Abstract
Nutrient intake is accompanied by increases in central sympathetic outflow, a response that has been mainly attributed to insulin. Insulin-mediated sympathoexcitation appears to be blunted in insulin-resistant conditions, suggesting that aside from peripheral insulin insensitivity, such conditions may also impair the central action of insulin in mediating sympathetic activation. What remains unclear is whether an insulin-sensitive state, such as that induced by chronic endurance training, alters the central sympathetic effects of insulin during postprandial conditions. To examine this question plasma insulin and glucose, muscle sympathetic nerve activity (MSNA), heart rate, and arterial blood pressure were measured in 11 high-fit [HF; peak oxygen uptake (V̇o2peak) 65.9 ± 1.4 ml·kg−1·min−1] and 9 average-fit (AF; V̇o2peak 43.6 ± 1.3 ml·kg−1·min−1) male subjects before and for 120 min after ingestion of a mixed meal drink. As expected, the insulin response to meal ingestion was lower in HF than AF participants (insulin area under the curve0–120: 2,314 ± 171 vs. 4,028 ± 460 μIU·ml−1·120−1, HF vs. AF, P < 0.05), with similar plasma glucose responses between groups. Importantly, following consumption of the meal, the HF subjects demonstrated a greater rise in MSNA compared with the AF subjects (e.g., 120 min: Δ21 ± 1 vs. 8 ± 3 bursts/100 heart beats, HF vs. AF, P < 0.05). Furthermore, when expressed relative to plasma insulin, HF subjects exhibited a greater change in MSNA for any given change in insulin. Arterial blood pressure responses following meal intake were similar between groups. Collectively, these data suggest that, in addition to improved peripheral insulin sensitivity, endurance training may enhance the central sympathetic effect of insulin to increase MSNA following consumption of a mixed meal.
Keywords: central insulin sensitivity, peripheral insulin sensitivity, muscle sympathetic nerve activity
it is well recognized that acute nutrient intake is accompanied by robust increases in central sympathetic outflow (5, 12, 56). Presumably, the physiological relevance of an increase in sympathetic neural activity is to facilitate blood flow redistribution and for the overall maintenance of arterial blood pressure (5, 12, 56, 60). In addition, increases in sympathetic outflow will stimulate facultative thermogenesis (e.g., diet-induced thermogenesis) (2, 45, 61) and may enhance glucose uptake in skeletal muscle and adipose tissue (25, 27, 29, 35, 50). While a variety of factors may be implicated, numerous findings have indicated that the large rise in plasma insulin concentrations following meal intake is an important mediator of the postprandial increase in central sympathetic outflow (13, 56).
A central sympathoexcitatory action of insulin is drawn from studies in experimental animals, whereby intracerebroventricular administration of insulin elicits robust increases in peripheral sympathetic nerve activity (34, 39). In line with these findings, a mixed meal (9, 13), glucose load (6, 14, 47–49), and acute euglycemic hyperinsulinemia (3, 7, 17, 57, 60) have been shown to stimulate increases in muscle sympathetic nerve activity (MSNA) in humans. Importantly, the increase in MSNA is also present during low-dose euglycemic hyperinsulinemia, in which peripheral vasodilation does not occur (17), consistent with the hypothesis that hyperinsulinemia-induced increases in sympathetic outflow are primarily due to a central effect (i.e., non-baroreflex-mediated). Overall, these studies, utilizing a variety of maneuvers to alter plasma insulin concentrations, demonstrate that elevations in insulin stimulate increases in central sympathetic outflow.
Recent studies have suggested that insulin-mediated sympathoexcitation may be impaired in insulin-resistant conditions. Indeed, Vollenweider et al. (59) demonstrated that obese individuals exhibited attenuated MSNA responses to euglycemic hyperinsulinemia, despite higher insulin concentrations, compared with lean subjects. Findings in insulin-resistant elderly subjects (14) and insulin-resistant metabolic syndrome patients (48, 49) have similarly reported a blunted sympathetic response to increases in plasma insulin following a glucose load. As such, in addition to peripheral insulin resistance, insulin-resistant conditions may also exhibit a central resistance to the actions of insulin. In line with this concept, recent studies indicate that insulin-resistant states are accompanied by reduced cerebrospinal fluid insulin concentrations, likely emanating from an attenuated transport of insulin into the central nervous system (19–21, 44). However, while an insulin-resistant condition appears to impair insulin-mediated sympathoexcitation, the influence of enhanced insulin sensitivity on insulin-induced increases in central sympathetic outflow remains unclear.
To begin to examine this question we recruited healthy endurance-trained (high fit, HF) and normally active (average fit, AF) subjects. It is well characterized that chronic endurance training results in enhanced peripheral insulin sensitivity (4, 8, 24, 26, 42). Therefore, our rationale was that inclusion of two healthy subject groups, with distinct differences in insulin sensitivity, would allow us to investigate how enhanced insulin sensitivity influences insulin-mediated changes in central sympathetic outflow. Direct measurements of central sympathetic outflow to skeletal muscle (i.e., MSNA) were recorded, and a mixed meal was used as a physiological method to evoke robust and sustained increases in MSNA, which have been primarily attributed to insulin (13, 56). We hypothesized that HF subjects would have a greater MSNA response, for a given plasma insulin concentration, following consumption of a mixed meal (i.e., greater central insulin sensitivity).
