by sulf-4-hydroxy-2-nonenal (HNE) was determined by immunoblotting as described in Ref. 39. Samples (16 μg protein per lane) were added on 12% polyacrylamide gels (Bio-Rad, Hercules, CA), separated by SDS-PAGE, transferred by electroblotting to a PVDF membrane at 100 V for 180 min and blocked in TBS with 5% nonfat milk. Membranes were blotted overnight at 4°C with primary polyclonal antibodies (dilution 1:1,000) against HNE adducts (Alpha Diagnostics, San Antonio, TX). After being washed three times in TBS buffer with 0.1% Tween-20 (TBST), the membranes were incubated with secondary antibodies conjugated with HRP (anti-goat, dilution 1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were washed again in TBS and incubated with the chemiluminescence detection reagent, enhanced chemiluminescence (Amersham, Piscataway, NJ). Quantifications were performed with Quantity One 1-D Analyzing software (Bio-Rad) using the profile trace with lane-based background subtraction option.
For determination of adenine nucleotide translocase (ANT), the membranes used for detection of HNE adducts were stripped 1 h at 37°C in stripping buffer (Pierce) and blotted with goat anti-ANT polyclonal antibodies [ANT (Q-18) sc-9300, Santa Cruz], dilution 1:200. The membranes were then washed three times in TBST buffer and incubated with secondary antibodies as above.
For cytochrome c determination, the membrane was stripped again and blotted with mouse anti-cytochrome c monoclonal antibodies (BD Pharmingen, San Diego, CA), dilution 1:2,500. Secondary antibody was here anti-mouse (Pierce).
Enzyme Activities
Creatine kinase (CK, EC 2.7.3.2) activity was measured in plasma by Synchron LX Systems, Beckman (Fullerton, CA). Citrate synthase (CS, EC 4.1.37) activity was measured in skeletal muscle homogenate as previously described (42). SOD (EC 1.15.1.1) was assayed spectrophotometrically by monitoring the rate of acetylated cytochrome c reduction by superoxide radicals generated by the xanthine + xanthine oxidase system (13). Glutathione peroxidase (GPX, EC 1.11.1.9) (12) and glutathione reductase (GR, EC 1.6.4.2) (5) were analyzed as previously described.
Thiol Group Content
Determination of total thiol groups in skeletal muscle homogenate was based on the ability of thiols to develop a colored complex (maximum absorbance peak at 412 nm) when reacting with 5,5-Dithio-bis 2-nitrobenzoic acid (DTNB, Ellman's reagent) (37). Homogenate or standard (l-cysteine) was added to 750 μl buffer [30 mM Tris·HCl, 3 mM EDTA (pH 8.2) containing 10 mM DTNB and 80% methanol]. After development of color (15 min) followed by centrifugation at 3,000 g for 5 min, the absorbance of the supernatant was measured.
Statistics
Data are presented as means ± SE from eight subjects. Differences between time points (insulin, glucose, FFA) were tested with one-way repeated measures ANOVA. If a difference was detected, the location of significance was determined with Newman-Keuls post hoc test. Two-sided Student's paired t-test was used to test the differences between muscle samples. Correlation between two variables was tested with correlation analysis. Statistical significance was accepted at P ≤ 0.05.
RESULTS
Eight subjects at the elite level in adventure racing completed 24 h of exercise composed of running, cycling, and kayaking. The average work intensity over the whole 24-h period was 55% of V̇o2peak. A detailed report of the changes in cardiorespiratory parameters during exercise has been published elsewhere (27). There was a marked increase in plasma CK activity from 175 ± 19 U/l (preexercise) to 4,232 ± 1,436 U/l at postexercise (P < 0.025 vs. preexercise) and 2,006 ± 687 U/l at recovery (P = 0.031 vs. preexercise), which demonstrates the severity of the physiological stress imposed by the exercise.
