Abstract
Ventilatory sensitivity to hypercapnia is greater in Dahl salt-sensitive (SS) rats than in Fawn Hooded hypertensive (FHH) and Brown Norway (BN) inbred rats. Since pH-sensitive potassium ion (K+) channels are postulated to contribute to the sensing and signaling of changes in CO2-H+ in chemosensitive neurons, we tested the hypothesis that there are more pH-sensitive K+ channel-immunoreactive (ir) neurons within the medullary raphé nuclei of the highly chemosensitive SS rats than in the other two strains. Medullary tissues from male and female BN, FHH, and SS rats were stained with cresyl violet or with antibodies targeting TASK-1, Kv1.4, and Kir2.3 channels. K+ channel-ir neurons were quantified and compared with the total neurons in the region. The total number of neurons in the medullary raphé 1) was greater in male FHH than the other male rats, 2) did not differ among the female rats, and 3) did not differ between sexes. The average number of K+ channel-ir neurons per section was 30–60 neurons higher in the male SS than in the other rat strains. In contrast, for the females, the number of K+ channel-ir neurons was greatest in the BN. We also found significant differences in the number of K+ channel-ir neurons between sexes in SS (males > females) and BN (females > males) rats, but not the FHH strain. Our findings support the hypothesis for males but not for females, suggesting that both genetic background and sex are determinants of K+ channel immunoreactivity of medullary raphé neurons, and that the expression of pH-sensitive K+ channels in the medullary raphé does not correlate with the ventilatory sensitivity to hypercapnia.
Keywords: potassium channels, CO2 sensitivity, raphé nucleus
we (6, 9, 11) and others (25) have previously reported differences in ventilatory sensitivity to hypercapnia between inbred rat strains. Male and female Dahl salt-sensitive (SS) rats increase pulmonary ventilation ∼183% after switching the inspired gas from room air to a mixture with 7% CO2, while both male and female Fawn Hooded hypertensive (FHH) and Brown Norway (BN) rats only increase ventilation 94% and 37%, respectively, with the same challenge (11). This large difference in pulmonary ventilation is a result of differences in both the tidal volume and breathing frequency response to inhaled CO2. Despite the large interstrain variation, there were no differences in the hypercapnic ventilatory response between sexes in any strain. Likewise, there were no differences between these strains in breathing during eupnea or in the ventilatory responses to hypoxia or exercise (6, 9, 11); thus the strain differences appear specific to the CO2-H+ chemoreception and/or chemosensory integration.
Respiratory CO2-H+ chemoreceptors are thought to be located at widespread sites in the brain (12, 17, 18, 21, 30). The sensing and intracellular signaling mechanism(s) involved in chemotransduction are not well understood but are likely driven by changes in intracellular and extracellular CO2 and [H+] (8, 21). For many chemosensitive neurons, acidosis-dependent depolarization apparently depends on the inhibition of multiple pH-sensitive K+ channels (2, 8, 19, 31), acting to increase discharge frequency and thus excitatory input to the respiratory network to ultimately increase pulmonary ventilation. These K+ channels include select inwardly rectifying (Kir2.3) (19, 31), voltage-activated (Kv1.4) (8), and twik-related acid-sensitive [TASK-1; (2)] potassium channels. TASK-1 channels are sensitive to changes in extracellular pH over a range of pH = 6.5–7.8 (5), while Kir2.3 and KV1.4 channels are sensitive to both intracellular and extracellular pH, with pKa values centered on pH ∼ 6.8–7.2 (31) and pH = 6.3–7.5, respectively (21). However, it is not known if differences in the expression of these pH-sensitive K+ channels within chemoreceptive nuclei relate to differences in the ventilatory response to hypercapnia in vivo, or what exactly determines the output of a chemosensitive region to the respiratory network. One possibility is the potential for differences or changes in expression of CO2-H+-sensitive ion channels, as has been suggested by Nichols et al. (18). They postulated that “a chronic hypoxia-induced increase in the number of CO2-H+ inhibited neurons in the solitary complex (SC) is due to the change in the expression of CO2-H+-sensitive ion channels converting CO2-insensitive SC neurons into CO2-inhibited neurons.”
Since the role in CO2 sensitivity of the number or percentage of neurons expressing K+ channels is unknown, the objective of the present study was to determine whether there are differences between the three inbred rat strains in the number of K+ channel-ir neurons. Our observations were focused on the medullary raphé nuclei, a documented site of CO2-H+ chemosensitive neurons (12, 13, 22, 30). We reasoned that CO2-H+ inhibition of K+ channels in a relatively greater number of neurons would lead to greater excitation of the respiratory network and a relatively greater hypercapnic ventilatory response. Thus we hypothesized that we would detect a greater number of K+ channel-ir neurons in the medullary raphé of SS rats than in the other rat strains.
