Abstract
AMP-activated protein kinase (AMPK) and the histone/protein deacetylase SIRT1 are fuel-sensing molecules that have coexisted in cells throughout evolution. When a cell's energy state is diminished, AMPK activation restores energy balance by stimulating catabolic processes that generate ATP and downregulating anabolic processes that consume ATP but are not acutely needed for survival. SIRT1 in turn is best known historically for producing genetic changes that mediate the increase in longevity caused by calorie restriction. Although the two molecules have been studied intensively for many years, only recently has it become apparent that they have similar effects on diverse processes such as cellular fuel metabolism, inflammation, and mitochondrial function. In this review we will examine the evidence that these similarities occur because AMPK and SIRT1 both regulate each other and share many common target molecules. In addition, we will discuss the clinical relevance of these interactions and in particular the possibility that their dysregulation predisposes to disorders such as type 2 diabetes and atherosclerotic cardiovascular disease and is a target for their therapy.
Keywords: adenosine 5′-monophosphate-activated protein kinase, sirtuin 1, metabolic syndrome, mitochondrial function, insulin resistance, peroxisome proliferator-activated receptor-γ coactivator-1α, type 2 diabetes, atherosclerosis
amp-activated protein kinase (AMPK) is a fuel-sensing enzyme that is activated by decreases in a cell's energy state as reflected by an increased AMP/ATP ratio. When activated, it initiates metabolic and genetic events that restore ATP levels by stimulating processes that generate ATP (e.g., fatty acid oxidation) and inhibiting others that consume ATP but are not acutely required for survival (e.g., triglyceride and protein synthesis, cell proliferation) (40). In addition, AMPK sets in motion changes in mitochondrial biogenesis and function (37) that could more chronically increase the ability of a cell to generate ATP and diminish oxidative stress and other potentially adverse cellular events (78). The sirtuins are a family of evolutionarily conserved NAD+-dependent histone/protein deacetylases that are also widely regarded as fuel-sensing molecules. They have many actions (see below) but are perhaps best known for their role in mediating the increase in longevity caused by caloric restriction in various species, including yeast, worms, and possibly mammals (21, 85). Seven sirtuins have been identified in mammalian cells. Of these the most studied and the focus of this review is silent information regulator T1 (SIRT1).
AMPK and the sirtuins are present in all eukaryotic cells and probably have coexisted throughout evolution (7, 29). Although both molecules have been studied intensively, the similarities in their regulation and in their actions on such diverse processes as cellular metabolism, inflammation, and mitochondrial function have only recently become apparent. In this review we will examine the evidence that these similarities occur, at least in part, because AMPK and SIRT1 both regulate each other and share many common target molecules. In addition, we will discuss the clinical ramifications of the interaction of these molecules and in particular the possibility that their dysregulation predisposes humans and experimental animals to the metabolic syndrome and associated disorders and is a target for their therapy.
REGULATION OF AMPK AND SIRT1
AMPK
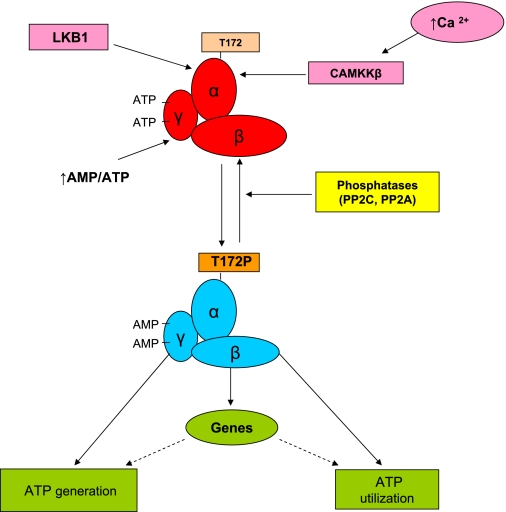
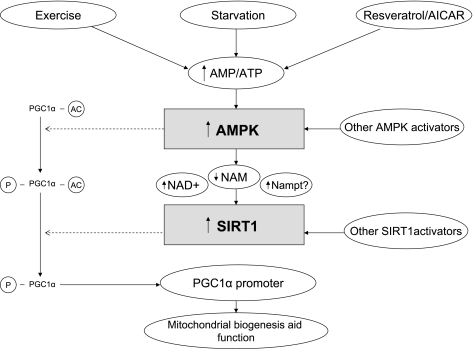
The currently accepted mechanisms of AMPK activation are depicted in Fig. 1 (29, 88, 99). Two upstream kinases, serine-threonine liver kinase B1 (LKB1; the primary AMPK kinase) and calcium/calmodulin kinase kinase-β (CaMKKβ; an AMPK kinase), activate AMPK by phosphorylating a threonine residue (Thr172) on its catalytic α-subunit (9). LKB1 is the principal AMPK kinase that catalyzes this phosphorylation when it occurs in response to a decrease in energy state, such as that produced by nutrient deprivation or increased energy expenditure (e.g., exercise). CaMKKβ phosphorylates Thr172 and activates AMPK in response to increases in intracellular Ca2+ caused by various hormones and possibly shear stress, and it can do so in the absence of LKB1 (32). It is a major regulator of AMPK in brain and also appears to function in vascular endothelium (15), B lymphocytes (95), and possibly, under some circumstances, skeletal muscle (75). LKB1, which was first identified as a tumor suppressor, regulates AMPK in almost all cells (100). In addition, it activates 13 other downstream kinases that are collectively referred to as AMPK-related kinases (ARKs) and whose functions are incompletely understood (1). Transforming growth factor-β-activated kinase-1) has also been identified as an AMPK kinase; however, its physiological role has been much less studied (99). LKB1 is necessary for AMPK activation by many factors, including metformin in liver (84) and exercise in skeletal muscle (81). On the other hand, several studies have suggested that LKB1 may be constitutively active, at least in skeletal muscle (82), and that AMPK activation in response to an increase in the AMP/ATP ratio results from a change in its conformation that makes phosphorylated AMPK resistant to the action of protein phosphatases. As shown in Fig. 1, when activated in an energy-stressed cell, AMPK sets in motion a wide variety of events that both acutely and subacutely increase ATP. The latter is due to its effects on various transcriptional activators and coactivators, including peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), as will be discussed in more detail later.
