Abstract
In this review, we examine why blood pressure (BP) and sympathetic nerve activity (SNA) increase during a rise in central nervous system (CNS) Pco2 (central chemoreceptor stimulation). CNS acidification modifies SNA by two classes of mechanisms. The first one depends on the activation of the central respiratory controller (CRG) and causes the much-emphasized respiratory modulation of the SNA. The CRG probably modulates SNA at several brain stem or spinal locations, but the most important site of interaction seems to be the caudal ventrolateral medulla (CVLM), where unidentified components of the CRG periodically gate the baroreflex. CNS Pco2 also influences sympathetic tone in a CRG-independent manner, and we propose that this process operates differently according to the level of CNS Pco2. In normocapnia and indeed even below the ventilatory recruitment threshold, CNS Pco2 exerts a tonic concentration-dependent excitatory effect on SNA that is plausibly mediated by specialized brain stem chemoreceptors such as the retrotrapezoid nucleus. Abnormally high levels of Pco2 cause an aversive interoceptive awareness in awake individuals and trigger arousal from sleep. These alerting responses presumably activate wake-promoting and/or stress-related pathways such as the orexinergic, noradrenergic, and serotonergic neurons. These neuronal groups, which may also be directly activated by brain acidification, have brainwide projections that contribute to the CO2-induced rise in breathing and SNA by facilitating neuronal activity at innumerable CNS locations. In the case of SNA, these sites include the nucleus of the solitary tract, the ventrolateral medulla, and the preganglionic neurons.
Keywords: central chemoreceptors, cardiorespiratory integration
a rise in central nervous system (CNS) Pco2 (central chemoreceptor stimulation) increases breathing, blood pressure (BP), and sympathetic nerve activity (22, 96). Beyond a certain level, CNS Pco2 also produces arousal from sleep (9, 82) and elicits a conscious sensation that is perceived as aversive in the awake state (69). In this short review we consider how a rise in CNS Pco2 activates the circulation. The focus is on the primary response of the vasomotor sympathetic system to central chemoreceptor stimulation (the central sympathetic chemoreflex, CSC). The parasympathetic control of the heart and the secondary circulatory reflexes that result from changes in pulmonary ventilation are not discussed.
As a preamble, it may be useful to recall that the brain performs two interrelated tasks that are critical to circulatory control. The first one is BP stabilization. This function is largely independent of breathing intensity and relies primarily on the arterial baroreflex with an important contribution of vestibular/cerebellar mechanisms in larger mammals. These mechanisms protect the brain and other sensitive organs against hypotension/hypoperfusion that could potentially result from postural changes, fluid loss, or excessive muscle vasodilatation during exercise. The second task, which is highly integrated with breathing, is the regulation of tissue perfusion. This regulation is behavior dependent and occurs via adjustments of the cardiac output, regulated rises in BP, and differential activation of the sympathetic tone to various organs. Blood gas delivery to the tissues requires that the cardiac output roughly covary with alveolar ventilation. The neural control of the cardiac output (cardiovagal and sympathetic divisions) is therefore highly integrated with that of breathing. The medulla oblongata, especially the ventrolateral medulla, plays a key role in both BP stabilization and tissue perfusion.
ROLE OF THE VENTROLATERAL MEDULLA IN VASOMOTOR CONTROL
The rostral ventrolateral medulla (RVLM) is a ventrolateral quadrant of the medullary reticular formation that overlaps with the Bötzinger and pre-Bötzinger subdivisions of the ventral respiratory column defined by respiratory physiologists (3). The RVLM contains a large collection of neurons that innervate the preganglionic sympathetic neurons (SPGNs) monosynaptically (presympathetic neurons) (Fig. 1A). These neurons are an important convergence point for sympathetic reflexes elicited by cardiopulmonary receptors and descending inputs from the hypothalamus and higher in the neuraxis (28). The presympathetic neurons of the RVLM are presumably glutamatergic (71, 99), but a large subset of them, defined as C1 neurons, also express the entire complement of catecholamine-biosynthetic enzymes inclusive of the epinephrine-producing phenylethanolamine N-methyl transferase (PNMT) (35, 88). In addition, the presympathetic neurons differentially express several neuropeptides such as substance P, enkephalin, and neuropeptide Y (56). The C1 neurons are not exclusively presympathetic as many C1 cells do not innervate sympathetic preganglionic neurons but target instead the hypothalamus, basal forebrain, and periaqueductal gray matter. These rostrally projecting neurons contribute to the neuroendocrine and autonomic responses to somatic stresses such as hypotension, hemorrhage, nociception, and infection (28, 90). RVLM presympathetic neurons are functionally as well as neurochemically heterogeneous. A subset of these neurons seems to regulate skeletal muscle vascular beds preferentially whereas other subsets probably regulate primarily the splanchnic circulation, the heart, the kidney, and so on. This arrangement, defined as “organotopy,” presumably underlies the ability of the RVLM to differentially regulate the cardiac output, BP, and organ perfusion (65).
