Abstract
Aqueous humor is formed by fluid transfer from the ciliary stroma sequentially across the pigmented ciliary epithelial (PE) cells, gap junctions, and nonpigmented ciliary epithelial (NPE) cells. Which connexins (Cx) contribute to PE-NPE gap junctional formation appears species specific. We tested whether small interfering RNA (siRNA) against Cx43 (siCx43) affects bovine PE-NPE communication and whether cAMP affects communication. Native bovine ciliary epithelial cells were studied by dual-cell patch clamping, Lucifer Yellow (LY) transfer, quantitative polymerase chain reaction with reverse transcription (qRT-PCR), and Western immunoblot. qRT-PCR revealed at least 100-fold greater expression for Cx43 than Cx40. siCx43 knocked down target mRNA expression by 55 ± 7% after 24 h, compared with nontargeting control siRNA (NTC1) transfection. After 48 h, siCx43 reduced Cx43 protein expression and LY transfer. The ratio of fluorescence intensity (Rf) in recipient to donor cell was 0.47 ± 0.09 (n = 11) 10 min after whole cell patch formation in couplets transfected with NTC1. siCx43 decreased Rf by ∼60% to 0.20 ± 0.07 (n = 13, P < 0.02). Dibutyryl-cAMP (500 μM) also reduced LY dye transfer by ∼60%, reducing Rf from 0.41 ± 0.05 (n = 15) to 0.17 ± 0.05 (n = 20) after 10 min. Junctional currents were lowered by ∼50% (n = 6) after 10-min perfusion with 500 μM dibutyryl-cAMP (n = 6); thereafter, heptanol abolished the currents (n = 5). Preincubation with the PKA inhibitor H-89 (2 μM) prevented cAMP-triggered current reduction (n = 6). We conclude that 1) Cx43, but not Cx40, is a major functional component of bovine PE-NPE gap junctions; and 2) under certain conditions, cAMP may act through PKA to inhibit bovine PE-NPE gap junctional communication.
Keywords: aqueous humor formation; connexin 43; adenosine 3′,5′-cyclic monophosphate; protein kinase A; small interfering RNA knockdown
the intraocular pressure(IOP) depends directly on the rate of inflow of aqueous humor and the resistance to outflow from the eye. Lowering IOP is the only approach proven to delay the onset and slow progression of glaucomatous blindness (15, 16, 34, 46), so that reducing the inflow rate is a strategy in treating glaucoma.
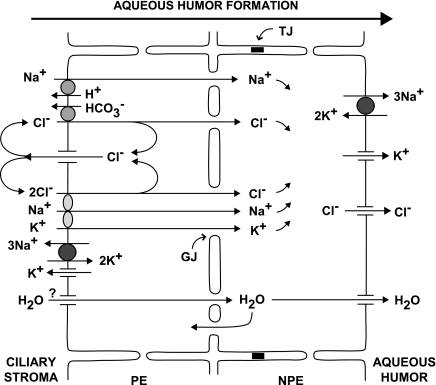
The ciliary epithelium secretes the aqueous humor. Solute, primarily Na+ and Cl−, and water are transferred from the extracellular stroma of the ciliary processes to the posterior chamber by sequential passage through the pigmented ciliary epithelial (PE) cells, gap junctions, and nonpigmented ciliary epithelial (NPE) cells (Fig. 1). Na+ is ejected through Na+-K+-activated ATPase, and Cl− is released through Cl− channels at the basolateral surface of the NPE into the aqueous humor. Water is released at that surface through aquaporin AQP1 and AQP4 channels (10, 45). The aquaporins at the apical surface of the NPE cells may provide an uptake pathway for water under conditions when the virtual space between the PE and NPE cells accumulates fluid (42). Aquaporins have not yet been identified in PE cells (11).
Fig. 1.
Pathways for aqueous humor formation. For clarity, only the major transporters subserving secretion are indicated. Mechanisms that could permit ionic recycling at the basolateral membranes have been omitted. Na+, Cl−, and water from the stroma enter pigmented epithelial (PE) cells through electroneutral symports and antiports, cross gap junctions (GJ) into the nonpigmented epithelial (NPE) cells, and are released through Na+-K+-activated ATPase, Cl− channels, and AQP1 and AQP4 aquaporin water channels, respectively. Aquaporins have not yet been identified in PE cells. Tight junctions (TJ) connect neighboring NPE cells.
