Abstract
In this study, we examined chronic norepinephrine suppression of insulin secretion in sheep fetuses with placental insufficiency-induced intrauterine growth restriction (IUGR). Glucose-stimulated insulin secretion (GSIS) was measured with a square-wave hyperglycemic clamp in the presence or absence of adrenergic receptor antagonists phentolamine (α) and propranolol (β). IUGR fetuses were hypoglycemic and hypoxemic and had lower GSIS responsiveness (P ≤ 0.05) than control fetuses. IUGR fetuses also had elevated plasma norepinephrine (3,264 ± 614 vs. 570 ± 86 pg/ml; P ≤ 0.05) and epinephrine (164 ± 32 vs. 60 ± 12 pg/ml; P ≤ 0.05) concentrations. In control fetuses, adrenergic inhibition increased baseline plasma insulin concentrations (1.7-fold, P ≤ 0.05), whereas during hyperglycemia insulin was not different. A greater (P ≤ 0.05) response to adrenergic inhibition was found in IUGR fetuses, and the average plasma insulin concentrations increased 4.9-fold at baseline and 7.1-fold with hyperglycemia. Unlike controls, basal plasma glucose concentrations fell (P ≤ 0.05) with adrenergic antagonists. GSIS responsiveness, measured by the change in insulin, was higher (8.9-fold, P ≤ 0.05) in IUGR fetuses with adrenergic inhibition than controls (1.8-fold, not significant), showing that norepinephrine suppresses insulin secretion in IUGR fetuses. Strikingly, in IUGR fetuses, adrenergic inhibition resulted in a greater GSIS responsiveness, because β-cell mass was 56% lower and the maximal stimulatory insulin response tended (P < 0.1) to be higher than controls. This persistent norepinephrine suppression appears to be partially explained by higher mRNA concentrations of adrenergic receptors α1D, α2A, and α2B in a cohort of fetuses that were naïve to the antagonists. Therefore, norepinephrine suppression of insulin secretion was maintained, in part, by upregulating adrenergic receptor expression, but the β-cells also appeared to compensate with enhanced GSIS. These findings may begin to explain why IUGR infants have a propensity for increased glucose requirements if norepinephrine is suddenly decreased after birth.
Keywords: pancreas, islets of Langerhans, hypoxia, glucose-stimulated insulin secretion, fetal growth restriction
increasing epidemiological and experimental evidence has demonstrated a relationship between fetal malnutrition and later life metabolic disorders such as insulin resistance, glucose intolerance, and overt type 2 diabetes mellitus (1, 15, 18, 19, 36, 47). Placental insufficiency is a common ailment in human pregnancy where the maternal supply of glucose, oxygen, and other nutrients to the fetus are restricted (11, 34), causing intrauterine growth restriction (IUGR). IUGR fetuses also have lower plasma insulin concentrations, impaired β-cell responsiveness, and, in more severe cases, less β-cell mass (6, 31, 54). Together, these findings indicate that fetal β-cells are susceptible to nutrition deficiencies in utero, which appear to alter their developmental program and lead to lifelong inadequacies.
Consistent with human data, animal models indicate that insulin-producing β-cells undergo altered fetal programming in response to fetal malnutrition (9, 13, 17, 33, 47, 48). An ovine model of IUGR with hyperthermia-induced placental insufficiency possesses all of the complications previously reported in humans (5, 37, 55), including impaired insulin secretion (18% of normal) and reduced β-cell mass (24% of normal) near term (24, 27). Although these fetuses are hypoglycemic, our research indicates that factors in addition to glucose deficiency likely contribute to the impaired β-cell responsiveness. These studies showed that sheep fetuses made experimentally hypoglycemic for 2 wk have only a 45% reduction in glucose-stimulated insulin secretion (GSIS), less than half that of the sheep fetuses with placental insufficiency, despite similar plasma glucose concentrations (23, 27). Additional causative factors might include time of glucose deficiency onset, duration of glucose deficiency, or other nutrient deficiencies.
One important difference between these two sheep models of IUGR is fetal blood oxygen content, which was significantly lower in fetuses with placental insufficiency (23, 27, 39). During acute fetal asphyxia or hypoxia caused by maternal hypoxemia, umbilical cord occlusion, or severing the umbilical cord at delivery, plasma norepinephrine concentrations rise (8, 20, 21, 29). In fetal sheep, as in several other species, catecholamines act on the β-cells predominantly via the α2-adrenergic receptors to suppress insulin secretion (16, 20, 21). We found that plasma norepinephrine concentrations on average were fourfold greater in near-term sheep fetuses with placental insufficiency-induced IUGR compared with normoxic controls (27). In addition, norepinephrine concentrations were negatively associated with fetal blood oxygen content (27), indicating that, during chronic exposure to hypoxia, norepinephrine release is tightly regulated by fetal blood oxygen content. In contrast to the placental insufficiency group, fetal sheep exposed only to chronic hypoglycemia did not have decreased blood oxygen content or elevated plasma norepinephrine concentrations (23). Therefore, we postulated that the disparity in insulin secretion between fetuses with placental insufficiency and those with experimental hypoglycemia reflects a suppressive action from norepinephrine.
To investigate chronic norepinephrine suppression of insulin secretion in placental insufficiency-induced IUGR sheep fetuses, we acutely blocked norepinephrine's effects with pharmacological antagonists phentolamine (an α-adrenergic receptor antagonist) and propranolol (a β-adrenergic receptor antagonist) and measured GSIS. The insulin secretion responsiveness with the adrenergic inhibition was compared with a GSIS study without adrenergic inhibition in the same fetus, and the differences were compared between IUGR and control fetuses. We also measured adrenergic receptor mRNA concentrations in fetal islets isolated from a separate cohort of fetuses to eliminate any confounding effects from the exposure to adrenergic receptor antagonists.
MATERIALS AND METHODS
Ovine model of IUGR.
Columbia-Rambouillet crossbred ewes (average body wt 51.7 ± 1.7 kg) carrying twin pregnancies were purchased from Nebeker Ranch (Lancaster, CA), and litter size was confirmed by ultrasonic scanning. All animal care and use were conducted with institutional approval at the University of Arizona Agricultural Research Complex, Tucson, AZ, which is accredited by the National Institutes of Health, the United States Department of Agriculture, and the American Association for Accreditation of Laboratory Animal Care. IUGR fetuses were created by exposing pregnant ewes to elevated ambient temperatures with a diurnal pattern of 40°C for 12 h and 35°C for 12 h, from 40 ± 1 days gestational age (dGA; mean ± SD) until 119 ± 2 dGA, as previously described (25, 26, 52). Normal control fetuses were from gestational age-matched pregnant ewes maintained at 25°C, and the ewes were pair fed to the average feed intake of the ewes exposed to hyperthermia. Ewes received alfalfa pellets, which had a dry matter composition of 19.5% crude protein, 32.6% acid detergent fiber, 42.6% neutral detergent fiber, 1.23 Mcal/kg net energy of maintenance, and 0.66 Mcal/kg net energy for reproductive processes (Dairy One Forage Testing Laboratory, Ithaca, NY). Twelve pregnant ewes were divided equally into the treatment groups IUGR and control. All but one pair of the control fetuses survived treatment, surgical procedures, and in vivo studies, as one ewe miscarried for an unknown reason prior to the in vivo study. Ewes exposed to hyperthermia exhibited a greater abortion rate during the treatment and surgical preparations (50% loss). At surgery, a control ewe and an IUGR ewe were found to have singletons. At necropsy, necrotic remains of fetal and placental tissues were found in the uterus of the IUGR ewe, but there was no indication of a second fetus in the control ewe, indicating a diagnostic error. The total number of animals in each treatment group that completed both in vivo experiments was nine control fetuses from five ewes and five IUGR fetuses from three ewes.
