Abstract
Developing clinical work suggests that vascular stiffening plays a role in the progression of pulmonary hypertension (PH), while recent studies in animal models of hypoxic PH have found significant proximal vascular stiffening in the diseased population. Here, we develop and validate a minimally invasive, clinically realizable method to estimate the local elastic modulus of the proximal pulmonary arteries from pressure-diameter (PD) data. PD measurements were made in the main pulmonary arteries of 16 calves; lumen diameter was assessed using color M-mode tissue Doppler imaging ultrasound, while pressure was measured via catheter. Two methods corresponding to thin-walled pressure vessel theory (“thin”) and Lame's equation for a thick-walled cylinder (“thick”) were used to approximate the artery elastic modulus from PD measurements. The harvested arteries were tested independently to determine their “true” ex vivo elastic modulus and stiffness. Both approximations displayed excellent correlation with ex vivo elastic modulus of the calf main pulmonary artery (thin r2 = 0.811; thick r2 = 0.844; both P < 0.01). Bland-Altman analysis indicated that the thick-walled approximation has better overall agreement with ex vivo modulus. The approximations displayed quantitatively distinct regression slopes that were statistically different (P = 0.02). The elastic modulus of the main pulmonary artery can be reasonably estimated from combined color M-mode tissue Doppler imaging ultrasound and catheter pressure measurements in calves. Such measurements may be a valuable tool in the diagnosis and treatment of human PH.
Keywords: pulmonary hypertension, hypoxia, animal model, pulmonary vascular stiffness
pulmonary hypertension (PH) has been historically associated with only distal events, such as sustained vasoconstriction, occlusive vascular remodeling, and in situ thrombosis. These pathologies can result in increased pulmonary vascular resistance (PVR), a physiological parameter that is the principal diagnostic for PH in the clinical setting (3). PVR is traditionally thought to be the principal determinant of right ventricular (RV) afterload that, when chronically increased, can lead to right heart failure. However, recent evidence suggests that, despite its relative importance in PH diagnosis, PVR is only a modestly useful prognostic tool (2, 28). PVR quantifies only the static component of pulmonary hemodynamics to the neglect of the dynamic component, described by pulmonary vascular stiffness (PVS). PVS refers to an elastic pulmonary artery's innate resistance to deformation under a pressure load: it can change with the dimensions of vessel or due to changes in the vessel's inherent material properties.
Several recent clinical studies have suggested that global PVS is a better prognostic than PVR alone, in that elevations in PVS are associated with increased adult mortality (14, 29) and poorer pediatric outcomes (18). This association between vascular stiffening and disease severity is believed to be due to several factors: 1) stiffening reduces the windkessel effect of the elastic arteries, leading to greater flow inefficiency (33, 34); 2) stiffening increases RV afterload independent of PVR (30, 42); and 3) changes in the flow and pressure waveforms due to stiffening in both the systemic (39, 45) and pulmonary circulations (26, 27) have been shown to stimulate cellular signaling pathways in distal vessels and the lungs that exacerbate and perpetuate existent vascular pathologies.
Despite this increased appreciation that vascular stiffness plays an important role in vascular pathology, the contribution of stiffening of the great arteries [specifically the main (MPA), right, and left pulmonary arteries] to the pathophysiology of PH is not fully understood. The great arteries were recently found to chronically increase in stiffness in PH due predominantly to elastin-based extracellular matrix remodeling in an ex vivo study of severe PH in the neonatal calf (22). It has also been found that the stiffness of the proximal pulmonary vasculature of the mouse model increases in response to chronic hypoxia, with similar mechanical consequences (20, 21). It remains unclear whether significant proximal remodeling in the human produces similar mechanical consequences, and whether such stiffening is important to disease outcomes and/or pathogenesis.
To answer clinical questions about proximal stiffening, we sought to assess it in a minimally invasive way in a large animal model of severe PH (36). While the most comprehensive means of measuring mechanical properties, such as vascular stiffness, or elastic modulus, which quantifies only the inherent material properties, involves in vitro uniaxial or multiaxial stress-strain testing (10–12, 22, 44), such a method is obviously not possible for serial monitoring or clinical studies. Methods do exist, however, to approximate mechanical properties from the in vivo response of an artery distending during systole; this response is measured by the artery's pressure-diameter (PD) curve. From PD curves and an estimate of thickness, previous work has obtained estimates of stiffness and elastic modulus (16, 23, 31), incremental elastic modulus (7), distensibility (1, 4, 24, 35), and compliance (43).
The novelty of our approach here is in the use of noninvasive ultrasound color M-mode tissue Doppler imaging (CMM-TDI) to accurately obtain instantaneous diameter of the pulmonary artery during cardiac catheterization (8, 13, 25); combining these two measurements, echo-assessed diameter and catheter-assessed pressure, the possibility of routinely obtaining modulus in a clinical setting emerges. From a structural mechanics viewpoint, the modulus of the arterial wall should be a useful parameter because it reflects basic material properties. Given the link between stiffness quantification (with pulmonary vascular input impedance) and improved prognostication (18), we speculate that this measure could provide similar prognostic benefits. In this study, we 1) develop two approximations to compute incremental elastic modulus from PD data; 2) apply these to obtain PD data in the chronically hypoxic neonatal calf model; and 3) compare the in vivo elastic modulus approximations thus obtained to direct (ex vivo) measures of tissue stiffness to assess the method's accuracy.
METHODS
Equations to compute elastic modulus.
Equations for the approximation of elastic modulus from PD data are well known (10, 15); here, we briefly review our implementation specific for use with ultrasound data, which provides lumen diameter. The typical simplifying assumptions for use of these approximations are isotropy, indicating no variation in mechanical properties with direction; homogeneity, indicating similar material throughout the arterial wall; and local linearity in mechanical behavior, which assumes that the stress is a linear function of the strain for strain ranges of interest. In addition, for our echo-based acquisition of diameter, we must assume the thickness of the arterial wall (as a percentage of the arterial diameter); this will be addressed in greater detail below.
