Abstract
The time course and mechanisms of adjustment of pulmonary oxygen uptake (V̇o2) kinetics (time constant τV̇o2p) were examined during step transitions from 20 W to moderate-intensity cycling in eight older men (O; 68 ± 7 yr) and eight young men (Y; 23 ± 5 yr) before training and at 3, 6, 9, and 12 wk of endurance training. V̇o2p was measured breath by breath with a volume turbine and a mass spectrometer. Changes in deoxygenated hemoglobin concentration (Δ[HHb]) were measured by near-infrared spectroscopy. V̇o2p and Δ[HHb] were modeled with a monoexponential model. Training was performed on a cycle ergometer three times per week for 45 min at ∼70% of peak V̇o2. Pretraining τV̇o2p was greater (P < 0.05) in O (43 ± 10 s) than Y (34 ± 8 s). τV̇o2p decreased (P < 0.05) by 3 wk of training in both O (35 ± 9 s) and Y (22 ± 8 s), with no further changes thereafter. The pretraining overall adjustment of Δ[HHb] was faster than τV̇o2p in both O and Y, resulting in Δ[HHb]/V̇o2p displaying an “overshoot” during the transient relative to the subsequent steady-state level. After 3 wk of training the Δ[HHb]/V̇o2p overshoot was attenuated in both O and Y. With further training, this overshoot persisted in O but was eliminated after 6 wk in Y. The training-induced speeding of V̇o2p kinetics in O and Y at 3 wk of training was associated with an improved matching of local O2 delivery to muscle V̇o2 (as represented by a lower Δ[HHb]/V̇o2p). The continued overshoot in Δ[HHb]/V̇o2p in O may reflect a reduced vasodilatory responsiveness that may limit muscle blood flow distribution during the on-transient of exercise.
Keywords: aging, muscle blood flow, near-infrared spectroscopy
the rate of adjustment of the primary component (phase II) of pulmonary oxygen uptake (V̇o2p) closely reflects the adjustments of oxidative metabolism at the skeletal muscle level (25, 39, 49). The phase II V̇o2p kinetics during the on-transient of moderate-intensity exercise is slower in older compared with younger adults (2, 5, 8, 12). A slower adjustment of oxidative metabolism results in a larger O2 deficit and greater reliance on substrate-level phosphorylation to provide ATP in sufficient amounts to sustain any given activity. As such, older adults may experience premature fatigue and reduced tolerance to exercise (15).
In some conditions (e.g., hypoxia, β-adrenergic receptor blockade, reduced arterial perfusion) and subject groups (e.g., those with chronic heart failure, peripheral vascular disease, diabetes) it has been suggested that O2 delivery may constrain V̇o2 kinetics (34, 47, 55). Although bulk delivery of O2 to the exercising limb does not seem to limit the rate of adjustment of V̇o2 kinetics (6), the local distribution of blood flow within the active muscles appears to be critical in the matching of O2 delivery to O2 utilization during the kinetic phase of adjustment to the increased energy demand with exercise (19). Near-infrared spectroscopy (NIRS) data in young adults have shown that the rate of deoxygenation is very rapid (14) and suggest that the rate of increase in microvascular blood flow in the active tissues is slow compared with the increase in muscle O2 utilization (V̇o2m) (14, 31). An age-related reduction in local (microvascular) blood flow is reflected by a greater ratio of change in deoxygenated hemoglobin to change in V̇o2p (Δ[HHb]/ΔV̇o2p) in older compared with younger men (13) as well as a transiently greater fall in microvascular Po2 (Po2mv) in older compared with young rats (4). Thus older adults rely more on O2 extraction during the on-transient of exercise, probably because of a lower local (microvascular) blood flow-to-V̇o2m ratio.
Endurance training results in faster V̇o2p kinetics in both older (1, 6) and younger (7, 21, 40, 46) individuals. However, the mechanisms underlying that faster response have not been clearly elucidated, nor has the time course of adaptation of V̇o2p been established in older adults. Information on the time course of the training-induced speeding of V̇o2 kinetics may shed light on factors limiting the adjustment of V̇o2m and on how and whether their contributions are influenced by aging and training status. As such, the main goal of this study was to determine the time course and mechanism of adaptation for phase II V̇o2p in older and younger men throughout a 12-wk endurance training program. We hypothesized that there would be an improved microvascular O2 delivery in the exercise transient in response to the endurance training that would be associated with a faster adjustment of V̇o2p kinetics observed early in training in both older and young adults. Improvements in microvascular O2 delivery would be indicated by a better matching between the rate of adjustment of muscle deoxygenation relative to phase II V̇o2p (i.e., the Δ[HHb]-to-ΔV̇o2p ratio), which represents a decreased reliance on O2 extraction for a given V̇o2p.
METHODS
Subjects.
Eight older (O; 68 ± 7 yr; mean ± SD) and eight young (Y; 23 ± 5 yr) adult men volunteered and gave written consent to participate in the study. Descriptive and baseline data from these subjects were given in a separate report looking at central and peripheral adaptations to endurance training in the same men (Ref. 43; the reader is referred to this paper for further information on increases in maximal V̇o2p, cardiac output, and arteriovenous O2 difference). All procedures were approved by the University of Western Ontario Research Ethics Board for Health Sciences Research Involving Human Subjects. All subjects were nonobese (body mass index ≤30 kg/m2) nonsmokers and were physically active, but none had been involved in any type of endurance training program for at least 12 mo before the study. Additionally, no subjects were taking medications that would affect the cardiorespiratory or hemodynamic responses to exercise. Subjects had no history of cardiovascular, respiratory, or musculoskeletal diseases, and older subjects were medically screened by a physician and underwent a maximal exercise stress test.
Protocol.