Of the many potential physiologic regulators of ciliary epithelial secretion, 3′,5′-cyclic adenosine monophosphate (cAMP) has been most intensively studied (21), and blockers of cAMP formation are clinically used to reduce inflow and thereby IOP (3, 47). The antagonists of β-adrenergic receptors used for this purpose do reduce both cAMP production and IOP, but whether the relationship is causal has long been uncertain (5, 36, 38, 52). Unexpectedly, inflow has been reported to be reduced by either increasing (5, 36) or decreasing (3, 47) cAMP production. Perhaps related to this apparent contradiction are reports that cAMP acts on multiple transport components that could favor either stimulation or inhibition of the secretory rate (10, 21). The direct effect of cAMP has long been under study with in vitro ciliary epithelia (9). On the basis of transepithelial measurements of bovine ciliary epithelium, the proposal has been made that cAMP possibly uncouples the intercellular gap junctions between PE and NPE cells (23). Substantial, cAMP-triggered inhibition of gap junctional transmission would be expected to markedly reduce inflow (Fig. 1). However, connexin 43 (Cx43) is well recognized to form a major component of the PE-NPE gap junctions in this tissue (4, 13, 14, 50), and cAMP has generally been found to increase or not affect Cx43 gap junctional communication in other preparations (33, 35, 51).
In the present work, we have directly studied the effect of cAMP on electrical and dye communication in excised native bovine PE-NPE couplets and have also estimated the contribution of Cx43 to PE-NPE communication by small interfering RNA (siRNA) knockdown.
MATERIALS AND METHODS
Cellular model.
The previously described procedure for cell isolation and preparation (24) was slightly modified to increase the yield of PE-NPE cell couplets. Briefly, fresh bovine eyes were obtained from a local abattoir. After removal of the cornea and iris along the limbus, ciliary processes were excised in small pieces and rinsed with Dulbecco's phosphate-buffered saline (PBS; Invitrogen-GIBCO, Grand Island, NY). The dissociated PE-NPE cell couplets were obtained by incubating the preparation with 0.15% trypsin in a shaker for 20 min at 200 rpm and 37°C. PE cells were readily distinguished from NPE cells by the presence of abundant pigment granules. The cells were washed twice with PBS and plated on coverslips (Fisher Scientific, Pittsburgh, PA) in medium 199 (Invitrogen-GIBCO) for at least 2 h at 37°C in 5% CO2 before the experiment. The medium contained 10% fetal bovine serum and 0.1% gentamicin (Invitrogen-GIBCO). The bovine preparations were prepared ∼1.5 h after death.
Patch-clamp measurements.
Micropipettes for whole cell measurements were prepared using a Flaming/Brown micropipette puller (P-97; Sutter Instrument, San Raphael, CA). Micropipettes were fire polished with a microforge (MF-830; Narishige, Tokyo, Japan) to resistances of 3–7 MΩ. The micropipettes were advanced to the cell surfaces with piezoelectric micromanipulators (models PCS-5400 and PCS-6200; Burleigh, Victor, NY). Couplets chosen for patching displayed a relatively large area of contact between the PE and NPE cells. Cell pairs with minimal areas of contact were avoided in the expectation that such couplets might have arisen from randomly associated cells following harvesting, and were less likely to arise from physiologically coupled pairs in vivo.
To minimize mechanical stress to the cell couplets, we initially tried to conduct whole cell measurements in the perforated-patch mode (31, 41). However, in contrast to experience with certain other cells, both β-escin (26) and gramicidin (1) caused death of the ciliary epithelial cells. As we have previously found (2), perforated patches can be formed with amphotericin or nystatin. However, the probability of success was so low with these cells, that forming perforated patches with both cells of the NPE-PE couplets was deemed unfeasible. Instead, ruptured patches were formed after first establishing gigohm seals in both cells of the couplet.
Currents in couplets of PE and NPE cells were measured by dual whole cell patch clamping (49) with a Multiclamp 700B (Axon Instruments, Foster City, CA) coupled to an external Bessel filter (model 900; Frequency Devices, Haverhill, MA). Data were acquired and analyzed with a digital interface (Digidata 1220 with Clampex 9 software; Molecular Devices, Union City, CA). At the beginning of each experiment, both cells were clamped at the same holding potential (0 mV) to eliminate any transjunctional electrical driving force. Then one of the cells (the donor cell) was stepped to different voltages (from either −110 mV to +110 mV or from −80 mV to +80 mV, in 20-mV increments). The junctional current values were determined by averaging the current in the recipient cell at ±10 mV throughout the entire voltage step.
Lucifer Yellow dye transfer.
The PE cell of each PE-NPE couplet was chosen to be the donor cell and was patched with a micropipette containing Lucifer Yellow (1 mg/ml) (48) for 10 min. Diffusion of dye was imaged at 5 or 10 min after break-in, using a digital camera (Nikon coolpix C4500, Japan) connected to a computer. The fluorescence intensities in recipient cells, donor cells, and background were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD). The total measured intensities were corrected by subtracting the background intensities. Relative intensities were calculated as the ratio of the corrected fluorescent intensities of the recipient to the source cell.