Thick-walled plane strain modulus.
The thick-walled plane-strain model is based on the two-dimensional plane strain approximation for a thick-walled elastic tube, which allows for variation in stress across the thickness and is appropriate when an artery's walls are greater than 10% of its diameter. The geometry of the tube cross section is described by thickness h, diastolic inner and outer diameters DID and DOD, respectively, and the instantaneous incremental transmural pressure PI. The instantaneous inner diameter at any time is referred to as DI. Simplification of this model's stress equilibrium equation provides a linear relationship between the incremental pressure and instantaneous diameter of the form DI = m PI + DID, in which m is a function (40) of the elastic modulus and the DID and DOD. Because the parameter m also corresponds to the slope of the incremental PD curve, we may equate this slope (obtained from experimental measurements) to the function and solve for the modulus,
| (1) |
where ETWPS is thick-walled plane strain modulus, and υ is Poisson's ratio (set to 0.45 to model the nearly incompressible nature of the artery wall). Because the diastolic diameters are constants in this expression for each PD curve, and the slope m may be extracted from linear regression of the PD data, ETWPS is readily obtained from Eq. 1.
Pressure-strain modulus.
The modulus Ep = (PS − PD)DID/(DIS − DID) has been used in prior studies of arterial mechanics (10, 15, 23, 31), where PS and PD are systolic and diastolic pressures (their difference being the pulse pressure), and DIS and DID are the artery inner diameter at systole and diastole, respectively. Applied to the outer diameter, it is known as the Peterson modulus (31). Ep is not a true elastic modulus (it lacks thickness information and is thus a ratio of pressure to strain rather than stress to strain) and is not investigated herein; however, an especially simple elastic modulus approximation based on Ep can be calculated as follows. The thin-walled model circumferential stress is defined as σθ = PID/2h; this yields an incremental change between systole and diastole of σθ = (PS − PD)DID/2h, while the same incremental change in the circumferential strain is εθ = (DIS − DID)/DID. We thus define the pressure-strain elastic modulus (EPS) as:
| (2) |
EPS is thus understood as Ep scaled by the wall-thickness-normalized diameter.
Ultrasound-based acquisition of diameter.
Institutional Animal Care and Use Committee approval was obtained for all animal studies. Sixteen newborn male dairy calves (Holstein), weighing between 35 and 50 kg, were used. Nine of the calves were put into a large hypobaric chamber at an atmospheric pressure of 445 mmHg, equivalent to an elevation of 4,300 m, between 1 and 3 days of age. These calves were maintained at this simulated altitude for 2 wk and developed hypoxic PH; the remaining animals remained at local ambient pressure (630 mmHg; Ft. Collins, CO) while awaiting measurement. Daily care for all animals proceeded as described previously (36) under the supervision of large-animal veterinarians. Both animal groups were at ambient pressure for at least 1 h before testing and underwent testing at ambient pressure to minimize the potential for residual hypoxic proximal vasoconstriction.
To begin each study, a calf was placed on its right side on an animal examination table. The animal's eyes were covered during the entire procedure to induce calm, and sedation was not required. A solid-state (Millar SPC-350) catheter was inserted percutaneously into the jugular vein to access the RV and MPA; placement was continuously assessed via evaluation of the pressure waveform. Pressure was recorded digitally by a laptop-computer-based data-acquisition system (LabView, National Instruments, Austin, TX) and by a commercial ultrasound machine through its PHYSIO port (Vivid 5, GE Medical Systems, Waukesha, WI). To allow echo measurements, a 15 × 15-cm patch was shaved on the right side of the chest, between the third and fifth intercostal spaces; a 3.5-MHz FPA probe accessed the animal through a hole in the underside of the examination table. A caudal, short-axis view at the fourth intercostal space, 3–6 cm dorsal to the elbow, was used to obtain CMM-TDI measurements of instantaneous diameter and velocity of both walls of the MPA; before acquisition, the ultrasound beam was swept through the long axis of the MPA to determine maximal diameter. Both pressure and ECG time histories were recorded with the echo images to ease offline processing.
Echo image processing.
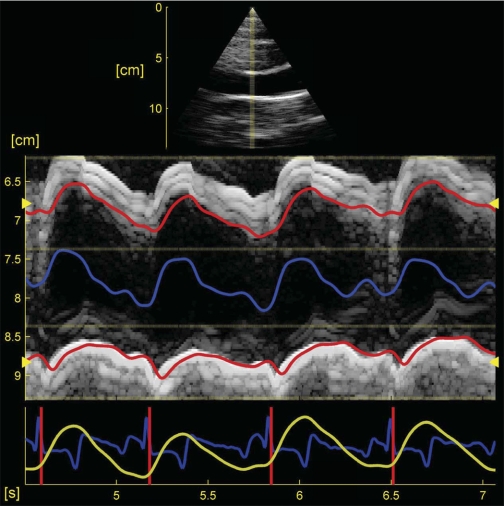
Analysis of the combined CMM-TDI data pressure was performed offline using custom-written MATLAB V7.7 analysis software (The Mathworks, Natick, MA; code based on EchoMAT version 2.1, GE Medical Systems, Waukesha, WI). The program displays the data visually; an example screenshot is shown in Fig. 1 and displays the two-dimensional echocardiogram (top plot) and M-mode image from a normoxic calf (the TDI data is not shown), as well as the electrocardiogram trace and pressure time history (blue and yellow traces, respectively, bottom plot). The program uses an image decimation-based method [described in detail elsewhere (17, 18)] to initially find the upper and lower wall boundaries as seen in the M-mode image. After this first pass, the code then time integrates the velocity data (obtained from the TDI image) along the initial trace to obtain an improved, final wall definition. In this way, both the M-mode and TDI data are incorporated into calculation of the final trace, while the integration yields a smoother wall definition than would M-mode data alone. The typical results for the upper and lower boundary are shown in Fig. 1 as solid red lines, whereas the computed diameter is shown in blue floating between the two wall boundaries (not to scale).
