Abstract
Retinoids are absolutely required for normal growth and development during the postnatal period. We studied the delivery of retinoids to milk, availing of mouse models modified for proteins thought to be essential for this process. Milk retinyl esters were markedly altered in mice lacking the enzyme lecithin:retinol acyltransferase (Lrat−/−), indicating that this enzyme is normally responsible for the majority of retinyl esters incorporated into milk and not an acyl-CoA dependent enzyme, as proposed in the literature. Unlike wild-type milk, much of the retinoid in Lrat−/− milk is unesterified retinol, not retinyl ester. The composition of the residual retinyl ester present in Lrat−/− milk was altered from predominantly retinyl palmitate and stearate to retinyl oleate and medium chain retinyl esters. This was accompanied by increased palmitate and decreased oleate in Lrat−/− milk triglycerides. In other studies, we investigated the role of retinol-binding protein in retinoid delivery for milk formation. We found that Rbp−/− mice maintain milk retinoid concentrations similar to those in matched wild-type mice. This appears to arise due to greater postprandial delivery of retinoid, a lipoprotein lipase (LPL)-dependent pathway. Importantly, LPL also acts to assure delivery of long-chain fatty acids (LCFA) to milk. The fatty acid transporter CD36 also facilitated LCFA but not retinoid incorporation into milk. Our data show that compensatory pathways for the delivery of retinoids ensure their optimal delivery and that LRAT is the most important enzyme for milk retinyl ester formation.
Keywords: fatty acid, lactation, vitamin A
retinoids (vitamin A and its natural and synthetic analogs) are required for maintaining many essential physiological processes within the body (30, 40). This is especially true for processes important to the normal growth and development of infants, including the development of the immune system, the brain, and other organ systems (30, 40). Retinoids act as potent transcriptional regulators; more than 500 genes may be responsive to these compounds (2, 8, 15). The transcriptional effects of retinoids are mediated primarily by all-trans- and 9-cis-retinoic acid acting through six distinct ligand-dependent transcription factors: the three retinoic acid receptors (RARα, RARβ, and RARγ) and the three retinoid X receptors (RXRα, RXRβ, and RXRγ) (2, 8, 15).
Mammals are incapable of synthesizing retinoids de novo. Consequently, all retinoids must be acquired from the diet either as preformed retinoid (primarily as dietary retinol or retinyl ester) or as proretinoid carotenoids (primarily as carotenes) (6, 32, 40). Different dietary retinoid forms are processed to retinol within the intestinal enterocytes and packaged into nascent chylomicrons as retinyl esters (6, 32, 40). The majority of retinyl esters are formed from retinol through the actions of lecithin:retinol acyltransferase (LRAT) (3, 33, 55). Approximately 66–75% of dietary retinoid is taken up and stored as retinyl ester in the liver, primarily in the nonparenchymal hepatic stellate cells (also called Ito cells, lipocytes, or fat-storing cells) (6, 17, 19). The remainder of dietary retinoid is taken up by other tissues, where it is either stored or converted directly to retinoic acid. Retinoids stored in the liver are relied upon to meet peripheral tissue needs and are mobilized into the circulation as retinol bound to plasma retinol-binding protein (RBP; also known as RBP4) (18, 48). Tissues can acquire retinol from either the circulating retinol-RBP complex or postprandially from chylomicrons and are capable of enzymatically oxidizing retinol to retinal, which in turn is oxidized to retinoic acid (6, 14, 31). Most tissues also possess some capacity to esterify and thus store retinol prior to its use for the synthesis of retinoic acid (3, 32, 33).
Newborns have very low retinoid stores at birth and are dependent on their mother's milk for acquiring retinoid needed for normal growth and development (40). Two complementary pathways appear to be involved in delivery of plasma retinoids for milk formation. Green et al. (20) estimated that ∼40–70% of milk retinoid from lactating rats comes from holo-RBP, with the rest arriving postprandially in chylomicrons and their remnants. Retinyl esters are good substrates for lipoprotein lipase (LPL), the rate-limiting enzyme required for hydrolysis of lipoprotein-associated triglycerides. We have demonstrated previously that LPL acts to facilitate postprandial retinoid uptake into adipose tissue and muscle (51). Lactating mammary gland can acquire retinyl esters from chylomicrons, although the importance of LPL in this process is uncertain (52).
Despite progress in understanding the biochemistry of retinoid incorporation into milk, much remains to be learned regarding how retinoid is delivered to, taken up by, and enzymatically incorporated into milk. To this end, we have used transgenic and knockout mouse models to help define the actions of LRAT, RBP, LPL, and the plasma membrane free fatty acid transporter CD36 in incorporating retinoid and fatty acids into the milk of lactating dams. In this article, we identify LRAT as the most important enzyme within mammary tissue for the formation of retinyl esters, prove that chylomicron retinyl ester can completely compensate for Rbp deficiency, and show that both LPL and CD36 play a role for long-chain fatty acid (LCFA) incorporation into milk. Our data demonstrate that mammary tissue possesses a very high degree of metabolic flexibility for incorporating retinoids into the milk, even in the face of the complete absence of a retinoid delivery pathway.
MATERIALS AND METHODS
Animals, animal husbandry, and diet.
All mutant mouse lines have been described in the literature and include Rbp−/− (38), Lrat−/− (3, 33), and CD36−/− (9, 16) mice. Lrat−/−, Rbp−/−, and CD36−/− mice were bred in a mixed C57Bl/6J/129sv genetic background. Wild-type mice were obtained from crosses of mice that were heterozygous for the disrupted alleles. MCK-LpL0 mice, transgenic mice totally lacking LPL but rescued from this lethal mutation through expression of human LPL in skeletal muscle, have also been described in the literature (28). Genotypes of the mice, aside from those harboring a disrupted Rbp allele, were determined by published PCR protocols using tail clip DNA, as described in the literature. Screening for the disrupted Rbp allele was done by Southern and/or Western blots, as reported previously (38). Mice were routinely maintained on a standard rodent chow diet containing 25 IU retinol/g. For our dietary studies, either a purified nutritionally complete control retinoid-deficient diet (Purified Test Diet 5822; W. F. Fisher and Son) containing <0.04 IU retinol/g, a control diet based on the same formulation but containing 22 IU retinol/g (Test Diet 5755), or an excess retinoid diet (Test Diet 5713) based on the same formulation but containing 220 IU retinol/g was employed. These diets are otherwise nutritionally complete, providing nutritionally sufficient levels of all other macro- and micronutrients. For all animal studies, both diet and water were made available to the animals on an ad libitum basis. Mice were maintained on an alternating 12:12-h dark-light cycle, with the period of darkness between 7 PM and 7 AM. All animal experimentation reported in this study was conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (7th ed., Washington, DC: National Academy Press, 1996) and was approved by the Columbia University Institutional Animal Care and Use Committee.