Surgical preparation.
At ∼126 ± 1 dGA, indwelling polyvinyl catheters were surgically placed in the fetus for blood sampling and glucose infusion as described previously (23, 27). Fetal catheters for blood sampling were placed in the abdominal aorta via hindlimb pedal arteries, and infusion catheters were placed in the femoral veins via the saphenous veins. Maternal catheters were placed in the femoral artery and vein for arterial sampling and venous infusions. All catheters were tunneled subcutaneously to the ewe's flank, exteriorized through a skin incision, and kept in a plastic mesh pouch sutured to the ewe's skin. Ewes were allowed to recover for 5–7 days before the first GSIS study was conducted.
Experimental design.
Control and IUGR fetuses were studied on two separate occasions to measure their GSIS responsiveness in the absence or presence of α- and β-adrenergic receptor antagonist. Within a ewe, twin fetuses were studied simultaneously. An initial GSIS study was performed on five control and five IUGR fetuses with saline as the vehicle control. After a 24- to 48-h recovery, a second GSIS study was conducted with adrenergic receptor antagonists phentolamine (α-adrenergic receptor antagonist) and propranolol (β-adrenergic receptor antagonist). Phentolamine was infused into the fetus at 40 μg·min−1·kg estimated fetal weight−1 following an 870-μg priming dose, and propranolol was infused at 14 μg·min−1·kg estimated fetal weight−1 after a 300-μg priming dose (12, 20, 49). Preliminary studies in two control fetuses from different ewes using an intravenous glucose tolerance test during a constant infusion of epinephrine at 1 μg/min (16) showed that the adrenergic receptor antagonists alleviated catecholamine suppression of insulin. Infusion of the adrenergic receptor antagonists or saline was started 45 min before the baseline blood sampling. Four control fetuses were exposed to the GSIS plus adrenergic antagonists first, and then a GSIS study with saline was performed after 48 h. No residual effects were observed as a result of the order and temporal arrangement of the GSIS studies. After completion of the in vivo studies, the pregnant ewes and their fetuses were returned to basal conditions and killed within 20 h. Fetus weights were measured, and tissues were dissected and weighed. Pancreas tissues from the fetuses exposed to adrenergic antagonists only in the final study before necropsy were collected for histology as reported previously (9).
GSIS study.
A square-wave hyperglycemic clamp was used to determine insulin secretion in response to glucose, as previously reported (23, 27). Briefly, a continuous transfusion of maternal blood into the fetus (12 ml/h) was started 45 min prior to baseline sampling and maintained for the duration of the study to compensate for blood collection and to stabilize fetal hematocrit. All sample times are presented relative to the start of the fetal glucose bolus and continuous glucose infusion (time = 0). Baseline plasma glucose and insulin concentrations were determined at −35, −25, −15, and −5 min. Whole blood collected in syringes lined with EDTA (Sigma Chemicals) was centrifuged (13,000 g) for 2 min at 4°C. Plasma was aspirated from the pelleted red blood cells and stored at −80°C for hormone measurements. Blood gas and oxygen saturations were measured in blood collected in syringes lined with heparin (Elkins-Sinn, Cherry Hill, NJ). The hyperglycemic clamp was initiated with a dextrose bolus directly into fetal circulation followed by a constant infusion of 33% dextrose in saline to maintain fetal arterial plasma glucose concentration at 2.4 mmol/l, which in preliminary experiments using various glucose concentrations (2–11 mmol/l) was found to produce maximal insulin concentrations in fetal sheep (27). At the onset of the glucose infusion, fetal arterial plasma samples were collected every 5–10 min for the initial 30 min to establish the hyperglycemic clamp, after which fetal blood and plasma samples were collected at 45, 60, 75, and 90 min during steady-state conditions. During basal (time = −35 to 0 min) and steady-state hyperglycemic clamp (45–90 min) periods, fetal blood was collected for blood gas and oximetry measurements, and plasma was collected for glucose, insulin, and catecholamine measurements.
Biochemical analysis.
Blood oxygen saturation and hemoglobin concentrations were measured with an ABL 520 with values temperature corrected at 39°C (Radiometer, Copenhagen, Denmark). Plasma glucose concentrations were measured immediately using a YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin concentrations were measured with an ovine insulin ELISA (ALPCO Diagnostics, Windham, NH; intra- and interassay coefficients of variation, 5.6 and 2.9%, respectively), and catecholamines, stored in a final concentration of 0.5 mM EDTA and 0.33 mM reduced glutathione, were determined by a 2-CAT ELISA (Rocky Mountain Diagnostic, Colorado Springs, CO; intra- and interassay coefficient of variation, 18 and 19% for epinephrine, 19 and 6% for norepinephrine).
Pancreas morphology.
Tissue sections (6 μm) for histological evaluation were cut from the tail of the pancreas (n = 5 controls, 4 twins and 1 single; n = 5 IUGR, 4 twins and 1 single). Procedures for fluorescent immunostaining and morphometric analysis on cryosections were performed as reported previously (9, 24). Insulin-positive β-cells were identified with guinea pig anti-porcine insulin (Dako, Carpinteria CA; 1:500) and detected with an affinity-purified secondary antiserum conjugated to 7-amino-4-methylcoumarin-3-acetic acid (AMCA; Jackson ImmunoResearch Laboratories, West Grove, PA). α-cells, δ-cells, and F cells were detected together with mouse anti-porcine glucagon (Sigma-Aldrich, St. Louis, MO; 1:500), rabbit anti-human somatostatin (Dako; 1:500), and rabbit anti-human pancreatic polypeptide (Dako; 1:500) and detected with affinity-purified secondary antiserum conjugated to Texas Red (Jackson ImmunoResearch Laboratories). Fluorescent images were visualized on a Leica DM5500 microscope system and digitally captured with a Spot Pursuit 4 Megapixel CCD camera (Diagnostic Instruments, Sterling Heights, MI). Morphometric analysis was performed with ImagePro 6.0 software (Media Cybernetics, Silver Spring, MD). Positive areas were determined for 25 fields of view (FOV = 0.39 mm2) on two pancreas sections per animal separated by ≥100-μm intervals (total area = 19.4 mm2, coefficient of variation between slides, 13%). Data are expressed as a percentage of total pancreas area, and β-cell mass was obtained by multiplying the pancreas weight by the percent insulin-positive area.