Fig. 1.
Software interface for the processing of in vivo color M-mode tissue Doppler images of wall motion. The brightness-mode image appears as a triangular sector at the top of the image, M-mode data is at the center, while pressure (yellow) and ECG (blue) traces appear at the bottom. Also seen in the M-mode image are upper and lower main pulmonary artery wall definitions (red lines) and superimposed diameter (blue line).
After wall detection is complete, the PD data for each cardiac cycle are separated using the ECG trace and stored for analysis. PD extrema and linear regression slopes of the PD and stress-strain curves are computed for the end-diastole to end-systole portion of each cycle. During postprocessing, we found that using only this portion of the curve (corresponding to loading) was both consistent with our ex vivo protocol (see Ref. 22) and more reproducible cycle to cycle compared with using the whole cycle. Means of the PD extrema and the error-weighted averages (9) of the PD and stress-strain regression slopes are then found and used to compute the reported values of in vivo elastic modulus.
Calculation of in vivo wall thickness.
While the lumen of the proximal arteries is well defined in the CMM-TDI image, the limit of the adventitia is often more difficult to discern. As a result, we chose to estimate wall thickness for the elastic modulus approximations based on an average ex vivo measured value; this avoids the uncertainties associated with determining the outer wall in the echo image and simplifies postprocessing. We avoided using individual thicknesses measured from each artery, because such measurements will not be available in a clinical application of this method.
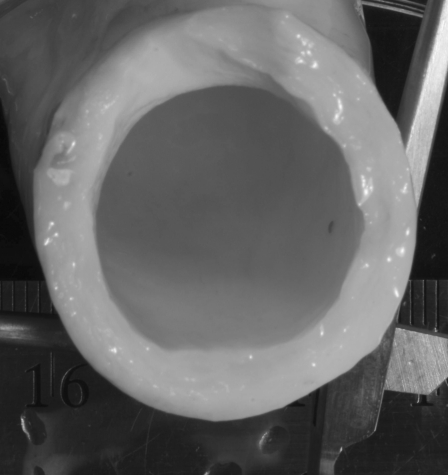
By processing high-resolution photographs (see Fig. 2) of the fresh MPA from the 16 animals and direct caliper measurements, we found diameter and thickness values and calculated wall thickness to diameter ratios (h/D) for each animal. While the hypertensive animal MPAs displayed a significant (P = 0.018) 13% increase in thickness with respect to the controls, their h/D values were not significantly different from the control h/D (P = 0.121), and thus we chose the mean of all animals, h/D = 0.178 (± 0.018), to represent both groups. An animal's estimated wall thickness was then obtained by multiplying its diameter at diastole (as determined from the echo image) by this group-average h/D.
Fig. 2.
Example cross-sectional image of hypertensive calf main pulmonary artery used for determination of diameter and wall thickness.
Ex vivo measurements.
To validate our elastic modulus approximations obtained from PD measurements, we compared them to ex vivo measurements of the tissue elastic modulus (22). Briefly, after death, the heart and lungs were removed from the animal in-block, and the right pulmonary artery, left pulmonary artery, and MPA were processed into circumferential strips and tested on a standard material testing system (MTS Insight II, Eden Prairie, MN) to obtain bench-top or “true” ex vivo elastic modulus (Eex) over a wide range of strains. As noted earlier, stiffness is the total measure of an elastic material's total resistance to mechanical deformation and may change due to both dimensional and inherent material factors, while elastic modulus quantifies only the inherent material property change. Here, Eex is the measured force normalized by the test sample area (22). We note that residual stress was not considered in the calculation of ex vivo parameters.
To compare these ex vivo measures to the in vivo approximations, values for Eex from the MPA must be found at relevant physiological strains (i.e., at the same relative operating conditions that existed in vivo). These physiological strains are found by relating an in vivo pressure measurement to an ex vivo strain state with Lame's equation, as described previously (22). We then find average “whole-cycle” values for Eex from the stress-strain linear regression slopes over the physiological strain range; our intent is to obtain an average modulus and stiffness representative of the physiological process of arterial distension. During this process, no regression had an r2 < 0.59, with the majority (88%) having r2 > 0.95, suggesting the use of this linear approximation to obtain slope was acceptable.
Statistical analysis.
The F-test and appropriate Student's t-test were used to detect differences between animal groups. Two tests were used to assess validation: 1) univariate linear regression analysis was performed using SAS 9.1 (SAS Institute, Cary, NC) to find relationships (and confidence limits) between the in vivo measured quantities and their corresponding ex vivo counterparts; and 2) Bland-Altman analysis (5) was used to assess limits of agreement (LoA). Analysis of covariance (32) was performed in MATLAB to examine differences in the correlations between the two approximations and ex vivo measures. All results are reported as statistically significant at the 95% confidence level, and P values refer to the two-tailed value. Finally, propagation of error analysis (9) was used to determine bias and random errors of the direct measurements and to estimate slope uncertainty (6); the details of this analysis are omitted. This propagated uncertainty is shown as error bars in each regression plot of the results.