Before training began, subjects reported to the laboratory on two separate occasions. On day 1, a maximal cycle ergometer ramp test (O 15–20 W/min; Y 25 W/min) was performed (Lode Corival 400; Lode, Groningen, The Netherlands) for determination of peak V̇o2 (V̇o2peak) and the estimated lactate threshold (θL). θL was defined as the V̇o2 at which CO2 production (V̇co2) began to increase out of proportion in relation to V̇o2 with a systematic rise in minute ventilation-to-V̇o2 ratio and end-tidal Po2 whereas minute ventilation-to-V̇co2 ratio and end-tidal Pco2 were stable. After this test, subjects returned to the laboratory on a different day to perform step transitions in work rate (WR) from 20 W to a moderate-intensity WR that elicited a V̇o2 corresponding to 90% θL. Each subject performed two sets, each including two step transitions consisting of 6 min of pedaling at 20 W, 6 min at 90% θL, 8 min at 20 W, and another 6 min at 90% θL. Each set was separated by a 30-min period of rest sitting on a chair. Identical procedures were repeated after weeks 3, 6, 9, and 12 of training. However, since the step transitions were performed at the same absolute intensity as the initial tests, the order for the ramp test and the step transitions was randomly assigned. At least 24 h was allowed between the ramp test and the step transitions.
Training.
The endurance training program consisted of three exercise sessions per week on a stationary cycle ergometer (Monark Ergomedic 874E; Monark Exercise, Varberg, Sweden) for a total duration of 12 wk. Training intensity was adjusted at 3-wk intervals to reflect changes in fitness level. During the first 10 wk, each session consisted of continuous training (CT) for 45 min at a power output that elicited ∼70% of the V̇o2peak observed during the incremental ramp test. During the final 2 wk of training (6 exercise sessions), each individual in each group (O and Y) was assigned to one of two subgroups: 1) CT as described above or 2) high-intensity interval training (HIT), performing 10–12 exercise bouts each lasting 1 min at 90–100% of the peak power output achieved during the incremental ramp test, with 1-min rest separating bouts. Since V̇o2peak was likely to plateau after ∼8 wk of CT (45), and this study was also designed to look at changes in V̇o2peak with training (see Ref. 43; data not further considered in the present study), HIT was used as a strategy for progressive and continued gains in the exercise program resulting in further increases in V̇o2peak favored by peripheral adaptations (11).
Measurements.
Gas exchange measurements were similar to those previously described (2). Briefly, inspired and expired flow rates were measured with a low-dead-space (90 ml) bidirectional turbine (Alpha Technologies VMM 110) that was calibrated before each test by using a syringe of known volume. Inspired and expired gases were sampled continuously (every 20 ms) at the mouth and analyzed for concentrations of O2, CO2, and N2 by mass spectrometry (PerkinElmer MGA-1100) after calibration with precision-analyzed gas mixtures. Changes in gas concentrations were aligned with gas volumes by measuring the time delay for a square-wave bolus of gas passing the turbine to the resulting changes in fractional gas concentrations as measured by the mass spectrometer. Data collected every 20 ms were transferred to a computer, which aligned concentrations with volume information to build a profile of each breath. Breath-by-breath alveolar gas exchange was calculated by using algorithms of Beaver et al. (3).
Heart rate (HR) was continuously monitored by electrocardiogram using PowerLab (ML132/ML880; ADInstruments, Colorado Springs, CO) with a three-lead arrangement. Data were recorded with LabChart v4.2 (ADInstruments) on a separate computer.
Local muscle oxygenation profiles of the quadriceps vastus lateralis muscle were made with NIRS (Hamamatsu NIRO 300, Hamamatsu Photonics, Hamamatsu, Japan). Optodes were placed on the belly of the muscle midway between the lateral epicondyle and greater trochanter of the femur. The optodes were housed in an optically dense plastic holder, secured on the skin surface with tape, and then covered with an optically dense black vinyl sheet, thus minimizing the intrusion of extraneous light. The thigh was wrapped with an elastic bandage to minimize movement of the optodes.
The physical principles of tissue spectroscopy are described in detail by Elwell (20), and the manner in which these are applied have been explained by DeLorey et al. (14). Briefly, one fiber-optic bundle carried the NIR light produced by the laser diodes to the tissue of interest while a second fiber-optic bundle returned the transmitted light from the tissue to a photon detector (photomultiplier tube) in the spectrometer. Four different wavelength laser diodes (775, 810, 850, and 910 nm) provided the light source. The diodes were pulsed in a rapid succession, and the light was detected by the photomultiplier tube for online estimation and display of the concentration changes from the resting baseline oxyhemoglobin (HbO2), deoxyhemoglobin (HHb), and total hemoglobin (Hbtot). In this study, we used an interoptode spacing of 5 cm. Given the uncertainty of the optical path length in the vastus lateralis at rest and during exercise, NIRS data are presented as delta (Δ) arbitrary units (a.u.). NIRS-derived signal was zero set before the onset of exercise while subjects were quietly seated on the cycle ergometer. The raw attenuation signals (in optical density units) were transferred to a computer for later analysis. Changes in light intensities were recorded continuously at 2 Hz.
Data analysis.
V̇o2 data were filtered by removing aberrant data points that lay outside 4 SD of the local mean. The data for each transition then were linearly interpolated to 1-s intervals and time aligned such that time zero represented the onset of exercise. Data from each transition were ensemble averaged to yield a single, averaged response for each subject. This transition was further time averaged into 10-s bins to provide a single time-averaged response for each subject. The on-transient response for V̇o2 was fitted with a monoexponential model of the form
| (1) |
where Y(t) represents V̇o2 at any time (t); YBsln is the baseline V̇o2 during 20-W cycling; Amp is the steady-state increase in V̇o2 above the baseline value; τ is the time constant defined as the duration of time for V̇o2 to increase to 63% of the steady-state increase; and TD is the time delay (such that the model is not constrained to pass through the origin). The phase I-phase II transition was constrained to a constant of 25 s. Data were modeled from the beginning of phase II to 4 min (240 s) of the step transition. The model parameters were estimated by least-squares nonlinear regression (Origin, OriginLab, Northampton, MA) in which the best fit was defined by minimization of the residual sum of squares and minimal variation of residuals around the y-axis (Y = 0). The 95% confidence interval for the estimated time constant was determined after preliminary fit of the data with YBsln, Amp, and TD constrained to the best-fit values and the τ allowed to vary.