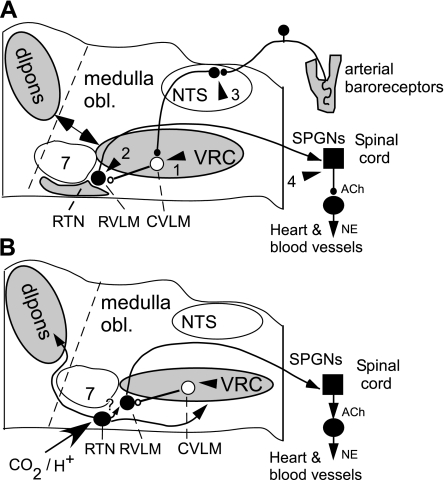
Fig. 1.
Cardiorespiratory coupling. A: arrowheads 1–4 indicate 4 lower brain stem or spinal locations where components of the central respiratory pattern generator may influence the sympathetic outflow to the heart and blood vessels. The sites are ranked according to the strength of the available supporting evidence from most plausible (1) to less documented (4). Site 1 is the main inhibitory relay of the baroreflex located in the caudal ventrolateral medulla (CVLM), a region that is coextensive with the pre-Bötzinger and rostral ventral respiratory group subdivisions of the ventral respiratory column. Site 2, the rostral ventrolateral medulla (RVLM), contains the main source of direct excitatory drive to the sympathetic vasomotor preganglionic neurons. The respiratory modulation of these neurons derives largely from the respiratory gating of their CVLM input but direct inputs from the central respiratory pattern generator (CRG) or from the retrotrapezoid nucleus (RTN) may also contribute to this modulation. Site 3 is the nucleus of the solitary tract (NTS). The NTS receives inputs from arterial baroreceptors and from the CRG. Site 4 is the spinal cord. Excitatory inputs from collaterals of bulbospinal inspiratory neurons were thought to contribute to the inspiratory modulation of SNA in cats either directly or via spinal interneurons. The required anatomic pathway has not been fully documented, and the theory is inconsistent with the phase-spanning nature of the respiration modulation of SNA present in rodents. Each arrow (1–4) describes one or a set of respiratory modulated inputs. The most plausible anatomic origins of these inputs are the ventral respiratory column (VRC), the dorsolateral pons (dlpons), and the RTN, which are highlighted in gray. B: possible role of the RTN in the central sympathetic chemoreflex. The RTN is a chemosensitive group of glutamatergic neurons that has a phase-spanning respiratory modulation (in rats) when the respiratory network is active and a tonic CO2-dependent activity below the apneic threshold or when the CRG is silenced pharmacologically. The RTN drives the CRG and probably integrates much of the chemical drive to breathe. The RTN also innervates the region that contains the vasomotor neurons (RVLM) and their CVLM antecedent neurons. The RTN could therefore contribute a respiratory-independent drive to the sympathetic outflow when CO2 is below the respiratory recruitment threshold and a respiratory-modulated drive to the sympathetic outflow when Pco2 exceeds this threshold. This theory remains to be thoroughly tested. NE, norepinephrine; SPGNs, preganglionic sympathetic neurons; medulla obl, medulla oblongata “7”, facial motor nucleus.
The presympathetic neurons of the RVLM are very active at rest in vivo (11). This characteristic is attributed to a combination of intrinsic properties (autoactivity) and synaptic drives (28, 45, 58). Under most anesthetics, the activity of the presympathetic neurons is very high (up to 35 Hz), presumably because of the depressant effect of these drugs on their main source of inhibition, the CVLM (Fig. 1A). The excitatory drives unmasked by this disinhibition are not mediated to a substantial degree by conventional ionotropic glutamatergic transmission (28). Their exact location and the nature of the participating transmitters are unknown. This mystery is a major limitation to current understanding of BP control in health and disease. In anesthetized rodents, the bulk of the activity of sympathetic nerves and of the RVLM presympathetic neurons persists under hyperoxia and at extremely reduced levels of Pco2; therefore this basal activity is clearly not dependent on central or peripheral chemoreceptor tone nor does it depend on an active central respiratory network. Human SNA likewise persists in the complete absence of respiratory activity (e.g., 97).
