Abstract
The major objective of this review is to evaluate existing information and reach conclusions regarding whether there is interaction between Pco2/H+ stimulation of carotid (peripheral) and intracranial (central) chemoreceptors. Interaction is defined as a ventilatory response to simultaneous changes in the degree of Pco2/H+ stimulation of both chemoreceptors that is greater (hyperadditive) or less (hypoadditive) than the sum of the responses when stimulation of each set of chemoreceptors is individually altered. Simple summation of the simultaneous changes in stimuli results in no interaction (i.e., additive interaction). Knowledge of the nature of central/peripheral interaction is crucial for determining the physiological significance of newer models of ventilatory control based on recent neuroanatomic observations of the circuitry of key elements of the ventilatory control system. In this review, we will propose that these two sets of receptors are not functionally separate but rather that they are dependent on one another such that the sensitivity of the medullary chemoreceptors is critically determined by input from the peripheral chemoreceptors and possibly other breathing-related reflex afferents as well. The short format of this minireview demands that we be somewhat selective in developing our ideas. We will briefly discuss the limitations of experiments used to study CO2/H+ sensitivity and interaction to date, traditional views of the relative contributions of peripheral and central chemoreceptors to CO2/H+ sensitivity, the evidence for and against different types of interaction, and the effect of tonic carotid chemoreceptor afferent activity on central control mechanisms.
Keywords: carotid chemoreceptors, interaction, carbon dioxide, central chemoreceptors, breathing
EXPERIMENTAL LIMITATIONS OF CO2/H+ SENSITIVITY AND INTERACTION STUDIES
Anesthetized/Decerebrate Preparations
Despite many insights provided by studies of interaction in anesthetized and decerebrate preparations, it must be acknowledged that such preparations also have major limitations. Chief among these is that the use of anesthesia inevitably obtunds reflexes to some degree and also induces acidosis in plasma and cerebrospinal fluid (CSF) that could confound the observations (e.g., 27, 39). To avoid anesthesia, some studies utilized a decerebrate preparation, which can be problematic because the loss of suprapontine structures has been shown to produce a tachypneic hyperventilation in spontaneously breathing preparations (25). This effect is a result of increased neural activity in the ventilatory control system that would also increase the likelihood of neural saturation on a superimposed chemoreceptor stimulation (14). In both anesthetized and decerebrate preparations, the animals are often vagotomized, which may have influenced central responsiveness as a result of removal of an inhibitory influence of vagal stimulation on chemosensitive retrotrapezoid nucleus (RTN) neurons (37). Finally, in many of these preparations, the animals are inhaling a hyperoxic gas mixture in an attempt to chemically denervate the carotid chemoreceptors. However, paradoxically, hyperoxia stimulates ventilation centrally if inhaled for prolonged periods, which obviously would affect the data obtained (13).
Unanesthetized Preparations
To overcome the difficulties of reduced preparations, some investigators have turned to unanesthetized preparations using variations of three basic approaches.
1) Carotid body denervation.
It has long been assumed that denervation of the carotid bodies (CBD) simply eliminates afferent input from the carotid body chemoreceptors, unmasking pure central responses. Within the last decade evidence has accumulated to suggest that this is not the case. CBD has been shown to i) reduce glutamate turnover in the CSF of dogs and therefore probably reflects a reduction in neuronal glutamatergic activity (30); ii) affect the levels of cytochrome oxidase in the pre-Bötzinger complex in the rat (33); and iii) reduce the ventilatory sensitivity to focal acidosis at multiple sites in the medullary raphe (29). Moreover, it is well known that peripheral chemosensitivity can return over time following CBD in some species particularly neonates (55, 56) possibly due to upregulation of the afferent and/or efferent limbs of the reflex (2, 31, 36). However, in adult ponies (2) and goats (45) there was no or minimal recovery of peripheral chemosensitivity following CBD even though resting arterial Pco2 (PaCO2) had returned to normal. Ventilatory responses in CBD preparations are therefore time dependent, which can increase the uncertainty of any observations.