RESULTS
Nine hypertensive (Hy1–Hy9) and seven control animals (Cn1–Cn7) were studied, and in vivo and ex vivo mechanical measures were obtained (see Table 1). As expected for this model of PH, the animal groups had significantly different (P < 0.001) mean pulmonary artery pressures, with the hypertensive group having a mean pressure (58 mmHg) more than double that of the control group (26 mmHg). Only one animal displayed small incremental strains (Hy2: 7.7%), although even this small deformation was over 22 times the M-mode resolution, and the TDI image data were readily used to obtain the wall definitions from echo for all animals. Interestingly, the incremental strain (assessed purely from the echo data) was also significantly different between groups (also see Table 1, P = 0.003).
Table 1.
Calf hemodynamic, mechanical, and ultrasound image data grouped by condition (control, hypertensive)
| Calf ID | mPAP, mmHg | Eex, kPa | ETWPS, kPa | EPS, kPa | Mean Incremental Strain, % | Average Deformation Divided by M-mode Resolution |
|---|---|---|---|---|---|---|
| Hyptertensive | ||||||
| Hy1 (59) | 75 | 216.1 | 174.1 | 108.8 | 10.3 | 19.0 |
| Hy2 (67) | 65 | 184.8 | 167.8 | 105.2 | 7.7 | 22.8 |
| Hy3 (68) | 40 | 112.6 | 100.0 | 65.5 | 10.1 | 35.4 |
| Hy4 (71) | 66 | 251.9 | 147.9 | 99.8 | 15.2 | 37.9 |
| Hy5 (72) | 83 | 267.1 | 194.6 | 126.2 | 10.3 | 28.5 |
| Hy6 (73) | 55 | 187.1 | 123.3 | 82.7 | 12.4 | 26.0 |
| Hy7 (78) | 69 | 244.9 | 223.2 | 161.1 | 12.1 | 26.9 |
| Hy8 (79) | 32 | 136.2 | 69.0 | 48.6 | 22.0 | 41.5 |
| Hy9 (83) | 40 | 131.8 | 92.7 | 71.5 | 22.6 | 53.3 |
| Mean ± SD | 58 ± 18 | 192 ± 56.7 | 143 ± 51.2 | 96.6 ± 34.2 | 13.6 ± 5.3 | |
| Control | ||||||
| Cn1 (65) | 23 | 72.7 | 56.7 | 39.6 | 38.6 | 61.9 |
| Cn2 (66) | 30 | 136.4 | 104.3 | 43.0 | 28.9 | 53.6 |
| Cn3 (74) | 46 | 137.6 | 71.7 | 54.6 | 22.1 | 44.9 |
| Cn4 (75) | 27 | 104.7 | 68.4 | 52.3 | 51.1 | 75.4 |
| Cn5 (77) | 19 | 64.1 | 40.8 | 30.3 | 30.4 | 48.0 |
| Cn6 (80) | 16 | 77.2 | 69.0 | 47.6 | 20.7 | 35.0 |
| Cn7 (81) | 21 | 86.0 | 36.2 | 23.9 | 43.9 | 60.6 |
| Mean ± SD | 26 ± 10 | 97 ± 30.1 | 63.9 ± 22.7 | 41.6 ± 11.3 | 33.7 ± 11.3 | |
Hy1–Hy9, hypertensive animal 1–9; Cc1-Cn7, control animal 1–7 (nos. in parenthese are calf ID nos.). mPAP, mean pulmonary artery pressure; Eex, ex vivo elastic modulus; ETWPS, thick-walled plane strain modulus; EPS, pressure-strain modulus.
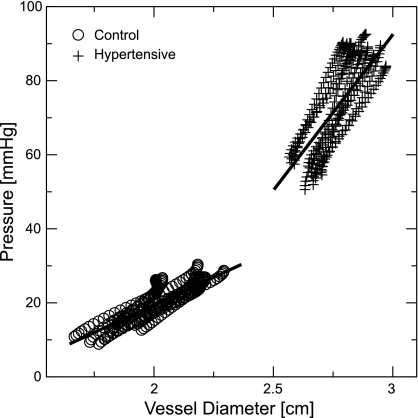
Representative measured PD curves from control (Cn3) and hypertensive (Hy1) animals are shown in Fig. 3 during systole of multiple cardiac cycles, along with regression lines, obtained by averaging the individual linear regression slope and intercept parameters from each cardiac cycle. Notably, these individual correlations all had r2 > 0.98 (and for a majority of measured curves of all animals), indicating the PD curves may be reasonably represented by a linear approximation. The control curves are readily distinguished from the hypertensive curves by both their greater range of displacement (and thus greater incremental strain) and the lower operating pressure (and thus lower incremental stress).
Fig. 3.
Representative measured pressure-diameter (PD) curves obtained from control (○) and hypertensive (+) calves.
Error of prediction: validation of in vivo measurements.
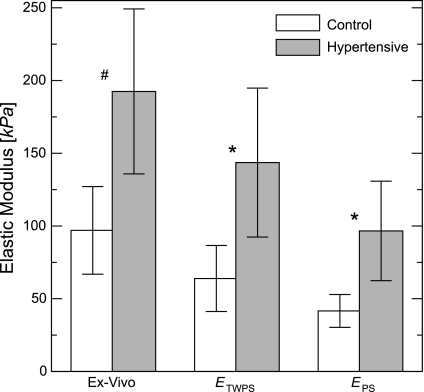
As shown previously through postmortem bench-top measurements (22), hypoxia-induced PH induces statistically significant increases in the elastic modulus of the calf MPA (Fig. 4, ex vivo, P < 0.001). We see that our in vivo approximations allow these increases to be assessed in the intact animal: both show significant differences between groups (ETWPS, P = 0.001; EPS, P = 0.001). However, increases in in vivo modulus are not identical to increases in the Eex: from control to hypertensive condition, the means of ETWPS and EPS increase 125 and 132%, respectively, while Eex increases only 99%. The in vivo means from control and hypertensive animals are, on average, 46 and 38% smaller than the means of Eex for controls and hypertensive animals, respectively.