METHODS
Twenty healthy men volunteered for participation in the study. Subjects were asymptomatic for cardiovascular, respiratory, or metabolic disease and were not taking any medications. The subject population consisted of 11 HF and 9 AF men. HF subjects were all competitive endurance athletes (i.e., marathon runners and triathletes) and had been competing in endurance events for 9 ± 1 yr with weekly exercise regimens including 12 ± 2 h/wk of training. In contrast, AF subjects were only recreationally active, engaging in aerobic activities for <30 min, ≤3 days/wk, reporting physical activities amounting to 45 ± 23 min/wk. When recruiting HF subjects a peak oxygen uptake (V̇o2peak) of ≥60 ml·kg−1·min−1 was used as the cutoff for inclusion. For the AF subjects we initially attempted to enroll subjects with a V̇o2peak ≤ 45 ml·kg−1·min−1; however, this proved difficult for young healthy lean subjects and therefore we had two subjects with a V̇o2peak of 47 ml·kg−1·min−1 in this group. A greater number of HF compared with AF subjects were recruited due to difficulty with MSNA measurements in this group (see MSNA below). All experimental procedures and protocols were approved by the University of Missouri Health Sciences Institutional Review Board. After receiving a detailed verbal and written explanation of the intended experimental protocol and measurements, each subject provided written informed consent before participation.
Experimental Measurements
General measurements.
Heart rate was continuously monitored using a lead II electrocardiogram (Quinton Q710, Bothell, WA). An automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY) was used to measure arterial blood pressure by auscultation of the brachial artery of the right arm. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position around the abdomen (Pneumotrace, UFI, Morro Bay, CA).
Plasma insulin and glucose.
Venous blood samples were drawn from an antecubital intravenous catheter for the measurements of plasma glucose and insulin. Glucose was analyzed using the glucose oxidase method (Thermo, Waltham, MA), and insulin was determined by chemiluminescent enzyme immunoassay (Immulite 1000 Analyzer, Diagnostic Products, Los Angeles, CA). The areas under the glucose and insulin curves (AUC0–120) were calculated from values measured at baseline, using the trapezoidal method (38).
MSNA.
Multiunit recordings of postganglionic MSNA were obtained as described previously (10, 11, 36, 37, 55). Briefly, a unipolar tungsten microelectrode was inserted percutaneously through the intact, unanesthetized skin and positioned into muscle nerve fascicles of the peroneal nerve near the fibular head of the left leg. Postganglionic sympathetic action potentials were amplified, filtered (bandwidth 700–2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. MSNA recordings were identified by their characteristic pulse-synchronous burst pattern and increased neural activity in response to an end-expiratory apnea or Valsalva maneuver, without any response to arousal stimuli or stroking of the skin (10, 11, 55). MSNA was not obtained in four HF subjects because an acceptable recording was not found in two subjects and recordings could not be maintained for the duration of the protocol in two others.
MSNA was first identified by visual inspection, independently by two investigators, and was then analyzed using custom-designed software (MatLab, The Math Works, Natick, MA) (16). This program first detects peaks in the integrated neurogram based on a latency window centered on each R wave from the electrocardiogram. After the peaks are chosen, the nearest minimum value on either side of the peak is detected, which is then denoted as the start and end of a sympathetic burst. An algorithm is applied to validate each burst, which takes into account the slope of the rise and fall of the burst, as well as the magnitude. The amplitude of the largest burst at baseline was assigned a value of 1,000 (arbitrary units; AU), and all other bursts within a trial were normalized with respect to this value. Sympathetic activity was quantified using standard measures, including burst frequency (bursts/min), burst incidence (bursts/100 heart beats), burst strength (burst area), and total activity (product of burst frequency and mean burst area). However, because the absolute area of a burst is dependent on the location of the microelectrode in relation to the nerve fibers that are being recorded, which cannot be determined, direct comparisons of burst strength and total activity between groups are typically not performed. Although various normalization procedures have previously been applied, these cannot completely account for the limitation associated with potential differences in electrode placement between individuals and, ultimately, subject groups (23, 51, 52). For example, calculating a percentage change in total activity from baseline will be affected by differences in resting total activity (i.e., microelectrode placement) and not necessarily reflect a difference in sympathetic responsiveness between two groups. In this manuscript, multiple MSNA data are included in an effort to be complete; however, we focused our results on measures of sympathetic bursts, which have been shown to be reproducible over time in the same subject and comparable between groups (50). Furthermore, because of group differences in heart rate and the inherent cardiac synchronicity of MSNA (10, 11, 55), we used MSNA burst incidence for our main comparison between groups. In addition, the area under the MSNA burst incidence curve (AUC0–120) was calculated for each subject using the trapezoidal method (38) and related to the insulin AUC0–120 as described previously (49).
Femoral artery blood flow.
Femoral blood flow (FBF) was obtained in the right leg using a duplex Doppler ultrasound unit (Logiq 7, GE Medical Systems, Milwaukee, WI) equipped with a linear array transducer operating at a frequency of 10 MHz. The common femoral artery was imaged 2–3 cm proximal to the bifurcation of the superficial and deep branches. Femoral blood velocity was obtained using the same probe in pulsed-wave mode, operating at a linear frequency of 5 Hz and at an insonation angle of 60°. Arterial diameter and mean blood velocity (Vmean) were calculated using commercially available software (Logiq 7, GE Medical Systems, Milwaukee, WI). Using femoral artery diameter and Vmean, FBF was calculated as: FBF = Vmean·π·(femoral artery diameter/2)2·60. FBF was normalized to right leg lean mass for group comparisons. Femoral vascular conductance (FVC) was calculated using the formula: FVC = FBF/mean arterial blood pressure.