2) Temporal separation.
Some investigators have attempted to take advantage of the differences in response times of central and peripheral chemoreceptors to separate central from peripheral effects (7, 49, 58, 61). The major limitations of the temporal separation approach are i) the temporal uncertainty introduced by short-term potentiation (STP) of ventilation following a ventilatory stimulus; ii) the transient nature of the responses; and iii) the potential for temporal overlap between the responses of the two sites.
3) Independent control of central and peripheral stimuli.
While challenging technically, independent control of central and peripheral stimuli in an unanesthetized preparation perhaps offers the most physiological way to quantify central/peripheral interaction at the integrative level. The general approach has been to control the blood gas environment of the carotid body chemoreceptors by means of perfusion with blood from an extracorporeal gas exchanger in which Pco2, Po2, and pH were controlled by the investigators (e.g., 5, 59). The systemic circulation, and therefore the environment of central chemoreceptors, can be altered by means of changes in fractional inspired oxygen (FiO2) and/or carbon dioxide content (FiCO2). This technique requires unilateral CBD, which has been shown to have a transient effect on CO2 sensitivity and PaCO2 while breathing room air (43) but appears to have no effect on eupneic ventilation or CO2 sensitivity after a few days (6, 59), which is the time when experiments of this sort typically begin. Retrograde perfusion of the carotid sinus region raises local blood pressure slightly and eliminates breathing-related blood gas oscillations, but this has no effect ventilation if the mean perfusate blood gases and pH are matched to a given animal's eupneic values.
RELATIVE CONTRIBUTIONS OF PERIPHERAL AND CENTRAL CHEMORECEPTORS TO CO2-H+ SENSITIVITY
For many years, the prevailing view was that CO2/H+ sensitivity was due exclusively to the central chemoreceptors. The elegant studies in awake goats by Fencl, Pappenheimer, and colleagues (15, 46, 47) and studies using lesioning in the ventrolateral medulla (52, 53) lent strong support to this view. However, this view has changed as a result of recent studies that utilized different techniques such as transient CO2 administration, CBD, mathematical modeling, temporal separation, and independent control of central and peripheral stimuli. With these techniques the consistent finding has been that central chemoreceptors contribute about two-thirds of the ventilatory response to CO2/H+ while the carotid chemoreceptors contribute about one-third (18). Accordingly, despite limitations of each technique, the dominant current view is that both central and peripheral chemoreceptors contribute substantially to CO2/H+-mediated ventilatory responsiveness. Whether or not these two sets of chemoreceptors interact with each other, however, is still controversial.
Evidence For and Against Interaction: Studies in Anesthetized/Decerebrate Preparations
Additive interaction (no interaction).
Heeringa et al. (26) cannulated the vertebral arteries of anesthetized cats and used an extracorporeal circuit to perfuse the pons and medulla separately from the systemic circulation (including the peripheral chemoreceptors). They measured the ventilatory response to changes in PaCO2 (2–8% inspired CO2) at three different constant levels of central Pco2. All three peripheral CO2 response curves were linear and there was no difference in their slopes, but the curves were shifted progressively to higher ventilations as the central Pco2 was increased. The central CO2 response curve was also linear, and its slope was twice that of the peripheral slope. CBD did not affect the central sensitivity to Pco2, but it eliminated peripheral sensitivity. The authors concluded that the central and peripheral chemoreceptors contributed two-thirds and one-third, respectively, to overall CO2 sensitivity and that “interaction between central and peripheral chemical stimuli are negligible.” This type of simple additive effect of respiratory stimuli has also been observed between muscle afferents and central chemoreceptors (35) and between the effect of cold block of “area S” in the medulla and peripheral chemoreceptor stimuli (54).
Hypoadditive interaction.