HR data were determined from the R-R interval on a second-by-second basis and edited and modeled in the same manner as the V̇o2 data described above. The on-transient HR response was modeled from the onset of exercise to 240 s with the exponential model described in Eq. 1.
The NIRS-derived Δ[HHb] data were time aligned and ensemble averaged to 5-s bins to yield a single response for each subject. The Δ[HHb] profile has been described to consist of a time delay at the onset of exercise, followed by an increase in the signal with an “exponential-like” time course (14). The time delay for the Δ[HHb] response (TD-Δ[HHb]) was determined with second-by-second data and corresponded to the time between the onset of exercise and the first point at which the Δ[HHb] signal started to systematically increase. Determination of the TD-Δ[HHb] was made on individual trials and averaged to yield a single value for each individual. The Δ[HHb] data were modeled from the end of the TD-Δ[HHb] to 90 s of the transition with an exponential model as described in Eq. 1. The τ[HHb] described the time course for the increase in Δ[HHb], while the overall time course of Δ[HHb] from the onset of exercise was described by the effective Δ[HHb] (τ′Δ[HHb] = TD-Δ[HHb] + τΔ[HHb]).
The second-by-second Δ[HHb] and V̇o2p data were normalized for each subject (0–100% of the response). The normalized V̇o2p was left shifted by 20 s to account for the phase I-phase II transition so that the onset of exercise coincided with the beginning of phase II V̇o2p, which has been previously described to coincide with muscle V̇o2 within 10% (49). Data were further averaged into 5-s bins for statistical comparison of the rate of adjustment for Δ[HHb] and ΔV̇o2p. Additionally, an overall Δ[HHb]-to-ΔV̇o2p ratio for the adjustment during the exercise on-transient was derived for each individual as the average value from 20–150 s into the transition. The start point was selected to be 20 s to begin beyond the physiological TD-Δ[HHb] derived from NIRS. An end point of 150 s was selected to ensure that both the Δ[HHb] and ΔV̇o2p signals had already reached 100% of their amplitudes.
Statistics.
Data are presented as means ± SD. Paired and unpaired t-tests and repeated-measures analysis of variance (ANOVA) were used to determine statistical significance for the dependent variables. The ANOVA model was described as S16 × T5 × A2 such that subjects (S; no. of subjects) are crossed with testing time (T; 5 testing times: pretraining, week 3, week 6, week 9, and posttraining) and age (A; older and young adults). A Tukey post hoc analysis was used when significant differences were found for the main effects of each dependent variable. Pearson product moment correlation coefficients were used to determine the degree of association between key variables. The ANOVA and correlation coefficients were analyzed by SPSS version 15.0 (SPSS, Chicago, IL). Statistical significance was declared when P < 0.05.
RESULTS
Subject characteristics and pretraining peak exercise values are listed in Table 1. Compliance with the training program was 94 ± 1% (28/30 training sessions) and 95 ± 1% (29/30 training sessions) in O and Y, respectively. V̇o2peak was significantly increased by 3 wk in both O (by 10 ± 9%) and Y (by 12 ± 6%). Further training resulted in a total improvement from pre- to posttraining that represented 31 ± 10% in O and 18 ± 10% in Y. As noted in methods, groups were split after the 10th week of training; however, since training type (i.e., continuous vs. interval) did not affect any of the kinetic parameters, data are not presented separately.
Table 1.
Subject characteristics and peak exercise responses
| n | Age, yr | Height, cm | Body Mass, kg | Peak WR, W | Peak HR, beats/min | V̇o2peak, l/min | V̇o2peak, ml·kg−1·min−1 | |
|---|---|---|---|---|---|---|---|---|
| Older | 8 | 68 ± 7 | 177 ± 9 | 82 ± 8 | 188 ± 44 | 144 ± 22 | 2.3 ± 0.5 | 28 ± 7 |
| Young | 8 | 23 ± 5* | 178 ± 5 | 80 ± 8 | 314 ± 41* | 189 ± 7* | 3.8 ± 0.5* | 48 ± 6* |
Values are means ± SD for n subjects. WR, work rate; HR, heart rate; V̇o2peak, peak oxygen uptake.
P < 0.05 compared with older.
V̇o2 kinetics.
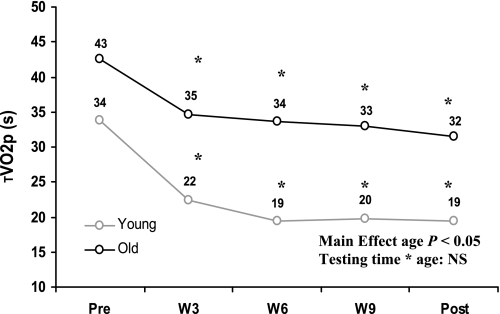
O had a greater phase II V̇o2 time constant (τV̇o2p) compared with Y. Pretraining τV̇o2p was 43 ± 11 s in O and 34 ± 8 s in Y. τV̇o2p decreased significantly by 3 wk of training in both O (35 ± 9 s) and Y (22 ± 8 s), with no further changes seen with continued training (Fig. 1, Table 2). After 3 wk of training, τV̇o2p in O was similar to that observed in Y before training. No testing time-by-age interactions were detected, reflecting a similar rate of adaptation of V̇o2p kinetics in both O and Y and a maintained difference between age groups across time.