Experimental Protocols
Visit 1.
Subjects were familiarized with the experimental protocols and procedures, after which a whole body dual-energy X-ray absorptometry scan (Hologic Delphi A, Waltham, MA) was performed to obtain right leg lean muscle mass, used to normalize blood flow measures between groups. In addition, to ascertain V̇o2peak, all subjects underwent a graded treadmill exercise test (Bruce Protocol) to exhaustion, and maximal effort was determined according to established criteria (1).
Visit 2.
Subjects reported to the laboratory on a separate occasion at least 5 days after completion of the first visit and were instructed to abstain from caffeinated beverages and food for 12 h, alcohol for 24 h, and physical activity for 48 h before the experimental session. The latter instruction was used to minimize the influence of acute physical activity effects on insulin sensitivity (18, 54). Subjects were placed in the supine position on a medical examination table and were instrumented for measures of heart rate, arterial blood pressure, MSNA, and FBF, and an intravenous catheter was placed in an antecubital vein. Baseline variables were collected for a period of 20 min and a premeal blood draw was taken, after which subjects ingested a mixed meal drink (Ensure Plus, Abbott Laboratories, Columbus, Ohio; 57% carbohydrate, 28% fat, 15% protein) corresponding to 20% of their estimated energy expenditure calculated from body weight (30). The calories consumed were similar between groups (521 ± 9 vs. 533 ± 14 kcal, HF vs. AF, P > 0.05). All variables were measured for a 5-min period, every 15 min, for 120 min following consumption of the mixed meal. Venous blood samples were also drawn every 15 min, and the resulting plasma was stored at −80°C for later analysis of plasma glucose and insulin concentrations.
Data Analysis
Heart rate and MSNA were sampled at 1,000 Hz and stored for off-line analysis (Chart v5.2 and Powerlab, ADInstruments, Bella Vista, NSW, Australia). Baseline heart rate, arterial blood pressure, MSNA, and FBF were calculated as mean values over a 6-min period. Following consumption of the mixed meal, 3-min averages were calculated from the 5-min data segments collected every 15 min. The ratio of a change in MSNA burst incidence to a change in insulin was evaluated at each time point for individual subjects as an index of the sympathetic response for a given concentration of plasma insulin.
Statistical Analysis
Statistical comparisons of physiological variables were conducted using a two-way ANOVA (fitness × time) with repeated measures, and a Tukey test was employed post hoc when significant main effects were found. Statistical significance was set at P < 0.05, and analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL) for Windows. Statistical comparisons were performed using all time points following meal intake. However, for presentation purposes, only 30-min time points are reported in some cases. Results are presented as means ± SE.
RESULTS
Subject Characteristics
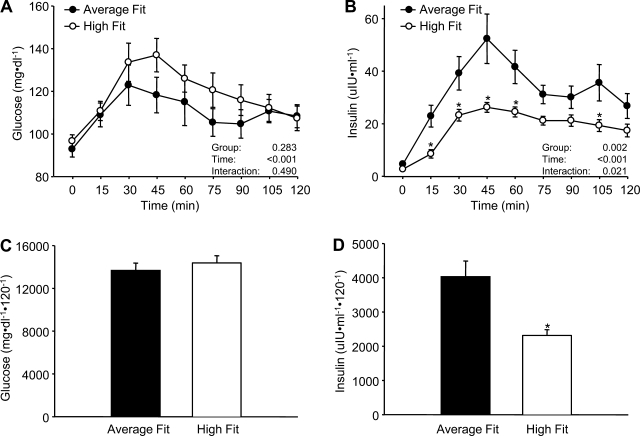
Age, height, weight, and body mass index did not differ between groups (Table 1). Similarly, fasting plasma glucose and insulin were not different between groups (Table 1 and Fig. 1). As anticipated, the HF subjects had a higher V̇o2peak than the AF subjects, with a mean group difference >20 ml·kg−1·min−1 (range: HF 60–73 ml·kg−1·min−1, AF 36–47 ml·kg−1·min−1). HF subjects exhibited a significantly lower heart rate at baseline, whereas resting systolic (SBP), diastolic (DBP), and mean arterial blood pressure (MAP) were similar between groups (Table 2).
Table 1.
Subject characteristics
| Average Fit | High Fit | |
|---|---|---|
| Age, yr | 26 ± 2 | 29 ± 2 |
| Height, cm | 179 ± 2 | 180 ± 1 |
| Weight, kg | 75 ± 4 | 74 ± 2 |
| Body mass index, kg/m2 | 23 ± 1 | 23 ± 1 |
| V̇o2peak, ml·kg−1·min−1 | 43.6 ± 1.3 | 65.9 ± 1.4* |
| V̇o2peak, l/min | 3.3 ± 0.2 | 4.9 ± 0.2* |
| Glucose, mg/dl | 92.9 ± 3.7 | 96.7 ± 2.9 |
| Insulin, μIU/ml | 3.6 ± 0.9 | 2.7 ± 0.3 |
Values are means ± SE. V̇o2peak, peak oxygen uptake. μIU, micro-international units.