METHODS
In-house SS (6 male and 6 female), BN (6 male and 6 female each), and FHH (7 male and 7 female) rat strains were studied. Both male and female rats were studied due to previously observed sex differences in physiological phenotypes, including the control of breathing (6, 20, 23, 24). All rats were generated and continuously housed at the Medical College of Wisconsin (MCW) Biomedical Resource Center Transgenic Barrier facility before participation in experimental protocols. All animals were provided a diet of Purina Rat Chow and water ad libitum. None of the rats were used for any physiological studies. All aspects of the protocols were reviewed and approved by the MCW Animal Welfare Committee.
Histology.
At 70 days of age, all rats were euthanized with pentobarbital. The heart was exposed and the ascending aorta was cannulated via left ventricular puncture. The lungs were tied with silk suture to ensure the direction of flow toward the brain, the descending aorta was clamped, and the right atrium was cut to allow flow. The brain was then perfused with 0.2 M PBS via a roller pump for 20 min, followed by perfusion with 4% paraformaldehyde (PFA) in PBS for 30 min (10 ml/min). The medulla was extracted, postperfusion fixed in 4% PFA for 2 h, and dehydrated over 2 days with 20% and 30% sucrose solutions, respectively. The medulla was then frozen at −80°C, and transverse sections (25 μm) were generated serially in four series and mounted on coated slides from obex and continuing +2.0 mm rostral. The first series was stained with cresyl violet (Nissl) to determine the total number of neurons within the medullary raphé. The remaining three series were stained with polyclonal antibodies (1:100, Alomone Labs, Jerusalem, Israel) raised in rabbits targeting rat (residues 589–655 for KV1.4, or residues 418–437 for Kir2.3) or human (residues 252–269 for TASK-1) proteins. The specificity of these antibodies has been demonstrated by the supplier (Kir2.3) and others [KV1.4 and TASK-1 (10, 14–16)]. The sections were first rinsed with Tris buffer, avidin and biotin blocked (30 min each), and peroxidase and protein blocked (20 min each) before exposure to the primary antibodies for 48 h. The tissues were then washed (20 min × 2) in PBS and incubated with a secondary antibody (biotinylated anti-rabbit antibody) for 3 h, rinsed, and reacted with diaminobenzidine (10 min), and finally rinsed with Tris buffer, dehydrated, and coverslipped.
Data acquisition and statistical analysis.
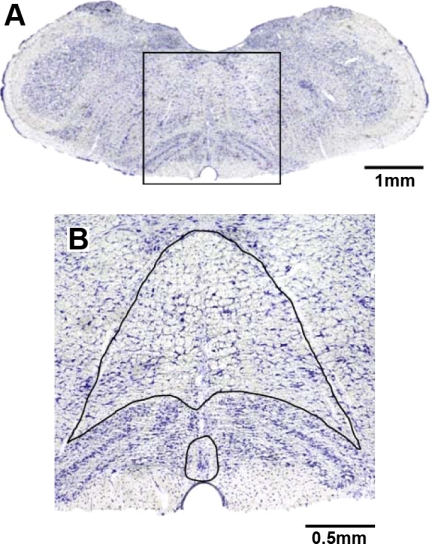
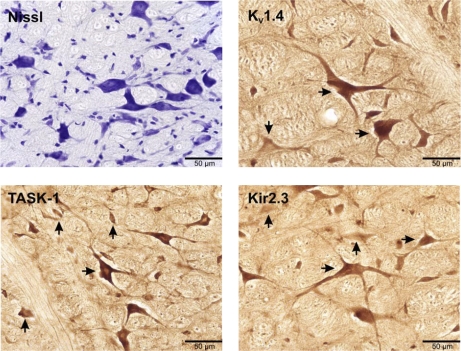
For the purposes of cell counts, we defined the raphé pallidus as ventral to the olivary nuclei and at the midline between the olivary nuclei (Fig. 1). Raphé obscurus was identified as dorsal to the olivary nuclei and medial and ventral to the hypoglossal nerves. Stained tissue was visualized microscopically (40×), imaged with acquisition software (SPOT Basic, Diagnostic Instruments), and the count area determined using analysis software (Metamorph, Molecular Devices). Within the designated area of interest, neurons were included in the counts based on the following criteria: degree of staining, neuronal size and shape, and section quality. Neurons that were 15–20 μm in diameter, with a clearly identifiable soma, nucleus, and dendritic or axonal processes were determined to be immunopositive or immunonegative based on the degree of antibody staining. The degree of neuronal staining was variable in individual neurons within the raphé (and other nuclei) for each of the K+ channels, where some neurons were faintly stained (negative) and others stained heavily (positive) compared with background levels. Examples of images of positively and negatively stained neurons are shown in Fig. 2. Finally, neuron counts were not made if the tissue sections were folded, bubbled, or damaged during processing.