Fig. 1.
AMP-activated protein kinase (AMPK) activation and its regulation (29). AMPK is a heterotrimer consisting of a catalytic α-subunit (α1, α2) and regulatory β- and λ-subunits (β1, β2, α1, α2, and α3), all of which are required for its activity. Heterotrimers containing the α1 subunit are exclusively cytoplasmic; however, α2-containing AMPK is also found in the nucleus, where its presence may increase after exercise (56). The γ-subunit contains several cystathione β-synthase domains that under baseline conditions predominantly bind ATP. When a cell is energy stressed and the AMP/ATP ratio increases, AMP replaces ATP on 2 of these domains (83). This results in a conformational change that 1) causes a modest increase in AMPK activity (2- to 10-fold) and 2) enhances the phosphorylation of Thr172 on the α-subunit, which results in a much greater activation of the enzyme. The active enzyme then phosphorylates multiple molecules (enzymes, transcriptional activators, and coactivators), with the end result a restoration of the cell's energy state. Serine-threonine liver kinase B1 (LKB1) is required for this phosphorylation; however, it is less clear whether changes in LKB1 activity specifically regulate it. Thus studies, predominantly in skeletal muscle, have suggested that LKB1 is constitutively active and that the conformational change induced by an increase in the AMP/ATP ratio increases phosphorylation at Thr172 by making AMPK resistant to the action of phosphatases (82). On the other hand, as is described in the text, activation of LKB1 and subsequently AMPK has been observed in various cells and tissues when silent information regulator T1 (SIRT1) is activated, and conversely, decreases in SIRT1 are associated with diminished LKB1 and AMPK activity (34, 48, 90). Not shown in the figure is that nutrient and O2 deprivation, increased energy expenditure (exercise), and various hormones typically initiate AMPK activation, whereas nutrient excess (e.g., high glucose) and other hormones (e.g., glucocorticoids) downregulate AMPK, leading to decreases in fatty acid oxidation and increases in lipid and protein synthesis (75). CaMKKβ, calcium/calmodulin kinase kinase-β; PP2A and PP2C, protein phosphatases 2A and 2C, respectively.
SIRT1
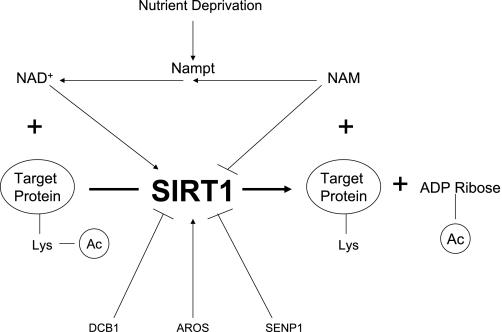
SIRT1 (21, 46, 85) is widely expressed in mammalian cells and has been studied in many tissues, including liver, skeletal muscle, adipose tissue, pancreas (β-cells), brain (101), and endothelium (69). Its regulation is somewhat less clear than that of AMPK; however, a substantial body of evidence suggests that, like AMPK, SIRT1 responds to increases and decreases in nutrient availability (caloric restriction or starvation) (10, 13, 60) and energy expenditure (8, 93). Regulation of SIRT1 has been attributed to changes in NAD+ abundance and the NAD+/NADH ratio, the concentration of nicotinamide (NAM), an end product of the deacetylase reaction and a SIRT1 inhibitor, and the activity of NAM phosphoribosyltransferase (Nampt; visfatin), which catalyzes the reconversion of NAM to NAD+ (see Fig. 2) (53, 57, 73). In addition, there appears to be genetic regulation of SIRT1 by the action of forkhead box-containing protein and p53 (60) on the SIRT1 promoter as well as regulation by various other factors (see Fig. 2 and its legend).
Fig. 2.