P < 0.05 vs. average fit.
Fig. 1.
Mean plasma glucose (A) and insulin (B) at baseline (time 0) and for 120 min following consumption of the mixed meal in the average-fit and high-fit groups, as well as the area under the curve for glucose (C) and insulin (D). Values are means ± SE. *P < 0.05 vs. average fit.
Table 2.
Cardiovascular and hemodynamic responses to a mixed meal
| Time |
ANOVA, P value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 60 min | 90 min | 120 min | Group | Time | Interaction | |
| Average fit | ||||||||
| SBP, mmHg | 116 ± 3 | 119 ± 4 | 118 ± 3 | 118 ± 4 | 120 ± 3 | 0.824 | <0.001 | 0.994 |
| DBP, mmHg | 68 ± 3 | 68 ± 3 | 68 ± 3 | 69 ± 3 | 71 ± 3 | 0.915 | 0.023 | 0.741 |
| MAP, mmHg | 84 ± 3 | 85 ± 2 | 85 ± 3 | 86 ± 3 | 87 ± 3 | 0.908 | 0.002 | 0.879 |
| HR, beats/min | 61 ± 2 | 70 ± 3 | 68 ± 2 | 71 ± 2 | 72 ± 3 | <0.001 | <0.001 | 0.284 |
| Femoral blood flow, ml·min−1·kg−1 | 29.84 ± 2.02 | 34.77 ± 4.07 | 43.30 ± 5.43 | 43.75 ± 6.56 | 39.30 ± 6.52 | 0.819 | <0.001 | 0.229 |
| Femoral vascular conductance, ml·min−1·kg−1·mmHg−1 | 0.36 ± 0.03 | 0.41 ± 0.05 | 0.52 ± 0.07 | 0.52 ± 0.08 | 0.45 ± 0.07 | 0.910 | <0.001 | 0.214 |
| High fit | ||||||||
| SBP, mmHg | 116 ± 2 | 120 ± 2 | 120 ± 2 | 120 ± 2 | 121 ± 2 | |||
| DBP, mmHg | 69 ± 2 | 69 ± 2 | 69 ± 2 | 70 ± 2 | 70 ± 2 | |||
| MAP, mmHg | 84 ± 2 | 86 ± 2 | 86 ± 2 | 86 ± 2 | 87 ± 2 | |||
| HR, beats/min | 51 ± 2 | 57 ± 2 | 56 ± 2 | 57 ± 2 | 58 ± 2 | |||
| Femoral blood flow, ml·min−1·kg−1 | 36.56 ± 2.91 | 33.28 ± 2.44 | 42.46 ± 5.05 | 41.80 ± 5.03 | 43.88 ± 4.81 | |||
| Femoral vascular conductance, ml·min−1·kg−1·mmHg−1 | 0.43 ± 0.03 | 0.39 ± 0.03 | 0.49 ± 0.06 | 0.48 ± 0.06 | 0.50 ± 0.05 | |||
Values are means ± SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HR, heart rate.
Metabolic Responses to a Mixed Meal
Mixed meal intake induced a significant rise in plasma glucose, with similar responses between groups at each time point (Fig. 1A) and when quantified as glucose AUC (Fig. 1C). In contrast, HF subjects demonstrated much less of an increase in plasma insulin compared with the AF subjects (Fig. 1B). This enhanced insulin sensitivity in the HF subjects was illustrated by an ∼45% lower insulin AUC0–120 (Fig. 1D).
MSNA Responses to a Mixed Meal
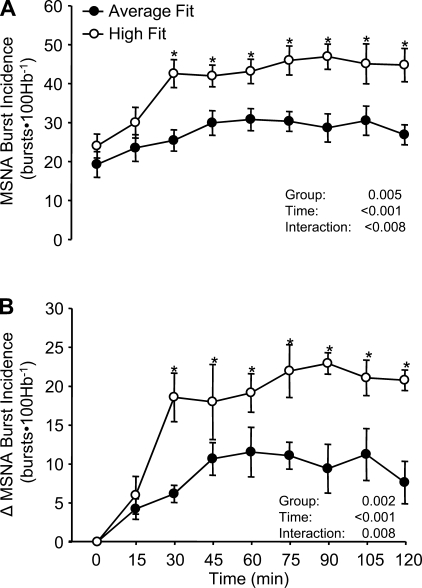
At rest, MSNA burst incidence and burst frequency were comparable between groups (Figs. 2 and 3 and Table 3). Following ingestion of the mixed meal, the HF subjects demonstrated a greater rise in MSNA burst incidence, as well as burst frequency, compared with AF subjects. Results obtained for MSNA total activity and burst strength are also provided in Table 3.
Fig. 2.
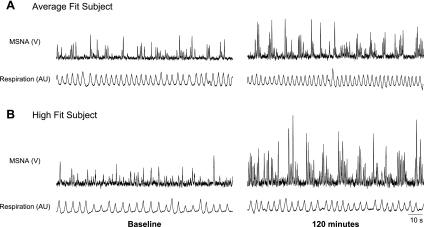
Original records from an average-fit (A) and high-fit (B) subject illustrating muscle sympathetic nerve activity (MSNA) and respiratory tracings at baseline and 120 min following consumption of the mixed meal. In response to the mixed meal, MSNA burst incidence increased 11 and 23 bursts/100 heart beats in the average-fit and high-fit subject, respectively. V, volts; AU, arbitrary units.