Fig. 1.
Area of the medulla analyzed for total neurons and K+ channel immunoreactive neurons. A: transverse section (1× magnification) at obex from a Dahl salt-sensitive (SS) rat that was stained for Nissl substance. B: the box in A at 10× magnification, wherein the neuron count area is outlined. The small area is raphé pallidus ventral to the olivary nuclei and at the midline between the olivary nuclei. The larger enclosed area is raphé obscurus dorsal to the olivary nucleus and medial and ventral to the hypoglossal nerves.
Fig. 2.
Medullary sections (25 μm) at 40× magnification that were Nissl stained (top left) or immunostained with antibodies for voltage activated (Kv1.4; top right), TASK-1 (bottom left), or inward rectifying (Kir2.3; bottom right) K+ channels. Horizontal arrows indicate positively stained neurons, whereas vertical arrows indicate negatively stained neurons.
Neurons were manually counted exclusively by one investigator. However, periodically a second investigator would also count a limited number of sections. In a systematic comparison between two investigators each counting neurons on 40 sections of each stain, the average difference between the counts was <1% (P > 0.10) of the total number of neurons and the SD of the differences were 5.7%, 6.5%, 7.8%, and 5.6% of the total number of Nissl, Task-1, Kv1.4, and Kir2.3 stained neurons, respectively. Nissl-stained neurons were used as an index of the total number of neurons, and these were compared with neurons immunostained for the K+ channel on serially juxtaposed section. The density of the neurons was calculated by dividing for each section the number of neurons by raphé area.
A two-way ANOVA was used to determine whether there were significant (P < 0.05) differences in variables measured for each rat strain within the distance 0–2 mm rostral to obex and to determine whether there were differences between the strains in raphé area, neuron number, and neuronal density. If the two-way ANOVA P value was <0.05, a Bonferroni post hoc analysis was used to identify pairwise differences. For each sex of each strain, the average number of neurons and average density of neurons for all Nissl and all K+ channel stained sections were computed and then an unpaired t-test was used to determine whether there were sex differences.
RESULTS
Body weight.
The body weights of the male SS (300.8 ± 10.3 g) and FHH (285 ± 7.6 g) rats did not differ, but these strains weighed more (P < 0.001) than the male BN (236 ± 4.5 g) rat strain. Similarly, the body weights of the female SS (200.2 ± 2.9 g) and FHH (193.6 ± 1.2 g) did not differ, but these strains weighed more (P < 0.001) than the female BN (156 ± 6.0 g) strain. Male rats consistently weighed more (P < 0.001) than the female rats within each strain.
Area of Raphé nucleus examined.
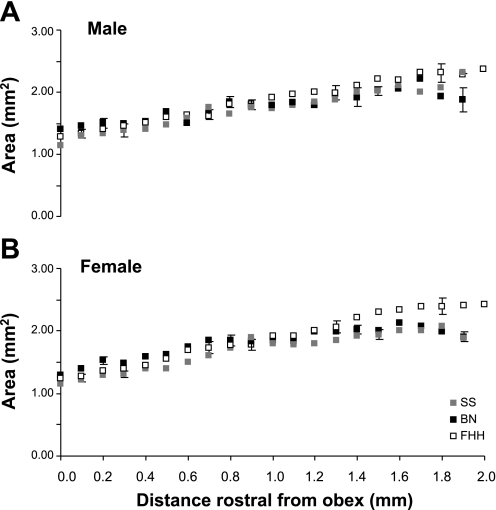
For both sexes of each strain, the cross-sectional area of the raphé increased (P < 0.001) from 1.25 mm2 at obex to nearly 2.0 mm2 at 2 mm rostral to obex (Fig. 3). Even though there were strain and sex differences in body weight, there were no significant (P > 0.05) differences in raphé area between the three strains and there were no sex differences for any strain.
Fig. 3.
In SS, Brown Norway (BN), and Fawn Hooded hypertensive (FHH) male (A) and female (B) rat strains, the area of the medullary raphé nucleus increases (P < 0.001) between 0 and 2 mm rostral to obex. There were no significant (P > 0.05) differences in medullary raphé area between the 3 strains, and there were no sex differences for any strain.