In contrast to the above, data from other isolated perfusion studies suggest hypoaddition of central and peripheral chemoreceptor activity. In anesthetized cats, Giese et al. (20) denervated one carotid complex and used an extracorporeal circuit to isolate the perfusion of the other complex from the systemic and thus central circulation. The major finding was that the ventilatory response to hypoxia in the carotid circulation was decreased when PaCO2 (and thus central Pco2) was increased from 35 to 65 mmHg. Similarly, Riedstra (48) cannulated a vertebral artery of anesthetized cats to isolate perfusion of the brain from the systemic and thus carotid perfusion. He found that increasing central Pco2 shifted the ventilation-PaO2 response relationship upward but the slope of the response to hypoxia was decreased as the central Pco2 was increased.
Data from other studies also suggest hypoaddition between central and peripheral chemoreceptor activity. In anesthetized cats, Berger et al. (1) perfused the ventral surface of the brain stem with different levels of [H+] in artificial CSF. They found the ventilatory response to stimulation of the carotid chemoreceptors by intra-arterial injections of NaCN was greater at an alkaline than at an acid CSF [H+]. Hypoaddition was also suggested by findings of Kiwull et al. (32) and Eldridge et al. (14), who found that the phrenic nerve activity or ventilatory responses to peripheral chemoreceptor stimulation elicited by electrical stimulation of the sinus nerve became progressively smaller as central Pco2 stimulation was progressively increased. Eldridge, however, did not think his observations were due to a specific interaction but rather to a general property of a neural component of the central pathway. Additional apparent demonstrations of hypoaddition were obtained by Kiwull et al. (32) and Gesell et al. (19) in anesthetized rabbits and dogs, respectively. Both groups found that the reduction in ventilation after cold block of sinus nerve activity decreased as central CO2 was increased. Conversely, when central CO2 was low, then cold block of the sinus had a large effect, leading to the conclusion that normally the peripheral “chemoreceptors exert an important tonic stimulation of breathing.” The idea of hypoaddition has received recent support from Day and Wilson (11, 12). These authors used a sophisticated dual perfusion decerebrate rat preparation to control the environments of the carotid body chemoreceptors and central chemoreceptors independently and observed smaller responses in phrenic output as the Pco2 of the central chemoreceptors was increased.
Hyperadditive interaction.
Hyperaddition of peripheral and central chemoreceptor activity has also been observed. Loeschcke et al. (34) electrically stimulated the carotid sinus nerve in anesthetized cats. He found that the tidal volume response to increased voltage of stimulation increased as end-tidal Pco2 (PetCO2) was increased and also when he increased [H+] in the subarachnoid space of the medulla. Loeschcke speculated that “the interaction of CO2 with sinus nerve impulses could then be a summation in a group of synapses of the impulses originating from this receptor with the impulses running in the sinus nerve. This summation would resemble the facilitation observed in the spinal cord as described by Sherrington, in which submaximal stimulation of two afferent nerves produces a total response that is greater than the sum of their individual effects.”
In summary, the bulk of the evidence in anesthetized/decerebrate preparations supports the concept of hypoaddition although there is enough evidence in support of hyperaddition or simple additive interaction to prevent consensus.
Evidence For and Against Interaction: Studies in Unanesthetized Preparations
The controversy remains even if one examines only data from intact, unanesthetized preparations. Additive (i.e., no) interaction has been observed in humans (8, 9, 61) utilizing techniques that relied on temporal separation of the central and peripheral chemoreceptors. Additive interaction has also been observed in the goat utilizing reversible isolation and perfusion of the carotid body chemoreceptors to isolate the peripheral and central stimuli (10). Hyperadditive interaction has been observed in one study in humans again using techniques that relied on temporal separation of the central and peripheral chemoreceptors (49). Consistent with the concept of hyperaddition are observations from the unanesthetized dog with a reversibly isolated and perfused carotid body in which specific, maximal physiological inhibition of the carotid body resulted in a marked inhibition of ventilation that persisted throughout the steady state (many minutes), suggesting that the gain of the central chemoreceptors had been reduced since they appeared not to compensate fully for the persistent respiratory acidosis (4, 59). Hypoadditive interaction has been observed in goats using perfusion of artificial CSF into the cisterna magna to alter the acid-base environment of the medullary chemoreceptors while stimulating the carotid body chemoreceptors with boluses of sodium cyanide (57). Again, comparisons among studies are difficult due to the diverse experimental approaches used.