Milk collection.
Milk was collected between days 10 and 15 postpartum. Female mice were mated (2 dams, 1 male/cage) at ∼60–80 days of age, and for most studies mice were kept on breeder chow (25 IU retinol/g diet) during this period. For studies investigating the effects of differing amounts of retinoid in the diet, female mice were mated as above and maintained on the control breeder chow diet until the birth of their litters. The diet of the dams was then changed to one of the three purified diets listed above when pups were first observed in the cage. Milk was collected as described previously (52). Briefly, each mouse was weighed and then anesthetized by intraperitoneal injection of a cocktail (1:1, vol/vol) of xylazine (100 mg/ml) and ketamine HCl (100 mg/ml) given at a dose of 100 μl/30 g of body weight. About 10 min after the anesthetic was administered, a dose of oxytocin (10 units/ml, Pitocin; Parkedale Pharmaceuticals, Rochester, MI) diluted 1:4 in PBS was injected into the tail vein (0.25 units of oxytocin/mouse). Milk was collected ∼5 min after the oxytocin injection using mild suction generated by a small pump. A standard 200-μl pipette tip was placed over the nipple, and the tip was connected by a small tube to two inverted 1,000-μl pipette tips that had holes drilled into them to allow us to control the suction intensity from a vacuum pump. Collected milk was then rinsed from the tip into 1.5-ml microcentrifuge tubes containing 180 μl of 0.005 M EDTA. On average, ∼20–25 μl of milk was collected over a 5-min period. Milk was immediately placed on ice and covered with foil to protect it from exposure to light. Subsequently, the milk sample was stored at −80°C prior to assay.
Gavage experiments.
For studies of postprandial delivery of retinol to milk, lactating mice at 15 days postpartum were separated from their pups and given an oral bolus of a physiological dose of [3H]retinol (6 μg and ∼106 3H-cpm in 50 μl of peanut oil). Milk was then collected 2, 4, and 6 h postgavage. Radioactivity present in milk samples was measured in a Beckman LS 1800 liquid scintillation counter. To assess the contribution of lipase activity to retinol uptake, the lipase inhibitor P-407 (1 g/kg body wt) was given to the mice the night before the experiment. P-407 is known to block the activity of LPL as well as other lipases such as hepatic lipase and endothelial lipase, thus effectively blocking lipoprotein clearance in treated mice (25, 33, 53).
High-performance liquid chromatography analysis of retinoids.
Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in milk and maternal serum was performed as described elsewhere (5, 54). Briefly, all samples were flash-frozen in liquid N2 immediately after collection. Livers were first homogenized in 10 volumes of PBS (10 mM sodium phosphate, pH 7.2, 150 mM sodium chloride) using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) set at half-maximal speed for 10 s. Two hundred microliters of a homogenate, 200 μl of serum, or ∼20–25 μl of milk was treated with an equal volume of absolute ethanol containing a known amount of retinyl acetate as an internal standard. After homogenization of the tissues or vigorous vortexing of the fluids, retinoids were extracted into 3 ml of hexane, evaporated under N2, and redissolved in bezene prior to being injected into the high-performance liquid chromatograph. The redissolved retinoids were separated on a 4.6 × 250-mm Ultrasphere C18 column (Beckman, Fullerton, CA) preceded by a C18 guard column (Supelco, Bellefonte, PA), using 70% acetonitrile-15% methanol-15% methylene chloride as the running solvent and flowing at 1.8 ml/min. Retinol and retinyl esters (routinely retinyl palmitate, oleate, linoleate, and stearate) were identified using a Waters 2996 photodiode array detector to compare retention times and spectral data of experimental compounds with those of authentic standards. Concentrations of retinol and retinyl esters in these samples were quantitated by comparing integrated peak areas of the unknowns against those of known amounts of purified standards. An internal standard, retinyl acetate, added immediately after homogenization of the samples, was used to correct for losses during extraction.
Triglyceride and fatty acid determinations.
Milk triglyceride levels were assessed colorimetrically using a kit, following the manufacturer's instructions (ThermoFisher). Analysis of the fatty acyl composition of milk triglycerides was performed with a Hewlett-Packard 5890 gas chromatograph equipped with a flame ionization detector. Extracts from milk samples were chromatographed as methyl esters on a 30-m fused silica column wall coated with 0.2 μm SP-2380 and having an internal diameter of 0.25 mm. Helium was used as carrier gas. The split ratio was 17:1. The injector and detector temperatures were 200 and 250°C, respectively. The column temperature was held at 80°C for 5 min and raised in a stepwise fashion until it reached a plateau of 220°C. A standard mixture of free fatty acids (Sigma, St. Louis, MO) was used for calibration. A correction factor was used to compensate for the lower detector response to unsaturated fatty acids relative to their saturated fatty acid counterparts. Amounts of individual fatty acid methyl esters were calculated by comparing their peak areas with that derived from a known quantity of an added C15:0 internal standard. Results were normalized for weight percent of each fatty acid (27, 34).
Statistical analyses.
All data were analyzed for statistically significant differences by employing an unpaired t-test or an ANOVA followed by pairwise comparisons using Bartlett's t-test (assuming equal variance among groups).
RESULTS
LRAT is the primary enzyme responsible for the synthesis of retinyl esters incorporated into milk.