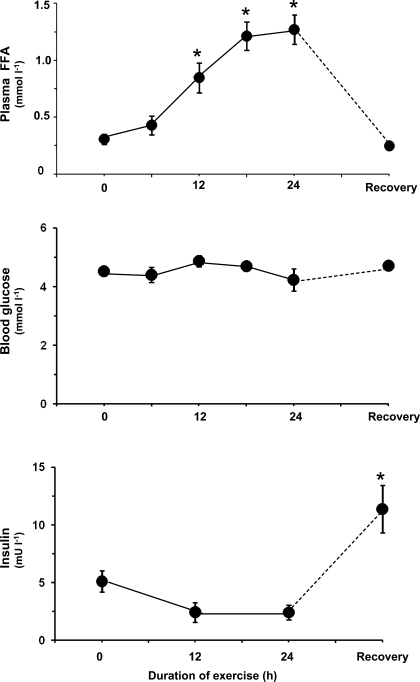
Plasma FFA increased successively during exercise, and during the last 6 h average FFA exceeded 1.2 mmol/l (Fig. 1). In one subject, plasma FFA was close to 2 mmol/l at the end of exercise. Although average blood glucose remained stable (Fig. 1), two subjects were hypoglycemic and reached a blood glucose level lower than 3 mmol/l at the end of exercise. Insulin tended to decrease during exercise (Fig. 1) and was at the end of exercise significantly correlated with blood glucose (r = 0.78, P < 0.05). After 28 h of recovery, insulin increased and was more than twofold higher (P < 0.001) than before exercise despite similar blood glucose.
Fig. 1.
Effect of exercise on blood glucose and plasma levels of free fatty acids (FFA) and insulin. Values are means ± SE from 8 subjects. *P < 0.001 vs. preexercise. Part of these data has been presented elsewhere (10); see materials and methods.
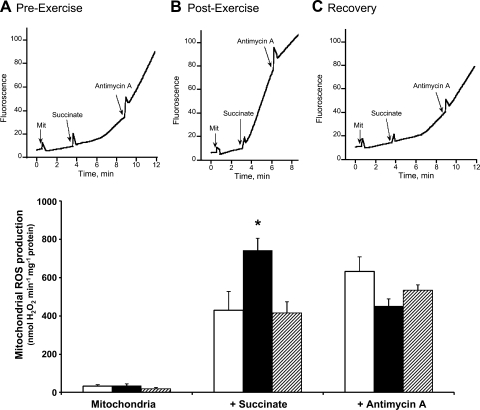
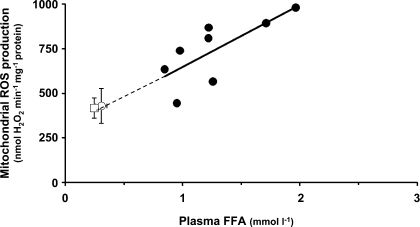
Muscle biopsies were analyzed for mitochondrial ROS production and related parameters. ROS production in isolated mitochondria in the absence of substrate was low and without difference between conditions (Fig. 2). After a lag period, addition of succinate resulted in a large increase in the rate of ROS production. The lag period was longer in preexercise biopsies (2.0 ± 0.4 min) and at 28 h of recovery (1.6 ± 0.3 min) than in postexercise biopsies (0.8 ± 0.7 min; P < 0.025 vs. preexercise and 28 h of recovery). The presence of a lag period may relate to the time taken to reduce the antioxidative capacity of mitochondria. The shorter lag period in postexercise muscle samples may thus be a consequence of the higher ROS production in these samples. Succinate-supported ROS production (i.e., the linear phase after the lag period) was almost twofold higher after exercise (P = 0.025 vs. preexercise) but was reversed to the initial level after 28 h of recovery. An interesting observation was that the succinate-supported mitochondrial ROS production in postexercise muscle samples was significantly correlated to the level of plasma FFA reached at the end of exercise (r = 0.74, P < 0.05; Fig. 3). Addition of antimycin A (an inhibitor of complex III) increased ROS production in preexercise biopsies (P < 0.01) but decreased ROS production in postexercise biopsies (P < 0.001). ROS production with succinate-antimycin A was not significantly different between conditions.
Fig. 2.
Effect of exercise on H2O2 production in isolated mitochondria. Top: representative original fluorometric traces from muscle biopsies taken from one athlete before exercise (preexercise) (A), immediately after exercise (postexercise) (B), and after a 28-h recovery period (recovery) (C). Isolated mitochondria were incubated with Amplex red and horseradish peroxidase to detect H2O2. Mitochondrial (Mit) protein concentrations were 0.27–0.29 mg/ml, and the final concentrations of succinate and antimycin A were 5 mmol/l and 3 μg/ml. Bottom: mitochondrial production of H2O2 in isolated mitochondria before and after additions of succinate and antimycin A. Values are means ± SE from 8 subjects. Muscle biopsies were taken preexercise (open bar), after 24 h of exercise (solid bar), and after 28 h of recovery (hatched bar). *P < 0.025 vs. preexercise and recovery. ROS, reactive oxygen species.