Fetal pancreatic islet isolation.
A second cohort of pregnant ewes carrying singletons from Nebekar Ranch was produced and housed in conjunction with the animals used in Ref. 26 for the same treatment duration as the twin fetuses above. The purpose of the second cohort of animals was to isolate fetal sheep islets that were not previously exposed to the adrenergic receptor antagonists to avoid residual effects. The ewes and fetuses were anesthetized with ketamine (4.4 mg/kg) and diazepam (0.11 mg/kg) given intravenously. After a hysterotomy, the fetus was removed, blotted dry, and weighed. The ewe was then killed with intravenous concentrated pentobarbital sodium (10 ml; Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA). Islets were obtained from the fetal pancreas with a retrograde perfusion of digestive solution into the pancreatic ducts, as described previously (24). The digestion solution was Liberase BlendZyme III (0.175 mg/ml; Roche Diagnostics, Indianapolis, IN) in Krebs-Ringer buffer (KRB; 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, KH2PO4, 25 mM NaHCO3, pH 7.3) supplemented with 0.2% DNase I (Roche). The pancreas was dissected free, placed into an additional 20 ml of digestion buffer, and incubated at 37°C for 35–45 min. The partially digested tissue was washed three times with 5 volumes of KRB containing 0.5% BSA. Islets were purified over a discontinuous gradient of 10 ml of a 2:1 solution of Histopaque (1.119 g/ml; Sigma-Aldrich) and KRB-/BSA and centrifuged at 800 g for 20 min. Cell clusters were removed from the interface and rinsed once in KRB-BSA medium with a 1-min centrifugation (800 g). Islets were hand picked and cultured overnight at 37°C in 95%O2-5%CO2 in RPMI 1640 medium (Sigma-Aldrich) containing 2.8 mmol/l glucose supplemented with 1% fetal bovine serum and 1× penicillin-streptomycin-neomycin solution (50 U-50 μg-100 μg, Sigma-Aldrich) and then frozen for RNA extraction.
RNA preparation and analysis.
Total RNA was extracted from purified islet of Langerhans (9, 27, 40) using the RNeasy Micro Kit (Qiagen, Valencia, CA) or from perirenal adipose tissue (positive control) as previously described (9, 26). The quality and concentration of the RNA were determined by measuring absorbance at 260 and 280 nm with the NanoDrop ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE), and RNA integrity was evaluated with an Experion Automated Electrophoresis System (Bio-Rad Laboratories, Hercules, CA). Synthetic oligonucleotide primers were designed with the aid of OligoPerfect Designer (Invitrogen Life Technologies, Carlsbad, CA) software and purchased from Eurofins MWG Operon (Huntsville, AL) (primer sequences are available upon request). PCR products for ovine adrenergic receptors, α1 (A, B, D), α2 (A, B, C), and β (1, 2, 3), were amplified from perirenal fat mRNA by reverse transcription-PCR using Superscript III reverse transcriptase and Taq DNA polymerase (Qiagen) according to the manufacturer's instructions (Chen and Limesand, unpublished data). The PCR products of the correct size were inserted into the TOPO TA cloning expression vector pCRII (Invitrogen Life Technologies) and transformed into One Shot Mach T1 Phage-Resistant Chemically Competent E. coli (Invitrogen Life Technologies). Plasmids were prepared for nucleotide sequencing with a QIAprep Spin Miniprep Kit (Qiagen) and sequenced at the University of Arizona DNA Sequencing Service. Nucleotide sequences were submitted to GenBank (α1A, EU723257; α1B, EU851039, α1D, EU723258; α2A, EU726635; α2B, EU741650; α2C, EU723259; β1, AF072433, β2, EU723260; β3, AF314205.1).
The relative mRNA expression for each adrenergic receptor was determined by quantitative PCR using SYBR Green (Qiagen) in an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories) as reported previously (26). After the initial denaturation at 95°C for 15 min, all reactions went through 40 cycles of 96°C (30 s), annealing temperature of 60–62°C (30 s), and 72°C (10 s), at which point the fluorescence was measured. Melt curve analysis was performed at the end of the amplification to confirm product homogeneity. PCR efficiency was determined with islet cDNA and was linear over six orders of magnitude. Samples were run in triplicate for each qPCR reaction. The results were normalized to the reference gene S15 for each RT-PCR, and the average ΔCT (cycle threshold) was analyzed by the comparative ΔCT method (CT gene of interest − CT reference gene), with fold change being calculated with the 2−ΔΔCT method (28). Standard curves for each gene product were run concurrently to determine the absolute mass by linear regression analysis.
Statistical analysis.
All data are expressed as means ± SE. Period means for each animal were used for biochemical and hematological value comparisons. Statistical analyses for in vivo biochemical and hematological values were conducted by two-way ANOVA (treatment and study). Maternal temperatures, feed intake, fetal weights, and qPCR (ΔCT values) were analyzed by one-way ANOVA (treatment) using the general linear means procedure in SAS Proc GLM, and differences were determined with a post hoc least significant difference test or Student's t-test (43). β-Cell mass data were nonnormally distributed, and effects of IUGR were analyzed by a Wilcoxon two-sample test using the SAS NPAR1WAY procedure (43).
During the experimentally controlled steady-state periods for glucose (basal and hyperglycemic periods), insulin concentrations were also found to be constant; therefore, the four samples were averaged for that steady-state period within a fetus and used for subsequent analysis. Because there were repeated measures for each fetus, general linear mixed models (GLMM) was used. GLMM uses all data contributions for a fetus regardless of missing values and accounts for within-animal correlation related to longitudinal measures (7). Two random effects were fitted in the two final models: fetuses nested within ewe and the study arm (saline or adrenergic receptor antagonists). The latter was necessary because all fetuses were included in more than one study arm. Best fitting models and best fitting covariance patterns were assessed using likelihood ratio tests and/or the Akaike information criterion (AIC). One model was used to look at basal insulin concentration (statistical model 1) and a second model to look at hyperglycemic insulin concentration (statistical model 2). The final model used the difference between hyperglycemic and basal insulin concentrations (statistical model 3). Analyses were carried out using Stata 10 (StataCorps 2007; Statistical software, Release 10.0, College Station, TX).
RESULTS
Experimental parameters during treatment.
Within 10 days of exposure to elevated ambient temperatures, maternal core body temperatures rose from 39.0 ± 0.2 to 39.8 ± 0.3°C, reaching an average plateau of 39.7 ± 0.2°C for the 80 days of treatment, greater (P < 0.05) than the control ewes' average of 39.1 ± 0.2°C. After removal of the pregnant ewes from treatment conditions, their average core body temperature decreased to 39.3 ± 0.2°C, not different from the control average of 39.3 ± 0.2°C posttreatment. During the treatment, the average feed intake was 1.3 ± 0.1 kg/day. After removal of ewes from treatment conditions, pair feeding the average intake for IUGR fetuses continued with an average food intake of 1.3 ± 0.1 kg/day for the IUGR and control animals.