DO CAROTID BODY CHEMORECEPTORS MODULATE THE CENTRAL SENSITIVITY TO CO2/H+?
The studies summarized above do not provide conclusive evidence on the nature of interaction between central and peripheral chemoreceptors. Although perhaps not generally appreciated, the existence of other-than-additive central/peripheral interactions demands modulation of one set of chemoreceptors by the other, or possibly even mutual modulation (13, 14, 34). As discussed below, the collective evidence from several CBD studies and studies using isolation and perfusion of the carotid bodies strongly suggests that the carotid body chemoreceptors modulate the ventilatory response to changes in central Pco2/H+, and also contribute substantially to the eupneic drive to breathe.
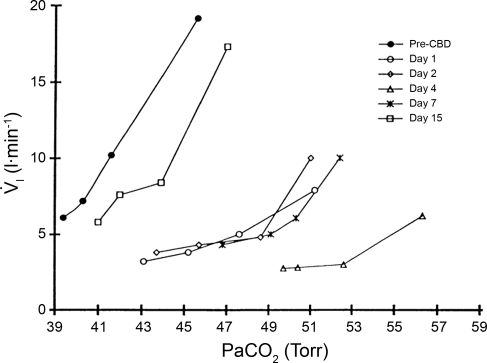
Over the first few days after CBD in goats, there is a decrease in eupneic breathing, an increase in PaCO2 during eupnea, and a decrease in CO2 sensitivity (Fig. 1 and Ref 45). The reduced CO2 sensitivity must reflect in part a decrease in CO2 sensitivity centrally. As shown in Fig. 1, before CBD, increasing PaCO2 by inhaling in order 3, 5, and 7% inspired CO2 increased PaCO2 from 39 to 46 mmHg, which increased inspired pulmonary ventilation (V̇i) from 6 to 20 l/min. However, 4 days after CBD, V̇i was 2–3 l/min below pre-CBD, and PaCO2 was 10 mmHg above pre-CBD. Not shown are data indicating that after CBD, hypercapnia and an acidosis also exist centrally (3). Accordingly, the loss of tonic carotid chemoreceptor afferent input must have reduced the sensitivity or gain of the central chemoreceptors or else the elevated Pco2 would have created a marked hyperpnea. Rodman et al. (50) made similar observations in the dog in response to hyperoxic hypercapnia before and after CBD. Indeed this change has been shown directly in the CBD model by findings in awake goats that the V̇i increase in response to focal acidosis (via microdialysis) within the medullary raphe was reduced by 50% post- vs. pre-CBD (29). A concern here is whether this reduction in central gain is representative of ventilatory responses when carotid chemoafferents are intact (see experimental limitations of co2/h+ sensitivity and interaction studies).
Fig. 1.
Goats hypoventilate and have reduced sensitivity to CO2 for at least 7 days after carotid body denervation (CBD), but thereafter, there is a normalization of breathing. Depicted are data from a single, awake goat and on each day data were obtained while breathing room air (initial point) and while inhaling in order 3, 5, and 7% CO2 gas mixtures. Note the reduced ventilation and increased arterial Pco2 (PaCO2) in eupnea and the reduced ventilatory response slope over the first 7 days after CBD. Data are from Ref. 45. V̇i, inspired pulmonary ventilation.