Regulation of SIRT1 (21). SIRT1 is an NAD+-dependent histone/protein deacetylase whose activity is regulated by nutrient availability. It has been proposed that nutrient deprivation (shown in the figure) increases SIRT1 activity by increasing the abundance of NAD+ and decreasing the abundance of nicotinamide (NAM), a product of the reaction, and NADH, both of which inhibit SIRT1. NAM phosphoribosyltransferase (Nampt) catalyzes the conversion of NAM to NAD+; therefore, it activates SIRT1 both by increasing cellular NAD+ and diminishing NAM. Exercise has been shown to increase Nampt activity in human muscle (14). Nutrient excess appears to have opposite effects on SIRT1 activity and these regulatory factors. Nampt, sometimes referred to as visfatin, is also found in the circulation and has been reported to increase insulin sensitivity (92). AMPK may mediate the activation of SIRT1 caused by fuel deprivation and other stimuli by its effects on these molecules (8, 23). SIRT1 can also be activated directly by binding the nuclear protein to active regulator of SIRT1 (AROS) (42) and inhibited by interaction with DBC1 (deleted in breast cancer; an inhibitor SIRT1 deacetylation) (43) and binding to SUMO1/snetrin-specific peptidase (SENP1) (102), which catalyzes its desumoylation. SIRT1 expression and abundance may also be upregulated by endothelial nitric oxide synthase (eNOS)/nitric oxide, the mRNA binding protein HuR (28), and a p53:FOXO3a complex and downregulated by p53 and a H1C1:CtBP corepressor complex (not shown). Of the latter molecules, AMPK has been demonstrated to influence eNOS, p53, and FOXO activity by causing their phosphorylation (60); however, the physiological relevance of this with regard to SIRT1 regulation is unclear. Finally, SIRT1 can be found in both the nucleus and the cytoplasm, depending on cell type and conditions, and movement between these compartments could be another determinant of its actions (94). Lys and Ac.
AMPK Regulation by SIRT1
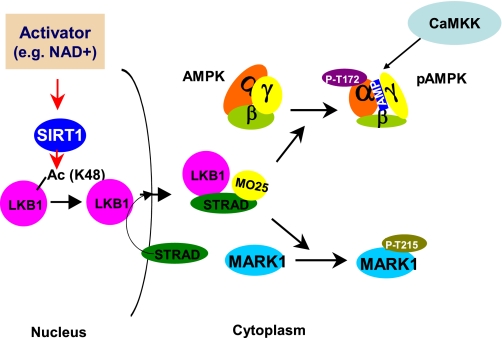
Downregulation of AMPK in response to high glucose exposure and in the apparent absence of a change in energy state was first shown to occur in an incubated rat extensor digitorum muscle preparation (36). Several years later, a similar change in response to high glucose, together with a decrease in SIRT1 activity, was observed in cultured HepG2 cells (90, 107). In both situations an increase in the release of lactate occurred, suggesting a decrease in the NAD+/NADH ratio, which could have contributed to the decrease in SIRT1 activity. These findings and the concurrent demonstration by many laboratories of common activators, actions, and target molecules of SIRT1 and AMPK (Fig. 3) led to an examination of a possible linkage between SIRT1 and the AMPK kinase LKB1. In one study, Lan et al. (48) demonstrated that overexpression of SIRT1 in human embryonic kidney-293T cells diminished lysine acetylation of LKB1 (K48) and caused its movement from the nucleus to the cytoplasm, where LKB1 can associate with the adaptor proteins STE20-related adaptor protein and mouse embryo scaffold protein, resulting in its own activation and subsequently that of AMPK (Fig. 4). In contrast, transfection with short hairpin (sh)RNAi for SIRT1 had opposite effects on these parameters and also decreased the phosphorylation of another LKB1 target, the AMPK-related kinase MARK1. Consistent with these findings in cultured cells, the same group found decreased LKB1 and AMPK activity and increased lysine acetylation of LKB1, suggestive of SIRT1 downregulation, in the liver of 48-h-starved rats 24 h after refeeding.
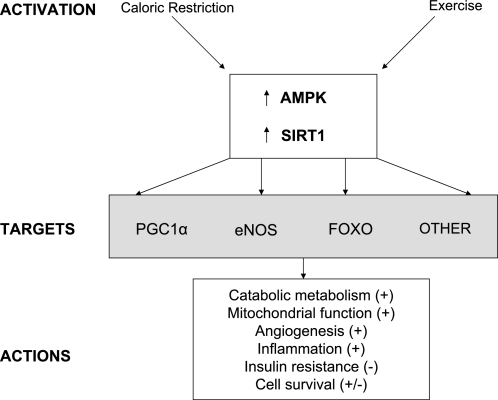
Fig. 3.
Commonalities between AMPK and SIRT1. Both AMPK and SIRT1 are activated in vivo in many tissues by caloric restriction and exercise as well as treatment with resveratrol and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (see below). In addition, they have many common target molecules and biological actions (7, 22, 46). A case in point: the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which in muscle (26) and other tissues is a master regulator of mitochondrial biogenesis and function, is illustrated in Fig. 5. FOXO, forkhead box-containing protein.
Fig. 4.