Fetal body and organ weights.
At autopsy, the gestational age was not different between control (133.8 ± 0.9 days) and IUGR (132.2 ± 0.5 days) fetuses. In the IUGR group, 80% of the fetuses were males compared with 44% males in the control group. IUGR fetuses weighed less than controls (1.51 ± 0.11 vs. 3.02 ± 0.17 kg, P < 0.05), and their placenta weights were lower (119 ± 20 vs. 187 ± 20 g, P < 0.05). Actual fetal weights for control and IUGR fetuses were 1 ± 6% higher and 34 ± 8% lower, respectively, than estimated weights. Average brain weight (41.1 ± 1.1 vs. 46.6 ± 1.9 g, P = 0.05) and liver weight (43.4 ± 4.2 vs. 78.2 ± 7.8 g, P < 0.01) were lower in IUGR fetuses than in controls, and the brain-to-liver ratio was increased (0.99 ± 0.10 vs. 0.63 ± 0.07, P < 0.05).
Fetal hematological values and catecholamine concentrations.
Fetal pH, blood gas, and oximetry variables at baseline (prior to infusing dextrose) during the GSIS study are shown in Table 1. In the saline GSIS study, no differences were found for pH, pco2, hematocrit, or bicarbonate between IUGR and control fetuses. IUGR fetuses were hypoxemic compared with controls and had a blood oxygen content that was 52% of that of controls.
Table 1.
Fetal hematology parameters for the baseline period
| AR Antagonists | Control Fetuses |
IUGR Fetuses |
||
|---|---|---|---|---|
| − | + | − | + | |
| pH | 7.36 ± 0.01 | 7.35 ± 0.01 | 7.37 ± 0.01 | 7.31 ± 0.01† |
| pco2, mmHg | 53.5 ± 1.2 | 55.6 ± 1.1 | 55.7 ± 1.0 | 60.0 ± 1.3 |
| po2, mmHg | 20.5 ± 0.6 | 17.4 ± 0.8† | 12.2 ± 1.1* | 10.6 ± 0.4* |
| O2 content, mmol/l | 3.3 ± 0.2 | 2.7 ± 0.2† | 1.7 ± 0.2* | 1.0 ± 0.1*† |
| O2 saturation, % | 49.9 ± 1.3 | 39.6 ± 3.3† | 25.0 ± 3.2* | 15.9 ± 1.1* |
| Hematocrit, % | 31.5 ± 1.4 | 32.5 ± 0.9 | 33.9 ± 0.9 | 31.0 ± 1.0 |
| Bicarbonate, mmol/l | 28.8 ± 0.3 | 29.2 ± 0.4 | 30.7 ± 0.3 | 28.4 ± 0.9 |
Values are means ± SE. AR, adrenergic receptor.
Significant differences, P < 0.05, for Control vs. IUGR fetuses in their respective study;
significant differences, P < 0.05, within a treatment group for without (–) vs. with (+) AR antagonists.
During the adrenergic receptor blockade GSIS study, the partial pressure of oxygen, blood oxygen content, and oxygen saturation fell significantly (P < 0.05) in control fetuses compared with baseline values in the GSIS study with saline (Table 1). Similar trends were observed in the IUGR fetuses, but only oxygen content was reduced significantly (P < 0.05) by the administration of adrenergic receptor antagonists. In control fetuses, no changes in pH, hematocrit, or bicarbonate concentrations were observed during the administration of adrenergic antagonists, which was similar for IUGR fetuses except that their pH was lower (P < 0.05; Table 1).
During the hyperglycemic period, similar patterns were observed between the GSIS studies with and without adrenergic antagonist in control fetuses (Table 2). In IUGR fetuses, oxygen content was not different between the saline and adrenergic blockade studies; however, an increase in the partial pressure of CO2 and decrease in bicarbonate concentrations were identified.
Table 2.
Fetal hematology parameters for the hyperglycemic period
| AR Antagonists | Control Fetuses |
IUGR Fetuses |
||
|---|---|---|---|---|
| − | + | − | + | |
| pH | 7.34 ± 0.01 | 7.33 ± 0.01 | 7.33 ± 0.01 | 7.20 ± 0.02*† |
| pco2, mmHg | 53.7 ± 1.2 | 56.7 ± 1.1 | 58.4 ± 1.8 | 66.1 ± 2.0*† |
| po2, mmHg | 19.9 ± 0.9 | 16.9 ± 0.8† | 11.4 ± 1.0* | 11.6 ± 0.6* |
| O2 content, mmol/l | 3.1 ± 0.2 | 2.4 ± 0.2† | 1.3 ± 0.2* | 0.9 ± 0.1* |
| O2 saturation, % | 47.3 ± 2.2 | 34.9 ± 3.0† | 21.6 ± 2.6* | 15.1 ± 1.1* |
| Hematocrit, % | 32.0 ± 1.2 | 32.6 ± 0.9 | 30.8 ± 1.6 | 29.3 ± 1.2 |
| Bicarbonate, mmol/l | 27.7 ± 0.4 | 27.8 ± 0.3 | 29.0 ± 0.3 | 23.9 ± 1.0*† |
Values are means ± SE.
Significant differences, P < 0.05, for Control vs. IUGR fetuses in their respective study;
significant differences, P < 0.05, within a treatment group for without (–) vs. with (+) AR antagonists.
Plasma epinephrine and norepinephrine concentrations were negatively associated with oxygen content, as reported previously for norepinephrine (27). At baseline during the saline GSIS, plasma epinephrine concentrations were 2.7-fold greater in IUGR than in controls, and the regression for epinephrine (x) vs. oxygen content (y) is y = −70.1x + 282.3, R2 = 0.54 (P < 0.01). During the same period, plasma norepinephrine concentrations were 5.7-fold greater in IUGR fetuses than in controls, with a regression line for norepinephrine (y) vs. oxygen content (x) of y = −1373x + 5,242, R2 = 0.62 (P < 0.01). Similar associations between catecholamine concentrations and oxygen were present during adrenergic receptor blockade study, explaining their rise with adrenergic antagonists. Basal epinephrine and norepinephrine concentrations (y) were also negatively associated with glucose (x), as described by the regression equations y = −140x + 249, R2 = 0.313 (P < 0.05) and y = −4138x + 6,024, R2 = 0.546 (P < 0.01), respectively. Epinephrine and norepinephrine concentrations for all steady-state periods are presented in Table 3.
Table 3.