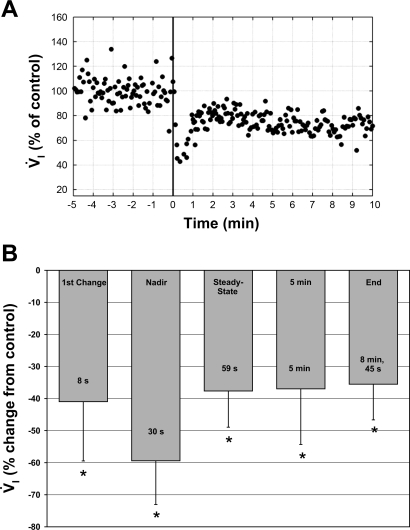
Some very recent data from Blain et al. (4) in the unanesthetized dog have attempted to address this question. In awake dogs, perfusion of the isolated carotid body chemoreceptors with hypocapnic and hyperoxic blood caused, within 30 s, a 60% decrease in V̇i and a 10 mmHg increase in PaCO2. Subsequently, within 1 min, V̇i rebounded ∼1 l/min to ∼70% of control (Fig. 2). Assuming this compensation of V̇i was secondary to hypercapnia at the central chemoreceptors, central CO2 sensitivity was then 100 ml·min−1·mmHg−1. In this preparation, the carotid hyperoxia and hypocapnia presumably attenuated tonic carotid chemoreceptor input to the respiratory control network. In a previous study from this laboratory (58) using similar techniques, the isolated carotid body was maintained normocapnic and normoxic; thus tonic activity was presumably normal when central CO2 sensitivity was assessed and found to be 400 ml·min−1·mmHg−1. Thus central CO2 sensitivity was reduced to 25% of normal by attenuating tonic carotid activity. Although these data are from two different studies using two different groups of dogs and, therefore, somewhat indirect, they support the idea that carotid body chemoreceptor afferents can modulate the sensitivity of the central chemoreceptor.
Fig. 2.
A: breath-by-breath V̇i from one trial of carotid body (CB) chemoreceptor inhibition via hyperoxia and hypocapnia. Inhibition begins at time 0 (vertical line). Note the initial 60% decrease in ventilation followed by only partial compensation to the hypoventilation-induced respiratory acidosis. B: bar graph of the time course of ventilation during CB chemoreceptor inhibition. Data are normalized to control (where control is normal, endogenous CB perfusion, i.e., CB not inhibited) and presented as means ± SD. Control is arbitrarily set to zero to more clearly indicate the direction of change. “1st Change” represents the first V̇i response > 3 SDs below the control mean. “Nadir” represents the 3-breath mean consisting of the breath with the lowest V̇i and the preceding and subsequent breaths. “Steady-state” represents the mean values of the first 3 consecutive breaths within 1 SD of the mean V̇i from the last 30 s of the experiment (> 7 min of perfusion). “5 min” represents the mean ventilatory values of the last 30 s of the 5th minute of CB inhibition. “End” represents the mean ventilatory values from the last 30 s of each experiment regardless of duration. Times in the bottom of each bar denote the average time for that response. Note that the nadir ventilatory response to CB inhibition averaged 60% below control and the maintained steady-state response was 38% below control. *Significant difference from control values, P < 0.05. Data are from Ref. 4.
There are at least three brain stem regions whereby carotid afferents may affect central chemosensitivity. One could be interaction within the nucleus tractus solitarius (NTS), which is the site of the first central synapse for carotid afferents and which is also a site of CO2/H+ chemoreceptor neurons (40). Interaction within the NTS may then result in augumented signaling to other parts of the respiratory network. A second proposed mechanism is based on neuroanatomic and neurophysiological evidence from reduced preparations indicating that carotid body chemoreceptor inputs and possibly many other ventilatory-related reflex inputs converge primarily on a putative chemosensitive/integrating region in the parafacial respiratory group (pFRG)/RTN. The pFRG/RTN is characterized by chemosensitive glutamatergic interneurons that strongly express Phox2b (21–24, 37, 38, 51, 60, 62, 63). These Phox2b neurons have been shown to mark “an uninterrupted chain of sensors and neurons involved in the integration of peripheral and central chemoreception. This circuit includes