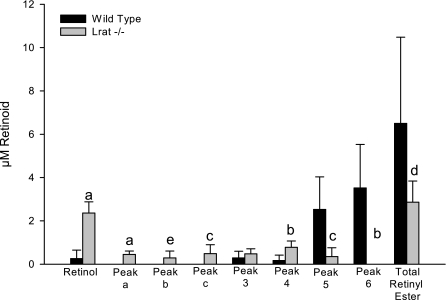
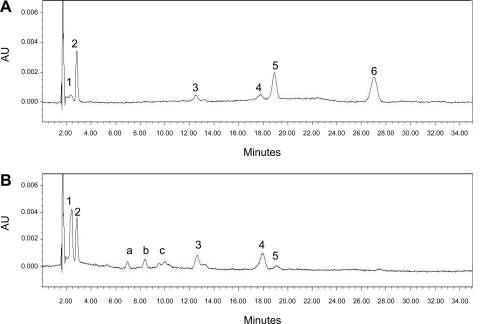
The preponderant retinoid species in the milk is retinyl ester. To determine the importance of LRAT in mammary tissue, we assessed milk retinyl esters in Lrat−/− mice. Although we did not observe a difference in the total retinoid (retinol plus combined retinyl esters) concentration present in Lrat−/− and wild-type milk, Fig. 1 shows that there was a significant reduction (P < 0.001) in the level of the total retinyl esters and a significant increase (P < 0.000001) in the level of free retinol in the milk from Lrat−/− compared with wild-type dams. Free (unesterified) retinol accounted for 47.1 ± 22.0% of the total retinol in the milk of Lrat−/− mice vs. 4.3 ± 7.0% in the wild-type controls. Figure 1 also shows changes in Lrat−/− milk long-chain retinyl ester composition, with a significant reduction in the level of retinyl palmitate (P < 0.005) and a complete absence of retinyl stearate. This reduction was accompanied by the presence of retinyl esters not observed in wild-type milk. These are probably esters of medium-chain acyl groups (C8–C12), which we refer to as peaks a, b, and c (see below). It had been proposed in earlier work that milk retinyl ester formation involves a fatty acyl-CoA-dependent enzyme, i.e., an acyl-CoA:retinol acyltransferase (ARAT) (41, 42). But the fatty acyl composition of retinyl esters present in milk of wild-type and all the other mouse strains we have examined (see Fig. 2A) suggests that this may not be the case, since the most abundant retinyl ester present in the milk of wild-type mice is retinyl palmitate and stearate, not retinyl oleate or retinyl linoleate, as would be expected if retinol esterification involved an acyl-CoA-dependent process. This observed retinyl ester composition suggests that LRAT, which catalyzes the transesterification of a fatty acyl group from the A1 position of phosphatidyl choline (which is most often a palmitate or stearate moiety) to retinol (44), acts importantly in generating retinyl esters in lactating mice. The retinyl ester composition of milk obtained from Lrat−/− dams, shown by the HPLC profiles presented in Fig. 2B, shows a markedly different milk retinyl ester composition from wild-type dams fed the same diet (shown in Fig. 2A). For wild-type dams, the predominant retinyl esters present in the milk were retinyl palmitate and retinyl stearate, each accounting for ∼40% of the total retinyl esters (80% of total), with the remaining 20% consisting of retinyl oleate and retinyl linoleate. Milk from Lrat−/− dams showed novel HPLC peaks (peaks a, b, and c) with shorter retention times than those observed for long-chain retinyl esters. The UV-VIS spectra obtained for each of the unidentified peaks are identical to those of retinol/retinyl ester (see Supplemental Fig. S1; Supplemental Material for this article can be found at the AJP-Endocrinology and Metabolism web site). We conclude on the basis of their HPLC retention times that these peaks represent retinyl esters with shorter acyl chain lengths (C8–C12).
Fig. 1.
Lecithin:retinol acyltransferase (LRAT) is normally the predominant enzyme responsible for the formation of milk retinyl esters. Retinoid concentrations are shown for wild-type (n = 9; black bars) and Lrat−/− (n = 9; gray bars) mice. The figure shows the levels of all-trans retinol; peak 3, retinyl linoleate; peak 4, retinyl oleate; peak 5, retinyl palmitate; peak 6, retinyl stearate. Also shown are peaks a, b, and c, which are unknown retinyl esters found only in milk from Lrat−/− dams. These peak designations are identical to the ones shown in Fig. 2. All values are shown as the mean ± 1 SD. Statistical significances were determined by t-tests: aP < 0.00001, bP < 0.001, cP < 0.005, dP < 0.01, and eP < 0.05 compared with wild-type mice.
Fig. 2.
Retinyl esters are the predominant retinoid in milk of wild-type but not Lrat−/− mice, which contains considerable unesterified retinol. Typical high-performance liquid chromatography (HPLC) profile for 30 μl of milk from wild-type (A) and Lrat−/− (B) dams maintained on a chow diet was collected at 10–15 days postpartum. HPLC peaks were identified on the basis of retention times and UV-Vis spectra compared with those of known standards. Peak 1, all-trans retinol; peak 2, retinyl acetate (internal standard); peak 3, retinyl linoleate; peak 4, retinyl oleate; peak 5, retinyl palmitate; peak 6, retinyl stearate; peaks a, b, and c are unknowns. Peaks a, b, and c are found only in milk from Lrat−/− dams. We conclude on the basis of their spectra (see Supplemental Fig. S1) and retention times that these peaks are retinyl esters, probably consisting of medium-chain fatty acyl groups.
Because milk total lipid levels were the same for Lrat−/− and wild-type mice, we wanted to establish whether this change in retinyl ester composition reflected an altered triglyceride fatty acyl-CoA composition. As shown in Table 1, the triglycerides in milk from Lrat−/− mice had significantly lower oleate and elevated palmitate concentrations compared with wild-type mice, a mirror image of the retinyl ester profile. We also observed a trend toward an increase in stearate concentrations, but this did not reach statistical significance. These data suggest that, in the absence of LRAT, an ARAT activity capable of using available fatty acyl groups is expressed in mammary tissue.
Table 1.
Total fatty acid composition of milk from wild-type and Lrat−/− mice
| Fatty Acid | %Total |
|
|---|---|---|
| Wild type | Lrat−/− | |
| Myristic (14:0) | 6.7 ± 0.9 | 7.7 ± 1.5 |
| Palmitic (16:0) | 22.7 ± 2.0 | 27.7 ± 3.3a |
| Stearic (18:0) | 4.5 ± 1.4 | 7.2 ± 3.8 |
| Oleic (18:1) | 33.7 ± 2.5 | 27.4 ± 1.9b |
| All others combined | 32.4 ± 7.2 | 30.1 ± 4.5 |
| Total | 100 | 100 |
Values are given as means ± 1 SD; n = 3 for all groups. Lrat, lecithin:retinol acyltransferase. Statistical significance:
P < 0.05 compared with wild type;
P < 0.0001 compared with wild type.
The absence of LPL or CD36 affects LCFA but not retinoid incorporation into milk.