Fig. 3.
Mean MSNA burst incidence responses (A) at baseline (time 0) and for 120 min following consumption of the mixed meal in the average-fit and high-fit groups. Panel B shows the changes (Δ) in MSNA burst incidence from baseline in both groups. Hb, heart beats. Values are means ± SE. *P < 0.05 vs. average fit.
Table 3.
Muscle sympathetic nerve activity responses to a mixed meal
| Time |
ANOVA, P value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 60 min | 90 min | 120 min | Group | Time | Interaction | |
| Average fit | ||||||||
| MSNA burst frequency, bursts/min | 11 ± 2 | 18 ± 2 | 21 ± 2 | 20 ± 3 | 20 ± 2 | 0.199 | <0.001 | 0.049 |
| MSNA total activity, AU/min | 293 ± 52 | 1096 ± 191 | 1261 ± 151 | 1138 ± 171 | 1124 ± 132 | 0.009 | <0.001 | <0.001 |
| Δ, AU/min | 0 | 804 ± 175 | 969 ± 149 | 845 ± 158 | 831 ± 139 | 0.004 | <0.001 | <0.001 |
| %Δ | 0 | 335 ± 78 | 447 ± 95 | 353 ± 75 | 408 ± 100 | 0.393 | <0.001 | 0.274 |
| MSNA burst strength, AU | 25 ± 2 | 62 ± 11 | 60 ± 0 | 54 ± 2 | 59 ± 4 | 0.014 | <0.001 | 0.017 |
| Δ, AU | 0 | 37 ± 11 | 35 ± 3 | 29 ± 3 | 34 ± 4 | 0.044 | <0.001 | 0.017 |
| %Δ | 0 | 145 ± 40 | 139 ± 14 | 118 ± 13 | 132 ± 14 | 0.246 | <0.001 | 0.049 |
| High fit | ||||||||
| MSNA burst frequency, bursts/min | 12 ± 2 | 23 ± 1 | 24 ± 1 | 27 ± 2* | 26 ± 3* | |||
| MSNA total activity, AU/min | 346 ± 49 | 1451 ± 144 | 1699 ± 155 | 2216 ± 250* | 2238 ± 350* | |||
| Δ, AU/min | 0 | 1105 ± 143 | 1353 ± 128 | 1870 ± 220* | 1892 ± 312* | |||
| %Δ | 0 | 364 ± 68 | 426 ± 57 | 578 ± 94 | 556 ± 80 | |||
| MSNA burst strength, AU | 29 ± 1 | 62 ± 4 | 70 ± 3 | 81 ± 4* | 83 ± 7* | |||
| Δ, AU | 0 | 33 ± 4 | 42 ± 3 | 53 ± 4* | 55 ± 7* | |||
| %Δ | 0 | 117 ± 15 | 147 ± 13 | 186 ± 19* | 195 ± 29 | |||
Values are means ± SE. Muscle sympathetic nerve activity (MSNA) total activity and burst strength are only included for completeness (see text for details). AU, arbitrary units.
P < 0.05 vs. average fit.
MSNA:Insulin Relationships
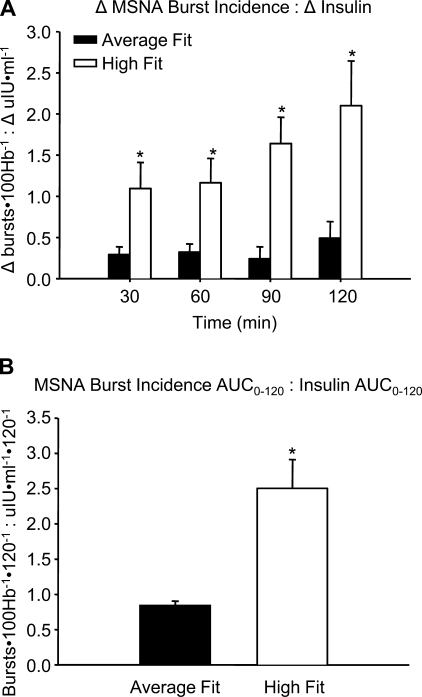
Figure 4 illustrates the ratios of a change in MSNA burst incidence to a change in plasma insulin, indicating that for a given change in insulin HF subjects had a greater change in MSNA burst incidence. Similar results were found when relating the MSNA burst incidence AUC0–120 to the insulin AUC0–120 (Fig. 4B).
Fig. 4.
Group averages for the ratios relating changes from baseline in MSNA burst incidence (A) to changes in plasma insulin (Δinsulin) following mixed meal intake. Panel B shows the ratio of MSNA burst incidence area under the curve (AUC0–120) to the insulin AUC0–120. Values are mean ± SE. *P < 0.05 vs. average fit.
FBF Responses to a Mixed Meal
Resting FBF was not different between groups. In response to the mixed meal, FBF was increased above baseline values, with similar increases in the HF and AF subjects. Likewise, resting FVC was the same between groups and increased similarly in response to mixed meal ingestion (all Table 2).
Cardiovascular Responses to a Mixed Meal
Overall there were no differences in the arterial blood pressure responses to mixed meal intake between groups. Following the mixed meal, SBP, DBP, and MAP were slightly but significantly increased in both groups. Heart rate responses to the mixed meal were not different between groups and therefore heart rate was lower throughout the entire protocol in the HF compared with the AF subjects, owing primarily to baseline differences (all Table 2).