the carotid bodies, chemoreceptor afferents, chemoresponsive NTS projections to the ventrolateral medulla (VLM), and pFRG/RTN central chemoreceptors” (62). Guyenet et al. (21) have shown that the activity of these chemosensitive neurons can be altered by changes in carotid chemoreceptor activity and by altered input from hypothalamic “central command” neurons. They postulate 1) that pFRG/RTN neurons integrate information from these multiple sources; and 2) that normal neuronal activity of these neurons is required for normal breathing awake and during sleep and also for normal responses to hypercapnia, hypoxia, and exercise. Supportive of this concept are data showing that cooling-induced neuronal dysfunction of this VLM area in awake goats uniformly decreases breathing during eupnea, hypoxia, hypercapnia, and exercise and this depressant effect is uniformly exaggerated by CBD (16, 17, 42–44). A third proposed mechanism for carotid/central chemoreceptor interaction is based on studies in decerebrate, vagotomized cats using multiarray technology and spike train analysis to identify functional connectivity among neurons in the ventral respiratory column (VRC), dorsal raphe, and pontine respiratory group (PRG) (41). Data were obtained during sequential activation of either carotid or central (raphe, 28) chemoreceptors via injection of CO2-saturated saline into either the carotid or vertebral arteries, respectively. Stimulation of each set of chemoreceptors increased activity of some VRC, raphe, and PRG respiratory neurons. Some raphe neurons stimulated by carotid activation were functionally inhibited by central chemoreceptor stimulation, whereas other raphe neurons were stimulated by activation of both carotid and central chemoreceptors. Correlations between neural spike trains indicated paucisynaptic interactions among neurons within the VRC, raphe, PRG network. The findings suggest “interactions that differentially promote and limit interactions” (41) between the two sets of chemoreceptors. The authors concluded the results “support the hypothesis that midline brain stem neurons are connected to the PRG and VRC in ways appropriate for shaping the physiological responses evoked by both types of chemoreceptors” (41).
In summary the collective evidence from studies in unanesthetized mammals strongly suggests that tonic carotid activity affects the centrally mediated ventilatory response to hypercapnia. This effect might be mediated through interactions within the NTS and/or through NTS projections to pFRG/RTN integrating neurons and/or through projections to neurons of a raphe-pontomedullary respiratory network. It is difficult to reconcile the findings in unanesthetized preparations with those of anesthetized preparations in which the preponderant finding is hypoaddition. We suggest that this qualitative difference is most likely explained by the limitations imposed by anesthesia and/or decerebration discussed above.
IMPLICATIONS
If the physiological significance of the neural circuitry of the NTS, pFRG/RTN, and/or raphe-pontomedullary respiratory network are confirmed it will change the way physiologists think about the interplay between central and peripheral chemical stimuli in fundamental ways. If the carotid body chemoreceptors can effectively and rapidly modulate the gain of the central chemoreceptors/integrators then it is no longer useful to think of the central and peripheral chemoreceptors as independent sensors each contributing some fraction of the ventilatory response to CO2/H+. Rapid modulation of the gain of the central chemoreceptors could be of particular importance in understanding ventilatory control in the face of transient stimuli such as in sleep apnea or in conditions in which carotid body chemoreceptor activity is known to be upregulated such as heart failure or chronic hypoxia.