Fetal catecholamine concentrations
| Period | Saline |
Blocker |
||
|---|---|---|---|---|
| Baseline | Hyperglycemic | Baseline | Hyperglycemic | |
| Control epinephrine, pg/ml | 60.3 ± 11.8 | 78.2 ± 19.1 | 75.9 ± 8.7 | 75.2 ± 12.8 |
| IUGR epinephrine, pg/ml | 163.5 ± 31.7* | 152.1 ± 52.6 | 1847 ± 856* | 2332 ± 1134* |
| Control norepinephrine, pg/ml | 570 ± 86 | 1171 ± 287 | 1090 ± 226 | 1683 ± 444 |
| IUGR norepinephrine, pg/ml | 3264 ± 614* | 3518 ± 1084* | 6758 ± 1802* | 11558 ± 3430* |
Values are means ± SE.
Significant differences, P < 0.05, in catecholamine concentrations for Control vs. IUGR fetuses in their respective study periods.
GSIS response to an adrenergic blockade.
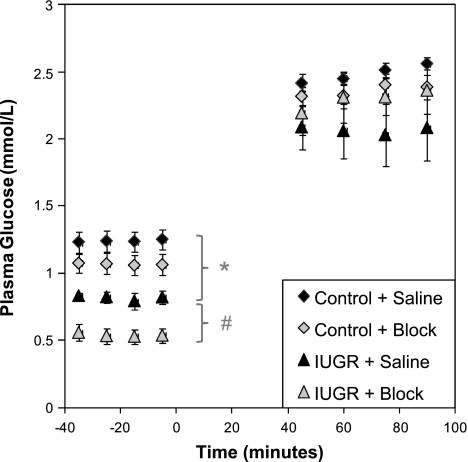
Fetal plasma glucose concentrations at basal and hyperglycemic steady-state periods are presented in Fig. 1. In IUGR fetuses at baseline, the mean plasma glucose concentration was lower (P < 0.05) than the control mean for the same GSIS study type (saline or block). Administration of adrenergic receptor antagonists reduced (P < 0.05) the average plasma glucose concentration in IUGR fetuses by 33% at baseline, whereas in control fetuses, plasma glucose concentrations were only marginally decreased (13%, P < 0.1). In accordance with the experimental design, mean plasma glucose concentrations during the GSIS hyperglycemic period were not different between treatments and GSIS studies.
Fig. 1.
Fetal glucose concentrations during glucose-stimulated insulin secretion (GSIS) clamp periods. Steady-state glucose concentrations for control (n = 9) and intrauterine growth-restricted (IUGR) (n = 5) fetuses during the Saline and adrenergic inhibition (Block) GSIS studies are presented (means ± SE). Time relative to the start of the exogenous dextrose bolus, time 0 min, is presented on the x-axis. *In IUGR fetuses, basal mean glucose concentrations were significantly less than in controls for the respective GSIS study. #Adrenergic blockade decreased (P < 0.05) basal glucose concentrations in IUGR fetuses. No differences in mean glucose concentrations were found during the hyperglycemic steady-state clamp.
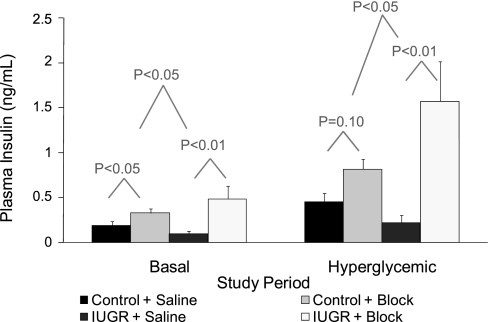
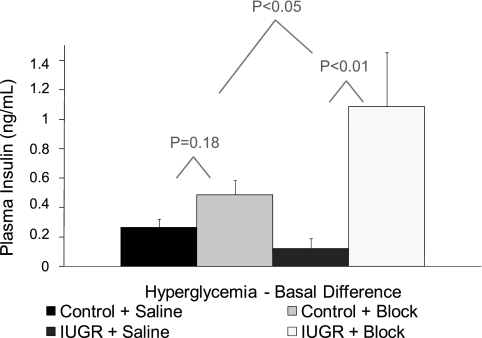
The average fetal plasma insulin concentrations during basal and hyperglycemic steady-state periods are presented in Fig. 2. Basal and glucose-stimulated plasma insulin concentrations were 48% (not significant) and 52% (P ≤ 0.05) lower in IUGR fetuses than in controls. Similar to our previous findings for this model (27), GSIS responsiveness, measured by the difference in insulin concentrations between hyperglycemic and baseline steady-state periods, during the saline-GSIS study was 54% lower (P ≤ 0.05) in IUGR fetuses than in control fetuses (Fig. 3).
Fig. 2.
Fetal insulin concentrations during GSIS steady-state periods. Mean plasma insulin concentrations are presented for control (n = 9) and IUGR (n = 5) fetuses (treatment groups) during basal and hyperglycemic steady-state periods of the Saline or Block (adrenergic inhibited) GSIS study. Differences between means within the steady-state period were determined independently (statistical models 1 and 2). The first tier of P values indicates comparisons between GSIS studies (Saline vs. Block) within a treatment group. The second tier of P values indicates comparisons between treatment groups for differences between GSIS studies.
Fig. 3.
GSIS responsiveness. Mean change in insulin is presented for control and IUGR treatments during the Saline or adrenergic inhibition (Block) GSIS study. Linear contrasts for Saline and Block GSIS studies within treatment groups were determined, and P values are presented in the 1st row above the bars being compared. The P value for the difference between treatments in response to adrenergic inhibition (Block − Saline-GSIS) is presented in the 2nd row.
The objectives of the present study were to determine the effect of elevated norepinephrine on fetal plasma insulin concentrations and fetal insulin secretion responsiveness to glucose in control and IUGR fetuses. In Fig. 2, the difference between the adrenergic inhibition (block) and saline-GSIS studies was examined during baseline (basal; statistical model 1) and hyperglycemic (statistical model 2) steady-state periods for each treatment condition. In control fetuses, plasma insulin concentrations increased 1.7-fold (P < 0.05) during the basal period in response to the adrenergic inhibition, but during the hyperglycemic period the 1.8-fold increase did not reach statistical significance (P = 0.10). In the IUGR fetuses with adrenergic inhibition, the average fetal insulin concentration increased 4.9-fold (P < 0.01) during basal conditions and 7.1-fold (P < 0.01) during the hyperglycemic period compared with the saline studies. Compared with control fetuses, mean plasma insulin concentrations in IUGR fetuses were elevated to a greater extent in response to the adrenergic receptor antagonists at both basal (P < 0.05) and hyperglycemic (P < 0.05) steady-state conditions (Fig. 2).