Nissl-stained neurons in medullary raphé as an index of total neuron number.
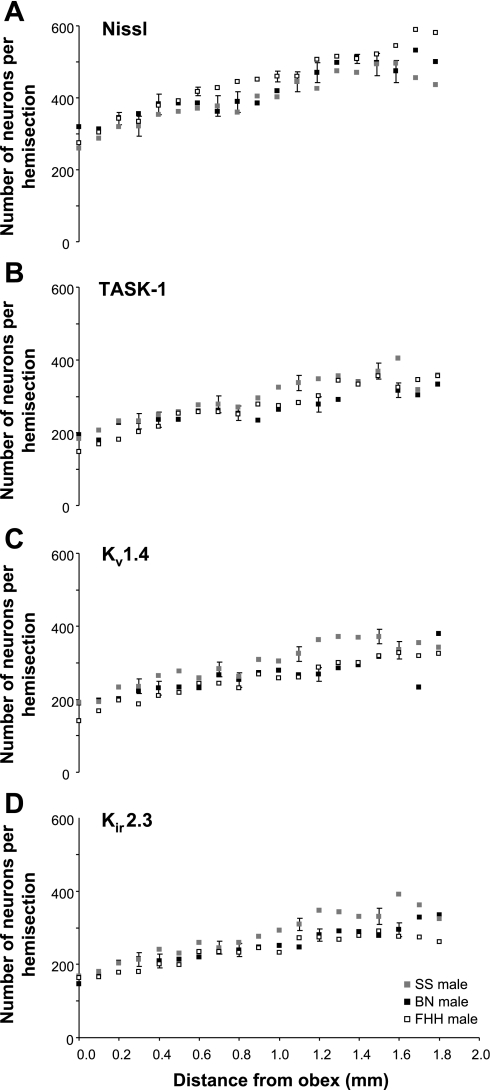
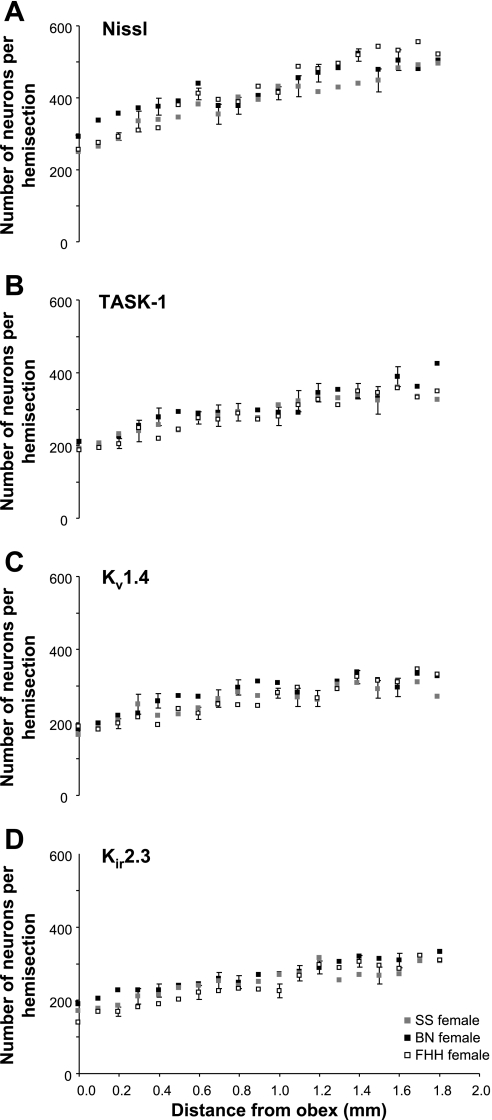
For both sexes of each strain, the number of Nissl-stained neurons in the raphé nucleus at obex was between 250 and 300 neurons (Figs. 4A and 5A). The number of neurons increased (P < 0.05) rostrally reaching values between 400 and 550 neurons on the section 2 mm rostral to obex. Over the 2 mm distance of the raphé studied, the average number of neurons of all the tissue sections varied from a low of 400 neurons in male SS to a high of 448 neurons in the male FHH (Fig. 6). The average number of neurons was higher (P < 0.05) in male FHH than in male SS and BN strains, but there were no strain differences among the females and there were no sex differences for any strain (Fig. 6, A–C).
Fig. 4.