Massive increases in sympathetic tone occur in response to even slight decreases in systemic BP (28, 104). This sympathetic “reserve” is independent of the activity of the central respiratory network and probably results from the dishinhibition of the RVLM presympathetic neurons rather than from an increase in their excitatory drive. This “reserve” underlies the BP stabilization role of the RVLM and has probably little to do with cardiorespiratory integration per se although, as will be seen later, both regulations may converge through a common pool of GABAergic interneurons that keeps the activity of RVLM presympathetic neurons in check at all times. This inhibitory input originates from interneurons located more caudally within a region of the VLM called the caudal ventrolateral medulla (CVLM, Fig. 1A) (91, 101, 110). The CVLM is roughly coextensive with the pre-Bötzinger complex and the rostral ventral respiratory group (rVRG) defined by respiratory physiologists (3, 14, 91, 109). The CVLM GABAergic interneurons are driven to a large extent by the ongoing activity of the arterial baroreceptors (Fig. 1A); therefore their firing is highly synchronized with the pressure pulse (43, 91). These neurons probably receive a monosynaptic excitatory input from the second-order cells in the arterial baroreflex pathway, which reside in the dorsolateral region of the caudal solitary tract nucleus (NTS) (8, 109). The NTS-CVLM-RVLM-SPGN pathway (Fig. 1A) is considered crucial to the operation of the sympathetic baroreflex, hence to the short-term stability of BP (28).
OTHER PATHWAYS THAT CONTROL VASOMOTOR SNA
The RVLM is not the only source of innervation of the SPGNs that control the circulation. These SPGNs also receive prominent innervation from spinal interneurons, the midline medulla oblongata (including from serotonergic neurons), the pontine A5 noradrenergic cell group, the dorsolateral pons, and the hypothalamus (42). Hypothalamic inputs include projections from the paraventricular nucleus and from the orexinergic neurons of the perifornical region (42, 59).
Several of these structures (the cord, the midline medulla oblongata, perhaps even the paraventricular nucleus of the hypothalamus) are sources of fast synaptic inputs to the SPGNs (ionotropic GABA, glutamate or glycine synapses) (30, 50, 98). Other regions such as the raphe, the A5 cell group, and the paraventricular nucleus of the hypothalamus are also, or predominantly (A5, raphe), sources of metabotropic transmitters (serotonin, norepinephrine, vasopressin, oxytocin, orexin) (28, 41, 42). These transmitters alter the intrinsic properties of the SPGNs, causing, among other effects, large changes in their accommodation to repetitive stimuli, which presumably modulate (amplify or attenuate) the efficacy of the conventional ionotropic receptor-mediated synapses (e.g., 37, 38).
THE CENTRAL RESPIRATORY NETWORK AS INTERFACE BETWEEN CENTRAL CHEMORECEPTORS AND THE SYMPATHETIC OUTFLOW
A rise in CNS Pco2 increases sympathetic nerve discharge and BP in anesthetized and awake animals, and in humans (67, 79, 95). The sympathoactivation occurs in waves that are synchronized with the central respiratory cycle (31, 67). The respiratory pattern of SNA is very similar regardless of whether central or peripheral chemoreceptors are being activated. This respiratory pattern is species specific and, within a given species, varies between different functional classes of sympathetic efferents (40). For example, in rats the respiratory pattern of the sympathetic nerves that target splanchnic vessel beds is generally different from that of the sympathetic efferents to the skeletal muscle vasculature (31, 78). These characteristics suggest that an increase in central respiratory drive caused by central chemoreceptor stimulation may exert a nonuniform influence over the various vascular beds, the heart, etc. Similar observations have been made with respect to the influence of peripheral chemoreceptors on the various sympathetic outflows (64).
THE PRESYMPATHETIC NEURONS OF THE RVLM AS PRINCIPAL RELAY OF THE CSC
In anesthetized adult rats subjected to a bilateral vagotomy and section of the four buffer nerves (aortic nerves and carotid sinus nerves), a procedure that eliminates input from arterial baroreceptors, cardiopulmonary receptors, and peripheral chemoreceptors, the discharge rate of the RVLM presympathetic neurons increases in parallel with SNA and to a comparable degree (70). Furthermore, the activation of these RVLM neurons is patterned by the central respiratory cycle in the same differentiated manner as the SNA (32, 63, 70). Thus, under anesthesia, the RVLM presympathetic neurons undoubtedly contribute to the sympathoactivation caused by CNS acidification. The simplest explanation of the phenomenon would be that CNS acidification activates subsets of respiratory neurons that, in turn, excite the RVLM presympathetic neurons (Fig. 1A, arrowhead 2). Connections between respiratory neurons of the Bötzinger region and RVLM presympathetic cells have been proposed, but the supportive evidence remains preliminary (102). Connections between the dorsal pons and the ventral respiratory column (Fig. 1A) are essential to the generation of the three-phase “eupneic” respiratory pattern (93). These connections are also necessary for the respiratory modulation of SNA (7). Unfortunately, this observation does not clarify the location of the respiratory neurons that are immediately antecedent to the RVLM vasomotor neurons because the dorsolateral pons innervates every medullary region involved in respiratory control and the connections are reciprocal.