Fig. 4.
Group comparisons of two elastic modulus approximations [thick-walled plane strain (ETWPS), pressure-strain (EPS)] and the ex vivo elastic modulus (Eex). Values are means ± SD. *P < 0.005; #P < 0.001.
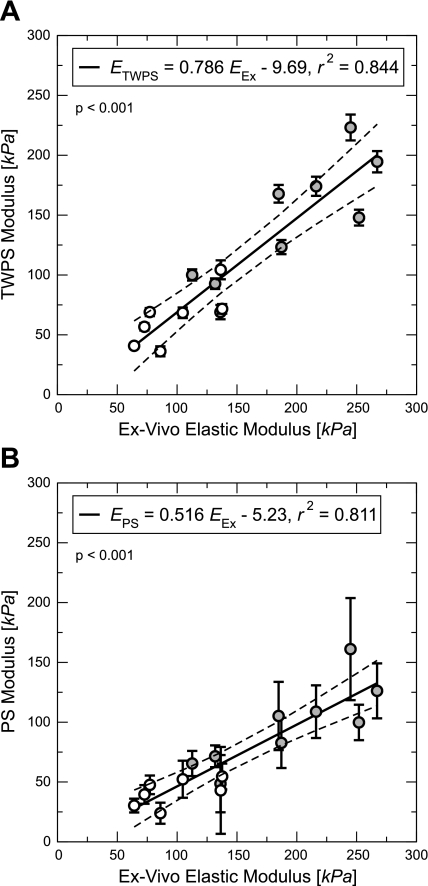
Table 2 provides Pearson correlation coefficients for regressions of the elastic modulus approximations against Eex. Regressions of the two approximations against Eex are shown in Fig. 5, A (ETWPS = 0.786 Eex − 9.69, r2 = 0.844, P < 0.001) and B (EPS = 0.516 Eex − 5.23, r2 = 0.811, P < 0.001). Also shown are dashed lines, showing the 95% confidence limits for the predicted value of Eex. Neither regression displays “exact validation” (e.g., slope = 1, intercept = 0), although both have significant correlation and goodness of fit is of good quality overall. Comparing Fig. 5, A and B, we see qualitatively that the thin-walled approximation (EPS) regression slope appears distinct from that of the ETWPS vs. Eex regression slope; this is confirmed by analysis of covariance, which reveals that the two slopes are significantly different (P = 0.023).
Table 2.
Pearson correlation coefficients and P values for regressions comparing the in vivo elastic modulus approximations to the ex vivo elastic modulus (all for measurements on the main pulmonary artery)
| Eex |
||
|---|---|---|
| r2 | P | |
| ETWPS | 0.844 | <1.0e-6 |
| EPS | 0.811 | 2.0e-6 |
Fig. 5.
The two elastic modulus approximations [ETWPS (A), EPS (B)] regressed against Eex of the calf MPA. Error bars refer to propagated measurement uncertainty. Open and shaded circles are indicative of control and hypertensive animals, respectively.
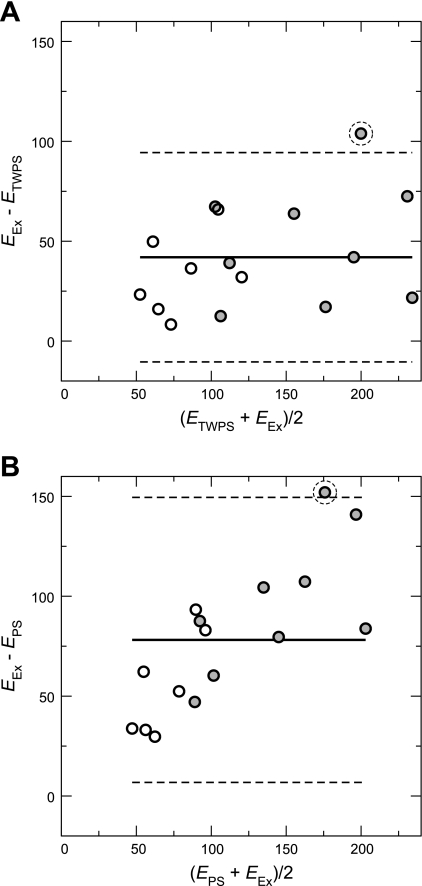
Bland-Altman plots are shown for ETWPS and EPS vs. Eex in Fig. 6, A and B, respectively. The thick-walled approximation has a roughly uniform bias of −42.0 kPa and LoA of 52.4 kPa, while the thin-walled approximation has both greater and nonuniform bias (mean of −78.2 kPa) that increases proportionally with average modulus and higher agreement limits (71.3 kPa). Each plot has a single outlier, indicated by a dashed circle: calf Hy4, which displayed substantially larger Eex compared with ETWPS (70.3% larger) and EPS (152.3% larger). When this outlier is removed, ETWPS bias and LoA improve to −37.8 and 42.6 kPa, respectively, while EPS bias and LoA improve to −73.2 and 62.1 kPa, respectively.
Fig. 6.
Bland-Altman agreement analysis between the elastic modulus approximations [ETWPS (A), EPS (B)] and the Eex of the calf MPA. Each solid middle line is the bias, and the bounding dashed lines indicate the limits of agreement (± 1.96 SD). Open and shaded circles are indicative of control and hypertensive animals, respectively. Each plot has a single outlier, indicated by a dashed circle (see results for details).