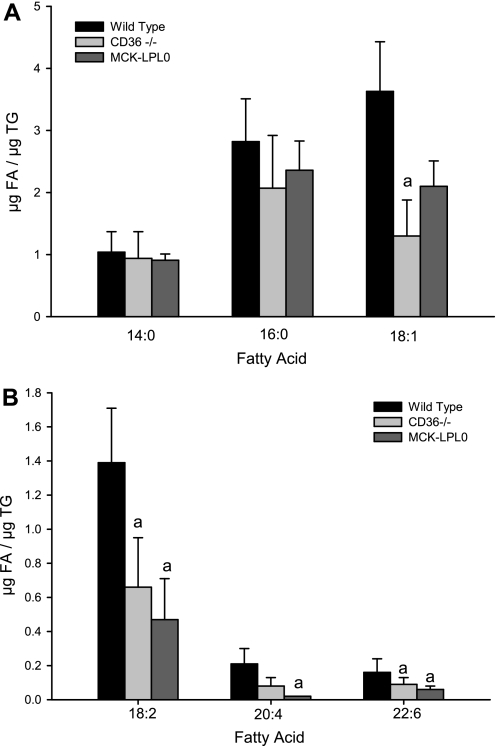
The major physiological role of LPL is to hydrolyze triglyceride that is present in the postprandial circulation following consumption of a fat-rich meal. It is well established that LPL facilitates fatty acid uptake into tissues (26). LPL is expressed in mammary tissue at increased levels throughout the entire period of lactation (23, 24). This upregulation is proposed to facilitate incorporation of dietary lipids from the circulation into mammary tissue, enhancing mammary tissue acquisition of fatty acids that are incorporated into milk triglycerides (23, 24). CD36 is a plasma membrane primary fatty acid transporter that is known to have a key role in facilitating uptake of fatty acids into tissues, although its role during lactation has yet to be studied (13, 16, 21). We assessed the fatty acid composition of milk obtained from MCK-LpL0 [a mouse that expresses LPL only in skeletal muscle and not in mammary tissue (28)] and wild-type mice fed chow diets. For both MCK-LpL0 and CD36−/− mice, milk triglyceride concentrations did not differ from those of matched wild-type mice. However, milk from MCK-LpL0 mice exhibited significantly lower levels of linoleic acid (18:2), arachidonic acid (ARA; 20:4), and docosahexaenoic acid (DHA; 22:6) in milk triglycerides. We further explored whether the absence of the fatty acid transporter CD36 influenced the fatty acid composition of milk. We observed a significant reduction in the concentration of oleic acid (18:1), linoleic acid (18:2), and docohexanoic acid (22:6) (Fig. 3, A and B) in milk obtained from CD36−/− mice. Thus, although the absolute triglyceride concentrations were not different, we did observe a relative reduction in the longer-chain fatty acids in the milk from both MCK-LpL0 and CD36−/− mice, resulting in a relative increase in medium-chain fatty acids compared with wild-type milk (Table 2).
Fig. 3.
Lipoprotein lipase and CD36 contribute significantly to the fatty acid (FA) composition of milk triglycerides (TG). Milk was collected from wild-type (black bars; n = 4), CD36−/− (light gray bars; n = 5), and MCK-LpL0 (dark gray bars; n = 5) mice, and the FA composition of the TG was analyzed. Values represent means ± 1 SD. A: levels of the relatively abundant myristic (14:0), palmitic (16:0), and oleic acids (18:1). B: levels of the less abundant linoleic (18:2), arachidonic (20:4), and docosahexaenoic acids (22:6). Statistical significance: aP < 0.05 compared with wild type.
Table 2.
Fatty acid compositions of milk triglycerides for wild-type, CD36−/−, and MCK-LpL0 mice
| Fatty Acid | μg Fatty Acid/μg TG |
||
|---|---|---|---|
| Wild type | CD36−/− | Mck-LpL0 | |
| 10:0 | 0.00 ± 0.00 | 0.03 ± 0.02 | 0.01 ± 0.01 |
| 12:0 | 0.51 ± 0.15 | 1.45 ± 0.21 | 1.45 ± 0.06 |
| 14:0 | 1.04 ± 0.33 | 0.94 ± 0.43 | 0.91 ± 0.10 |
| 16:0 | 2.82 ± 0.69 | 2.07 ± 0.85 | 2.36 ± 0.47 |
| 16:1 | 0.47 ± 0.15 | 0.35 ± 0.10 | 0.50 ± 0.09 |
| 18:0 | 0.32 ± 0.09 | 0.11 ± 0.06 | 0.17 ± 0.03 |
| 18:1 | 3.63 ± 0.80 | 1.30 ± 0.58 | 2.10 ± 0.41 |
| Unknown | 0.44 ± 0.19 | 0.17 ± 0.11 | 0.35 ± 0.08 |
| 18:2 | 1.39 ± 0.32 | 0.66 ± 0.29 | 0.47 ± 0.24 |
| 18:3 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 |
| 20:4 | 0.21 ± 0.09 | 0.08 ± 0.05 | 0.02 ± 0.02 |
| Unknown | 0.04 ± 0.02 | 0.00 ± 0.00 | 0.12 ± 0.11 |
| 22:6 | 0.16 ± 0.08 | 0.09 ± 0.04 | 0.06 ± 0.02 |
| Total | 11.04 ± 2.91 | 7.25 ± 2.74 | 8.53 ± 1.65 |
Values are given as means ± 1 SD; n = 5 for each group. TG, triglycerides
We investigated retinoid uptake into milk in the MCK-LpL0 mice, but we found no significant differences in the retinoid concentrations in MCK-LpL0 milk (Table 3) nor qualitatively in the retinyl ester composition of this milk compared with milk from wild-type mice. We also examined milk from CD36−/− mice and similarly found no differences in retinyl ester concentration (Table 3) or in the retinyl ester composition (data not shown) compared with wild-type dams. This is not unexpected since both of these mutant mouse models are able to acquire retinoid for incorporation into milk via delivery as retinol-RBP.
Table 3.
Milk retinoid concentrations do not differ between CD36−/−, MCK-LpL0, and wild-type dams maintained on a chow diet
| Total Retinoid, μM | |
|---|---|
| Wild type | 11.0 ± 6.4 |
| CD36−/− | 10.5 ± 6.7 |
| MCK-LpL0 | 15.6 ± 7.8 |
Values are given as means ± 1 SD; n = 5 for each group.
Postprandial acquisition of retinoid by mammary tissue for incorporation into milk.