DISCUSSION
The major finding of the present study was that HF subjects demonstrated a greater increase in MSNA burst incidence compared with AF subjects following consumption of a mixed meal. Importantly this greater sympathetic activation occurred despite lower plasma insulin concentrations, with comparable plasma glucose between groups. Furthermore, when MSNA responses were expressed relative to plasma insulin, HF subjects exhibited a greater change in MSNA for any given change in insulin. Collectively, these data suggest that, in addition to improved peripheral insulin sensitivity, endurance training may also enhance the central actions of insulin to increase MSNA following consumption of a mixed meal.
Nutrient intake is accompanied by increases in central sympathetic outflow, a response that has been mainly attributed to insulin (13, 56). In the present study we used chronic endurance training as a model to establish two distinct subject groups. An enhancement in the peripheral sensitivity to the actions of insulin (i.e., glucose disposal) is well characterized with chronic endurance training (4, 8, 24, 26, 42), and therefore we rationalized that endurance-trained individuals would exhibit a lower insulin response with similar glucose concentrations following the mixed meal. Furthermore, if enhanced central insulin sensitivity was present, we reasoned that the HF subjects would exhibit a similar or greater increase in MSNA despite the lower plasma insulin. Indeed, following consumption of the mixed meal, the HF subjects demonstrated a significantly larger increase in MSNA compared with the AF subjects.
From our direct sympathetic recordings, an increase in central sympathetic outflow to skeletal muscle can be characterized by either an increase in efferent sympathetic impulses (i.e., burst incidence) or an increase in the strength of the sympathetic bursts (i.e., burst area) or a combination of the two (22). Interestingly, the rise in MSNA following nutrient intake was evident when examining both burst occurrence (i.e., burst incidence), as well as burst strength in both groups. These data suggest that the increase in central sympathetic outflow to skeletal muscle following a mixed meal is due to an enhanced sympathetic firing rate as well as enhanced neuronal recruitment (22). The larger increase in MSNA burst incidence in the HF compared with AF subjects clearly demonstrates a greater postprandial central sympathoexcitation. In addition, the greater increases in burst strength in the HF group suggests that this fitness effect on MSNA may include greater neuronal recruitment (28); however, group comparisons of burst strength need to be interpreted cautiously because the area of a burst is dependent on the location of the recording microelectrode, which cannot be determined (10, 53). Therefore, we focused our results on measures of sympathetic bursts, which have been shown to be reproducible over time in the same subject and comparable between groups (53). Furthermore, the use of MSNA burst incidence was warranted because of group differences in heart rate and the inherent cardiac synchronicity of MSNA (10, 11, 55).
Following food intake, coordinated cardiovascular, metabolic, and neural responses occur to distribute, absorb, and store nutrients. Postprandial activation of the sympathetic nervous system is crucial for blood flow redistribution and overall maintenance of arterial blood pressure (5, 12, 56, 61). Although a number of factors have been suggested (56), several lines of evidence indicate that the elevation in plasma insulin following a meal plays a prominent role in stimulating the increases in central sympathetic outflow (13, 56). First, a strong positive correlation has been reported between plasma insulin and the increases in MSNA after a glucose meal in healthy subjects (6, 46). Second, a mixed meal and glucose load have been shown to elicit the greatest changes in MSNA compared with fat or protein intake alone, in which insulin secretion is minimal (13). In addition, euglycemic hyperinsulinemia within the postprandial range increases MSNA in healthy humans to a similar extent as that following a mixed meal (17). Third, recent animal studies have provided clear evidence indicating a direct action of insulin to increase central sympathetic outflow (34, 39). Last, alterations in the sympathetic response to a mixed meal and glucose load have been identified in insulin-resistant conditions (14, 48, 49, 59). Overall, although insulin-independent mechanisms cannot be completely discounted, these data indicate that a mixed meal represents a physiological method to investigate insulin-mediated stimulation of central sympathetic outflow.
In the present study, when the MSNA burst incidence responses to the mixed meal were expressed relative to insulin, a greater change in sympathetic outflow for a given change in plasma insulin concentrations was noted in the HF group. This held true whether the values were expressed at each time point or as a ratio of the MSNA AUC0–120 to the insulin AUC0–120. These data suggest that an enhancement in insulin sensitivity, even in otherwise healthy individuals, improves insulin-stimulated sympathoexcitation to skeletal muscle (i.e., enhanced central insulin sensitivity). Of note, MSNA burst frequency data also suggest that HF subjects exhibit greater central insulin sensitivity. Although the physiological consequence of a greater increase in central sympathetic outflow in the HF subjects is beyond the scope of the present project, it may be that an augmentation in sympathetic outflow following mixed meal intake allows for greater control over redistribution of blood flow and absorption of nutrients, thus contributing to the enhanced peripheral insulin sensitivity.