Fig. 3.
Relationship between rate of mitochondrial ROS production and plasma FFA. Correlation between succinate-supported mitochondrial ROS production in postexercise muscle samples and plasma FFA at the end of exercise (r = 0.743, P < 0.05). Correlation has been calculated for postexercise samples. Unfilled symbols correspond to the means ± SE of 8 subjects preexercise (○) and after 28 h of recovery (□).
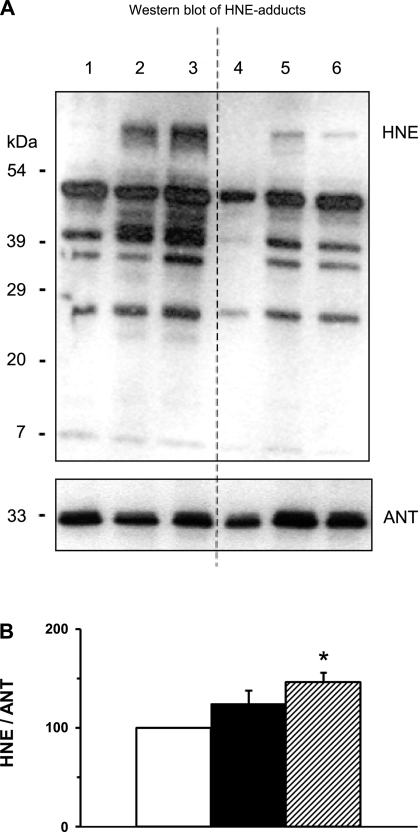
The contents of mitochondrial proteins such as ANT and cytochrome c remained unchanged by exercise (Table 1). The occurrence of mitochondrial oxidative damage was investigated by analysis of HNE/protein adducts with ANT as an internal reference base. Immunoblots of mitochondria analyzed with a specific antibody against HNE/protein adducts showed many bands with different molecular weights, indicating that several mitochondrial proteins had been modified by HNE already before exercise (Fig. 4A). There was a large variation in the number and intensity of HNE/protein adduct bands between the athletes. Average of HNE/protein adducts was higher postexercise but did not reach significance until 28 h postexercise (Fig. 4B). Thus strenuous exercise increased the level of HNE/protein adducts in skeletal muscle mitochondria, but this mainly occurred during the recovery phase.
Table 1.
Mitochondrial protein contents and muscle antioxidative enzyme activities
| Preexercise | Postexercise | Recovery | |
|---|---|---|---|
| ANT, AU vs. preexercise | 100 | 95.5 ± 5.3 | 101.7 ± 2.6 |
| Cytochrome c, AU vs. preexercise | 100 | 92.2 ± 12.2 | 86.1 ± 14.4 |
| Cytochrome c/ANT, AU vs. preexercise | 100 | 95.4 ± 9.5 | 83.7 ± 13.6 |
| SOD, U/mg protein | 6.40 ± 0.19 | 6.39 ± 0.15 | 6.31 ± 0.09 |
| GPX, U/g protein | 10.1 ± 0.7 | 11.0 ± 0.6 | 11.9 ± 0.6* |
| GR, U/g protein | 4.2 ± 0.1 | 4.4 ± 0.3 | 4.5 ± 0.5 |
| Thiols (SH groups), µmol/mg protein | 119.1 ± 7.5 | 109.4 ± 7.4 | 133.9 ± 3.0† |
Applicable values are means ± SE. Adenine nucleotide translocase (ANT), cytochrome c, and SH groups were analyzed in isolated mitochondria. Superoxide dismutase (SOD), glutathione peroxidase (GPX), and glutathione reductase (GR) were analyzed in muscle homogenate. AU, arbitrary units.
P < 0.025 vs. preexercise;
P < 0.025 vs. postexercise.
Fig. 4.
Effect of exercise on oxidative modification of proteins [sulf-4-hydroxy-2-nonenal (HNE)/protein adducts] in isolated mitochondria. A: immunoblot of skeletal muscle mitochondria. Top: HNE adducts. Lanes 1–3 are from one subject, and lanes 4–6 are from another subject (preexercise, lanes 1 and 4; postexercise, lanes 2 and 5; recovery, lanes 3 and 6). Bottom: adenine nucleotide translocase (ANT) as above. B: quantification of HNE adducts. For each athlete the level of HNE adducts was normalized to ANT protein level, and this ratio was set to 100% in mitochondria isolated from preexercise biopsy. *P < 0.05 vs. preexercise.