In addition to monitoring absolute differences in circulating insulin concentrations, we also determined GSIS responsiveness (i.e., the change in insulin responsiveness to glucose) in control and IUGR fetuses with and without adrenergic inhibition (Fig. 3; statistical model 3). In control fetuses, the glucose-induced change in insulin was not different (P = 0.18) between GSIS studies with or without adrenergic receptor antagonists. In contrast, the GSIS responsiveness was significantly increased, 8.9-fold, in IUGR fetuses with the adrenergic inhibition compared with the saline study (P < 0.01). Moreover, this increase in GSIS responsiveness in IUGR fetuses due to adrenergic inhibition was greater (P < 0.05) than the 1.8-fold increase in controls, showing that insulin secretion was blunted by norepinephrine in the IUGR fetuses (Fig. 3). Furthermore, GSIS responsiveness in IUGR fetuses with adrenergic inhibition was greater than in controls with saline (P < 0.01) and marginally higher than in controls with adrenergic inhibition (P < 0.1).
Pancreas endocrine area and β-cell mass.
The mean pancreatic insulin+ area was not different between IUGR (1.9 ± 0.2%) and control fetuses (2.3 ± 0.5%). The estimated β-cell mass was 56% lower (P < 0.05) in IUGR pancreases (29.2 ± 2.5 mg) compared with controls (65.9 ± 18.9 mg), indicating that the reduction was primarily due to smaller pancreas mass. The combination of glucagon-, somatostatin-, and pancreatic polypeptide-positive area was not different between IUGR (2.0 ± 0.3%) and controls (2.2 ± 0.1%), but the mass was less (P < 0.05) in IUGR pancreases (25.2 ± 4.6 mg) than in controls (62.8 ± 20.1 mg).
Adrenergic receptor expression in fetal sheep islets.
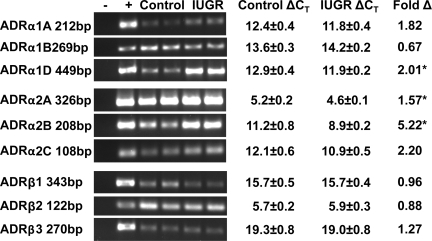
All nine adrenergic receptor subtypes were cloned for sheep, and each showed a high degree of identity with previously defined orthologs. In the pancreases collected from the fetuses used for the in vivo studies, mRNA concentrations for the predominant adrenergic receptors isoforms in the whole pancreas, α1 (A,B, and D), α2 (A,B, and C), and β2 were not different between twins and a previously collected group of control singleton fetuses (24). We also found no significant difference in adrenergic receptor expression levels between control and IUGR pancreas tissue, which indicates that changes in adrenergic receptor expression may be limited to the islets of Langerhans.
All of the adrenergic receptor isoforms were identified in isolated fetal sheep islets (Fig. 4). To avoid residual effects, adrenergic receptor concentrations were determined in fetal sheep islets collected from a second cohort of IUGR (n = 7) and control (n = 6) singleton fetuses that had not previously been exposed to the adrenergic receptor antagonists. Fetal tissues were collected at a similar age (132 ± 2 dGA controls and 134 ± 2 dGA IUGRs). IUGR fetuses were hypoxic (1.4 ± 0.3 vs. 3.4 ± 0.1 mmol/l), hypoglycemic (0.64 ± 0.05 vs. 1.13 ± 0.07 mmol/l plasma glucose concentrations), and growth restricted (1.7 ± 0.3 vs. 3.2 ± 0.3 kg), similar to the IUGR twin cohort. The sex ratios for the singleton control and IUGR groups were 50 and 29% males, respectively. The relative expression of adrenergic receptors α1D, α2A, and α2B were higher (P < 0.05) in the IUGR islets than in controls. No differences were found for the other six subtypes (Fig. 4).
Fig. 4.
Adrenergic receptor expression in fetal sheep islets. Adrenergic receptors (ADR) were cloned from sheep tissues, and their expression was determined for fetal sheep islets isolated from control or IUGR islets at 135 dGA. PCR products were separated by agarose gel electrophoresis and stained with ethidium bromide. Lanes for each gel include a negative control (no cDNA; −) and positive control (adipose; +) alongside 2 representative samples from islet mRNA extracted from control or IUGR islets. Quantitative PCR was performed (n = 6 controls and n = 7 IUGRs). ΔCT ± SE are presented for control and IUGR islets. Fold change (fold Δ) in IUGR islets from control islets was calculated by the 2−ΔΔCT method for all adrenergic receptor isoforms, and results are presented. Statistical analysis was performed on ΔCT values with ribosomal protein S15 as the reference gene (*P < 0.05 between treatment groups).
DISCUSSION
We have previously shown (23, 27) that insulin secretion was blunted in IUGR fetuses and that the insulin secretion deficiency was greater for fetuses with placental insufficiency than for those with chronic hypoglycemia alone, indicating that additional factors contribute to the defect in placental insufficiency-induced IUGR fetuses. In the current study, we confirm that plasma norepinephrine concentrations are elevated and act to suppress insulin secretion in IUGR fetuses because adrenergic inhibition reestablishes GSIS responsiveness. These findings, combined with the reduction in β-cell mass, indicate that insulin secretion is enhanced in β-cells of the IUGR fetus with acute adrenergic inhibition. Previous reports have shown that prolonged administration of catecholamines to fetal sheep result in diminished adrenergic responsiveness, where several metabolites and hormones returned to normal values, except insulin which remained lower (2, 4). Although adrenergic desensitization is a known phenomenon that has been shown to result from reductions in receptor expression (10), previous studies indicate that the islets of Langerhans respond differently (2, 30, 35, 53). Our present study supports this assertion. We show that transcripts for all adrenergic receptors are present in fetal sheep islet preparations; however, those primarily responsible for suppressing insulin secretion, α2-adrenergic receptors (21, 35), are expressed more robustly in IUGR fetuses, suggesting that receptor levels are maintained or even increased in IUGR islets. These data indicate that β-cells are chronically suppressed by elevated norepinephrine concentrations in the IUGR fetus. However, these islets appear to compensate for this chronic suppression by augmenting their insulin secretion-coupling pathway rather than by decreasing adrenergic receptor expression, thus explaining the predicted enhancement in GSIS responsiveness.
Most experimental conditions that were previously used to examine catecholamine suppression of insulin secretion were induced by acute fetal hypoxia or exogenous epinephrine administration (16, 20, 50). By contrast, placental insufficiency-induced IUGR fetuses are chronically hypoxic, and the severity worsens as gestation progresses. At 103 dGA, we have observed mild hypoxia in IUGR fetuses (28% less than control, P ≤ 0.05, n = 6/treatment; SW Limesand and WW Hay Jr, unpublished data), which by 133 dGA increased to 48% (Table 1). In control fetuses, blood O2 content was constant during this period. In addition, plasma norepinephrine concentrations were elevated in IUGR fetuses at 103 dGA (635 ± 104, n = 5 IUGR vs. 191 ± 91 pg/ml, n = 3 controls, P < 0.05; SW Limesand and WW Hay Jr, unpublished data) and at 133 dGA (this study and Ref. 27). At both gestational ages, the plasma norepinephrine concentrations were tightly associated with blood O2 content, which indicates that the adrenal medulla maintains its capacity to respond to low oxygen (42). Thus, our placental insufficiency-induced IUGR sheep model creates both chronic hypoxia and chronic exposure to elevated norepinephrine during the final trimester.