Recent investigations have suggested that the central stimulatory actions of insulin may be blunted in insulin-resistant conditions (14, 48, 49, 59). Indeed, the sympathoexcitation following a mixed meal, glucose load, or euglycemic hyperinsulinemic clamp is blunted in elderly insulin-resistant individuals (14), insulin-resistant metabolic syndrome patients (48, 49), and obese subjects (59), respectively. In addition, Straznicky et al. (49) recently demonstrated that diet- and exercise-induced weight loss in insulin-resistant metabolic syndrome subjects improved the blunted sympathetic responsiveness to a glucose load, as measured by whole body norepinephrine kinetics. Moreover, a positive correlation between maximal oxygen uptake and the insulin-induced increases in whole body norepinephrine spillover has been noted in metabolic syndrome patients (48). Taken together these findings suggest that an insulin-resistant state may blunt insulin-mediated sympathetic neural responses and that potential improvements in insulin sensitivity may restore insulin's ability to increase sympathetic outflow. The present findings are in agreement and extend this work by directly assessing MSNA in two groups of young, lean healthy individuals, who display distinct differences in insulin sensitivity (i.e., different postprandial insulin responses to a meal). Importantly, in these previous investigations, the populations studied were all characterized by resting sympathetic overactivity. Therefore, it is possible that the sympathetic responses to insulin may be influenced by higher basal sympathetic activity (i.e., a ceiling effect) (43). The present findings allowed us to determine if differences in insulin-stimulated sympathoexcitation occur without confounding factors such as elevated resting sympathetic activity, body mass index, or age.
While the exact mechanism(s) contributing to alterations in insulin-stimulated changes in central sympathetic outflow to skeletal muscle remain unknown, several possibilities are worthy of consideration. First, it is possible that alterations in insulin transport across the blood-brain barrier occur with changes in insulin sensitivity. In support of this concept, a reduced cerebrospinal fluid-to-plasma insulin ratio has been reported in obese individuals, with the ratio being highly negatively related to the degree of insulin resistance (21). Moreover, pharmacologically induced insulin resistance in animal models has been shown to reduce central nervous system insulin uptake (19, 20), whereas brain endothelial cells of obese rats demonstrate impaired insulin binding (44). Thus our findings demonstrating a more robust increase in MSNA in the HF compared with the AF subjects may be due to differences in insulin delivery and/or rate of transport into the brain. In addition, it is possible that central insulin-mediated signaling cascade pathways may be altered with changes in central insulin sensitivity. Findings from rodent models suggest that insulin-stimulated increases in sympathetic outflow primarily occur within hypothalamic regions through both the phosphoinositide 3-kinase and mitogen-activated protein kinase pathways (33, 34, 39). Given that both of these pathways have been shown to be modified in the periphery in insulin-resistant states (54) and in response to endurance training (15), it is plausible that alterations in either of these signaling pathways within the central nervous system occur with changes in insulin sensitivity.
A role for insulin in mediating peripheral vasodilation has been well documented (31, 32, 58). Although not the main focus of our study, considering the known increases in peripheral insulin sensitivity with endurance training (4, 8, 24, 26, 42), one may have anticipated a greater insulin-mediated increase in blood flow in the HF compared with the AF subjects following the mixed meal. However, we did not observe any group differences in absolute FBF or FVC responses following ingestion of the mixed meal. In line with our findings, the peripheral blood flow responses following a glucose load in insulin-resistant and insulin-sensitive metabolic syndrome subjects have also been shown to not differ between groups with differences in insulin sensitivity (48, 49). It may be that the majority of insulin-stimulated vasodilation following meal intake occurs within the microvasculature. As discussed by Renkin and colleagues (40, 41), enhancing capillary surface area will greatly increase nutrient delivery in direct proportion to the surface area available for nutrient exchange (e.g., microvascular recruitment), whereas increasing total limb blood flow (i.e., FBF) would minimally enhance nutrient exchange. Therefore, although we did not see a difference between the HF and AF groups in large-conduit-vessel blood flow, we cannot rule out differences in postprandial microvascular blood flow responses between groups.
In summary, we found that HF subjects demonstrated a greater increase in MSNA burst incidence following ingestion of a mixed meal compared with AF subjects. Moreover, when the MSNA responses were expressed relative to changes in plasma insulin, the HF subjects had a greater increase in MSNA for any given change in insulin. Collectively, these data suggest that, in addition to improved peripheral insulin sensitivity, endurance training may also enhance the central actions of insulin to increase MSNA following consumption of a mixed meal.
GRANTS
This research is the result of work supported with resources by National Heart, Lung, and Blood Institute Grant HL-093167.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Charla Jay for technical assistance and Dr. David Keller for constructive comments regarding data analyses and interpretation.