Antioxidative enzymes were determined in muscle homogenate. GPX was significantly higher 28 h postexercise (P < 0.025 vs. preexercise), whereas SOD and GR were unchanged (Table 1). Mitochondrial thiols (SH groups) were significantly increased 28 h post-exercise (P < 0.025 vs. Post-exercise).
DISCUSSION
We have here for the first time shown that prolonged exercise increases ROS production in isolated mitochondria from human muscle and that this is reversed after 28 h of recovery. The increased ROS production was not correlated with oxidative modification of mitochondrial proteins at a global level.
Effect of Exercise on Mitochondrial ROS Production
It is well established that strenuous exercise increases ROS production in contracting muscle (17, 34). Studies in rats have demonstrated an increased ROS production in mitochondria isolated from the exercised muscle when measured during standardized conditions (4, 46). In contrast, both acute and chronic eccentric exercise resulted in a reduced ROS production (30). There are numerous studies also in humans to show that exercise induces an increased oxidative stress, but the source of ROS is under dispute. This is the first study to demonstrate an increased ROS production in isolated mitochondria from human skeletal muscle after exercise. The increased ROS production was observed in vitro under standardized conditions and demonstrates that exercise induces changes of mitochondria, which increase the potential to produce ROS. We have previously reported that prolonged exercise results in altered mitochondrial function such as reduced mitochondrial efficiency and increased capacity for lipid oxidation (10), but it is unclear whether these changes are related to the increased mitochondrial ROS production.
Source of ROS
In mitochondria, superoxide is produced by single electron reduction of oxygen by an electron carrier within the ETC, and the primary sites of ROS production are considered to be complex I and III (3, 22, 31, 41, 45). The presence of SOD converts superoxide into H2O2, which is detected by increased fluorescence. In line with previous studies (16), we could, in verifying control experiments, show that succinate-supported ROS production is blocked by rotenone. Rotenone blocks complex I, and these results indicate that the succinate-supported ROS production occurs through back flow of electrons through complex I.
The succinate-supported ROS production was increased postexercise in isolated mitochondria (Fig. 2). Our data therefore support the idea that complex I of the mitochondrial ETC could be an important source of ROS during exercise (9). Specific changes in complex I sites could be the explanation for the observed increase in ROS production. Several respiratory components in complex I are thermodynamically capable of transferring one electron to oxygen and generate superoxide (3, 22, 45), and further analyses are required to clarify which components of complex I undergo changes during exercise.
Antimycin A inhibits complex III and is known to stimulate the production of ROS within the Q-cycle of complex III (45). Experiments in mouse muscle show that mitochondrial ROS production in the presence of antimycin A is not affected by rotenone, whereas another inhibitor of complex III (myxothiazole) totally blocked ROS production induced by succinate-antimycin A (data not shown). These findings suggest that succinate-supported ROS production in the presence of antimycin A is derived entirely from complex III. The observed decrease in succinate-supported ROS production after addition of antimycin A in postexercise samples is consistent with this contention. The present results show that ROS production from complex III was not affected by exercise.
A critical point is whether the present findings of increased succinate-supported ROS production in vitro are relevant for in vivo conditions. Pyruvate and FFA are the major mitochondrial substrates and will primarily donate electrons to complex I. However, the first step in β-oxidation of FFA (fatty acyl-CoA dehydrogenase) will donate electrons to complex II (via the electron transfer flavoprotein), and, because succinate is an obligatory intermediate in the tricarboxylic acid cycle, electron supply to ETC will always be convergent (14), involving both complex I and complex II. It has been shown (31) that succinate increases ROS production in isolated mitochondria even in the presence of complex I substrate (e.g., pyruvate) and that this occurs by reversed electron flow through complex I (inhibited by rotenone). Muller et al. (31) also showed that this occurred at low physiological concentrations of succinate (<1 mM) and concluded that reverse electron transfer-mediated superoxide production can occur in vivo. Our finding of increased succinate-supported ROS production may therefore be relevant for in vivo conditions.