In male rats, there were significant (P < 0.05) strain differences in the total number of neurons stained for Nissl substance (A) and in the number of K+ channel-immunoreactive (ir) neurons (B–D) in the medullary raphé nucleus between 0 and 2 mm rostral to obex. The total number of neurons was greater (P < 0.05) in the FHH strain than in the SS and BN strains. The number of neurons immunoreactive for all 3 pH-sensitive K+ channels was greater (P < 0.05) in the SS strain than in the BN and FHH strains. The post hoc test did not reveal any significant differences at specific distances rostral to obex. For the total and all 3 K+ channel-ir neurons, there was a significant increase (P < 0.05) between 0 and 2 mm rostral to obex.
Fig. 5.
In female rats, there were relatively few significant strain differences in total neurons (A) and K+ channel-ir neurons (B–D) in the medullary raphé nucleus between 0 and 2 mm rostral to obex. The BN tended to have more K+ channel-ir neurons than the other strains, but the difference was only significant (P < 0.05) for the Kir2.3 channel. For the total neurons and all 3 K+ channel-ir neurons, there was a significant increase (P < 0.05) between 0 and 2 mm rostral to obex.
Fig. 6.
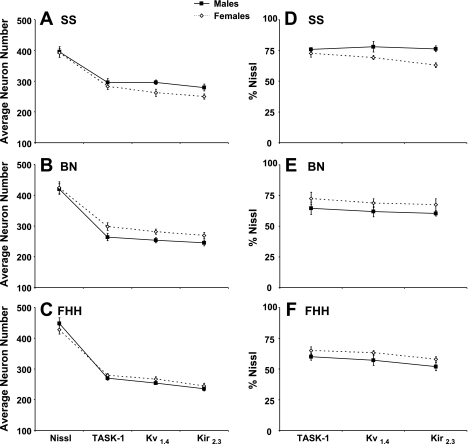
There were sex differences in the number (A–C) and percentage (D–F) of total neurons immunoreactive for K+ channels. For the SS strain, the number of K+ channel-ir neurons was ∼10% higher (P < 0.001 for Kv1.4 and Kir2.3) in males than females (A). In contrast in the BN strain, K+ channel-ir neurons were consistently more than 10% higher (P < 0.01) in females than in males, but in the FHH strain, there were no significant (P > 0.10) sex differences in the number of K+ channel-ir neurons. When K+ channel immunoreactivity is viewed as a percentage of the total number of neurons (i.e., Nissl stained), the sex differences stated above are apparent, but none of these differences reach statistical significance (D–F).
Number of K+ channel-ir neurons in the medullary raphé.
For both sexes of each strain, there were ∼200 K+ channel-ir neurons at obex (Fig. 4, B–D, and Fig. 5, B–D). The number of K+ channel-ir neurons increased (P < 0.05) rostrally reaching values generally over 300 neurons on sections 2 mm rostral to obex. Over the 2 mm distance of the raphé studied, the average number of K+ channel-ir neurons of all the tissue sections was 30–60 neurons greater (P < 0.01) in the male SS rats than in the male BN and FHH rats (Fig. 4, B–D). In contrast, for the females, the number of K+ channel-ir neurons was greater in the BN strain than in the other strains with the differences significant (P < 0.05) for the Kir2.3 channel. For the SS strain, the number of K+ channel-ir neurons was ∼10% higher (P < 0.001 for 2 of 3 channels) in the males than in females (Fig. 6A). However, in the BN strain, K+ channel-ir neurons were consistently more than 10% higher (P < 0.01) in females than in males (Fig. 6B). Finally, in the FHH strain there were no sex differences in number of K+ channel-ir neurons (Fig. 6C).
Density of neurons in the raphé nucleus.
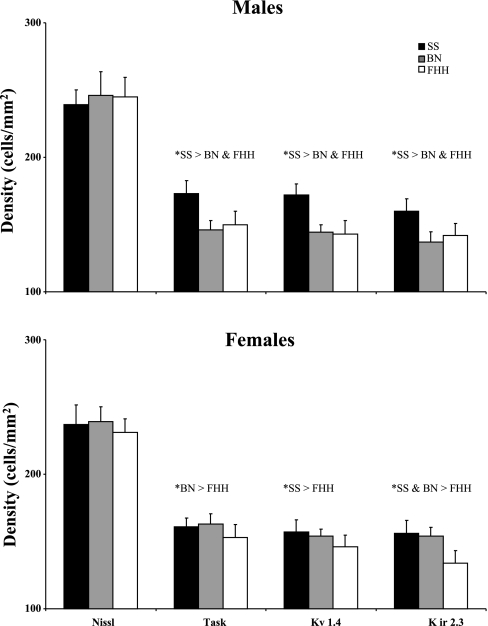
The density of Nissl-stained neurons and K+ channel-ir neurons did not change significantly (P > 0.10) in the region of interest. In other words, the difference in the number of neurons between 0 and 2 mm rostral to obex (Figs. 4 and 5) was due to the difference in area between 0 and 2 mm rostral to obex (Fig. 3). There were no differences (P > 0.10) between the strains in the density of Nissl-stained neurons (Fig. 7). However, the density of K+ channel-ir neurons was greater in (P < 0.01) the SS male than in the other two male strains whereas the density of K+ channel-ir neurons in the FHH females was less than one or both of the other strains (Fig. 7). Finally the density of K+ channel-ir neurons was generally lower in SS females than in males, but the opposite was true for the BN and there were no sex differences in the FHH strain.