Proposed mechanism for activation of LKB1 and LKB1 target molecules by SIRT1 Activation of SIRT1 by genetic or pharmacological means in human embryonic kidney-293T cells (and presumably others) leads to deacetylation of Lys48 and possibly other key lysine residues on LKB1. This in turn enhances LKB1 binding to STE20-related adaptor protein (STRAD) and mouse embryo scaffold protein (MO25), which activates its kinase activity and leads to the phosphorylation of AMPK. The scheme assumes that SIRT1 is primarily nuclear and that LKB1 acetylation occurs in the nucleus and in some way enhances its movement to the cytoplasm (where it binds to STRAD). Since under some circumstances SIRT1 may be found in the cytoplasm, it is also possible that LKB1 acetylation could be an extranuclear event. In addition to AMPK, LKB1 phosphorylates and activates MARK1 and 12 other AMPK-related kinases (ARKs). CaMK kinase (CaMKK), which phosphorylates and activates AMPK even in the absence of LKB1, presumably would not activate the ARKs unless the increase in AMPK activity activated SIRT1 and, secondarily, LKB1 (adapted from Ref. 48).
Further evidence for a SIRT/LKB1/AMPK signaling mechanism was concurrently obtained by Hou et al. (34), who demonstrated that the ability of polyphenols (resveratrol, SI 17834) to activate AMPK in cultured HepG2 cells and mouse liver in vivo required the presence of both SIRT1 and LKB1. Likewise, in studies carried out predominantly in HepG2 cells, Suchankova et al. (90) noted that incubation with 25 vs. 5 mM glucose (6 h) or the SIRT1 inhibitor NAM (10 mM, 2 h) downregulated the activity of both AMPK and SIRT1 (indicated by increased PGC-1α acetylation), whereas incubation with pyruvate and the SIRT1 activator quecertin increased both of their activities. Furthermore, where studied, these findings occurred in the absence of a change in whole cell energy state (90), although the presence of a local change in energy state could not be excluded. Similar effects of pyruvate and glucose on SIRT1 had been described previously in primary hepatocytes, suggesting that they are not unique to HepG2 cells (76).
ACTIVATION OF SIRT1 BY AMPK
Concurrent with the demonstration of AMPK activation by SIRT1, two groups reported that AMPK can function as a SIRT1 activator (Fig. 5). In the first of these studies, Fulco et al. (23) observed that glucose restriction (incubation with 5 vs. 25 mM glucose for 48 h) impaired the differentiation of C2C12 skeletal muscle myoblasts, an effect preceded by a much earlier decrease in cellular ATP and an increase in the activity of AMPK. They also found that incubation for 36 h with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), a more direct AMPK activator, produced a similar sequence of events. In both situations, the inhibition of myoblast differentiation was accompanied by an increased transcription of the NAD+ biosynthetic enzyme Nampt, which in turn increased the NAD+/NADH ratio and decreased the concentration of NAM. Conversely, both myoblasts derived from SIRT1+/− mice and cells transduced with shRNAi for SIRT1 were resistant to the effect of AMPK activation on muscle differentiation, strongly suggesting that it was SIRT1 mediated.
Fig. 5.
Proposed mechanisms by which AMPK both activates SIRT1 and cooperates with it in enhancing the ability of PGC-1α to stimulate mitochondrial biogenesis and function. This schema, based on studies carried out primarily in skeletal muscle and cultured myocytes, assumes that the activation of AMPK and the phosphorylation of PGC-1α are early events and that activation of SIRT1 and PGC-1α deacetylation occur later. It has been suggested that the phosphorylation of PGC-1α by AMPK makes it more susceptible to deacetylation by SIRT1 (8) and enhances its ability to activate its own promoter (37). Whether Nampt activation is pivotal for SIRT1 activation by AMPK is unclear (cf. Refs. 8 and 23).
In a subsequent study, Canto and Auwerx (7) demonstrated that AMPK activation by AICAR increased PGC-1α-mediated gene expression in a SIRT1-dependent manner in C2C12 myocytes and mouse embryonic fibroblasts. They went on to show that various AMPK activators, including AICAR, metformin, and the mitochondrial uncoupler dinitrophenol, none of which can directly activate SIRT1, increased NAD+ levels and the NAD+/NADH ratio and that this resulted in SIRT1 activation, as evidenced by the deacetylation and activation of PGC-1α (Fig. 5). Importantly, an increased NAD+/NADH ratio was also observed in skeletal muscle after an exhaustive bout of exercise, suggesting that a similar sequence of events may also take place in vivo (7). Thus two independent groups have shown that SIRT1 activity increases following AMPK activation. They differ in that Fulco et al. (23) proposed that upregulation of Nampt was the key linkage between increased AMPK and SIRT1 activities, whereas Canto et al. (8) found that AMPK could alter the NAD+/NADH ratio and activate SIRT1 apparently independently of Nampt.
THE PUTATIVE SIRT1/AMPK CYCLE AND ITS REGULATION
The observation that AMPK and SIRT1 can activate each other raises the possibility that they are components of a cycle. Such a notion is attractive since one molecule responds to changes in energy state and the other to alterations in NAD+, and they both appear to regulate cellular biology and metabolism in a similar manner. On the other hand, the relevant data supporting the existence of such a cycle are preliminary, and many questions need to be addressed. For instance, the well-known citric acid (Krebs) and urea cycles involve events that appear to be quite rapid (s/min). In contrast, in contracting muscle in vivo and cultured C2C12 cells incubated with AICAR and other AMPK activators, AMPK activation is an early event (s/min), but SIRT1 activation appears to take place much later (4–12 h) (8, 23), although an increase in SIRT1 protein expression has been observed in rat muscle 2 h after exercise (93). Presumably, if a cycle exists, the activation of SIRT1 in these circumstances, at least in muscle, is a later event and would presumably act to sustain the activation of AMPK. However, this remains to be proven.