THE CVLM NEURONS AS INTERFACE BETWEEN THE RESPIRATORY NETWORK AND THE SYMPATHETIC OUTFLOW
The CVLM neurons (Fig. 1A) rather than the RVLM presympathetic neurons may be the main interface between the respiratory network and the network that generates the vasomotor SNA. One argument is that of proximity. The CVLM neurons are located within the pre-Bötzinger and rVRG segments of the ventral respiratory column, which are most critical to define the respiratory rate and inspiratory amplitude. However, the principal evidence is that the discharge of the CVLM GABAergic interneurons is not driven just by the ongoing activity of the baroreceptors but it is also very powerfully modulated by the central respiratory network (61). The respiratory modulation of the CVLM neurons is phase-spanning in rats and occurs in many different patterns (61). Several of these respiratory patterns are the mirror image of those displayed by subsets of RVLM neurons and sympathetic efferents. Given that CVLM neurons are the main known source of inhibitory input to the RVLM neurons, the complementary nature of these respiratory patterns suggests that the respiratory fluctuations of the discharges of RVLM neurons and sympathetic efferent could largely originate from the respiratory gating of the baroreflex at the CVLM level (Fig. 1A, arrowhead 1).
THE NUCLEUS OF THE SOLITARY TRACT AS INTERFACE BETWEEN THE RESPIRATORY NETWORK AND THE SYMPATHETIC OUTFLOW
In theory, the central respiratory gating of the baroreflex could also occur upstream of the CVLM neurons on the sensory side, namely within the nucleus of the solitary tract (NTS), which provides the baroreceptor-related excitatory drive to the CVLM neurons (Fig. 1A, arrowhead 3). The evidence supporting this view is equivocal.
The NTS contains many neurons with respiratory-related activity, and this structure is innervated by neurons with central respiratory modulation (8, 51, 109). The NTS also contains a plethora of neurons that respond to the mass activation of arterial baroreceptors (20, 80, 81, 86, 113), and some of these neurons do have a respiratory modulation (66). However, the evidence that these dual (baroreceptor and respiratory) input neurons contribute to the sympathetic baroreflex is slim for the following reason. Robust pulse modulation is present on the sensory afferent side (baroreceptors) and at every step of the sympathetic baroreflex pathway beyond the NTS (CVLM, RVLM, sympathetic pre- and postganglionic neurons). Yet, virtually none of the NTS barosensitive neurons that have been recorded exhibit a pulse-modulated discharge (e.g., 89). These barosensitive NTS neurons are therefore unlikely to be those that mediate the sympathetic baroreflex because no known mechanism can account for the disappearance of the pulse modulation within the NTS and its reappearance downstream in the baroreflex pathway. Thus the question of whether the baroreflex is modulated by the central respiratory pattern generator at the level of the NTS is still unanswered.
The NTS contains neurons that respond directly to acid in vitro and may also detect changes in Pco2 in vivo (18, 74). These neurons have not been characterized from a functional and connectivity standpoint but the observation suggests that, in theory, CO2 could also regulate the sympathetic outflow at the level of the NTS.
The primary cardiovascular responses elicited by activation of central or peripheral chemoreceptors are modified to a considerable extent by the resulting changes in pulmonary ventilation (17, 96). The NTS presumably plays a critical role in these secondary circulatory reflexes. This important topic is not within the scope of this review.
RESPIRATORY NETWORK-INDEPENDENT STIMULATION OF THE SYMPATHETIC TONE BY BRAIN ACIDIFICATION
The respiratory gating of the CVLM neurons does not entirely explain the CSC for two reasons. First, it is not clear that this gating results in an increase or a decrease in the mean activity of the CVLM neurons. Second, silencing the pre-Bötzinger/rVRG/CVLM region with injections of the GABA-mimetic muscimol increases the sympathoexcitation elicited by central (or peripheral) chemoreceptor stimulation (48, 70). If the CSC was entirely caused by disinhibition of the RVLM, this reflex should have been reduced or eliminated, not increased, by silencing the CVLM region because muscimol injection into this region silences the respiratory network and eliminates the baroreflex (49, 111). After silencing the CVLM region, chemoreceptor stimulation produces a seemingly aperiodic (tonic) activation of SNA consistent with the elimination of the central respiratory drive (48, 70). This evidence suggests that brain acidification influences the sympathetic outflow in two ways. The first depends on the degree of activation of the central respiratory network and probably involves the gating of the baroreflex at the level of the CVLM. This mechanism presumably accounts for the respiratory synchrony of the sympathetic bursts evoked by chemoreceptor stimulation. CNS acidification is also capable of activating the sympathetic vasomotor tone via pathways that are independent of the central respiratory network. Consistent with this notion CO2 can activate the sympathetic outflow at partial pressures that are well below the apneic threshold in some in vivo preparations (107) and, in awake humans, SNA increases before a respiratory motor output can be detected as CO2 rises during posthyperventilation apnea (97). These observations, both in animals and in humans, support the existence of a line of communication between central chemoreceptors and the sympathetic network that bypasses the respiratory rhythm and generating network.