REFERENCES
- 1. ACSM ACSM's Guidelines for Exercise Testing and Prescription (6th ed.) Philadelphia, PA: Lippincott Williams and Williams, 2000 [Google Scholar]
- 2. Acheson KJ. Influence of autonomic nervous system on nutrient-induced thermogenesis in humans. Nutrition 9: 373–380, 1993 [PubMed] [Google Scholar]
- 3. Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berger M, Kemmer FW, Becker K, Herberg L, Schwenen M, Gjinavci A, Berchtold P. Effect of physical training on glucose tolerance and on glucose metabolism of skeletal muscle in anaesthetized normal rats. Diabetologia 16: 179–184, 1979. [DOI] [PubMed] [Google Scholar]
- 5. Berne C, Fagius J. Metabolic regulation of sympathetic nervous system activity: lessons from intraneural nerve recordings. Int J Obes Relat Metab Disord 17, Suppl 3: S2–S6; discussion S22, 1993 [PubMed] [Google Scholar]
- 6. Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration. Evidence from microelectrode nerve recordings. J Clin Invest 84: 1403–1409, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35: 873–879, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Bjorntorp P, Fahlen M, Grimby G, Gustafson A, Holm J, Renstrom P, Schersten T. Carbohydrate and lipid metabolism in middle-aged, physically well-trained men. Metabolism 21: 1037–1044, 1972 [DOI] [PubMed] [Google Scholar]
- 9. Cox HS, Kaye DM, Thompson JM, Turner AG, Jennings GL, Itsiopoulos C, Esler MD. Regional sympathetic nervous activation after a large meal in humans. Clin Sci (Lond) 89: 145–154, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 11. Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972 [DOI] [PubMed] [Google Scholar]
- 12. Fagius J. Sympathetic nerve activity in metabolic control—some basic concepts. Acta Physiol Scand 177: 337–343, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin Sci (Lond) 86: 159–167, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Fagius J, Ellerfelt K, Lithell H, Berne C. Increase in muscle nerve sympathetic activity after glucose intake is blunted in the elderly. Clin Auton Res 6: 195–203, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol 91: 1199–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hausberg M, Mark AL, Hoffman RP, Sinkey CA, Anderson EA. Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J Hypertens 13: 1015–1021, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55: 512–517, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D, Jr, Figlewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull 30: 571–575, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 49: 2790–2792, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimmerly DS, O'Leary DD, Shoemaker JK. Test-retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci 114: 61–71, 2004 [DOI] [PubMed] [Google Scholar]
- 24. LeBlanc J, Nadeau A, Boulay M, Rousseau-Migneron S. Effects of physical training and adiposity on glucose metabolism and 125I-insulin binding. J Appl Physiol 46: 235–239, 1979 [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Perusse F, Bukowiecki LJ. Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. Am J Physiol Regul Integr Comp Physiol 266: R914–R920, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Lohmann D, Liebold F, Heilmann W, Senger H, Pohl A. Diminished insulin response in highly trained athletes. Metabolism 27: 521–524, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Lupien JR, Hirshman MF, Horton ES. Effects of norepinephrine infusion on in vivo insulin sensitivity and responsiveness. Am J Physiol Endocrinol Metab 259: E210–E215, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516: 293–301, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marette A, Bukowiecki LJ. Stimulation of glucose transport by insulin and norepinephrine in isolated rat brown adipocytes. Am J Physiol Cell Physiol 257: C714–C721, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Muntzel M, Beltz T, Mark AL, Johnson AK. Anteroventral third ventricle lesions abolish lumbar sympathetic responses to insulin. Hypertension 23: 1059–1062, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 267: R1350–R1355, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43: 533–549, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Purves RD. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J Pharmacokinet Biopharm 20: 211–226, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Renkin E, Crone C. Microcirculation and capillary exchange. In: Comprehensive Human Physiology (1st ed.), edited by Greger R, Windhorst U. Berlin-Heidelberg: Springer, 1996, p. 1965–1979 [Google Scholar]
- 41. Renkin EMBW. Zweifach Award lecture. Regulation of the microcirculation. Microvasc Res 30: 251–263, 1985 [DOI] [PubMed] [Google Scholar]
- 42. Richard D, LeBlanc J. Effects of physical training and food restriction on insulin secretion and glucose tolerance in male and female rats. Am J Clin Nutr 33: 2588–2594, 1980 [DOI] [PubMed] [Google Scholar]
- 43. Schobel HP, Oren RM, Mark AL, Ferguson DW. Influence of resting sympathetic activity on reflex sympathetic responses in normal man. Clin Auton Res 5: 71–80, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11: 467–472, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Schwartz RS, Jaeger LF, Veith RC. Effect of clonidine on the thermic effect of feeding in humans. Am J Physiol Regul Integr Comp Physiol 254: R90–R94, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Scott EM, Greenwood JP, Vacca G, Stoker JB, Gilbey SG, Mary DA. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci (Lond) 102: 523–529, 2002 [PubMed] [Google Scholar]
- 47. Spraul M, Anderson EA, Bogardus C, Ravussin E. Muscle sympathetic nerve activity in response to glucose ingestion. Impact of plasma insulin and body fat. Diabetes 43: 191–196, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ, McGrane MT, Mariani JA, Socratous F, Chopra R, Esler MD, Schlaich MP, Lambert EA. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr 89: 27–36, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Straznicky NE, Lambert GW, McGrane MT, Masuo K, Dawood T, Nestel PJ, Eikelis N, Schlaich MP, Esler MD, Socratous F, Chopra R, Lambert EA. Weight loss may reverse blunted sympathetic neural responsiveness to glucose ingestion in obese subjects with metabolic syndrome. Diabetes 58: 1126–1132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol Endocrinol Metab 261: E298–E303, 1991 [DOI] [PubMed] [Google Scholar]
- 51. Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525–532, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272: 383–397, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol 294: R1103–R1110, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 56. van Baak MA. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav 94: 178–186, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Van De Borne P, Hausberg M, Hoffman RP, Mark AL, Anderson EA. Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am J Physiol Regul Integr Comp Physiol 276: R178–R183, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 3: 279–288, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 93: 2365–2371, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vollenweider P, Tappy L, Randin D, Schneiter P, Jequier E, Nicod P, Scherrer U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 92: 147–154, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Welle S. Sympathetic nervous system response to intake. Am J Clin Nutr 62: 1118S–1122S, 1995. [DOI] [PubMed] [Google Scholar]