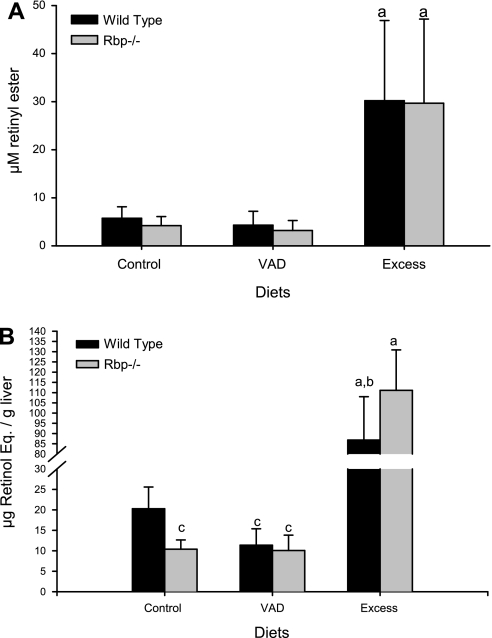
To understand the limitations and regulation of retinoid delivery to the lactating mammary gland, we investigated the effects of changing levels of dietary retinoid on the milk retinoid concentrations in Rbp−/− and wild-type dams. We further examined how this influences retinoid stores of pups nursed by these dams. Upon the births of their litters, Rbp−/− and wild-type dams were placed on either a nutritionally complete retinoid-sufficient purified diet (22 IU retinol/g diet), the same purified diet supplemented with excess retinoid (retinoid-excess diet, 220 IU retinol/g diet), or the same diet totally lacking retinoid (retinoid-deficient diet, <0.04 IU retinol/g diet). Figure 4A shows that milk total retinol levels were greater in both Rbp−/− and wild-type mice maintained on the retinoid-excess diet. However, the total retinol concentrations of milk were not statistically different between Rbp−/− and wild-type mice for any of the three diets. Also, the total retinol concentration of milk did not differ statistically between Rbp−/− and wild-type mice receiving the retinoid-deficient compared with the retinoid-sufficient diet, indicating that RBP absence failed to significantly affect milk retinoid content. We note that adipocytes present in mammary tissue contain retinoids that might serve as a local source of retinoid for incorporation into milk.
Fig. 4.
Alternate pathways act to maximize retinoid delivery and incorporation into milk. A: results of a diet study where both wild-type (black bars) and retinol-binding protein (Rbp)−/− (gray bars) dams were placed, at the time of birth of their litters, on either a retinoid-sufficient diet containing 22 IU retinol/g (control), a retinoid-deficient diet (VAD), or a diet containing 220 IU retinol/g (excess). The concentration of retinoid was measured in milk at 10–15 days postpartum (n = 10–18 lactating dams for each diet group and genotype). B: pup hepatic retinoid levels for the different diets. Retinol and retinyl ester levels were determined in the livers of wild-type (black bars) or Rbp−/− (gray bars) pups at weaning (21 days; n = 10–15 pups/group). Data are provided as means ± 1 SD. Statistical significance: aP < 0.00005 compared with all other diet groups; bP < 0.0005 compared with wild-type control; cP < 0.05 compared with wild-type control diet.
To determine whether the level of retinoid being fed to each dam affected her newborn pup, we measured hepatic total retinol levels for the pups at their time of weaning on postnatal day 21. Figure 4B shows that the hepatic total retinol levels of the pups directly correlated with dietary retinoid content fed to the dams. Pups nursed by dams receiving the retinoid-excess diet accumulated far more hepatic retinoid than was observed for pups nursed by dams maintained on a retinoid-sufficient diet. Interestingly, although we did not observe statistically significant diet-dependent differences in the milk total retinol levels for the dams receiving the retinoid-deficient diet vs. the retinoid-sufficient diet (Fig. 4A), hepatic total retinol levels of the wild-type pups maintained on this milk were significantly lower for the retinoid-deficient group compared with the retinoid-sufficient group (Fig. 4B). The Rbp−/− livers showed significantly higher retinoid stores than wild-type livers when their mothers were maintained on a vitamin A-excess diet (P < 0.005). This likely arises because the retinoid present within the Rbp−/− pup livers cannot be mobilized in the absence of RBP and, therefore, in the face of normal dietary intake, continues to accumulate. We did not observe any differences in either pup body or liver weights for any of the strains or treatments.
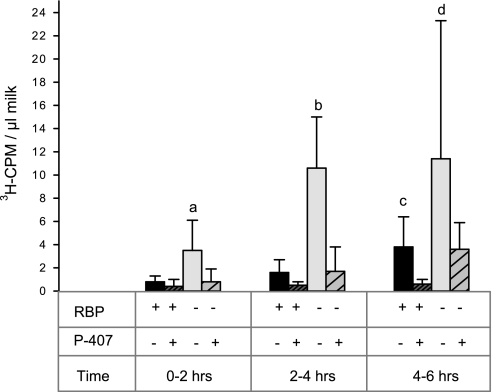
It is clear from this study and our previous study of chow-fed mice (52) that the absence of RBP does not lead to a decrease in milk retinoid content. This suggested that most if not all of the total retinol present in the milk of Rbp−/− mice can be derived postprandially from chylomicron lipolysis. To test this, we gave lactating Rbp−/− and wild-type mice an oral physiological dose of 6 μg [3H]retinol (∼106 3H-cpm) in 50 μl of peanut oil. This allowed us to distinguish postprandially derived retinol from unlabeled retinol already present in the circulation and/or mammary tissue. Milk samples were collected 2, 4, and 6 h after gavage, and differences in 3H-cpm incorporation of radioactivity into milk were assessed (Fig. 5). We found that following gavage administration the Rbp−/− mice showed 2–4 times higher 3H-cpm levels in their milk than wild-type mice at all time points (P < 0.005). Thus, the postprandial pathway does indeed facilitate maintenance of milk retinoid concentrations in Rbp−/− mice.
Fig. 5.
Lipase action is needed to assure retinoid incorporation into milk. 3H-cpm/μl milk is given for wild-type (dark bars; n = 6) and Rbp−/− mice (light bars; n = 5) 2, 4, and 6 h after administration of a gavage dose of [3H]retinol in 50 μl of peanut oil, with (+) and without (−) P-407 administration. Data are shown as means ± 1 SD. Statistical significance: aP < 0.005, untreated Rbp−/− mice different from all other treatment groups at 2 h; bP < 0.00001, untreated Rbp−/− mice different from all other treatment groups at 4 h; cP < 0.05 different from wild-type treated; dP < 0.05, untreated Rbp−/− mice different from all other treatment groups at 6 h.
Milk acquisition of retinoids during lipase inhibition.
To investigate the role of lipases in the delivery of postprandial retinoids to the milk of Rbp−/− mice, we repeated the same experiment in another group of lactating wild-type and Rbp−/− mice. However, this time one-half of the mice in each group received a dose of the lipase inhibitor P-407 (1 g/kg body wt), given by an intraperitoneal injection several hours before the experiment to functionally block the activity of LPL and other vascular lipases (25, 33, 53). Radioactivity in milk was lower in all groups of mice treated with the lipase inhibitor than was present in untreated mice, and 3H-cpms for the Rbp−/− group were now similar to those levels seen in the untreated wild-type animals (Fig. 5). Serum levels of postprandial radioactivity remained high in the P-407-treated mice and were equivalent for wild-type and Rbp−/− mice (Supplemental Fig. S2), as did serum triglycerides (126 ± 5.9 mg/dl without P-407 treatment compared with 2,639 ± 617 mg/dl with P-407 treatment), consistent with the known P-407 actions of inhibiting lipolysis and chylomicron clearance (25). We interpret these data to indicate that lipase activity is required for incorporation of postprandial retinoid (which is present in chylomicrons as retinyl ester) into milk of Rbp−/− mice.