GRANTS
Work cited from the Forster laboratory was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-25739 and by the Department of Veterans Affairs. Work cited from the Smith laboratory was supported by NHLBI Grants HL-50531 and HL-15469 and the American Heart Association.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Berger W, Berger K, Berndt J, Giese K. Interaction of peripheral and central respiratory drives in cats. I. Effects of sodium cyanide as a peripheral chemoreceptor stimulus at different levels of CSF pH. Pflügers Arch 374: 205–210, 1978 [DOI] [PubMed] [Google Scholar]
- 2. Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol 49: 964–970, 1980 [DOI] [PubMed] [Google Scholar]
- 3. Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J Appl Physiol 40: 184–190, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol 106: 1564–1573, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busch MA, Bisgard GE, Forster HV. Ventilatory acclimatization to hypoxia is not dependent on arterial hypoxemia. J Appl Physiol 58: 1874–1880, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Busch MA, Bisgard GE, Mesina JE, Forster HV. The effects of unilateral carotid body excision on ventilatory control in goats. Respir Physiol 54: 353–361, 1983 [DOI] [PubMed] [Google Scholar]
- 7. Carroll JL, Canet E, Bureau MA. Dynamic ventilatory responses to CO2 in the awake lamb: role of the carotid chemoreceptors. J Appl Physiol 71: 2198–2205, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Clement ID, Bascom DA, Robbins PA. An assessment of central-peripheral ventilatory chemoreflex interaction in humans. Respir Physiol 88: 87–100, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Clement ID, Pandit JJ, Bascom DA, Dorrington KL, O'Connor DF, Robbins PA. An assessment of central-peripheral ventilatory chemoreflex interaction using acid and bicarbonate infusions in humans. J Physiol 485: 561–570, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daristotle L, Bisgard GE. Central-peripheral chemoreceptor ventilatory interaction in awake goats. Respir Physiol 76: 383–391, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Day TA, Wilson RJ. Brainstem Pco2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol 578: 843–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Day TA, Wilson RJA. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol 587: 883–896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dean JB, Mulkey DK, Henderson TA, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species and hyperventilation: oxygen sensitivity of brainstem neurons. J Appl Physiol 96: 784–791, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol 311: 81–95, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol 210: 459–472, 1966 [DOI] [PubMed] [Google Scholar]
- 16. Forster HV, Ohtake PJ, Pan LG, Lowry TF. Effect on breathing of surface ventrolateral medullary cooling in awake, anesthetized and asleep goats. Respir Physiol 110: 187–197, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Forster HV, Ohtake PJ, Pan LG, Lowry TF, Korducki MJ, Aaron EA, Forster AL. Effects on breathing of ventrolateral medullary cooling in awake goats. J Appl Physiol 78: 258–265, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity, and PaCO2 during eupneic breathing. Adv Exp Med Biol 905: 322–326, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Gesell R, Lapides J, Levin M. The interaction of central and peripheral chemical control of breathing. Am J Physiol 130: 155–170, 1940 [Google Scholar]
- 20. Giese K, Berndt J, Berger W. Interaction of central and peripheral respiratory drives in cats. II. Peripheral and central interaction of hypoxia and hypercapnia. Pflügers Arch 374: 211–217, 1978 [DOI] [PubMed] [Google Scholar]
- 21. Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL, Moreira TS, Takakura AT. The retrotrapezoid nucleus and central chemoreception. Adv Exp Med Biol 605: 327–332, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90: 247–253, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Hayashi F, Sinclair JD. Respiratory patterns in anesthetised rats before and after anemic decerebration. Respir Physiol 84: 61–76, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Heeringa J, Berkenbosch A, de Goede J, Olievier CN. Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respir Physiol 37: 365–379, 1979 [DOI] [PubMed] [Google Scholar]
- 27. Heeringa J, de Goede J, Berkenbosch A, Olievier CN. Influence of the depth of anaesthesia on the peripheral and central ventilatory CO2 sensitivity during hyperoxia. Respir Physiol 41: 333–347, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97: 2303–2309, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol 98: 1234–1242, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Hoop B, Masjedi M, Shih VE, Kazemi H. Brain glutamate metabolism during hypoxia and peripheral chemodenervation. J Appl Physiol 69: 147–154, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiwull PH, Kiwull-Schone S, Klatt W. Interaction of central and peripheral respiratory drives: differentiation between the role of stimuli and afferents. In: Acid-Base Homeostasis of the Brain Extracellular Fluid and Respiratory Control System, edited by Loeschcke HH. Stuttgart, Germany: Thieme, 1976, p. 146–156 [Google Scholar]