Mechanisms
The present finding of an increased ROS production in isolated mitochondria after exercise cannot be explained by an increased oxygen flux because ROS production was measured in vitro, where the conditions were identical for all muscle samples. However, it is possible that a prolonged period of exercise-induced oxidative stress modifies mitochondrial components in such a way that ROS production measured in vitro increases.
In a recent study (1), it was shown that, 4 h after a high-fat meal, mitochondrial ROS production (with succinate) increased threefold and cellular redox state of glutathione became more oxidized (1). The mechanism for the increased ROS production after fat loading is unclear, but it was suggested that elevated FFA concentration could affect the ETC or the scavenging of H2O2 within mitochondrial matrix. In the present study, the concentration of plasma FFA was highly elevated for more than 6 h and was correlated with ROS production measured in isolated mitochondria (Fig. 4). It is tempting to speculate that prolonged exposure to high levels of FFA during exercise affects mitochondria in a similar way as dietary fat loading and therefore could be the cause of the increased ROS production. Further studies are required to investigate the mechanism of lipid-induced increase in mitochondrial ROS production.
Physiological Implications
Relation to insulin resistance.
Transient insulin resistance can be induced in lean subjects by short-term high-fat diet or by starvation (19), possibly related to an increased mitochondrial ROS production (1). The subjects in the present study experienced a severe metabolic and physiological stress, and the levels of plasma FFA during the last 6 h of exercise are similar to that after three days of starvation (19). It is therefore possible that the increased FFA load coupled to increased mitochondrial ROS production could evoke a transient state of insulin resistance. The twofold increase in plasma insulin observed 28 h postexercise (Fig. 1) despite similar blood glucose lends support to this line of reasoning. Furthermore, unpublished findings from our laboratory show that ultraendurance exercise for 6 days results in increased insulin levels even during the exercise period, which indicates a state of reduced insulin sensitivity. These findings are in agreement with the reduced insulin sensitivity observed after a marathon race (44). Lower insulin sensitivity may be of physiological advantage during ultraendurance exercise because this will reduce muscle utilization of blood glucose, spare carbohydrates, and secure the glucose demand by central nervous system. However, the link between oxidative stress and insulin sensitivity appears to be more complex because supplementation with antioxidants during training can prevent the improvement in insulin sensitivity after training (35).
No vicious cycle.
HNE is a major product of endogenous lipid peroxidation and can react with several functional groups of mitochondrial proteins (6, 33) in a concentration-dependent manner (39). The HNE/protein adducts are stable compounds, and analysis of adduct density in the proteins of isolated mitochondria can therefore be used to estimate the degree of oxidative damage that had occurred in vivo. Mitochondrial HNE adducts were not significantly changed at a time when ROS production was increased, and oxidative protein modification can therefore not be the primary reason for the observed increase in mitochondrial ROS production. The observed increase in HNE/protein adducts 28 h postexercise, at a time when mitochondrial ROS production was normalized, may seem paradoxical. However, the increase in HNE/protein adducts corresponds to the integral of oxidative protein modification incurred during the whole recovery period. Although not measured, it is possible that mitochondrial ROS production remains elevated during at least part of the recovery period, resulting in elevated HNE/protein adducts that remain after 28 h of recovery. Another possibility is that the period of strenuous exercise induces an inflammatory response with infiltration of neutrophils and subsequent ROS production, resulting in increased HNE/protein adducts (17).
A self-accelerating vicious cycle of ROS release from oxidative-damaged tissue has been implicated in ageing, mitochondrial neurodegenerative diseases, and apoptosis (3, 22, 45, 47). If a similar scenario would be valid for strenuous exercise, physical activity might cause self-inflicted oxidative damage, leading to muscle degeneration and reduced performance. However, the increased mitochondrial potential for ROS production associated with exercise was a reversible phenomenon and may instead be part of a normal physiological adaptation to training. The further implication of our observations is that the vicious-cycle hypothesis cannot have general significance because it clearly does not apply to the specific condition of this study and that the events occurring in ageing and other pathological states must demand more complex explanations.
Conclusions/Perspectives
ROS have traditionally been regarded as harmful products associated with tissue damage, ageing, and a number of diseases. However, it is now recognized that ROS have important functions in cell signaling and gene transcription such as glucose transport (35, 36) and mitochondrial biogenesis (40). This contention is supported by the finding that supplementation with antioxidants can inhibit the physiological adaptation to training (15, 35) and also affect performance negatively (26). The present study shows that ROS production in isolated mitochondria from human muscle is increased after exercise in a reversible manner and without indication of a detrimental vicious cycle. The exercise was of an extreme type with extraordinary duration. Further studies are required to investigate whether mitochondrial ROS production is increased also after exercise of less extreme nature and in less trained subjects.