Fig. 1.
Changes in the phase II pulmonary oxygen uptake (V̇o2) time constant (τV̇o2p) over the course of the endurance training program in older and young adults. Pre, pretraining; W3, W6, W9, weeks 3, 6, and 9 of training; Post, posttraining. *P < 0.05 compared with pretraining; NS, not significant.
Table 2.
Kinetics parameters for V̇o2, HR, and Δ[HHb] in O and Y from pretraining through posttraining
| Pretraining | Week 3 | Week 6 | Week 9 | Posttraining | |
|---|---|---|---|---|---|
| Phase II τV̇o2p, s | |||||
| O§ | 43 ± 10 | 35 ± 9* | 34 ± 8* | 33 ± 8* | 32 ± 7* |
| Y | 34 ± 8 | 22 ± 8* | 19 ± 6* | 20 ± 7* | 19 ± 7* |
| τHR, s | |||||
| O§ | 49 ± 15 | 35 ± 10* | 38 ± 10* | 31 ± 10* | 31 ± 11*† |
| Y | 45 ± 14 | 28 ± 10* | 27 ± 7*‡ | 29 ± 13* | 26 ± 7*† |
| τΔ[HHb], s | |||||
| O | 13 ± 11 | 15 ± 7 | 11 ± 4 | 13 ± 4 | 12 ± 4 |
| Y | 11 ± 4 | 12 ± 2 | 10 ± 2 | 11 ± 2 | 10 ± 1 |
| TD-Δ[HHb], s | |||||
| O§ | 7 ± 3 | 9 ± 2 | 8 ± 2 | 8 ± 1 | 9 ± 1 |
| Y | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 2 | 7 ± 1 |
| τ′Δ[HHb], s | |||||
| O§ | 20 ± 12‡ | 24 ± 8‡ | 19 ± 4‡ | 21 ± 4‡ | 19 ± 4‡ |
| Y | 17 ± 4‡ | 18 ± 2 | 16 ± 2 | 17 ± 3 | 16 ± 1 |
Values are means ± SD. τ, Time constant of response; V̇o2p, pulmonary V̇o2; Δ[HHb], change in deoxygenated hemoglobin concentration; TD, time delay; τ′Δ[HHb], sum of effective τΔ[HHb] and TD-Δ[HHb];
Significantly different from pretraining values (P < 0.05);
significantly different from week 6 (P < 0.05),
significantly different from phase II τV̇o2p at the same testing time (P < 0.05);
significantly different from Y (P < 0.05).
The amplitude of the increase in V̇o2p across all testing times was lower in O (0.55 ± 0.29 l/min) compared with Y (1.23 ± 0.20 l/min), reflecting the lower WR in O (i.e., O 68 ± 15 W, Y 128 ± 28 W). No changes in the functional V̇o2p gain were observed with training (with a mean overall ΔV̇o2p/ΔWR in O of 11.4 ± 1.3 ml·min−1·W−1 and in Y of 11.5 ± 0.9 ml·min−1·W−1).
HR kinetics.
The τHR was greater in O compared with Y. After 3 wk of training τHR decreased in O and in Y, and it remained decreased compared with pretraining for the remainder of the study (Table 2). In O, τHR was not different from τV̇o2p at any testing time. In Y, the only significant difference was a greater τHR compared with τV̇o2p at week 6.
Δ[HHb] kinetics.
The amplitudes of the increase in Δ[HHb] (O 10 ± 5 a.u., Y 13 ± 6 a.u.) as well as τΔ[HHb] (O 13 ± 6 s, Y 11 ± 2 s) were similar in both groups. The overall time course of Δ[HHb], as reflected by τ′Δ[HHb], was longer (P < 0.05) in O (21 ± 7 s) compared with Y (17 ± 3 s). No changes in response to training were observed for TD-Δ[HHb], τΔ[HHb], or τ′Δ[HHb] in either O or Y (Table 2).
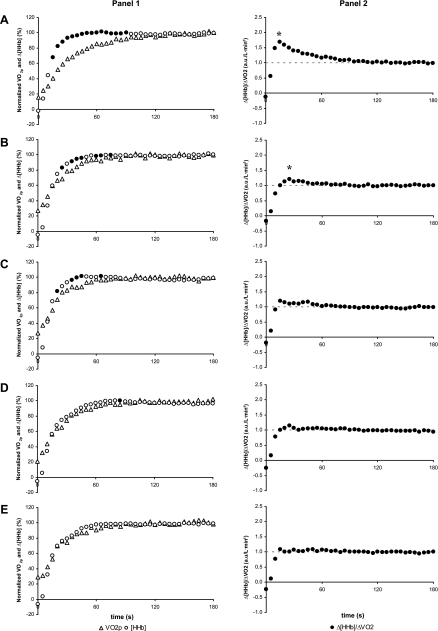
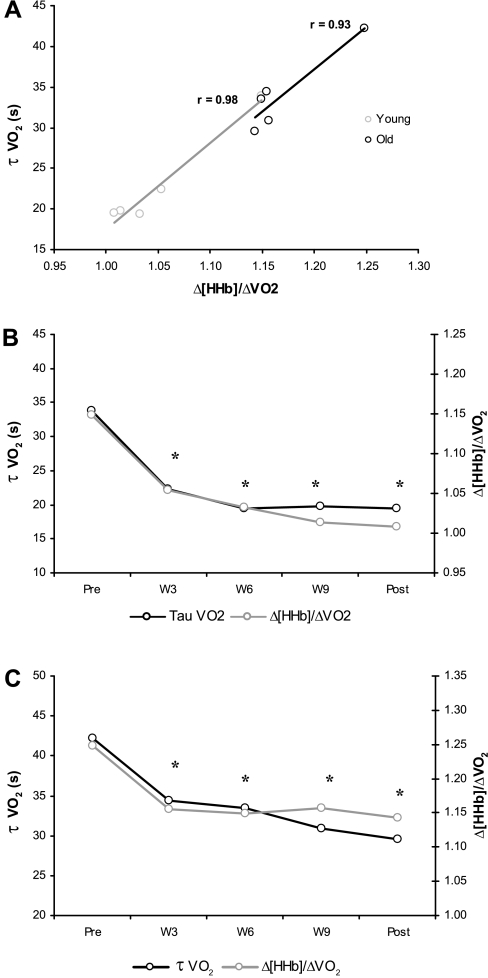
Before training, τ′Δ[HHb] adjustment was shorter than τV̇o2p (P < 0.05) in both O and Y (Table 2, Fig. 2A1 and Fig. 3A1), which resulted in the calculated Δ[HHb]-to-ΔV̇o2p ratio displaying a transient “overshoot” during the exercise on-transient relative to the subsequent steady-state level (Fig. 2A2 and Fig. 3A2). After 3 wk of training in Y, τ′Δ[HHb] and τV̇o2p were similar (Table 2), and the Δ[HHb]/ΔV̇o2p overshoot was attenuated (Fig. 3, B1 and B2). With further training, the overshoot was eliminated in Y (Fig. 3, C1–E1 and C2–E2). In O, after 3 wk of training the Δ[HHb]/ΔV̇o2p overshoot (Fig. 2B2) also was attenuated; however, in this group, the τ′Δ[HHb] remained shorter (P < 0.05) than the τV̇o2p (Table 2). No further attenuations in the Δ[HHb]/ΔV̇o2p overshoot were observed with continued training in O (Fig. 2, C2–E2). The reductions in τV̇o2p in both O and Y with training were closely associated with a lowered Δ[HHb]-to-ΔV̇o2p ratio during the exercise on-transient (r: O = 0.93, P < 0.05; Y = 0.98, P < 0.05; Fig. 4).
Fig. 2.