ARE THE RVLM PRESYMPATHETIC NEURONS ACID SENSITIVE?
This hypothesis would explain very simply why increasing brain Pco2 activates SNA even when the respiratory network is silenced. To test it, we recorded from RVLM presympathetic neurons of the C1 variety in brain slices from a tyrosine-hydroxylase-EGFP transgenic mouse (62) and we examined whether the activity of these neurons changes during acidification of the slice in the 6.9–7.5 pH range. The C1 cells have similar properties in mice as in rats of the same age (6–9 day old), namely a slow regular discharge rate (1.8 ± 0.3 Hz) and a profound inhibitory response to bath application of an α2-adrenergic agonist (57). The spontaneous discharge rate of the RVLM catecholaminergic neurons of the mouse was unaffected by acidification (54). Under the same experimental conditions (age, temperature, perfusion medium) the retrotrapezoid neurons of the mouse exhibited a robust response to acidification (0–5 Hz between pH 7.8 and 7.0) (54). Based on this evidence, the C1 neurons are unlikely to function as central chemoreceptors and their activation by hyperoxic hypercapnia in vivo is presumably mediated by synaptic inputs. This interpretation is subject to several caveats: the C1 cells are only a subset of the RVLM presympathetic neurons, albeit a large one (>70%), and these cells were studied at room temperature and, for technical reasons, in 6- to 10-day-old mice. In rats at least, the central chemoreflex is at a low ebb during this period of life although this phenomenon may not be caused by a major change in the pH sensitivity of individual brain neurons (100).
THE RETROTRAPEZOID NUCLEUS AS A PUTATIVE SOURCE OF CHEMORECEPTOR INPUT TO THE SYMPATHETIC OUTFLOW
The tonic activation of SNA caused by hypercapnia in the absence of central respiratory drive suggests that CO2 can be detected by CNS neurons that are not part of the respiratory rhythm and pattern generator and respond to CO2 in a tonic fashion when the respiratory network is silenced. Retrotrapezoid nucleus (RTN) neurons (Fig. 1B) are examples of neurons that fit this requirement. These cells are activated by hypercapnia in vivo (72), many of them have a CO2 threshold below that of the respiratory network, and they display several types of phase-spanning respiratory modulation when the respiratory network is strongly activated (10, 29, 75) but their CO2-induced discharge is tonic at low levels of CO2 or when the respiratory network is silenced by inhibition of the pre-Bötzinger/rVRG region (106). These glutamatergic neurons drive the respiratory rhythm and pattern generator (1). They innervate the entire ventrolateral medulla, which includes the ventral respiratory column and the regions that are especially critical to sympathetic tone generation such as the CVLM and the RVLM (1, 87). The diversity of phase-spanning respiratory patterns displayed by RTN neurons (in rats) could also, in theory, account for the variety of respiratory patterns exhibited by CVLM neurons in the same species (29, 61). Although RTN neurons have properties suited to contribute to the activation of the sympathetic tone by low levels of brain Pco2, the evidence supporting this theory is still modest.
THE WAKE-PROMOTING SYSTEMS AS ALTERNATE SOURCES OF CENTRAL CHEMORECEPTOR INPUT TO THE SYMPATHETIC OUTFLOW
Serotonergic, orexinergic, and noradrenergic neurons are wake-ON neurons that innervate the entire brain stem respiratory network down to the motor neurons (5, 39, 52). The state-dependent activity of these neurons most likely contributes to the “waking neural drive to breathe,” the brain mechanism that maintains breathing during waking regardless of the CO2 level (2, 76, 83, 92). These wake-ON neurons are activated by high levels of hypercapnia (25, 44, 103), presumably for several reasons. First, when brain Pco2 reaches levels that cause aversive sensations and/or arousal (9, 69, 82), many if not all the executive pathways involved in arousal are presumably recruited to some degree. Second, orexin neurons appear to be essential for the cardiorespiratory responses to stress and emotions (53) and severe hypercapnia is an especially aversive stimulus (69). Finally, subsets of serotonergic, catecholaminergic, and orexinergic neurons may also have central chemoreceptor properties (18, 19, 26, 34, 55, 74). Given that these three neuronal systems also target virtually all the CNS sites involved in generating the sympathetic tone (42, 52), their contribution to the sympathetic component of the central chemoreflex is plausible a priori.