Also requiring study is the relation between changes in SIRT1 and AMPK under different conditions and in tissues other than skeletal muscle. With respect to the latter, concurrent decreases in AMPK and apparently SIRT1 activity have been observed in the liver of 48-h-starved rats 24 h after refeeding (48), and both molecules appear to be activated by starvation in adipose tissue (65, 86). Likewise, in HepG2 cells (90), pyruvate activates and NAM inhibits both AMPK and SIRT1, and they do so within 1–2 h, suggesting that their interaction is much more rapid than in C2C12 cells. Finally, under some conditions, it is clear that the proposed cycle does not operate as described here. Thus, in mice carrying two null alleles, for SIRT1, decreased SIRT1 in liver was associated with increased rather than decreased AMPK phosphorylation (Thr172), a finding attributed to a decrease in energy state as a result of impaired mitochondrial function (5).
THE AMPK AND SIRT1 PARTNERSHIP: CLINICAL IMPLICATIONS
The Metabolic Syndrome
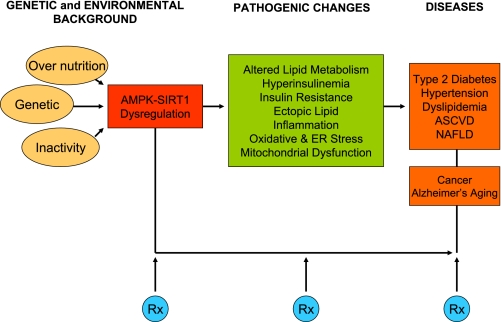
Just as research in the SIRT1 field has focused substantially on the notion that in some fashion SIRT1 and other sirtuins provide the connection between what we eat and how long we live (21), research on the clinical relevance of AMPK has focused on its relation to the metabolic syndrome and associated diseases (29, 40, 78, 79). As shown in Fig. 6, the metabolic syndrome is a disorder in which genetic factors coupled with overnutrition and inactivity produce pathogenic changes that predispose individuals to a wide array of common diseases, including type 2 diabetes, atherosclerosis, hypertension, nonalcoholic fatty liver disease, certain cancers, and possibly Alzheimer's disease. It is presently diagnosed clinically by the presence of at least three of the following: abdominal obesity, hyperglycemia (fasting glucose > 100 mg/dl), hypertension, hypertriglyceridemia, and decreased HDL cholesterol (18). The putative pathogenetic changes, including hyperinsulinemia, insulin resistance, and abnormalities in lipid metabolism and mitochondrial gene expression, may antedate these diagnostic criteria and even more so the diseases that follow their appearance by many years (77). A fundamental question is whether abnormalities in AMPK and SIRT1 accompany or precede these pathogenic changes. Another is whether therapies that activate AMPK and/or SIRT1 are useful for their treatment and prevention.
Fig. 6.
The metabolic syndrome: pathogenetic factors and associated diseases. A combination of overnutrition, inactivity, and genetic and other factors interact to produce a state of metabolic susceptibility that we are proposing leads to dysregulation of AMPK and SIRT1. This in turn could lead to pathogenetic factors for the metabolic syndrome, such as insulin resistance, hyperinsulinemia, and mitochondrial dysfunction, and abnormalities in cellular lipid metabolism. The latter is reflected typically by modest increases in plasma triglycerides and ectopic lipid deposition in muscle and liver. Systemic evidence of inflammation is often a later event (66). The transition from this preclinical stage to the clinically diagnosed metabolic syndrome to overt disease may take many years, with the rate of progression determined by environmental factors such as diet and exercise, genetic predisposition, and pharmacotherapy. Presumably, treatments (Rx) targeted at AMPK and SIRT1 would be useful at all stages but would be most effective early before abnormalities in tissues and cells such as oxidative modification of proteins and DNA, plaque formation in arteries, and capillary rarefaction in muscle and other tissues occur. ER, endoplasmic reticulum; ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease.
AMPK and SIRT1 Activity in the Setting of the Metabolic Syndrome
Humans.
Assays of AMPK and SIRT1 in humans with the metabolic syndrome have been limited. With respect to AMPK, studies in skeletal muscle of obese, insulin-resistant humans have yielded mixed results, with some showing decreased AMPK activity or impaired activation (2, 17, 87) and others no difference from control individuals (33). Recently, decreased AMPK activity has been found in adipose tissue of patients with high circulating glucocorticoids due to Cushing's Syndrome (11) and of markedly obese insulin-resistant individuals undergoing bariatric surgery (25). SIRT1 was not assayed in either study; however, it is noteworthy that, in another study, SIRT1 mRNA and AMPK activity were significantly increased (as was insulin sensitivity) in muscle of obese, insulin-resistant patients by 6 mo of caloric restriction that resulted in a 10% loss of body weight (12). Likewise, increased SIRT1 mRNA expression has been observed in subcutaneous adipose tissue of human volunteers after 6 days of total starvation, and in the same study, significantly greater SIRT1 expression was observed in lean compared with obese women (65).