DISCUSSION
Our studies of genetically modified mouse models allowed us to obtain new insights into factors and processes responsible for the incorporation of retinoids and fatty acids into milk. This research establishes the dual importance of two pathways for retinoid delivery through the blood to mammary tissue (postprandial chylomicrons and RBP) and the importance of LPL, CD36, and LRAT and ARAT activities on retinoid and fatty acid delivery to and incorporation into milk.
LRAT is responsible for the synthesis of most retinyl esters that are incorporated into mouse milk.
Although it is now generally accepted that retinol is esterified through the actions of LRAT in most tissues (6, 35, 45, 59), mammary tissue has long been proposed to be an exception (41, 42). Early work employing microsomes prepared from lactating rat mammary tissue suggested that retinyl esters formed for incorporation into milk arose through an acyl-CoA-dependent process involving an ARAT activity (41). However, it is now known that LRAT is highly expressed in mammary epithelium during lactation and that retinol bound to cellular retinol-binding protein type III, a protein that is highly expressed in mammary tissue, is an excellent substrate for LRAT (37). These data suggested that LRAT acts importantly within mammary tissue to catalyze retinyl ester formation. Our data in Figs. 1 and 2 convincingly establish that LRAT plays an important role in catalyzing the formation of retinyl ester that is incorporated into milk. When LRAT was absent, we observed a significant reduction in the concentration of milk retinyl ester and an increase in the concentration of free retinol, although serum retinol levels for these mutant mice were the same as for wild-type mice. This is not unlike the situation we observed previously in Lrat−/− mice for the intestinal absorption of dietary retinoid, a reduction in the concentration of retinyl esters and an increase in the concentration of free retinol in chylomicrons (33). Lrat−/− mice display the same serum retinol and RBP concentrations as wild-type mice (33). Moreover, adipose tissue of Lrat−/− mice is enriched in retinyl esters (33). Because the total retinoid (retinol plus retinyl ester) concentration in milk obtained from Lrat−/− mice is not different from that of wild-type mice, we do not believe the reduction of retinyl esters we observed in the milk of the Lrat−/− mice arises due to a reduction in postprandial retinoid delivery but rather due to the lack of LRAT activity within mammary tissue. Thus, we conclude that LRAT and not an ARAT activity normally acts to catalyze retinyl ester synthesis within the mammary epithelium.
The fatty acyl composition of the retinyl esters present in Lrat−/− milk is qualitatively different from that of wild-type mice. Figure 2 clearly shows a greater abundance of medium-chain retinyl ester groups in Lrat−/− mice compared with wild-type mice. The literature indicates that medium-chain fatty acids are relatively common in milk (1, 43). Our data suggest that these fatty acids can be used as a substrate for an ARAT enzyme, but only in the absence of LRAT. Moreover, the absolute amount of ARAT-synthesized retinyl ester found in milk from Lrat−/− mice is significantly lower than the retinyl ester level determined for milk of Lrat-expressing wild-type mice (see Fig. 1). We and others have shown that diacylglycerol acyltransferase 1 (DGAT1) is capable of synthesizing retinyl esters in vivo and is partially responsible for the residual retinyl esters found in the chylomicrons of Lrat−/− mice (33, 36, 55, 58). It is possible that some retinyl ester found in the milk of the Lrat−/− mice arises from DGAT1 activity. However, we were unable to confirm this possibility in vivo since Dgat1−/− mice fail to lactate due to inadequate mammary triglyceride synthesis (47). The retinyl ester profile of milk from Lrat−/− mice suggests that DGAT1 or other ARATs may use fatty acyl-CoAs, most notably oleyl-CoA, along with other medium-chain fatty acyl-CoAs that are abundant within the lactating mammary gland, and incorporate these into retinyl esters. The major long-chain retinyl ester present in the milk of Lrat−/− mice is retinyl oleate. We observed a change in the fatty acid composition of the milk triglycerides from Lrat−/− mice, a reduction in total oleate concentrations with an accompanying increase in their palmitate concentration. At present, we do not understand the basis for this change in triglyceride fatty acyl distribution.
Both LPL and CD36 have important roles in facilitating polyunsaturated fatty acid incorporation into milk.
Mouse milk contains a large amount of lipid, ∼30% by weight (1). Mice very efficiently package triglyceride into milk, secreting their entire body weight in lipid during their 21 days of lactation (∼30 g) (1). LPL is known to facilitate the uptake of fatty acids and other fat-soluble nutrients such as α-tocopherol by the mammary gland (29, 46). LPL activity and mRNA rise after birth and are elevated in mammary tissue and depressed in adipose tissue to redirect lipids for incorporation into milk fat (23, 24). We investigated the roles of LPL and the plasma membrane fatty acid transporter CD36 in fatty acid and retinoid uptake by mammary tissue for incorporation into milk. For this purpose, we employed MCK-LpL0 transgenic mice and CD36−/− mice and found that the fatty acid compositions of milk triglycerides are significantly different for these mice compared with wild-type mice (see Fig. 3, A and B). Interestingly, CD36−/− mice show normal serum fatty acid concentrations despite impaired intestinal absorption of LCFAs when challenged with a large fatty acid load (13, 16, 21). This suggests that, despite the normal serum levels, transfer of LCFAs, including polyunsaturated fatty acids (PUFAs), from serum to milk is impaired in CD36-deficient mammary tissue. Our data also imply that LPL acts in vivo in mammary tissue to facilitate uptake of LCFAs and PUFAs by mammary tissue. This finding in MCK-LpL0 mice is consistent with published observations made in humans where more than 70 LPL gene mutations that result in complete or partial loss of LPL function have been described. More medium- and short-chain fatty acids are found in milk obtained from humans harboring mutant LPL forms (4, 49). But humans with LPL deficiency are also reported to have less milk triglyceride (4, 49), a finding that differs from our mouse studies, but that may be a reflection of species differences in the milk composition (mice have extremely high fat contents in their milk, whereas human milk is relatively low in fat) (1). Alternatively, the human data may primarily reflect the low-fat diets of the LPL-deficient humans.