Orexinergic neurons, for example, innervate sympathetic preganglionic neurons and the presympathetic neurons of the RVLM and, at both sites, orexin exerts effects that somehow result in an increase in sympathetic tone (4, 15, 16, 23, 27, 94). Furthermore, orexin knockout (KO) mice are markedly hypotensive when awake (46). In addition, the respiratory component of the central chemoreflex is attenuated in orexin KO mice only during the period when orexin neurons are active, i.e., during waking hours (19). This observation could have several potential explanations, one of which being that orexin causes a widespread increase in the excitability of brain stem neurons, including the central respiratory controller, motor neurons, and the RTN, thereby potentiating the effect of brain stem acidification on breathing regardless of where the chemoreceptors are located (21). A second possibility is that the orexin neurons might be themselves activated by acidification. This notion is supported by experiments in vitro (19, 24, 112) but requires verification in vivo. Indeed, the in vitro pH sensitivity of orexin neurons is attributed to the presence of a TASK-like resting potassium conductance (24, 112), but TASK channel knockout produces no effect on the CRC (73). In addition, only a very small proportion of orexin neurons express c-Fos in mice exposed to as much as 10% CO2 (103). This result could mean that, in apparent contradiction with the in vitro data, only a very small subset of orexin neurons responds to acidification in vivo. It could also mean that the orexin neurons are not directly affected by Pco2 in vivo and that their mild activation by hypercapnia denotes the aversive and wake-promoting effect of this stimulus. Indeed, Fos expression by orexin neurons normally tracks the level of vigilance (60). Finally, the orexin neurons also activate the serotonergic system and the pontine noradrenergic neurons, which could indirectly change the activity of the sympathetic outflow (12, 36, 47).
Given the ubiquitous presence of serotonergic terminals in the lower brain stem and elsewhere, an equally large range of mechanisms could contribute to the known facilitation of the CRC by serotonergic neurons (34) and the increase of serotonin release caused by hypercapnia (44). One possibility is that specialized subsets of serotonergic neurons are directly activated by brain acidification in vivo. Many lower brain stem serotonergic neurons are indeed activated by acidification in vitro (84, 85), and a small subset of midline raphe, putatively serotonergic, neurons (∼25%) respond to hypercapnia in conscious cats (108). However, other subsets of serotonergic neurons including parapyramidal neurons with proven projections to the intermediolateral cell column are unresponsive to CO2 under anesthesia and therefore unlikely to be directly acid sensitive in vivo (72).
The pontine noradrenergic neurons of the locus ceruleus (LC) are modestly activated by severe hypercapnia in vivo (25, 33). This effect probably results from their intrinsic sensitivity to acid (26, 77) and from synaptic inputs. Indeed, the LC is innervated by C1 neurons and by the orexin system, which are CO2 responsive in vivo (36, 68, 70). The LC is not known to directly target the spinal and brain stem circuits that generate the sympathetic tone or the respiratory outflows and, under specific anesthetic conditions, LC stimulation even lowers BP (105). However, LC neurons have a very wide array of brain projections and are also activated by arousing stimuli (6). In that general sense the LC could potentially contribute to the rise in sympathetic tone caused by hypercapnia, especially if the stimulus is intense enough to be aversive and wake-promoting. The pontine A5 noradrenergic neurons selectively target the central circuitry that generates the sympathetic outflow (13, 42). These neurons have intrinsic properties that are very similar to those of the LC. Their acid sensitivity has not been investigated, and their pattern of activity in the absence of anesthesia remains unexplored.
CONCLUSIONS
Central chemoreceptors influence the sympathetic outflow via their effects on the central respiratory network and independently of it. A modest rise in CNS Pco2 increases sympathetic vasomotor tone in bursts that are synchronized with the central respiratory cycle. This synchronization occurs at least in part via a periodic respiratory gating of the baroreflex, which happens at the level of the CVLM or, possibly, within the NTS. This effect results in a respiratory synchronous disinhibition of the presympathetic neurons located in the RVLM. The same or different central chemoreceptors also contribute to the rise in SNA caused by CNS acidification in ways that are independent of their effects on the respiratory network. Some of these chemosensitive neurons are evidently responsive to small changes in Pco2 around the normal resting level of 40 mmHg. These neurons are not definitively identified but candidates include the retrotrapezoid neurons or neurons located within the NTS or the raphe. Many other brain neurons are activated by CO2 but, presumably, only in a range of concentration that triggers aversive sensations in the waking state and arousal from sleep. Their activation by CO2 may be partly network driven and partly due to an intrinsic sensitivity to acid. These neurons probably contribute to the increase in sympathetic tone associated with defensive mechanisms that restore breathing when the airways are obstructed. The LC, the serotonergic system, and the orexin neurons could be in this second category.