In the IUGR fetus at 103 dGA, the plasma norepinephrine concentrations, although higher than in age-matched controls, were within the normal range for control fetuses at later stages of gestation (8). A positive correlation was reported for norepinephrine and fetal age, showing that norepinephrine concentrations increase over the last third of gestation in sheep (8). To date, fetal norepinephrine action has not been fully evaluated to know whether these concentrations at 103 dGA are above threshold or, for that matter, whether β-cells are responsive to catecholamines, but these experiments are ongoing. In addition to developmental increases in norepinephrine secretion, as fetal outcomes to placental insufficiency progressively worsen (i.e., fetus becomes more hypoxic), norepinephrine concentrations will continue to rise, revealing that both developmental maturation and hypoxia will continued to increase plasma norepinephrine concentrations to suppress insulin secretion in IUGR fetuses, even if β-cell compensation is occurring.
Investigations in fetal sheep have shown that catecholamines inhibit insulin secretion by interacting with the α2-adrenergic receptors, because an α2-adrenergic receptor-specific antagonist, idazoxan, eliminates the β-cell response to epinephrine (21). An earlier study using a nonselective α-adrenergic receptor blocker, phentolamine, to block adrenergic receptors showed a sixfold increase in plasma insulin concentrations, which was not found when both α- and β-adrenergic receptors were blocked (Fig. 2), indicating direct and indirect actions for catecholamines on β-cells (20, 21, 49). Similarly, our findings show that the combination of pharmacological inhibitors does not substantially augment insulin secretion, as no difference was found in GSIS responsiveness in control fetuses (Fig. 3).
We also show that catecholamines suppress insulin secretion (∼40%) in the control fetuses at basal steady-state periods (Fig. 2). These findings differ from earlier reports, where adrenergic inhibitors had little effect in normal uncompromised pregnancies under basal conditions (21). One potential factor that might contribute to our observed difference includes the experimental design. In our study, all measurements were taken on each animal, and data were analyzed using repeated measures to reduce intra-animal variation as a confounding variable, at least within treatments, and this design might have increased our power to detect a difference.
Our studies show that a complete adrenergic blockade significantly increases GSIS responsiveness in IUGR fetuses compared with controls (Fig. 3). Moreover, the changes in insulin concentrations in IUGR fetuses almost exceed those of the controls with adrenergic inhibition and did exceed the normal responsiveness (saline-GSIS controls; Fig. 3). These findings, in combination with 56% less β-cell mass in IUGR fetuses, indicate that β-cells have compensated for the persistent norepinephrine suppression and are capable of secreting insulin more efficiently. Evidence supporting improvements in insulin release were also observed in static islet incubation experiments, where under stimulatory conditions the fraction of insulin released relative to the total insulin content was greater in IUGR islets than in controls (27). Therefore, insulin stimulus-secretion coupling is enhanced in the IUGR fetuses, but this overcompensation in GSIS responsiveness appears to be independent of norepinephrine responsiveness. In rat islets, compensatory actions to norepinephrine have been suggested to occur at the ATP-sensitive K+ channel, L-type voltage-gated Ca2+ channel, adenylate cyclase, and β-granule exocytosis processes of the stimulus-secretion coupling pathway (22, 46, 53).
Previously in IUGR singleton fetuses, insulin secretion responsiveness was found to be 82% lower than in controls (27), whereas in the current study using twin fetuses GSIS responsiveness was only 54% lower than in the controls. Two factors that could potentially contribute to this difference include fetal number and the uneven sex ratio. Differences have been found in pancreatic insulin secretion between twins and singletons (41). In twins, plasma glucose and insulin concentrations were lower, but the insulin profile and maximum concentration to an acute glucose bolus were similar, which resulted in greater area under the insulin curve (41). In contrast, insulin secretion in response to an arginine bolus was lower, which indicated that β-cell mass or insulin content is reduced in twins (38, 41). Comparisons between that study (41) and our current study, where insulin responsiveness in twins tended to be lower, are difficult to compare directly because they measure different phases of insulin secretion, which can differ (23). Our previous findings (24) showed that β-cell mass was less because both insulin+ area (insulin-positive area) and pancreas mass were decreased (24). In contrast, control twin fetuses have less (P < 0.01) pancreatic insulin+ area and β-cell mass compared with singleton control fetuses, which had an insulin+ area of 4.1 ± 0.3% and a β-cell mass of 160.8 ± 16.4 mg/g (24). The uneven sex ratio does not appear to have any impact on insulin secretion because when we examined control fetuses from the current and previous studies (23, 27) no difference was found for GSIS responsiveness between males (n = 16) and females (n = 18): 0.44 ± 0.7 and 0.41 ± 0.06 ng, respectively. Although, we show that pancreatic adrenergic receptor expression is not different between twins and singletons, we cannot rule out the possibility that there are changes in twin islets. Norepinephrine concentrations, however, are similar between control twins and singletons; therefore, we do not anticipate an upregulation in islet adrenergic receptor expression if it is a result of chronic exposure to elevated norepinephrine.
Studies with pharmacological agents and genetically engineered mice have shown that α2-adrenergic receptors are the predominant subtypes involved in inhibiting insulin secretion (14, 32, 35, 44). It is not surprising that all adrenergic receptor subtypes are present in our isolated fetal sheep islets (Fig. 4), because isolated islets are not devoid of acinar, endothelial, or other nonendocrine cells, which also express a variety of adrenergic receptor subtypes (45). However, it is surprising that adrenergic receptor expression of those receptors responsible for suppressing insulin secretion is greater in IUGR islets than in controls (Fig. 4). These findings support previous work showing that fetal sheep islets remain responsive to norepinephrine (2–4) and indicate that the β-cells themselves can contribute to this maintenance.
In addition to suppressing insulin secretion, norepinephrine might also serve a critical role in regulating fetal glucose metabolism and homeostasis via other physiological processes. IUGR fetuses with lower insulin concentrations appeared to have improved insulin sensitivity, because rates of glucose utilization were equivalent to those in uncompromised control fetuses (26). However, maintenance of glucose utilization in IUGR fetus appeared to rely on hepatic glucose production, as umbilical glucose uptake was ∼40% less than their glucose utilization rate (26). Therefore, we postulated that norepinephrine was at least partly responsible for increased hepatic glucose production in sheep fetuses with placental insufficiency (26, 27, 51). In the present study, adrenergic inhibition lowered glucose concentrations in IUGR fetuses (Fig. 1). This reduction might be the result of adrenergic actions on the liver via norepinephrine or glucagon, which were suggested to augment hepatic glucose production (26). It is difficult to discriminate between greater insulin sensitivity and catecholamine-stimulated hepatic glucose production, or both with the current study design.