LPL and CD36 appear to be especially important in providing PUFAs to milk. The potential benefits of higher levels of DHA (22:6, ω3) and ARA in milk are under especially intense scrutiny (22, 56). DHA is the major n-3 fatty acid in the central nervous system and retina and accumulates in mammals primarily in late gestation and early life (7). DHA can be synthesized in varying degrees from its precursor, α-linolenic acid, but levels of this fatty acid are very low in all of our mouse strains. It is well known that LPL and CD36 are importantly involved in lipid metabolism and fatty acid uptake in many tissues, and our studies demonstrate that both of these proteins have an important role in assuring PUFA incorporation into milk.
Dietary retinoid can be a major source for retinoid incorporated into milk.
Early work by Vahlquist and Nilsson (50) carried out in Rhesus monkeys concluded that about 90% of milk retinoid is derived from the retinol-RBP complex and that lipoprotein-mediated transfer of retinoid increases upon high dietary retinoid intake, a finding that was subsequently confirmed for ewes and rodents (12, 42). Our data agree with these studies. Mice lacking LPL in mammary tissue, MCK-LpL0 mice, do not show any alteration in milk retinoid concentrations upon changes in dietary retinoid intake, implying that mammary tissue in these mice receive retinol solely via the retinol-RBP pathway. Therefore, we were surprised to find that Rbp−/− milk retinoids do not differ from those of chow-fed wild-type mice. This indicates that either retinol-RBP or retinyl ester present in chylomicrons is sufficient to maintain normal milk retinoid concentrations despite fasting serum retinol levels that were greatly reduced in Rbp−/− mice (<10% those of wild-type mice) (38). When we challenged mice with diets containing various levels of retinoid, we found no difference in the milk retinoid levels of the Rbp−/− mice, which suggested to us that RBP is not playing a direct role in regulating the amount of retinoid delivered to the mammary tissue.
Retinoids are essential for the growth and development of the newborn, who has very limited stores at the time of birth (10, 11). Our studies lead us to the conclusion that there is a remarkable degree of metabolic plasticity to ensure delivery of this essential micronutrient to the offspring. We hypothesized that in order for the Rbp−/− mice to maintain their milk retinoid concentrations, the postprandial pathway must be very active. We reported previously that LPL also facilitates uptake of chylomicron retinoid into adipose and other tissues (51). Consequently, we wondered whether LPL might act similarly to facilitate retinoid uptake into mammary tissue in the absence of RBP. The Rbp−/− mice show greatly increased incorporation of 3H-cpm into their milk after receiving an oral gavage; increased postprandial delivery is in fact compensating in the absence of RBP. We were able to block this increase delivery by pretreating the mice with the lipase inhibitor P-407. Our data are consistent with the conclusion that LPL facilitates retinoid uptake into mammary tissue in the absence of RBP and that Rbp−/− mice maintain their milk retinoid levels by utilizing the postprandial pathway.
RBP is secreted by the liver and adipose tissue and, when its circulating levels are elevated, can interfere with proper insulin signaling (57). Conversely, lower or no RBP, as is the case in Rbp−/− mice, leads to enhanced insulin sensitivity (57). It is also known that LPL in milk and lactating mammary gland, like LPL in adipose tissue, is regulated by insulin (1, 39). The increased postprandial uptake and increased LPL activity that we noted in the Rbp−/− mice might therefore be attributable to an insulin-dependent upregulation of LPL activity, conveniently preserving the retinoid content of the mouse milk. We are currently investigating this relationship (postprandial/fasting) on retinoid delivery to other tissues in nonlactating mice.
In summary, our research provides several new key insights into how retinoids and fatty acids are incorporated into milk. First, we have shown that LRAT, and not an ARAT activity as proposed in the literature (41, 42), is normally responsible for the synthesis of milk retinyl esters. In the absence of LRAT the retinyl ester profile changes to reflect differences in fatty acyl groups available for use by an ARAT activity (or activities) for use in retinyl ester synthesis. Both LPL activity and the plasma membrane fatty acid receptor CD36 are required to facilitate optimal uptake of LCFAs and PUFAs for incorporation into milk triglyceride. We also have shown that mammary tissue has the ability to increase the delivery of retinoid via the postprandial pathway in the absence of RBP. Thus, mammary tissue has evolved in a manner that ensures optimal incorporation of retinoid into milk whether retinoid is delivered to the mammary gland as retinol-RBP or as retinyl esters within chylomicrons or both. Our data indicate that mammary tissue possesses a metabolic plasticity, enabling it to maintain milk retinoid concentrations for use by the newborn, even in the face of disruptions that markedly influence retinoid availability/abundance in other tissues.
GRANTS
This work was supported by grants R01-DK-068437, R01-DK-079221, R01-EY-009339, R01-HL-40404, and R01-HL-45095 from the National Institutes of Health.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Rajasekhar Ramakrishnan (Department of Pediatrics, Columbia University) for assistance in statistical matters and for useful comments and discussion.