GRANTS
This work was supported by research grants from the National Institutes of Health (HL-74011 and HL-28785 to P. G. Guyenet and F32-HL-096280 to S. D. Depuy) and by a fellowship from the French Hypertension Society (R. Kanbar).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29: 5806–5819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol 164: 3–11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281: R1801–R1807, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci 4: 732–738, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234: 734–737, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol 57: 59–67, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Bodineau L, Frugiere A, Marlot D, Wallois F. Effect of hypoxia on the activity of respiratory and non-respiratory modulated retrotrapezoid neurons of the cat. Auton Neurosci 86: 70–77, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457–459, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol 261: 529–542, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371–387, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 278: R692–R697, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Ciriello J, Li ZH, De Oliveira CVR. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res 991: 84–95, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Daly MdB, Ward J, Wood LM. Modification by lung inflation of the vascular responses from the carotid body chemoreceptors and other receptors in dogs. J Physiol 378: 13–30, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol 103: 1772–1779, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Deuchars J, Li YW, Kasparov S, Paton JFR. Morphological and electrophysiological properties of neurones in the dorsal vagal complex of the rat activated by arterial baroreceptors. J Comp Neurol 417: 233–249, 2000 [PubMed] [Google Scholar]
- 21. Dias MB, Li A, Nattie EE. Antagonism of orexin receptor 1 (OX1R) in the retrotrapezoid nucleus (RTN) inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587: 2059–2067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duffin J. Modelling the respiratory chemoreflex control of acid-base balance. Conf Proc IEEE Eng Med Biol Soc 6: 5836–5839, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, Chang JK. Orexins: a role in medullary sympathetic outflow. Regul Pept 96: 65–70, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Duprat F, Lauritzen I, Patel A, Honore E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci 30: 573–580, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and Hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res 222: 373–381, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol 102: 1577–1590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 122: 541–550, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guyenet PG, Stornetta RL, Weston MC, McQuiston T, Simmons JR. Detection of amino acid and peptide transmitters in physiologically identified brainstem cardiorespiratory neurons. Auton Neurosci 114: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Habler HJ, Janig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Progr Neurobiol 43: 567–606, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol 256: R739–R750, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol 105: 35–45, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hokfelt T, Fuxe K, Goldstein M, Johansson O. Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res 66: 235–251, 1974 [Google Scholar]
- 36. Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van den Pol AN. Hypocretin (Orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415: 145–159, 1999 [PubMed] [Google Scholar]
- 37. Inokuchi H, Yoshimura M, Polosa C, Nishi S. The effects of 5-hydroxytryptamine on cat sympathetic preganglionic neurons in vitro. Kurume Med J 37: 309–312, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Inokuchi H, Yoshimura M, Polosa C, Nishi S. Adrenergic receptors (alpha 1 and alpha 2) modulate different potassium conductances in sympathetic preganglionic neurons. Can Journal Physiol Pharmacol 70, Suppl: S92–S97, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 21: 9S–15S, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Janig W, Habler HJ. Neurophysiological analysis of target-related sympathetic pathways—from animal to human: similarities and differences. Acta Physiol Scand 177: 255–274, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Jansen ASP, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270: 644–646, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 683: 1–24, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Jeske I, Morrison SF, Cravo SL, Reis DJ. Identification of baroreceptor reflex interneurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 264: R169–R178, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Kanamaru M, Homma I. Compensatory airway dilation and additive ventilatory augmentation mediated by dorsomedial medullary 5-hydroxytryptamine 2 receptor activity and hypercapnia. Am J Physiol Regul Integr Comp Physiol 293: R854–R860, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Kangrga IM, Loewy AD. Whole-cell recordings from visualized C1 adrenergic bulbospinal neurons: Ionic mechanisms underlying vasomotor tone. Brain Res 670: 215–232, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R581–R593, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res 187: 1–16, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol 491: 859–869, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res 609: 174–184, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Krupp J, Bordey A, Feltz P. Electrophysiological evidence for multiple glycinergic inputs to neonatal rat sympathetic preganglionic neurons in vitro. Eur J Neurosci 9: 1711–1719, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kukkonen JP, Holmqvist T, Ammoun S, Åkerman KEO. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol 283: C1567–C1591, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci 142: 11–16, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid-sensitivity and ultrastructure of the retrotrapezoid nucleus in phox2b-eGFP transgenic mice. J Comp Neurol; doi:10.1152/japplphysiol.00712.