1: Group mean profiles for the adjustment of change in deoxygenated hemoglobin concentration (Δ[HHb]) and V̇o2p (left shifted such that data from phase I V̇o2p were not included) during the initial 180 s of a step transition in work rate in older adults pretraining (A), at week 3 (B), week 6 (C), and week 9 (D) of training, and posttraining (E). ●, Time points at which the relative increase of Δ[HHb] is greater than the relative increase of V̇o2p (P < 0.05). 2: Group mean profiles for the adjustment of Δ[HHb]/ΔV̇o2p during the initial 180 s of a step transition in work rate in older adults pretraining (A), at week 3 (B), week 6 (C), and week 9 (D) of training, and posttraining (E). a.u., Arbitrary units. *Δ[HHb]/ΔV̇o2p significantly different from 1.0 (P < 0.05).
Fig. 3.
1: Group mean profiles for the adjustment of Δ[HHb] and V̇o2p (left shifted such that data from phase I V̇o2p were not included) during the initial 180 s of a step transition in work rate in young adults pretraining (A), at week 3 (B), week 6 (C), and week 9 (D) of training, and posttraining (E). ●, Time points at which the relative increase of Δ[HHb] is greater than the relative increase of V̇o2p (P < 0.05). 2: Group mean profiles for the adjustment of Δ[HHb]/ΔV̇o2p during the initial 180 s of a step transition in work rate in young adults pretraining (A), at week 3 (B), week 6 (C), and week 9 (D) of training, and posttraining (E). *Δ[HHb]/ΔV̇o2p significantly different from 1.0 (P < 0.05).
Fig. 4.
A: correlation between changes in Δ[HHb]/ΔV̇o2p and τV̇o2p in response to training for older men (O) and young men (Y). B: time course of changes in Δ[HHb]/ΔV̇o2p and τV̇o2p in Y. C: time course of changes in Δ[HHb]/ΔV̇o2p and τV̇o2p in O. *P < 0.05 compared with pretraining.
DISCUSSION
This study examined the effects of a 12-wk endurance training program on the time course of adaptation of V̇o2p during transitions to moderate-intensity exercise in older and young healthy adults. The main findings were as follows: 1) The decrease in τV̇o2p in both O and Y occurred within the first 3 wk of training, with no significant changes seen thereafter. 2) After 3 wk of training, an attenuation in the Δ[HHb]/ΔV̇o2p overshoot during the on-transient, relative to the subsequent steady-state level, suggests a better matching of microvascular blood flow and O2 distribution and muscle O2 utilization in the exercise transient in both O and Y. 3) Continued training beyond 3 wk in both O and Y was not associated with further apparent improvement in the matching of blood flow to O2 utilization or with reductions in τV̇o2p. 4) In Y, by week 6 O2 utilization and phase II V̇o2p were closely matched (Δ[HHb]/ΔV̇o2p ∼1.0), suggesting that the possibility of a O2 delivery constraint to muscle V̇o2 kinetics was ameliorated, whereas in O further improvements in the matching of Δ[HHb]/ΔV̇o2p were not achieved in response to the training program and thus no additional changes in τV̇o2p were observed, indicating that O2 delivery remained a constraint.
Previous studies have demonstrated that endurance training results in a faster V̇o2p kinetics in both older (1, 6) and young (7, 21, 40, 46) adults. However, the time course of this adaptation has only been explored in young (40, 46) and middle-aged (21), but not older, adults. In younger groups a faster V̇o2p occurred in as little as 2–4 days of endurance training (40, 46), with improvements continuing up to 30 days after the start of the training program (46) and no further changes observed after 30, 60, and 90 days of training (21). The present data demonstrated faster V̇o2p kinetics with endurance training in older adults (as well as young) within the first 3 wk, with no further significant changes thereafter (6, 9, 12 wk). Taken together, these data show that the rate of adaptation of oxidative phosphorylation at exercise onset after the initiation of an endurance training program occurs within 3 wk of training regardless of age and may continue up to 30 days (4 wk), with no further change observed with up to 12 wk. Further studies are warranted to clarify the short-term (e.g., <3 wk of training) time course of adaptation of τV̇o2p in older adults.