Fig. 7.
There were differences among the rat strains in the density of K+ channel-ir neurons in the medullary raphé. Presented for both males and females is the average neuron density over all the sections 0–2 mm rostral to obex. In male rats the density of all 3 K+ channel-ir neurons was greater in (P < 0.05) in the SS males than in the other 2 male strains. In the female rats, there were also significant differences between strains in density of K+ channel-ir neurons with density least in the FHH strain. There were no significant (P > 0.10) strain differences in the density of Nissl-stained neurons.
Relative number of K+ channel-immunoreactive neurons.
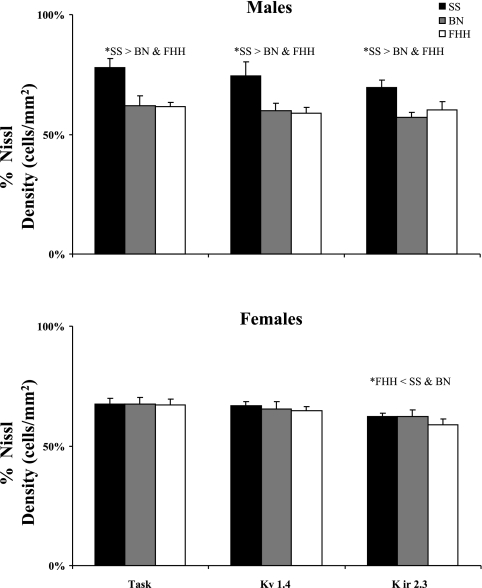
There were strain and sex differences in the percent of total neurons and density of neurons that were K+ channel-ir. In the male SS strain, 71–75% of the neurons were immunoreactive for K+ channels (Fig. 6D), which was significantly higher (P < 0.01) than the 58–63% in the BN (Fig. 6E) and the 52–60% expression in the FHH (Fig. 6F). Similarly, the density of K+ channel-ir expressed as a percentage of density of total neurons was also higher in SS males than in BN and FHH males (Fig. 8). In the females, there were no significant differences between the SS and BN, but the percentage of total and the density of neurons that were K+ channel-ir neurons in the FHH strain was less (P < 0.05) than one or both of the other strains (Fig. 8). There were no significant sex differences in the percentage of K+ channel-ir neurons, but expression in the SS males tended to be higher than in females while the opposite was true in the other two strains. In the SS females and in both male and female of the FHH strain, the percentage of Kir2.3 channel-ir was lower (P < 0.05) than expression of the TASK-1 K+ channel.
Fig. 8.
There were differences among rat strains in the density of K+ channel-ir neurons expressed as a percentage of total neurons density. Presented for both males and females is the average percent density over all the sections from 0 to 2 mm rostral to obex. In male rats, the percent density was consistently greater for the SS strain than for the other strains. The only significant difference in females was that the percent density in the Kir2.3 channel was less than in the SS and BN rats.
DISCUSSION
Previous observations indicated that the hypercapnic ventilatory responses in SS rats were greater than in BN and FHH rat strains. Accordingly, we hypothesized that there would be a greater number of CO2-H+-sensitive K+ channel-ir neurons in the medullary raphé nucleus of SS rats than in BN and FHH rat strains. The findings support the hypothesis for males but not for females.