DISCUSSION
Current clinical evaluation of PAH relies primarily on measurement of PVR; however, incipient clinical analysis has begun to demonstrate the importance of vascular stiffening on the prediction of outcomes and mortality in this disease (14, 18, 29). In this work, we examined the utility of two equations that use PD data to estimate proximal pulmonary vascular elastic modulus in the intact animal; these estimates were validated against “true” elastic modulus of these tissues measured with an industry-standard uniaxial material tester. We obtained pressure and diameter data from invasive measurements of pressure and noninvasive CMM-TDI ultrasound, respectively. Our results suggest that both thick- and thin-walled approximations may be used to estimate Eex, although the thick-walled approximation is preferred. The implications and limitations of these topics are discussed below.
Estimation equations.
Two basic equations to obtain elastic modulus from PD data corresponding to thin-walled and thick-walled theory were derived to determine if a thick-walled approximation was required to accurately estimate elastic modulus in the intact animal. Both equations needed wall thickness, which was estimated using an average of data obtained from extracted (ex vivo) tissues. Our analyses indicate that the thick-walled equation results are more accurate, have tighter agreement bounds, and are significantly different from the thin-walled equation results. Both underestimate the true modulus; this could be expected, given this application of infinitesimal deformation theory, which assumes constant thickness, to our clearly finite-displacement in vivo conditions, in which the wall must undergo thinning. Furthermore, the underestimation of circumferential stress even at smaller strains by thin-walled theory accounts for the uniformly smaller values of elastic modulus values obtained from the EPS approximation and for its increasing agreement bias as the vessels become thicker due to PH.
In practice, EPS is the easier approximation for which to obtain measurements and to compute, only requiring PD extrema and simple arithmetic, respectively. A recommendation seems premature, however, given the limited number of animals examined in this study. Preliminary application of this method in pediatric PAH patients (19) suggests that the tighter agreement limits of ETWPS are likely more suitable for differentiation of pediatric patient groups. These tighter agreement limits, along with its better correlation coefficient, suggest that ETWPS is the more accurate measurement. Ongoing work by our group seeks to determine whether either approximation is a better predictor of patient outcomes compared with PVR alone.
Diameter acquisition.
Diameter data were relatively easy to obtain using a standard clinical digital ultrasound machine with color M-mode tissue-Doppler imaging. For the GE system we used, pressure data were digitized directly into the auxiliary input of the scanner, and our MATLAB postprocessing program allows direct extraction of both for simultaneous comparison and analysis. The use of transthoracic ultrasound is clearly advantageous over previous, more invasive methods to obtain diameter and has already allowed these techniques to be routinely used in a clinical setting when catheterization is performed.
Our group has previously explored the use of intravascular ultrasound to obtain diameter measurements (43). This previous work recommended the use of compliance measures (the inverse of stiffness) due to considerable measurement uncertainty that can result from a small strain appearing in the denominator of a computed elastic modulus or stiffness. The CMM-TDI method did not display this problem: in our studies, we found that the smallest deformations (≈7% strain for Hy2) were associated with uncertainties on the order of 30% (EPS), using only M-mode images to acquire the wall trace. While this is larger than desired, it is much smaller than what was predicted previously with the intravascular ultrasound modality (43).
In vivo measurement.
To the authors' knowledge, this work represents the first effort to compare in vivo and ex vivo mechanical measurements of the pulmonary vasculature in an animal model of PH. Given our group's recent findings of significant increases in proximal vascular elastic modulus and stiffness in hypoxic PH (22) and preliminary results from application of this method in pediatric PAH patients (19), we hypothesize that this measurement could have significant diagnostic and/or prognostic value. As site-specific proximal measures of elastic modulus, increases in the approximations could be indicative of proximal extracellular matrix remodeling, which is associated with vascular stiffening in the calf model. Stiffening of the proximal vessels should be most detrimental to the vascular windkessel (as opposed to distal vessels) (15) and would significantly change the pulse flow seen by the distal vasculature.
The potential presence of smooth muscle cell (SMC) tone in our in vivo measurements, which has been shown to alter arterial stiffness in muscular arteries (10, 15), could be a major source of the error seen in comparing these to ex vivo measurements, conducted on passive (dead) tissue. To the contrary, several very recent studies have suggested that conduit artery SMC tone does not appear elevated after hypoxia-induced PH and has little impact on proximal mechanics (38, 41). Regardless, such SMC-associated differences between in vivo and ex vivo tissue mechanics, if present, should be most apparent in the hypoxic group. However, groupwise Bland-Altman ETWPS and Eex agreement analyses suggest that any differences are small: the hypoxic group bias was slightly larger (48.9 kPa) than the control group bias (33.1 kPa), although the difference between these biases (15.8 kPa) is smaller than the standard deviation of either group mean. Such difference might be attributed to SMC tone, but could also be due to the infinitesimal displacement approximation inherent in the Lame equation. The impact of proximal SMC tone on proximal mechanics is an important area of future research.
Mechanical studies by our group have shown that proximal PVS increases in both rat (12) and calf (22) models of hypoxia-induced PH. The cellular and molecular mechanisms responsible for these increases are a subject of current research; however, many studies have previously examined histological and morphological features of the proximal pulmonary arteries in disease (37). Briefly, small mammals, such as the rat, primarily display immediate adventitial thickening due to accumulation of cells and collagen upon exposure to hypoxia, while the media responds more slowly. In contrast, large mammals, such as the calf, exhibit early and substantial medial thickening due to accumulation of both elastin and collagen. It is hypothesized that these differences are due to a more complex cellular composition of the conduit vessels in the larger mammals. Much more detail regarding proximal changes in hypoxic PH may be found in a recent review (37).
Clinical application.