What mechanisms or regulatory factors might constrain the V̇o2 kinetics in both older and younger subjects and explain the faster V̇o2 kinetics with exercise training? A limitation in O2 delivery to the active tissues has been proposed as one of the likely mechanisms regulating the rate of adaptation of oxidative phosphorylation (34, 47, 55). Phillips et al. (46) hypothesized that a faster femoral artery blood velocity in the absence of increases in muscle oxidative enzyme activity (27) was the mechanism responsible for the reduction in τV̇o2p observed early in training in young subjects. From the present study, using the HR as an estimate of “central” blood flow, it was notable that τHR was similar to τV̇o2p in O and Y at any testing time (with the only exception being Y at week 6), suggesting that the time course of increase of central blood flow and thus central O2 delivery was matched to muscle O2 utilization. Training resulted in a decreased τHR in both O and Y. Importantly, τHR is only an indirect estimation of O2 delivery. Measures of muscle conduit artery blood flow kinetics during the transition to exercise show that the rate of adjustment is similar to or faster than that of V̇o2p (19, 31, 37). Indeed, in a training study of older adults that resulted in a faster V̇o2 kinetics, the kinetics of femoral artery mean blood velocity was unchanged (6). Thus bulk delivery of O2 to the exercising limb does not seem to be limiting τV̇o2p or the adaptation to training that speeds V̇o2 kinetics.
Despite evidence showing that the kinetics of bulk delivery of O2 in the limbs is appropriate to meet the metabolic requirements of the active tissues, blood flow responses to exercise are mediated not only by changes in cardiac output or conduit artery flow but also by the effects of the muscle pump and various vasoactive metabolites and hormones regulating the level of constriction and dilation within the microvascular resistance vessels (17, 18, 41, 48). Recent advances with NIRS have allowed continuous assessment of muscle deoxygenation as an index of the matching of microvascular O2 delivery to muscle O2 utilization. DeLorey et al. (13, 14), applying this measure in conjunction with measurements of V̇o2 kinetics, showed that the rate of adjustment of the NIRS-derived Δ[HHb] signal was faster than the adjustment of phase II V̇o2p and that this response was exacerbated in older adults, indicating a greater fractional O2 extraction and thus poorer blood flow distribution. Similarly, Harper et al. (31) reported that in young adults performing moderate-intensity knee-extension exercise femoral artery blood flow adjusted faster than the estimated capillary blood flow. Thus, although the rate of adjustment of blood flow in the conduit artery matches that observed for V̇o2, the kinetics of microvascular blood flow may have a slower time course and therefore limit the rate of adjustment of V̇o2 kinetics. With training of young subjects, based on a faster adjustment of phase II V̇o2p with unchanged adjustment in Δ[HHb], McKay et al. (40) speculated that training-induced decreases in the τV̇o2p were explained, at least in part, by better matching of muscle O2 delivery to O2 utilization. The present data showed that before training, τ′Δ[HHb] was shorter than τV̇o2p (P < 0.05) in both O and Y (Table 2, Fig. 2A1, and Fig. 3A1), which resulted in the Δ[HHb]-to-ΔV̇o2p ratio displaying a transient overshoot relative to the subsequent steady-state level (Fig. 2A2 and Fig. 3A2). This transient overshoot in the Δ[HHb]-to-ΔV̇o2p ratio (values > 1.0) is consistent with a greater microvascular fractional O2 extraction per unit V̇o2p compared with the exercise steady state (values = 1.0) and reflects a lower O2 delivery relative to muscle O2 utilization in the area of the NIRS probe (slower adjustment of microvascular blood flow).
The young subjects in this study displayed a “slower” V̇o2p kinetics than normally observed in relatively fit, young adults (τV̇o2p ∼20 s). The adjustment of V̇o2 in this group may be constrained by a mismatch between local muscle perfusion and metabolism that requires O2 extraction to increase rapidly during the exercise on-transient because of a slow increase in local muscle O2 delivery. In this regard, untrained, sedentary young (as well as older) adults, as used in the present study, may exhibit a reduced endothelium-dependent vasodilation compared with those who regularly perform aerobic exercise (16), which could contribute to a poorer microvascular blood flow and the transient overshoot in the Δ[HHb]-to-ΔV̇o2p ratio reported in this study. Animal studies have shown that endothelium-dependent vasodilation [using acetylcholine (ACh)] and flow (shear stress)-induced vasodilation were reduced in feed arteries and in 1A arterioles of soleus muscles (oxidative) of old but not of young rats (42), which could contribute to an impaired blood flow distribution in the older adults in this study. Interestingly, at rest and during steady-state submaximal exercise total hindlimb blood flow was similar in old and young rats, but blood flow distribution to oxidative muscles was reduced and distribution to glycolytic muscles was increased in older animals (44), suggesting that the matching of blood flow and O2 delivery to O2 utilization in active fibers of the old rats may be compromised. Δ[HHb]/ΔV̇o2p data presented in the present study support the idea of older individuals having a maldistribution of blood flow within the active muscles at the start of exercise.