The rationale for the present study was based on the following information. First, the medullary raphé nucleus is one of a handful of brain stem sites in which there are neurons sensitive to changes in CO2-H+ (4, 12, 30). Second, studies have shown that in many neurons, increased hypercapnic acidosis inhibits K+ channels, which depolarizes neurons and results in an increase in discharge frequency of the neurons (2, 8, 17, 19, 31). Presumably, the increase in discharge frequency increases the excitatory input to the respiratory control network to increase breathing. Third, TASK-1, KV, and inward rectifying K+ channels have all been shown to be CO2-H+ sensitive to varying degrees, and many medullary raphé neurons express all three of these K+ channels (Figs. 4 and 5). Finally, it has been shown that raphé 5-HT neurons in neonatal TASK-1 channel knockout mice lack H+ sensitivity in vitro (17). Accordingly, these cumulative findings provided the rationale for our hypothesis that since CO2-H+ ventilatory sensitivity is greater in SS rats compared with BN and FHH rats, there would be a relatively greater number of K+ channel-ir neurons in raphé neurons of the SS rats. Our hypothesis is not without precedent as Nichols et al. (18) recently postulated that a chronic hypoxia-induced increase in the number of CO2-H+ inhibited neurons in the solitary complex (SC) is due to the change in the expression of CO2-H+-sensitive ion channels converting CO2-insensitive SC neurons into CO2-inhibited neurons.
In the interpretation of these findings, it is important to emphasize that multiple factors influence the ventilatory response to changes in CO2-H+ (21). However, the factors accounting for the differences between these three rat strains must be specific to the CO2-H+ sensing and/or signaling pathway because the ventilatory responses to hypoxia and exercise and eupneic breathing do not differ between the three strains (6, 9, 11). In addition, introgression of BN chromosome 6 on the SS background attenuated the CO2-H+ ventilatory response of this consomic rat compared with the parental SS rat, but the ventilatory responses to hypoxia and exercise and eupneic breathing did not differ between the parental and consomic rat (6).
The data from the present and past studies provide three examples of absence of direct correlation between CO2 sensitivity and the number of K+ channel-ir neurons. First, in male rats, the expression of K+ channel-ir neurons is 11–24% higher in SS rats than in BN and FHH rats while the SS rat has a fivefold greater ventilatory sensitivity to hypercapnia. Second, in female rats the number of medullary raphé K+ channel-ir neurons was greater in the BN than in the other rat strains, but ventilatory sensitivity to hypercapnia is least in the BN strain. Third, the percentage of raphé neurons that were positive for the various K+ channels was 50–75% of the total number of neurons, but as shown by others, the percentage of medullary raphé neurons that have a firing rate response to hypercapnia is only a fraction (∼18%) of the total. These quantitative differences could reflect the contributions of 1) other chemoreceptors at widespread locations, 2) other pH-sensitive K+ channels, 3) other pH-sensitive ion channels, such as nonselective cation channels (21, 22), 4) the fact that we only quantified the number and percentage of K+ channel-ir neurons and not the magnitude of the response to hypercapnia of individual neurons, and/or 5) differences in downstream signaling mechanisms that affect overall chemosensitivity. The mismatch between the percentage of raphé K+ channel-ir neurons and percentage of chemosensitive raphé neurons suggests a large percentage of the K+ channel-ir neurons are insensitive to hypercapnia, perhaps because they contain isoforms of these channels that are insensitive to hypercapnia. Overall, these findings suggest that neither the number nor percentage of raphé K+ channel-ir neurons is a critical determinant of the ventilatory response to hypercapnia.
Our findings emphasize the important role of factors related to sex in physiological responses and cellular properties. We have previously observed sex differences in the effects of introgressing chromosome 6 of the BN rat onto the SS genetic background (6). This consomic rat's ventilatory response to CO2-H+ was attenuated compared with the SS rat in the females as a result of a reduced tidal volume response. In contrast, in the male consomic rat, the ventilatory response was the same as in the SS even though the tidal volume response was reduced. This reduction was offset by a greater breathing frequency response to increased CO2-H+ stimulation in the consomic male compared with the parental male. Important to note is that introgression of the BN Y chromosome onto the SS background increased the breathing frequency response to hypercapnia in males (6). This finding suggests that multiple genes and gene-gene interactions not only determine physiological responses but may also determine neuronal phenotypes such as expression of K+ channels.
We are not aware of any studies that have previously documented sex differences in the number of neurons expressing ion channels that could impact ventilatory sensitivity to CO2-H+. However, others have documented sex differences in factors that impact CO2-H+ sensitivity. For example, Schlenker and Hansen (24) found the density of neurons in the nucleus tractus solitarius and hypoglossal nucleus with the NR1 subunit of the NMDA receptor is greater in female than in male rats. Blocking this receptor subunit decreased the breathing frequency response to hypercapnia more in female than in males. Similarly, in the same nuclei the number of neurons expressing estrogen receptors-α and -β were greater in female than in male rats, leading to the conclusion that these differences “may be responsible for sex differences in control of breathing” (23). Finally, Polotsky et al. (20) found that leptin deficiency in female obese mice had a greater depressive effect on CO2-H+ sensitivity than on male obese rats.