Experimental animals.
In contrast to the findings in humans, clear-cut decreases in AMPK activity have been observed in multiple tissues of rodents with the metabolic syndrome, including ob/ob (105) and IL-6-knockout mice (41), fa/fa (80), Zucker diabetic fatty (105), and Otsuka Long-Evans Tokushima fatty (OLETF) rats (50), and rats and mice with various forms of diet-induced obesity (75). Decreased SIRT1 activity in turn has been observed in cardiac muscle of the OLETF rat (54) and adipocytes from ob/ob mice (72, 89); however, measurements of SIRT1 in other rodents with the metabolic syndrome and decreased AMPK activity appear to be lacking.
Effects of AMPK and SIRT1 Activation on the Metabolic Syndrome and Its Pathogenetic Factors
Humans.
Studies in humans have demonstrated that regular exercise, calorie restriction, metformin, and thiazolidinediones, all of which have been shown to activate AMPK in humans as well as in rodents, diminish many of the pathogenetic factors for the metabolic syndrome (75, 78, 98), as well as endothelial cell dysfunction, which is generally regarded as an early manifestation of atherosclerotic vascular disease (69). In keeping with this presumption, these treatments also have been shown to diminish the prevalence of metabolic syndrome-associated diseases, including type 2 diabetes, atherosclerosis, certain cancers, and possibly even Alzheimer's disease (see legend of Table 1) (75). Studies of the effects of drugs that activate SIRT1 in humans with the metabolic syndrome are currently in progress (49, 58). Interestingly, 3 wk of physical training has been shown to increase Nampt protein by 127% in muscle of previously sedentary individuals, and in the same study, twofold higher levels of Nampt protein were found in muscle of athletes compared with sedentary obese and nonobese individuals and patients with type 2 diabetes (14).
Table 1.
Pathogenetic factors for the metabolic syndrome that are improved by AMPK and SIRT1 and the target molecules that likely mediate their effects
| Pathogenetic Factors or Markers | AMPK | SIRT1 | Likely Target Molecules |
|---|---|---|---|
| Insulin resistance | −78 | (52, 91, 104) | PGC-1α, NF-κB, eNOS, p53 |
| Oxidative stress | (24, 45) | (52, 89) | PGC-1α |
| Inflammation | (6, 31) | (52, 103, 104) | NF-κB |
| Lipid abnormalities | (29, 78) | (21, 51) | PGC-1α, SREBP-1c, LXR |
| Mitochondrial dysfunction | (8, 35, 62, 74) | (8, 22, 52, 61) | PGC-1α, eNOS |
| Endothelial cell dysfunction | (35, 109) | (21, 55, 62, 64, 69) | eNOS |
| Decreased plasma adiponectin | (30, 39, 59) | (71, 72) | Unknown |
Relevant references are in parentheses. AMPK, AMP-activated protein kinase; SIRT1, silent information regulator T1; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; eNOS, endothelial nitric oxide synthase; SREBP-1c, sterol regulatory element-binding protein-1c; LXR, liver X receptor. Lipid abnormalities could include impaired fatty acid oxidation, increased diacylglycerol and ceramide synthesis, and ectopic lipid deposition, all of which are associated with the metabolic syndrome. Adiponectin is an AMPK activator, and decreases in its plasma concentration have been associated with a predisposition to both diabetes and coronary heart disease. SIRT1 has been shown to increase adiponectin synthesis by adipocytes. In keeping with the likely clinical relevance of these effects, multiple pharmacological and physiological AMPK and SIRT1 activators have been shown to diminish or prevent atherosclerosis (106, 108), type 2 diabetes (3, 67, 68, 105), hepatic steatosis (nonalcoholic fatty liver disease) (70, 78), and Alzheimer's disease (75) in experimental animals. Likewise, calorie restriction and exercise, and for some diseases pharmacological AMPK activators such as metformin, have shown similar benefits in humans (44, 75, 97).
Experimental animals.
With respect to AMPK, similar findings have been observed in experimental animals. Thus, in the Zucker diabetic fatty rat in which AMPK activity is decreased, various therapies that activate AMPK, including AICAR, exercise, metformin, thiazolidinediones, and calorie restriction, have been shown to improve insulin sensitivity, decrease ectopic lipid deposition, and prevent the development of diabetes and pancreatic β-cell damage (68, 105). Likewise, similar results have been obtained in other rodents in which the metabolic syndrome was accompanied by decreased AMPK activity (75, 98).
Positive effects of SIRT1 activation have also been observed in rodents with the metabolic syndrome. Thus, moderate SIRT1 overexpression produced with a bacterial artificial chromosome overexpressor was demonstrated to increase insulin sensitivity and prevent the development of diabetes in both C57BL6 mice fed a high-fat diet and db/db mice (3). Likewise, moderate overexpression of SIRT1 under the control of its natural promoter had similar effects in mice fed a high-fat diet (67). Interestingly, in the first study, increased levels of adiponectin (an AMPK activator) were observed in plasma and increased AMPK activity in liver, white adipose tissue, and muscle. In the second study (67), SIRT1 prevented glucose intolerance, hepatic steatosis, and various manifestations of inflammation, effects attributed to the induction of antioxidant proteins such as manganese superoxide dismutase, PGC-1α activation, and downmodulation of NF-κB. AMPK activity was not assessed (67). In other studies (20, 58), potent small molecule activators of SIRT1 lowered plasma glucose, improved insulin sensitivity, and increased mitochondrial capacity in adipose tissue, skeletal muscle, and liver of mice with diet-induced obesity. Where measured (20, 58), increased AMPK (phosphorylation) was reported as a late-occurring event.