GRANTS
The study was supported by grants from The Swedish National Centre for Research in Sport (CIF), The Swedish Research Council (project 13020), and The Swedish School of Sport and Health Sciences (GIH).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Gunilla Hedin and Alexandra Tonkonogi for excellent technical assistance and Vladimir Gogvadze (Karolinska Institute) and Mikhail Vyssokikh (Moscow University) for discussion.
REFERENCES
- 1. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol 287: H1689–H1699, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87: 465–470, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol 113: 484–490, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE). Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta 1380: 336–344, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med 40: 976–982, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept 10: 125–140, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fernstrom M, Bakkman L, Tonkonogi M, Shabalina IG, Rozhdestvenskaya Z, Mattsson CM, Enqvist JK, Ekblom B, Sahlin K. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol 102: 1844–1849, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr 36: 347–352, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 105: 114–121, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol 105: 93–104, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41: 1837–1845, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem 90: 405–421, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102: 1664–1670, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222: 283–292, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Johnson NA, Stannard SR, Rowlands DS, Chapman PG, Thompson CH, O'Connor H, Sachinwalla T, Thompson MW. Effect of short-term starvation versus high-fat diet on intramyocellular triglyceride accumulation and insulin resistance in physically fit men. Exp Physiol 91: 693–703, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Katz A. Modulation of glucose transport in skeletal muscle by reactive oxygen species. J Appl Physiol 102: 1671–1676, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Kettle AJ, Carr AC, Winterbourn CC. Assays using horseradish peroxidase and phenolic substrates require superoxide dismutase for accurate determination of hydrogen peroxide production by neutrophils. Free Radic Biol Med 17: 161–164, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279: 4127–4135, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Goldfarb AH, Rescino MH, Hegde S, Patrick S, Apperson K. Eccentric exercise effect on blood oxidative-stress markers and delayed onset of muscle soreness. Med Sci Sports Exerc 34: 443–448, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci USA 95: 510–514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magalhaes J, Ascensao A, Soares JM, Ferreira R, Neuparth MJ, Marques F, Duarte JA. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol 99: 1247–1253, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Malm C, Svensson M, Ekblom B, Sjodin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand 161: 379–384, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Mattsson CM, Enqvist JK, Brink-Elfegoun T, Johansson PH, Bakkman L, Ekblom B. Reversed drift in heart rate but increased oxygen uptake at fixed work rate during 24 h ultra-endurance exercise. Scand J Med Sci Sports. In press [DOI] [PubMed] [Google Scholar]
- 28. Maughan RJ, Donnelly AE, Gleeson M, Whiting PH, Walker KA, Clough PJ. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve 12: 332–336, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 97: 1477–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Molnar AM, Servais S, Guichardant M, Lagarde M, Macedo DV, Pereira-Da-Silva L, Sibille B, Favier R. Mitochondrial H2O2 production is reduced with acute and chronic eccentric exercise in rat skeletal muscle. Antioxid Redox Signal 8: 548–558, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J 409: 491–499, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med 37: 937–945, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Reid MB. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med 44: 169–179, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 575: 251–262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192–205, 1968. [DOI] [PubMed] [Google Scholar]
- 38. Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain dependent and independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shabalina IG, Petrovic N, Kramarova TV, Hoeks J, Cannon B, Nedergaard J. UCP1 and defense against oxidative stress. 4-Hydroxy-2-nonenal effects on brown fat mitochondria are uncoupling protein 1-independent. J Biol Chem 281: 13882–13893, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763: 969–976, 2006 [DOI] [PubMed] [Google Scholar]
- 41. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand 161: 345–353, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Tuominen JA, Ebeling P, Bourey R, Koranyi L, Lamminen A, Rapola J, Sane T, Vuorinen-Markkola H, Koivisto VA. Postmarathon paradox: insulin resistance in the face of glycogen depletion. Am J Physiol Endocrinol Metab 270: E336–E343, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5: 109–117, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Wei YH, Lu CY, Lee HC, Pang CY, Ma YS. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann NY Acad Sci 854: 155–170, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Med 35: 1045–1062, 2005. [DOI] [PubMed] [Google Scholar]