REFERENCES
- 1.Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res 9: 204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 43: 1773–1808, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem 279: 10422–10432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger GM, Spark A, Baillie PM, Huskisson J, Stockwell G, van der Merwe E. Absence of serum-stimulated lipase activity and altered lipid content in milk from a patient with type I hyperlipoproteinaemia. Pediatr Res 17: 835–839, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Blaner WS, Das SR, Gouras P, Flood MT. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J Biol Chem 262: 53–58, 1987. [PubMed] [Google Scholar]
- 6.Blaner WS, Olsen JA. Retinol and retinoic acid metabolism. In: The Retinoids, Biology, Chemistry and Medicine, edited by Sporn MB, Roberts AB, Goodman DS. New York: Raven, 1994, p. 229–256 [Google Scholar]
- 7.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr 89: 678S–684S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954, 1996 [PubMed] [Google Scholar]
- 9.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275: 32523–32529, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Dann WJ. The transmission of vitamin A from parents to young in mammals: The vitamin A and carotenoid contents of human colostrum and milk. Biochem J 30: 1644–1651, 1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davila ME, Norris L, Cleary MP, Ross AC. Vitamin A during lactation: relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J Nutr 115: 1033–1041, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Donoghue S. Vitamin A transport in plasma of ewes during late gestation and into milk during early lactation. Int J Vitam Nutr Res 58: 3–11, 1988 [PubMed] [Google Scholar]
- 13.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 283: 13108–13115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem 267: 4315–4324, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol 19: 1429–1438, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274: 19055–19062, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Geerts A, Bleser PD, Hautekeete ML, Niki T, Wisse E. Fat storing (Ito) cell biology. In: The Liver: Biology and Pathobiology, edited by Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter D, Shafritz DA. New York: Raven, 1994, p. 819–837 [Google Scholar]
- 18.Goodman DS. Retinol-binding protein. In: The Retinoids, edited by Sporn MB, Roberts AB, Goodman DS. Orlando, FL: Academic, 1984, p. 41–88 [Google Scholar]
- 19.Goodman DW, Huang HS, Shiratori T. Tissue distribution and metabolism of newly absorbed Vitamin A in the rat. J Lipid Res 63: 390–396, 1965 [PubMed] [Google Scholar]
- 20.Green MH, Green JB, Akohoue SA, Kelley SK. Vitamin A intake affects the contribution of chylomicrons vs. retinol-binding protein to milk vitamin A in lactating rats. J Nutr 131: 1279–1282, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Goudriaan JR, Dahlmans VE, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem 239: 199–202, 2002 [PubMed] [Google Scholar]
- 22.Heird WC, Lapillonne A. The role of essential fatty acids in development. Annu Rev Nutr 25: 549–571, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jensen DR, Bessesen DH, Etienne J, Eckel RH, Neville MC. Distribution and source of lipoprotein lipase in mouse mammary gland. J Lipid Res 32: 733–742, 1991 [PubMed] [Google Scholar]
- 24.Jensen DR, Gavigan S, Sawicki V, Witsell DL, Eckel RH, Neville MC. Regulation of lipoprotein lipase activity and mRNA in the mammary gland of the lactating mouse. Biochem J 298: 321–327, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston TP, Palmer WK. Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem Pharmacol 46: 1037–1042, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Goldberg IJ. Lipoprotein lipase-derived fatty acids: physiology and dysfunction. Curr Hypertens Rep 9: 462–466, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lepage G, Roy CC. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25: 1391–1396, 1984 [PubMed] [Google Scholar]
- 28.Levak-Frank S, Weinstock PH, Hayek T, Verdery R, Hofmann W, Ramakrishnan R, Sattler W, Breslow JL, Zechner R. Induced mutant mice expressing lipoprotein lipase exclusively in muscle have subnormal triglycerides yet reduced high density lipoprotein cholesterol levels in plasma. J Biol Chem 272: 17182–17190, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Martinez S, Barbas C, Herrera E. Uptake of alpha-tocopherol by the mammary gland but not by white adipose tissue is dependent on lipoprotein lipase activity around parturition and during lactation in the rat. Metabolism 51: 1444–1451, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Moore T. Vitamin A Amsterdam: Elsevier, 1957 [Google Scholar]
- 31.Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol 63: 139–188, 1999 [DOI] [PubMed] [Google Scholar]
- 32.O'Byrne SM, Blaner WS. Introduction to the retinoids. In: Carotenoids and Retinoids: Molecular Aspects and Health Issues, edited by Packer L, Obermueller-Jevic U, Kraemer K, Sies H. Champaign, IL: AOCS, 2004, p. 1–22 [Google Scholar]
- 33.O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 280: 35647–35657, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira FL, Rumsey SC, Schlotzer E, Hansen I, Carpentier YA, Deckelbaum RJ. Triglyceride hydrolysis of soy oil vs fish oil emulsions. JPEN J Parenter Enteral Nutr 21: 224–229, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Ong DE, MacDonald PN, Gubitosi AM. Esterification of retinol in rat liver. Possible participation by cellular retinol-binding protein and cellular retinol-binding protein II. J Biol Chem 263: 5789–5796, 1988 [PubMed] [Google Scholar]
- 36.Orland MD, Anwar K, Cromley D, Chu CH, Chen L, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim Biophys Acta 1737: 76–82, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Piantedosi R, Ghyselinck N, Blaner WS, Vogel S. Cellular retinol-binding protein type III is needed for retinoid incorporation into milk. J Biol Chem 280: 24286–24292, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 18: 4633–4644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos P, Martin-Hidalgo A, Herrera E. Insulin-induced up-regulation of lipoprotein lipase messenger ribonucleic acid and activity in mammary gland. Endocrinology 140: 1089–1093, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Ross AC. Introduction to vitamin A: a nutritional and life cycle perspective. In: Carotenoids and Retinoids: Molecular Aspects and Health Issues, edited by Packer L, Obermueller-Jevic U, Kraemer K, Sies H. Champaign, IL: AOCS, 2004, p. 23–41 [Google Scholar]
- 41.Ross AC. Retinol esterification by mammary gland microsomes from the lactating rat. J Lipid Res 23: 133–144, 1982 [PubMed] [Google Scholar]
- 42.Ross AC, Pasatiempo AM, Green MH. Chylomicron margination, lipolysis, and vitamin a uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood) 229: 46–55, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ, Stein T, Anderson SM, Neville MC. Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28: 323–336, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem 274: 3834–3841, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem 264: 8636–8640, 1989 [PubMed] [Google Scholar]
- 46.Sattler W, Levak-Frank S, Radner H, Kostner GM, Zechner R. Muscle-specific overexpression of lipoprotein lipase in transgenic mice results in increased alpha-tocopherol levels in skeletal muscle. Biochem J 318: 15–19, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87–90, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: The Retinoids: Biology, Chemistry and Medicine, edited by Sporn MB, Roberts AB, Goodman DS. New York: Raven, 1994, p. 229–256 [Google Scholar]
- 49.Steiner G, Myher JJ, Kuksis A. Milk and plasma lipid composition in a lactating patient with type I hyperlipoproteinemia. Am J Clin Nutr 41: 121–128, 1985 [DOI] [PubMed] [Google Scholar]
- 50.Vahlquist A, Nilsson S. Mechanisms for vitamin A transfer from blood to milk in rhesus monkeys. J Nutr 109: 1456–1463, 1979 [DOI] [PubMed] [Google Scholar]
- 51.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res 40: 565–574, 1999 [PubMed] [Google Scholar]
- 52.Vogel S, Piantedosi R, O'Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry 41: 15360–15368, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Wasan KM, Subramanian R, Kwong M, Goldberg IJ, Wright T, Johnston TP. Poloxamer 407-mediated alterations in the activities of enzymes regulating lipid metabolism in rats. J Pharm Pharm Sci 6: 189–197, 2003 [PubMed] [Google Scholar]
- 54.Wei S, Episkopou V, Piantedosi R, Maeda S, Shimada K, Gottesman ME, Blaner WS. Studies on the metabolism of retinol and retinol-binding protein in transthyretin-deficient mice produced by homologous recombination. J Biol Chem 270: 866–870, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem 283: 13510–13519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright K, Coverston C, Tiedeman M, Abegglen JA. Formula supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA): a critical review of the research. J Spec Pediatr Nurs 11: 100–112; discussion 112–113, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356–362, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res 46: 1502–1511, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Yost RW, Harrison EH, Ross AC. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. J Biol Chem 263: 18693–18701, 1988. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.