Resveratrol.
Similar effects have been described in rodents with diet-induced obesity and glucose intolerance when treated with resveratrol (4, 47, 106), another SIRT1 activator. In addition, treatment with resveratrol has been shown to diminish atherosclerosis in LDL receptor-deficient mice fed a high-fat, high-sucrose diet (106). However, resveratrol has also been shown to activate AMPK independent of SIRT1 (16), possibly by binding to and inhibiting mitochondrial ATP synthase and causing a decrease in energy state (98). Thus, the precise mechanism responsible for its benefits is unclear.
A list of some of the pathogenetic factors and/or markers for the metabolic syndrome that are diminished by both AMPK and SIRT1 and the putative target molecules through which they act is presented in Table 1. The table is constructed on the basis of studies carried out in humans and experimental animals and to some extent the effects of AMPK and SIRT1 and the factors that activate them in cultured cells. As already noted, exercise and calorie restriction have been demonstrated to diminish many of these pathogenetic factors in humans, as have, to some extent, pharmacological agents that activate AMPK, such as metformin and the thiazolidinediones (27, 63).
CONCLUDING REMARKS
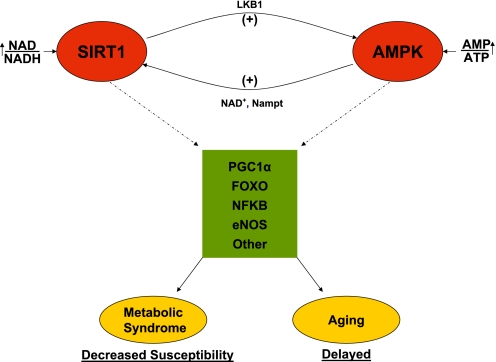
A schematic depiction of the hypothetical SIRT1/AMPK cycle and its physiological significance are presented in Fig. 7. The partnership between AMPK and SIRT1 is long standing, and it has many potential implications for our understanding of the pathogenesis and therapy of disorders associated with the metabolic syndrome and aging. On the other hand, many aspects of this partnership require further study. For instance, SIRT1 is one of seven class III histone/protein deacetylases (7, 21), and the relation of the others to AMPK is not well known. Such knowledge may be relevant since some of these sirtuins, in contrast to SIRT1, are mainly mitochondrial (SIRT3, -4, and -5) and others shuttle between the nucleus and cytoplasm (SIRT2), and their overlap in function with SIRT1 and their effects on AMPK are incompletely understood (7, 21). Likewise, LKB1, the principal upstream kinase for AMPK, activates 13 other ARKs, and their overlap in function with AMPK is unresolved. In this context, recent studies suggest that mice deficient in one of the ARKs [sucrose nonfermenting protein kinase/AMPK-related protein kinase (SNARK)] develop a metabolic syndrome phenotype (96), although at a molecular level SNARK has not been shown to mimic many of the actions of AMPK (19). Finally, it is likely that SIRT1 and AMPK have actions independent of the other, and these also need to be sorted out.
Fig. 7.
The hypothetical SIRT1/AMPK cycle and its significance. A decrease in energy state or activation of AMPK by other means leads to activation of SIRT1, perhaps by increasing NAD+ or the NAD/NADH ratio (8) and/or the activity of Nampt (23). SIRT1 then deacetylates and activates LKB1, which in turn activates AMPK. Alternatively, these events could be set in motion by factors that primarily increase SIRT1. The joint activation of SIRT1 and AMPK allows for the concurrent deacetylation and phosphorylation of the listed target molecules and presumably others. The predicted result would be a decreased susceptibility to metabolic syndrome-associated disorders and possibly delayed aging. Not shown is that a primary downregulation of SIRT1 or AMPK would presumably have opposite effects and predispose to the metabolic syndrome and accelerated aging.
These and other questions aside, the evidence that SIRT1 and AMPK can work in tandem raises some intriguing possibilities. For instance, SIRT1 abundance and activity (5, 13, 38) and AMPK activation (74) diminish in at least some mammalian tissues with aging. Thus, if treatment with an AMPK or a SIRT1 activator individually does not reverse aging-associated abnormalities, might dual therapy be more effective? If so, treatment with SIRT1 activators might diminish the impaired ability of exercise to activate AMPK that has been observed in skeletal muscle of aging rodents (74) and obese, insulin-resistant humans (17, 87).
It has also become increasingly apparent that both AMPK and SIRT1 mediate the biological response of cells to nutrient availability and that in doing so they regulate, often interdependently, metabolism, gene expression, and many aspects of cell biology. The resultant changes in enzymes, transcriptional activators and coactivators, and the genes they regulate are often quite complex. Hopefully, the relation of such changes to SIRT1 and AMPK, which appear to act predictably to maintain cellular function and integrity, will help to unravel this complexity. Finally, recent studies have revealed that various hormones (e.g., leptin, gherelin, adiponectin, and catecholamines) and paracrine factors (endocannabinoids) regulate AMPK and that AMPK in turn mediates their actions on individual tissues and/or whole body fuel homeostasis (40, 75). Whether SIRT1 is also regulated by these molecules and mediates their actions clearly warrants investigation.