After 3 wk of training, the τ′Δ[HHb] in Y was unchanged compared with pretraining values but now was similar to τV̇o2p (Table 2). Also, during the transition to exercise, the overshoot in the Δ[HHb]/ΔV̇o2p profile was attenuated in this group (Fig. 3, B1 and B2) and was not evident after further training (Fig. 3, C1–E1 and C2–E2), suggesting that local blood flow and O2 delivery were better matched to the O2 requirement of the active muscle. Older adults also showed an attenuated Δ[HHb]/ΔV̇o2p overshoot after 3 wk of training (Fig. 2B2); however, τ′Δ[HHb] adaptation remained faster than τV̇o2p (Table 2), and, unlike the response in Y, the overshoot in the Δ[HHb]/ΔV̇o2p profile was not eliminated with continued training in O (Fig. 2, C2–E2). What mechanism might explain the improvements in the matching of local O2 delivery to muscle O2 utilization within 3 wk in both older and younger individuals? Changes to ACh-mediated and flow-induced vasodilation represent an important factor that may have influenced the time course of adaptation of V̇o2 kinetics to training in older and younger groups. Older and middle-aged men who regularly perform endurance exercise (16, 54) or who have completed 3 mo of aerobic exercise (16) demonstrate a greater ACh-mediated vasodilatory response compared with their sedentary counterparts. Interestingly, exercise training was shown to restore both endothelium (52)- and flow (53)-dependent vasodilation in soleus muscle arterioles from old and young rats. However, training in young rats resulted in improvements beyond those observed in old trained animals (52, 53), supporting the finding of the present study that training adaptations did not continue beyond 3 wk in O, and with 12 wk of training, or even longer-term training (1), the τV̇o2 does not achieve values reached in the trained young. Rapid improvements in both endothelium- and flow-mediated vasodilation were reported 12–24 h after a single bout of exercise and were sustained for 1–2 days; however, unlike the acute response, chronic exercise training induces adaptations that are twofold higher and more long-lasting, remaining for up to 1 wk after exercise (26, 30). Therefore, enhancement of endothelium- and flow-mediated vasodilation in both O and Y adults may be mainly responsible for the faster blood flow adjustment at exercise onset, and thereby lead to an attenuation or elimination of the overshoot in the Δ[HHb]/ΔV̇o2p profile that is seen in the present study with exercise training. The high correlation and similar time course of changes in Δ[HHb]/ΔV̇o2p and τV̇o2p (Fig. 4) in both O and Y further support the notion that an improved O2 distribution within the microvasculature plays a major role in the changes observed in τV̇o2p.
Another mechanism that may affect the vasodilatory responses to exercise is increased accumulation of reactive oxygen species (ROS). Increased ROS have been proposed to affect the nitric oxide (NO)-mediated signaling and bioavailability in older subjects because of their binding affinity to NO (54). Taddei et al. (54) showed that treating older sedentary individuals with antioxidants restored the vasodilatory capacity of NO inhibited by NG-monomethyl-l-arginine; this suggests that the age-related endothelial dysfunction is at least in part caused by oxidative stress-induced reduction in NO bioavailability. Indeed, administration of antioxidants eliminated the Po2mv undershoot observed in older rats’ spinotrapezius muscle during a transition from rest to moderate-intensity exercise (33).
Age-related changes in capillary structure have been proposed to affect gas exchange between the capillary and the muscle fiber (9). However, recent data showed that the capillary structure is not compromised with age (38). In fact, the ratio of capillary-to-fiber surface contact to oxidative capacity has been shown to be substantially higher in older rats (32). As such, an O2 diffusion limitation in the old would not be explained by a reduced structural capacity for O2 transfer per se but rather depend on the flux and distribution of red blood cells (RBC) within the capillary bed. In this regard, older rats have a decreased lineal density of RBC-perfused capillaries lying adjacent to a fiber (which determines the potential for blood-myocyte O2 flux) and compensate for this, at least at rest, by increasing individual capillary RBC velocity and flux such that O2 delivery (as quantified by RBC·mm−1·s−1) is similar in both old and young (51). However, during electrically induced contractions older rats do not show the increased capillary RBC velocity and flux observed in young rats (10). These alterations in capillary hemodynamics in the older rats are likely to reduce the convective and diffusive transport of O2 to the myocyte.
Several studies have suggested that the locus of control for oxidative phosphorylation resides intracellularly (22, 23). At the onset of exercise, the rate of increase in V̇o2 is determined by the phosphocreatine shuttle attenuating the increase in ADP accumulation in the mitochondria (56). Additionally, NO production competing with O2 for the binding site of cytochrome-c oxidase has been proposed to play a role in regulating the rate at which V̇o2 adapts (35, 36). Substrate supply, in particular related to pyruvate dehydrogenase activity, has also been thought to be one of the mechanisms related to the rate of V̇o2 increase (24, 28, 29, 50). Although these intracellular factors (as cited above) may be the main ones controlling oxidative phosphorylation and eliciting a V̇o2 kinetics of less than ∼20 s, for those with slower V̇o2 kinetics the present data suggest that the matching of microvascular O2 delivery to the metabolic demand is a key factor determining or constraining the rate at which V̇o2p adjusts (i.e., τV̇o2). Nevertheless, we cannot eliminate the possibility that changes in mitochondrial oxidative capacity (with training) may alter phosphorylation and/or redox potential and thereby influence the driving of oxidative phosphorylation.
In conclusion, this study demonstrates that 3 wk of endurance training resulted in a significant decrease in τV̇o2p in both older and young adults, with no significant reductions in τV̇o2p seen during the subsequent 9 wk of training. Additionally, τV̇o2p measured after 3 wk of training in O was similar to that observed in Y before the start of training. Finally, an improved Δ[HHb]-to-ΔV̇o2p ratio (reflecting a better matching of O2 distribution within the tissues) was associated with the reduction in τV̇o2p observed in both O and Y. Although these data do not preclude that the basic control to V̇o2p kinetics resides within intracellular factors that were not measured in this study, it suggests that with “slower” V̇o2 kinetics the rate of adaptation of V̇o2 may be constrained by O2 availability related to the matching of microvascular O2 delivery to muscle V̇o2.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) research and equipment grants. Additional support was provided by Standard Life Assurance Company of Canada. J. M. Murias was supported by a doctoral research scholarship from the Canadian Institutes of Health Research (CIHR).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We express our gratitude to the subjects in this study and acknowledge the assistance provided by Brad Hansen, Matt Spencer, and Lisa Chin.