In summary, we have shown that chronically elevated norepinephrine during the final trimester continually suppresses insulin secretion in placental insufficiency and IUGR sheep fetuses. This suppression of insulin would benefit the IUGR fetus by increasing glucose availability to tissues that are not insulin sensitive. Acute adrenergic inhibition results in a full recovery of GSIS responsiveness, suggesting that fetal β-cells have in some way compensated for the chronic suppression by increasing their ability to release insulin once the inhibition is removed. These data may begin to explain why IUGR or small-for-gestational-age infants have a propensity toward increased glucose requirements. Chronically elevated norepinephrine can increase glucose and insulin sensitivity, which would be further compounded by higher GSIS responsiveness if norepinephrine were suddenly decreased.
GRANTS
The project described was supported by Award No. R01 DK-084842 (Principle Investigator S. W. Limesand) from the National Institute of Diabetes and Digestive and Kidney Diseases and an Endocrine Society Bridge Grant (Principle Investigator S. W. Limesand). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
DISCLOSURES
No conflicts of interest are reported by the author(s).
ACKNOWLEDGMENTS
We thank Prof. William W. Hay, Jr. and Dr. Paul J. Rozance at the University of Colorado-Denver Health Sciences Center for scholarly discussions concerning this research and critical reading of the manuscript. We also thank Prof. Duane Sherrill at the University of Arizona for supervision of our statistical analysis.
REFERENCES
- 1.Barker DJP. Mother, Babies and Health in Later Life Edinburgh: Churchill Livintstone, 1998 [Google Scholar]
- 2.Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol Regul Integr Comp Physiol 274: R1536–R1545, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bassett JM, Hanson C. Prevention of hypoinsulinemia modifies catecholamine effects in fetal sheep. Am J Physiol Regul Integr Comp Physiol 278: R1171–R1181, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bassett JM, Symonds ME. Beta2-agonist ritodrine, unlike natural catecholamines, activates thermogenesis prematurely in fetal sheep. Am J Physiol Regul Integr Comp Physiol 275: R112–R119, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9: 17–29, 1987 [PubMed] [Google Scholar]
- 6.Beringue F, Blondeau B, Castellotti MC, Breant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes 51: 385–391, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Brown H, Prescott R. Applied Mixed Models in Medicine West Sussex, UK: Wiley & Sons, 2009 [Google Scholar]
- 8.Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia-direct and neural components. Am J Physiol Regul Integr Comp Physiol 258: R1340–R1346, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Cole L, Anderson MJ, Antin PB, Limesand SW. One process for pancreatic β-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol 203: 19–31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins S, Caron MG, Lefkowitz RJ. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu Rev Physiol 53: 497– 508,: 497–508, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Creasy RK, Resnik R. Intrauterine Growth Restriction. In: Maternal-Fetal Medicine, edited by Creasy RK, Resnik R. Philadelphia, PA: Saunders, 1999, p. 569–584 [Google Scholar]
- 12.Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol 563: 611–620, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fagerholm V, Gronroos T, Marjamaki P, Viljanen T, Scheinin M, Haaparanta M. Altered glucose homeostasis in alpha2A-adrenoceptor knockout mice. Eur J Pharmacol 505: 243–252, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 88: 234–243, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fowden AL. Effects of adrenaline and amino acids on the release of insulin in the sheep fetus. J Endocrinol 87: 113–121, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia 40: 1231–1234, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35: 595–601, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson BT, Cohn HE, Morrison SH, Baker RM, Piasecki GJ. Hypoxia-induced sympathetic inhibition of the fetal plasma insulin response to hyperglycemia. Diabetes 42: 1621–1625, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol 279: R2179–R2188, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259: 3–17, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol 547: 95–105, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Limesand SW, Regnault TR, Hay WW., Jr Characterization of glucose transporter 8 (GLUT8) in the ovine placenta of normal and growth restricted fetuses. Placenta 25: 70–77, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Milley JR. Ovine fetal metabolism during norepinephrine infusion. Am J Physiol Endocrinol Metab 273: E336–E347, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Morgan NG, Montague W. Studies on the mechanism of inhibition of glucose-stimulated insulin secretion by noradrenaline in rat islets of Langerhans. Biochem J 226: 571–576, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Niddam R, Angel I, Bidet S, Langer SZ. Pharmacological characterization of alpha-2 adrenergic receptor subtype involved in the release of insulin from isolated rat pancreatic islets. J Pharmacol Exp Ther 254: 883–887, 1990 [PubMed] [Google Scholar]
- 33.Owens JA, Thavaneswaran P, De Blasio MJ, McMillen IC, Robinson JS, Gatford KL. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am J Physiol Endocrinol Metab 292: E1879–E1889, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, Buscaglia M, Battaglia FC. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med 328: 692–696, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Peterhoff M, Sieg A, Brede M, Chao CM, Hein L, Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur J Endocrinol 149: 343–350, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res 2: 139–143, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies. Placenta 23, Suppl A: S119–S129, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 56: 2420–2424, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res 65: 72–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozance PJ, Limesand SW, Hay WW., Jr Decreased Nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab 291: E404–E411, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rumball CW, Harding JE, Oliver MH, Bloomfield FH. Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose-insulin axis function in ovine pregnancy. J Physiol 586: 1399–1411, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rychkov GY, Adams MB, McMillen IC, Roberts ML. Oxygen-sensing mechanisms are present in the chromaffin cells of the sheep adrenal medulla before birth. J Physiol 509: 887–893, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SAS Institute Inc. SAS/STAT User's Guide Cary, NC: SAS Institute, 1999 [Google Scholar]
- 44.Savontaus E, Fagerholm V, Rahkonen O, Scheinin M. Reduced blood glucose levels, increased insulin levels and improved glucose tolerance in alpha2A-adrenoceptor knockout mice. Eur J Pharmacol 578: 359–364, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343: 230–238, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol Cell Physiol 271: C1781–C1799, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57: 107–118, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Sperling MA, Christensen RA, Ganguli S, Anand R. Adrenergic modulation of pancreatic hormone secretion in utero: studies in fetal sheep. Pediatr Res 14: 203–208, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Sperling MA, Ganguli S, Leslie N, Landt K. Fetal-perinatal catecholamine secretion: role in perinatal glucose homeostasis. Am J Physiol Endocrinol Metab 247: E69–E74, 1984 [DOI] [PubMed] [Google Scholar]
- 51.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol Regul Integr Comp Physiol 263: R578–R585, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Urano Y, Sakurai T, Ueda H, Ogasawara J, Sakurai T, Takei M, Izawa T. Desensitization of the inhibitory effect of norepinephrine on insulin secretion from pancreatic islets of exercise-trained rats. Metabolism 53: 1424–1432, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977 [DOI] [PubMed] [Google Scholar]
- 55.Wallace JM, Regnault TR, Limesand SW, Hay WW, Jr, Anthony RV. Investigating the causes of low birth weight in contrasting ovine paradigms. J Physiol 565: 19–26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]