Recent clinical studies have focused on the prognostic capabilities of invasive measurement of global stiffness [as assessed with pulmonary vascular input impedance (18) or pulmonary vascular compliance (29)] and MRI-based noninvasive measurement of pulmonary vascular relative area change (RAC) (14). These are all promising prognostics, but only the last is a site-specific measure, like the approximations explored herein. We used our echo-measured diameter alone to estimate RAC, which clearly correlates to our approximations (given the functional dependencies shown in Eqs. 1 and 2); however, RAC is a lesser correlate of Eex (r2 = 0.477, P = 0.003) in our animal model. Given the established link between proximal vascular remodeling and increasing vascular elastic modulus in animal models (7, 11, 12, 20–22, 38, 41, 44), we speculate that our approximations, as better correlates to true elastic modulus, could be superior clinical prognostics.
Although coupled in vivo and in vitro mechanics work remains to be done to establish their correlative relationship in humans, preliminary evidence exists that such information may not be necessary for this method to have clinical utility (19). Indeed, the three stiffness prognostics above have been used without such correlation. The ease with which the ultrasound acquisition may be added to the typical clinical catheterization workflow (requiring only 2–3 additional min to obtain an image), the minimally invasive nature of this addition, and the simplicity of calculation (specifically EPS) all enable straightforward clinical adoption.
Limitations.
There are several limitations that must be acknowledged. First, our use of linear regressions to obtain averages of ex vivo modulus and stiffness may not best represent the typical in vivo situation; other possibilities for comparison include the incremental secant elastic modulus or standard or weighted averages of the elastic modulus over the physiological range. However, given our use of regression slopes with the PD data, ex vivo linear regressions were consistent, and the high correlation coefficients (r2) found suggests that an incremental, linear slope was representative across each physiological pressure range. We neglected finite-strain effects and longitudinal deformation in the derivation of our elastic modulus approximation equations; however, our goal here was to obtain an estimate of in vivo stiffness behavior that would represent heart load and could guide clinical diagnosis, not to obtain an exact measurement of elastic modulus, as can be obtained with bench-top measurement methods. Finally, we note that use of PD curves to estimate vascular stiffness is not new; our contribution is the use of ultrasound to assess diameter, which makes the method more practical for serial measurements in animals and minimally invasive in a clinical setting.
Conclusion.
Through comparisons to true elastic modulus obtained from bench-top measurements, we have shown that PD data provide a reasonable means to estimate pulmonary vascular elastic modulus, a fundamental material property of the vasculature, in intact calves. Unlike previous work, we have used a noninvasive imaging modality, CMM-TDI ultrasound, to obtain arterial diameter, which should enable these measurements to be easily translated into a clinical environment. Our work provides evidence that simple mathematical models of the vessel are sufficient to extract an estimate of elastic modulus in these animals and that an estimate based on thick-walled theory provides the best accuracy. Finally, given the link between hypoxia-induced PH and proximal stiffening, we speculate that this measurement will be a useful prognostic tool in the treatment and management of human PH.
GRANTS
This project was supported in part by grants from the National Heart, Lung, and Blood Institute (R01-HL067393, T32-HL072738, K24-HL081506, and P50-HL084923) and the American Heart Association (09SDG2260194).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
K. S. Hunter expresses gratitude to Maria Frid, Sandi Walchak, and the many others who assisted with various aspects of the calf experiments.
REFERENCES
- 1. Armentano R, Simon A, Levenson J, Chau NP, Megnien JL, Pichel R. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 26: 48–54, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Balzer DT, Kort HW, Day RW, Corneli HM, Kovalchin JP, Cannon BC, Kaine SF, Ivy DD, Webber SA, Rothman A, Ross RD, Aggarwal S, Takahashi M, Waldman JD. Inhaled nitric oxide as a preoperative test (INOP Test I). The INOP Test Study Group. Circulation 106, Suppl S: I76–I81, 2002 [PubMed] [Google Scholar]
- 3. Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 43: 40S–47S, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Berger RMF, Cromme-Dijkhuis AH, Hop WCJ, Kruit MN, Hess J. Pulmonary arterial wall distensibility assessed by intravascular ultrasound in children with congenital heart disease. Chest 122: 549–557, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 6. Brown KK, Coleman HW, Steele WG. Estimating uncertainty intervals for linear regression (Abstract). In: Proceedings of the AIAA 33rd Aerospace Sciences Meeting and Exhibit 1995. Reston, VA: AIAA, 1995, p. 95–0796 [Google Scholar]
- 7. Chesler NC, Thompson-Figueroa J, Millburne K. Measurements of mouse pulmonary artery biomechanics. J Biomech Engr 126: 309–314, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Das BB, Lanning CJ, Dyer KL, Lee PF, Ivy DD, Valdes-Cruz L, Shandas R. Evaluation of pulmonary artery pulsatility in pulmonary hypertension using color M-model tissue Doppler imaging in infants and children. Pediatr Res 55: 51a–51a (289, Part 2, Suppl. 5), 2004 [Google Scholar]
- 9. Dieck RH. Measurement Uncertainty/Methods and Applications (4th Ed.). Research Triangle Park, NC: International Society of Automation, 2006 [Google Scholar]
- 10. Dobrin PB. Vascular mechanics. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, chapt. 3, p. 65–102 [Google Scholar]
- 11. Drexler ES, McCowan C, Wright J, Slifka AJ, Ivy DD, Shandas R. Comparison of strength properties of normotensive and hypertensive rat pulmonary arteries. Biomed Sci Instrum 40: 297–302, 2004 [PubMed] [Google Scholar]