We are also not aware of any previous studies documenting differences between genetically unique rat strains in expression of ion channels that could impact CO2-H+ sensitivity. However, several studies in addition to our own (6) have provided insights on the genetics of ventilatory control (20, 27–29). In addition to the role of leptin, Tankersley et al. have found that the interactions between hypoxia and hypercapnia are related to genes on mouse chromosomes 1 and 5 (29), and that variation in the acute response to hypoxia is linked to genes on mouse chromosome 9 (28). Finally, specific genetic mutations underlie the absence of chemosensitivity in human congenital central alveolar hypoventilation syndrome (1).
Previous studies have found sex and/or strain differences in K+ channel expression. For example, Brouillette et al. (3) found in mice that strain and sex differences in cardiac repolarization are related to differences in androgen levels that affect the expression of Kv1.5 channels. Also studies by Few and Zakon (7) showed that variation in Kv1 channels expressed in the electric organ of electric fish correlate with differences in and sexual dimorphism of the signal this species utilizes for communication. Finally, Takimoto and Levitan (26) found that many stimuli including steroid hormones, neurotransmitters, and electrical activity affect K+ channel subunit gene expression, leading to changes or differences in neuronal excitability. Accordingly, these data provide additional rationale for future studies on the contribution of variations in expression of K+ channels to variation in chemosensitivity and other aspects of ventilatory control.
Summary and conclusions.
One major finding of this study was the absence of a consistent relationship among rat strains in the number of medullary raphé K+ channel-ir neurons and the previously documented ventilatory sensitivity to CO2-H+ in rat strains. These findings suggest that the relative expression of pH-sensitive K+ channels in medullary raphé neurons is not a critical determinant of chemosensitivity. A second major and novel finding was the differences between rat strains and between male and female rats in the number of medullary raphé neurons immunoreactive for K+ channels. These findings indicate that genetic background and sex are determinants of K+ channel immunoreactivity in the medullary raphé nucleus of rats. Finally, the sex differences in K+ channel immunoreactivity varied among the rat strains, indicating gene-gene interaction in the determination of this neuronal phenotype.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-066579 and HL-25739 and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33: 459–461, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” K+ channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159–174, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 65: 148–157, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dwinell MR, Forster HV, Petersen J, Rider A, Kunert MP, Cowley AW, Jr, Jacob HJ. Genetic determinants on rat chromosome 6 modulate variation in the hypercapnic ventilatory response using consomic strains. J Appl Physiol 98: 1630–1638, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Few WP, Zakon HH. Sex differences in and hormonal regulation of Kv1 potassium channel gene expression in the electric organ: molecular control of a social signal. Dev Neurobiol 67: 535–549, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Forster HV, Dwinell MR, Hodges MR, Brozoski D, Hogan GE. Do genes on rat chromosomes 9, 13, 16, 18, and 20 contribute to regulation of breathing? Respir Physiol Neurobiol 135: 247–261, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gopel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting cells in intact mouse pancreatic islets. J Physiol 528: 497–507, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol 93: 974–983, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol 96: 1815–1824, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97: 2303–2309, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Meiri N, Sun MK, Segal Z, Alkon DL. Memory and long-term potentiation (LTP) dissociated: normal spatial memory despite CA1 LTP elimination with Kv1.4 antisense. Proc Natl Acad Sci USA 95: 15037–15042, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mienville JM, Maric I, Maric D, Clay JR. Loss of IA expression and increased excitability in postnatal rat Cajal-Retzius cells. J Neurophysiol 82: 1303–1310, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA 97: 3614–3618, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nichols NL, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats. Respir Physiol Neurobiol 168: 272–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med 164: 1470–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Schlenker EH, Hansen SN. Comparison of NMDA modulation of breathing and NR1 expression in medullary nuclei of weanling male and female rats. Respir Physiol Neurobiol 155: 203–212, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Strohl KP, Thomas AJ, St JP, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol 82: 317–323, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Takimoto K, Levitan ES. Altered K+ channel subunit composition following hormone induction of Kv1.5 gene expression. Biochemistry 35: 14149–14156, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Tankersley CG. Genetic aspects of breathing: on interactions between hypercapnia and hypoxia. Respir Physiol Neurobiol 135: 167–178, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Tankersley CG. Selected contribution: variation in acute hypoxic ventilatory response is linked to mouse chromosome 9. J Appl Physiol 90: 1615–1622, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Tankersley CG, Broman KW. Interactions in hypoxic and hypercapnic breathing are genetically linked to mouse chromosomes 1 and 5. J Appl Physiol 97: 77–84, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol 516: 699–710, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]