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li Q, Goodchild AK, Seyedabadi M, Pilowsky PM. Pre-protachykinin A mRNA is colocalized with tyrosine hydroxylase-immunoreactivity in bulbospinal neurons. Neuroscience 136: 205–216, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Li YW, Bayliss DA, Guyenet PG. C1 neurons of neonatal rats: Intrinsic beating properties and α2-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 269: R1356–R1369, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Lipski J, Kanjhan R, Kruszewska B, Rong WF. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study “in vivo”. J Physiol 490: 729–744, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett 351: 115–119, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 441: 589–594, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem 82: 295–304, 2002 [DOI] [PubMed] [Google Scholar]
- 63. McAllen RM. Central respiratory modulation of subretrofacial bulbospinal neurons in the cat. J Physiol 388: 533–545, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McAllen RM. Actions of carotid chemoreceptors on subretrofacial bulbospinal neurons in the cat. J Auton Nerv Syst 40: 181–188, 1992 [DOI] [PubMed] [Google Scholar]
- 65. McAllen RM, May CN, Shafton AD. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin Exp Hypertens 17: 209–221, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol 399: 349–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Millhorn DE. Neural respiratory and circulatory interaction during chemoreceptor stimulation and cooling of ventral medulla in cats. J Physiol 370: 217–231, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Milner TA, Abate C, Reis DJ, Pickel VM. Ultrastructural localization of phenylethanolamine N-methyltransferase-like immunoreactivity in the rat locus coeruleus. Brain Res 478: 1–15, 1989 [DOI] [PubMed] [Google Scholar]
- 69. Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol 94: 141–154, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morrison SF, Callaway J, Milner TA, Reis DJ. Rostral ventrolateral medulla—a source of the glutamatergic innervation of the sympathetic intermediolateral nucleus. Brain Res 562: 126–135, 1991 [DOI] [PubMed] [Google Scholar]
- 72. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nattie EE, Fung ML, Li A, St. John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol 94: 35–50, 1993 [DOI] [PubMed] [Google Scholar]
- 76. Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci 27: 4435–4442, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett 81: 279–284, 1987 [DOI] [PubMed] [Google Scholar]
- 79. Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci 117: 105–114, 2005 [DOI] [PubMed] [Google Scholar]
- 80. Paton JFR. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998 [DOI] [PubMed] [Google Scholar]
- 81. Paton JFR. Nucleus tractus solitarii: Integrating structures. Exp Physiol 84: 815–833, 1999 [PubMed] [Google Scholar]
- 82. Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis 115: 251–259, 1977 [DOI] [PubMed] [Google Scholar]
- 83. Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 85. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Rogers RF, Paton JFR, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. Am J Physiol Regul Integr Comp Physiol 265: R1355–R1368, 1993 [DOI] [PubMed] [Google Scholar]
- 87. Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. J Neurophysiol 76: 3750–3757, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol 502: 455–467, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Schreihofer AM, Guyenet PG. Baroactivated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol 89: 1265–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 92. Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci 28: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res 950: 261–267, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953–1957, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67: 2101–2106, 1989 [DOI] [PubMed] [Google Scholar]
- 97. St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res 85: 457–469, 1999 [DOI] [PubMed] [Google Scholar]
- 98. Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol 444: 207–220, 2002 [DOI] [PubMed] [Google Scholar]
- 100. Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127: 135–155, 2001 [DOI] [PubMed] [Google Scholar]
- 101. Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol Regul Integr Comp Physiol 249: R672–R680, 1985 [DOI] [PubMed] [Google Scholar]
- 102. Sun QJ, Minson J, Llewellyn-Smith IJ, Arnolda L, Chalmers J, Pilowsky P. Botzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 388: 23–31, 1997 [DOI] [PubMed] [Google Scholar]
- 103. Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol 166: 184–186, 2009 [DOI] [PubMed] [Google Scholar]
- 104. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sved AF, Felsten G. Stimulation of the locus coeruleus decreases arterial pressure. Brain Res 414: 119–132, 1987 [DOI] [PubMed] [Google Scholar]
- 106. Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Trzebski A, Kubin L. Is the central inspiratory activity responsible for Pco2-dependent drive of the sympathetic discharge? J Auton Nerv Syst 3: 401–420, 1981 [DOI] [PubMed] [Google Scholar]
- 108. Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol 460: 525–541, 2003 [DOI] [PubMed] [Google Scholar]
- 110. Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla. Identification by microinjection of l-glutamate. Neuropharmacology 22: 1071–1079, 1983 [DOI] [PubMed] [Google Scholar]
- 111. Willette RN, Krieger AJ, Barcas PP, Sapru HN. Medullary gamma-aminobutyric acid (GABA) receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther 226: 893–899, 1983 [PubMed] [Google Scholar]
- 112. Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA 104: 10685–10690, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang J, Mifflin SW. Responses of aortic depressor nerve-evoked neurones in rat nucleus of the solitary tract to changes in blood pressure. J Physiol 529: 431–443, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]