- 12. Drexler E, Bischoff JE, Slifka AJ, McCowan CN, Quinn TP, Shandas R, Ivy DD, Stenmark K. Stiffening of the extrapulmonary arteries from rats in chronic hypoxic pulmonary hypertension. J Res Natl Inst Stand Technol 113: 239–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyer K, Lanning CJ, Das B, Lee PF, Ivy DD, Valdes-Cruz L, Shandas R. Noninvasive Doppler tissue measurement of pulmonary artery compliance in children with pulmonary hypertension. J Am Soc Echocardiogr 19: 403–412, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gan CTJ, Lankhaar JW, Weterhof N, Marcus JT, Becker A, Twisk JWR, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noinvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 32: 1906–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Gow BS. Circulatory correlates: vascular impedance, resistance, and capacity. In: Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc., 1980, sect. 2, vol. II, chapt. 14, p. 353–408 [Google Scholar]
- 16. Greenfield JC, Griggs DM. Relation between pressure and diameter in main pulmonary artery. J Appl Physiol 18: 557–559, 1963 [DOI] [PubMed] [Google Scholar]
- 17. Hunter KS, Gross JK, Lanning CJ, Kirby KS, Dyer KL, Ivy DD, Shandas R. Noninvasive methods for determining pulmonary vascular function in children with pulmonary arterial hypertension: application of a mechanical oscillator model. Congenit Heart Dis 3: 106–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than PVR alone in pediatric patients with pulmonary hypertension. Am Heart J 155: 166–174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunter KS, Lanning CJ, Kirby KS, Ivy DD, Shandas R. In-vivo pulmonary vascular stiffness obtained from color M-mode tissue Doppler imaging and pressure measurements predicts clinical outcomes better than indexed pulmonary vascular resistance in pediatric patients with pulmonary arterial hypertension (Abstract). Circulation 118: S879, 2008 [Google Scholar]
- 20. Kobs RW, Chesler NC. The mechanobiology of pulmonary vascular remodeling in the congenital absence of eNOS. Biomech Model Mechanobiol 5: 217–225, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC. Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 288: H1209–H1217, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lammers SR, Kao P, Qi HJ, Hunter KS, Lanning CJ, Albietz JA, Hofmeister SE, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lanne T, Sonesson B, Bergqvist D, Bengtsson H, Gustafsson D. Diameter and compliance in the male human abdominal-aorta: influence of age and aortic aneurysm. Eur J Vasc Surg 6: 178–184, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Laurent S, Lacolley P, Girerd X, Boutouyrie P, Bezie Y, Safar M. Arterial stiffening: opposing effects of age- and hypertension-associated structural changes. Can J Physiol Pharmacol 74: 842–849, 1996. [PubMed] [Google Scholar]
- 25. Lee PF, Lanning CJ, Drexler ES, Ivy DD, Shandas S. Extracting Young's modulus of the pulmonary arteries from color M-mode tissue Doppler data of pediatric patients with pulmonary hypertension. In: Proceedings of the 2005 Summer Bioengineering Conference, Vail, CO, June 2005. New York: ASME, 2005 [Google Scholar]
- 26. Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng 37: 1082–1092, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Stenmark KR, Shandas R, Tan W. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J Vasc Res 46: 561–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr 19: 1045–1050, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47: 799–803, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Milnor WR, Conti CR, Lewis KB, O'Rourke MF. Pulmonary arterial pulse wave velocity and impedance in man. Circ Res 25: 637–649, 1969 [DOI] [PubMed] [Google Scholar]
- 31. Peterson LH, Jensen RE, Parnell J. Mechanical properties of arteries in vivo. Circ Res 8: 622–639, 1960 [Google Scholar]
- 32. Rutherford A. Introducing ANOVA and ANCOVA: A GLM Approach. London: SAGE, 2001 [Google Scholar]
- 33. Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107: 2864–2869, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Shadwick RE. Mechanical design in arteries. J Exp Biol 202: 3305–3313, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Stefanadis C, Tsiamis E, Vlachopoulos C, Stratos C, Toutouzas K, Pitsavos C, Marakas S, Boudoulas H, Toutouzas P. Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation 95: 31–38, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Stenmark KR, Fasules J, Hyde DM, Voelkel NF, Henson J, Tucker A, Wilson H, Reeves JT. Severe pulmonary hypertension and arterial adventitial changes in newborn caves at 4,300 m. J Appl Physiol 62: 821–830, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Tabima DM, Chesler NC. The effects of vasoactivity and disease on conduit pulmonary artery biomechanics (Abstract). In: Proceedings of the Pittsburgh International Lung Conference, Oct 9–10, 2009. Pittsburgh, PA: Univ. of Pittsburgh, 2009, p. 19 [Google Scholar]
- 39. Topper JN, Cai J, Falb D, Gimbrone MA. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cycoloxgenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 93: 10417–10422, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ugural AC, Fenster SK. Advanced Strength And Applied Elasticity (4th Ed). Upper Saddle River, NJ: Prentice Hall, 2003, p. 315–320 [Google Scholar]
- 41. Vanderpool RR, Chesler NC. Effect of acute rho kinase inhibition on pulmonary vascular structure in isolated mouse lungs following chronic hypoxia (Abstract). In: Proceedings of the Pittsburgh International Lung Conference, Oct 9–10, 2009. Pittsburgh, PA: Univ. of Pittsburgh, p. 15 [Google Scholar]
- 42. Weinberg C, Hertzberg J, Valdes-Cruz LM, Shandas R. Extraction of pulmonary vascular compliance, PVR and RV work from single-pressure and Doppler flow measurements in children with pulmonary hypertension–a new method for evaluating reactivity: in vitro and clinical studies. Circulation 110: 2609–2617, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Weinberg CE, Hertzberg JR, Shandas R. Use of intravascular ultrasound to measure local compliance of the pediatric pulmonary artery: in vitro studies. J Am Soc Echocardiogr 15: 1507–1514, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Wright JE, Drexler ES, Slifka AJ, McCowan CN, Ivy DD, Shandas R. Stress and strain in rat pulmonary artery material during a biaxial bubble test. Biomed Sci Instrum 40: 303–308, 2004 [PubMed] [Google Scholar]
- 45. Ziegler T, Bouzourène K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]