REFERENCES
- 1. Babcock MA, Paterson DH, Cunningham DA. Effects of aerobic endurance training on gas exchange kinetics of older men. Med Sci Sports Exerc 26: 447–452, 1994 [PubMed] [Google Scholar]
- 2. Babcock MA, Paterson DH, Cunningham DA, Dickinson JR. Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc 26: 440–446, 1994 [PubMed] [Google Scholar]
- 3. Beaver WL, Lamarra N, Wasserman K. Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51: 1662–1675, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146: 259–268, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bell C, Paterson DH, Kowalchuk JM, Cunningham DA. Oxygen uptake kinetics of older humans are slowed with age but are unaffected by hyperoxia. Exp Physiol 84: 747–759, 1999 [PubMed] [Google Scholar]
- 6. Bell C, Paterson DH, Kowalchuk JM, Moy AP, Thorp DB, Noble EG, Taylor AW, Cunningham DA. Determinants of oxygen uptake kinetics in older humans following single-limb endurance exercise training. Exp Physiol 86: 659–665, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Berger NJ, Tolfrey K, Williams AG, Jones AM. Influence of continuous and interval training on oxygen uptake on-kinetics. Med Sci Sports Exerc 38: 504–512, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Chilibeck PD, Paterson DH, Petrella RJ, Cunningham DA. The influence of age and cardiorespiratory fitness on kinetics of oxygen uptake. Can J Appl Physiol 21: 185–196, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47: B71–B76, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res 77: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev 23: 25–63, 1995 [PubMed] [Google Scholar]
- 12. DeLorey DS, Kowalchuk JM, Paterson DH. Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy-intensity exercise in young and older adults. J Appl Physiol 98: 1697–1704, 2005 [DOI] [PubMed] [Google Scholar]
- 13. DeLorey DS, Kowalchuk JM, Paterson DH. Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97: 165–172, 2004 [DOI] [PubMed] [Google Scholar]
- 14. DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 15. DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metab 32: 1251–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 16. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Kinetics of VO2 limb blood flow and regional muscle deoxygenation in young adults during moderate intensity, knee-extension exercise. Eur J Appl Physiol 108: 607–617, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Elwell C. A Practical Users Guide To Near-Infrared Spectroscopy. London: Hamamatsu Photonics, 1995, p. 1–155 [Google Scholar]
- 21. Fukuoka Y, Grassi B, Conti M, Guiducci D, Sutti M, Marconi C, Cerretelli P. Early effects of exercise training on on- and off-kinetics in 50-year-old subjects. Pflügers Arch 443: 690–697, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect V̇o2 on-kinetics in isolated in situ canine muscle. J Appl Physiol 85: 1394–1403, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect V̇o2 on-kinetics in isolated in situ canine muscle. J Appl Physiol 85: 1404–1412, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin-Teodosiu D, Gladden LB. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol 538: 195–207, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80: 988–998, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green HJ, Jones S, Ball-Burnett ME, Smith D, Livesey J, Farrance BW. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol 70: 2032–2038, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM. O2 uptake kinetics, pyruvate dehydrogenase activity, and muscle deoxygenation in young and older adults during the transition to moderate-intensity exercise. Am J Physiol Regul Integr Comp Physiol 294: R577–R584, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM. Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol 577: 985–996, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haram PM, Adams V, Kemi OJ, Brubakk AO, Hambrecht R, Ellingsen O, Wisloff U. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil 13: 585–591, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Human femoral artery and estimated muscle capillary blood flow kinetics following the onset of exercise. Exp Physiol 91: 661–671, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Hepple RT. Skeletal muscle: microcirculatory adaptation to metabolic demand. Med Sci Sports Exerc 32: 117–123, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Herspring KF, Ferreira LF, Copp SW, Snyder BS, Poole DC, Musch TI. Effects of antioxidants on contracting spinotrapezius muscle microvascular oxygenation and blood flow in aged rats. J Appl Physiol 105: 1889–1896, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Hughson RL, Tschakovsky ME, Houston ME. Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29: 129–133, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by l-NAME speeds phase II pulmonary VO2 kinetics in the transition to moderate-intensity exercise in man. J Physiol 552: 265–272, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol 132: 169–178, 2002 [DOI] [PubMed] [Google Scholar]
- 37. MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM. Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol 99: 1822–1834, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Mathieu-Costello O, Hepple RT. Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev 30: 80–84, 2002 [DOI] [PubMed] [Google Scholar]
- 39. McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol 81: 1331–1338, 1996 [DOI] [PubMed] [Google Scholar]
- 40. McKay BR, Paterson DH, Kowalchuk JM. Effect of short-term high-intensity interval training vs. continuous training on O2 uptake kinetics, muscle deoxygenation, and exercise performance. J Appl Physiol 107: 128–138, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation 13: 301–314, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol (January 7, 2010).10.1152/japplphysiol.01152.2009 [DOI] [PubMed] [Google Scholar]
- 44. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 45. O'Donovan G, Owen A, Bird SR, Kearney EM, Nevill AM, Jones DW, Woolf-May K. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol 98: 1619–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol 79: 1914–1920, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Poole DC, Barstow TJ, McDonough P, Jones AM. Control of oxygen uptake during exercise. Med Sci Sports Exerc 40: 462–474, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 518: 921–932, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ. Effects of dichloroacetate on V̇o2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol 95: 1105–1115, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 285: H251–H258, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol 86: 1101–1113, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Walsh B, Howlett RA, Stary CM, Kindig CA, Hogan MC. Determinants of oxidative phosphorylation onset kinetics in isolated myocytes. Med Sci Sports Exerc 37: 1551–1558, 2005 [DOI] [PubMed] [Google Scholar]