GRANTS
This work was supported in part by US Public Health Service Grants RO1-DK-019514, RO1-DK-067509, and PO1-HL-068758 and a mentor-based grant from the American Diabetes Association (N. Ruderman). L. Nelson was the recipient of a predoctoral fellowship from the National Institute of Diabetes and Digestive and Kidney Diseases and J. Cacicedo of a postdoctoral fellowship from T32-DK-007201.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Lenny Guarente for reviewing the manuscript and Amanda Andreyev, Elise Kofke, Michelle Simunovic, Meena Sivaraman, and Sharon Mosher for assistance with its preparation.
REFERENCES
- 1.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem 75: 137–163, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55: 2277–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8: 333–341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One 3: e1759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 324: 1204–1209, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Canto C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 32, Suppl 4: S55–S59, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22: 1753–1757, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, Feltrin D, Igreja SC, Ajodha S, Harvey-White J, Kunos G, Muller B, Pralong F, Aubert G, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing's syndrome. FASEB J 22: 1672–1683, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4: e76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 298: E117–E126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res 98: e39–e47, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 104: 7217–7222, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 294: E607–E614, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Egan B, Zierath JR. Hunting for the SNARK in metabolic disease. Am J Physiol Endocrinol Metab 296: E969–E972, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347–358, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finley LW, Haigis MC. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res Rev 8: 173–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14: 661–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthier MS, Bigornia S, Mott M, Gokce N, Apovian C, Ruderman NB. Decreased AMP-activated protein kinase activity is associated with markers of inflammation and infiltration of immune cells in visceral and subcutaneous adipose tissue, and with whole-body insulin resistance in obese patients (Abstract). Obesity (Silver Spring) 17: S93.(158-P), 2009 [Google Scholar]
- 26.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368: 1096–1105, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Gorospe M, de Cabo R. AsSIRTing the DNA damage response. Trends Cell Biol 18: 77–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett 582: 1719–1724, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab 286: E239–E244, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51: 159–167, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes 52: 1635–1640, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 68: 48–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582: 74–80, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 320: 449–454, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell 28: 277–290, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature 451: 583–586, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006 [PubMed] [Google Scholar]
- 46.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci 33: 517–525, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283: 27628–27635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7: 841–853, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol 25: 2488–2494, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 28: 91–106, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol 5: 367–373, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Makino N, Maeda T, Oyama J, Higuchi Y, Mimori K. Improving insulin sensitivity via activation of PPAR-γ increases telomerase activity in the heart of OLETF rats. Am J Physiol Heart Circ Physiol 297: H2188–H2195, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M. Exercise increases nuclear AMPK alpha2 in human skeletal muscle. Diabetes 52: 926–928, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281: 2654–2660, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306: 2105–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142: 611–619, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43: 571–579, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Pedersen SB, Olholm J, Paulsen SK, Bennetzen MF, Richelsen B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int J Obes (Lond) 32: 1250–1255, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104: 12587–12594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105: 9793–9798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54: 928–934, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Potente M, Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7: 2117–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9: 327–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27: 4698–4707, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281: 39915–39924, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 418: 261–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes 47: 699–713, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3: 340–351, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Ruderman N, Shulman G. The metabolic syndrome. In: Textbook of Endocrinology (6th ed.), edited by Jameson J, DeGroot L. Philadelphia, PA: Elsevier, 2010, chapt. 44, p. 822–839 [Google Scholar]
- 80.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun 314: 580–585, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am 294: 48–51, 54–57, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Sponarova J, Mustard KJ, Horakova O, Flachs P, Rossmeisl M, Brauner P, Bardova K, Thomason-Hughes M, Braunerova R, Janovska P, Hardie DG, Kopecky J. Involvement of AMP-activated protein kinase in fat depot-specific metabolic changes during starvation. FEBS Lett 579: 6105–6110, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab 293: E159–E164, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 378: 836–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6: 307–319, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Sun Q, Li L, Li R, Yang M, Liu H, Nowicki MJ, Zong H, Xu J, Yang G. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Ann Med 41: 311–320, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism 57: 986–998, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Tsuchihara K, Ogura T, Fujioka R, Fujii S, Kuga W, Saito M, Ochiya T, Ochiai A, Esumi H. Susceptibility of Snark-deficient mice to azoxymethane-induced colorectal tumorigenesis and the formation of aberrant crypt foci. Cancer Sci 99: 677–682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001. [DOI] [PubMed] [Google Scholar]
- 98.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 65: 3737–3755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab 17: 186–191, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol 9: 1253–1262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29: 1363–1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia 47: 2012–2021, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279: 47898–47905, 2004 [DOI] [PubMed] [Google Scholar]
- 108.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 80: 191–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY.AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 26: 1281–1287, 2006. [DOI] [PubMed] [Google Scholar]