- 33. Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MT. Carotid body denervation effect on cytochrome oxidase activity in pre-Bötzinger complex of developing rats. J Appl Physiol 94: 1115–1121, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF, Jr, Konig A. Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centers. Ann NY Acad Sci 109: 651–660, 1963 [DOI] [PubMed] [Google Scholar]
- 35. Millhorn DE, Eldridge FL, Waldrop TG. Effects of medullary area I cooling on respiratory response to muscle stimulation. Respir Physiol 49: 41–48, 1982 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell GS, Bach KB, Martin PA, Foley KT, Olson EB, Brownfield MS, Miletic V, Behan M, McGuirk S, Sloan HE. Increased spinal monoamine concentrations after chronic thoracic dorsal rhizotomy in goats. J Appl Physiol 89: 1266–1274, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol 580: 285–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Musch TI, Pelligrino A, Dempsey JA. Effects of prolonged N2O and barbiturate anesthesia on brain metabolism and pH in the dog. Respir Physiol 39: 121–131, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296: R763–R773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nuding SC, Segers LS, Shannon R, O'Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364: 2501–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohtake PJ, Forster HV, Pan LG, Lowry TF, Korducki MJ, Aaron EA, Weiss EM. Ventilatory responses to cooling the ventrolateral medullary surface of awake and anesthetized goats. J Appl Physiol 78: 247–257, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Ohtake PJ, Forster HV, Pan LG, Lowry TF, Korducki MJ, Smith K, Forster AL. Effect on breathing of neuronal dysfunction in the caudal ventral medulla of goats. J Appl Physiol 79: 1586–1594, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Pan LG, Forster HV, Ohtake PJ, Lowry TF, Korducki MJ, Forster AL. Effect of carotid chemoreceptor denervation on breathing during ventrolateral medullary cooling in goats. J Appl Physiol 79: 1120–1128, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Pan LG, Forster HV, Martinao P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol 85: 1299–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Pappenheimer JR. The ionic composition of cerebral extracellular fluid and its relation to control of breathing. Harvey Lect 6: 71–93, 1967 [PubMed] [Google Scholar]
- 47. Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol 208: 436–450, 1965 [DOI] [PubMed] [Google Scholar]
- 48. Riedstra JW. Influence of central and peripheral Pco2 (pH) on the ventilatory response to hypoxic chemoreceptor stimulation.. Acta Physiol Pharmacol Neerl 12: 407–452, 1963 [PubMed] [Google Scholar]
- 49. Robbins PA. Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man. J Physiol 401: 503–518, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol 91: 328–335, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Schlaefke ME, Kille JF, Loeschcke HH. Elimination of central chemosensitivity by coagulation of a bilateral area on the ventral medullary surface in awake cats. Pflügers Arch 378: 231–241, 1979 [DOI] [PubMed] [Google Scholar]
- 53. Schlaefke ME, Loeschcke HH. Lokalisation an der Regulation von Atmung und Kreislauf beteiligten Gebietes an der ventralen Oberfläche de Medulla oblongata durch Kälteblockade. Pflügers Arch 297: 201–220, 1967 [Google Scholar]
- 54. Schlaefke ME, See WR, Herker-See A, Loeschcke HH. Respiratory response to hypoxia and hypercapnia after elimination of central chemosensitivity. Pflügers Arch 381: 241–248, 1979 [DOI] [PubMed] [Google Scholar]
- 55. Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol 91: 1298–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R, Forster HV. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J Appl Physiol 92: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Smith CA, Jameson LC, Mitchell GS, Musch TI, Dempsey JA. Central-peripheral chemoreceptor interaction in awake cerebrospinal fluid-perfused goats. J Appl Physiol 56: 1541–1549, 1984 [DOI] [PubMed] [Google Scholar]
- 58. Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100: 13–19, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Smith CA, Saupe KW, Henderson KS, Dempsey JA. Ventilatory effects of specific carotid body hypocapnia in dogs during wakefulness and sleep. J Appl Physiol 79: 689–699, 1995 [DOI] [PubMed] [Google Scholar]
- 60. Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989 [DOI] [PubMed] [Google Scholar]
- 61. St Croix CM, Cunningham DA, Paterson DH. Nature of the interaction between central and peripheral chemoreceptor drives in human subjects. Can J Physiol Pharmacol 74: 640–646, 1996 [DOI] [PubMed] [Google Scholar]
- 62. Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]