Gene expression analysis.
Connexin 40 (Cx40), Cx43, and peptidylprolyl isomerase B (PPIB) mRNA expression was analyzed by quantitative polymerase chain reaction with reverse transcription (qRT-PCR) using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA). Total RNA from bovine ciliary epithelial cells, kidney medulla, and cerebral cortex was isolated using the RNeasy Mini Kit (QIAGEN, Valencia, CA) and reverse transcribed using Taqman Reverse Transcription reagents (Applied Biosystems) according to the manufacturers’ protocols. The TaqMan primers and probes (Applied Biosystems) used were as follows: Cx40 forward primer, GGCTGCCGAGTCATCCT; Cx40 reverse primer, ACGTTCTCGCAACCAGGTT; Cx40 probe, TATCACAGAGGAAATCAGC; Cx43 forward primer, TGGGTGACTGGAGTGC-CTTA; Cx43 reverse primer, GCCGTGGAGTAGGCTTGAA; Cx43 probe, CTTGTCA-AGGAGTTTGCC; PPIB forward primer, CCCGATGAGAACTTCAAGCTTAAAC; PPIB reverse primer, GAGCCGTTGGTGTCTTTGC; PPIB probe, TTGGCCATGCTCACCC. qRT-PCR was performed using the TaqMan Universal PCR Master Mix and the Prism 7500 sequence detection system (Applied Biosystems). The thermal cycling protocol for the PCR was 50°C for 2 min and 95°C for 10 min, followed by 40 repeat cycles of 95°C for 15 s and 60°C for 1 min. Cx40 and Cx43 mRNA expression was normalized to expression of an endogenous control (PPIB). The relative quantification values (RQ) of target-gene knockdown were calculated with the 2−ΔΔCT method, where CT is threshold cycle.
siRNA knockdown.
Freshly isolated primary bovine PE and NPE cells were transfected at 30 nM siRNA using XtremeGene (Roche, Indianapolis, IN) at a 5:1 XtremeGene:siRNA ratio immediately after the trypsinized cells were plated on coverslips. The siRNA against bovine Cx43 (siCx43) was a Custom SmartPool (Dharmacon, Lafayette, CO) comprising individual siRNAs targeting the sequences GAGCAAAACUGGGCCAAUU, CUGAGAACCUACA-UCAUCA, UAGGCAAACUCCUUGACAA, and GUACCUGGCUCAUGUGUUC. Nontargeting control siRNA no. 1 (NTC1) was used as a negative control (Dharmacon). Cx43 expression was determined by qRT-PCR and Western blot at 24–48 h posttransfection. Antibodies against Cx43 and tubulin were obtained from BD Biosciences (San Jose, CA) and Abcam (Cambridge, MA), respectively. Western immunoblots were scanned and bands were quantified with the ImageJ software.
Chemicals and solutions.
All chemicals were reagent grade. H-89 was purchased from Biomol (Plymouth Meeting, PA) and dibutyryl-cAMP from Sigma-Aldrich (St. Louis, MO). The compositions of the solutions in the micropipettes and external bath are provided in Table 1. Bicarbonate was omitted to obviate potential pH-dependent changes in gap junctional permeability triggered by redistributions of CO2 and HCO3−. Potassium was omitted to minimize uncontrolled shifts in plasma-membrane conductance, in part through cAMP-stimulated K+ channels of NPE cells (12). Solutions were filtered through 0.22 μm (Millipore) before use.
Table 1.
Composition of micropipette filling solutions and external bath
| Micropipette | |||
|---|---|---|---|
| Component | Voltage Clamp | Dye Transfer | External Bath |
| Na+ | 25.0 | 25.0 | 116.0 |
| NMDG | 120.0 | 110.0 | 0.0 |
| Cl− | 25.0 | 25.0 | 116.0 |
| Aspartate | 110.0 | 100.0 | 0.0 |
| Mg2+* | 1.0 | 1.0 | 1.2 |
| Ca2+† | 0.38 | 0.38 | 1.8 |
| HEPES | 12.0 | 12.0 | 12.0 |
| Glucose | 0.0 | 0.0 | 5.0 |
| Mannitol | 0.0 | 0.0 | ±67.0 |
| EGTA | 1.0 | 1.0 | 0.0 |
| ATP | 1.0 | 1.0 | 0.0 |
| GTP | 0.01 | 0.01 | 0.0 |
| pH | 7.2 | 7.2 | 7.4 |
| Osmolality, mosmol/kgH2O | 278 | 278 | 309 |
NMDG, N-methyl-d-glucamine.
Free Mg2+ concentration was 260 μM in micropipette. Except for pH and osmolality, all values are in mM.
Free Ca2+ concentration was 100 nM in micropipette.
Statistical analysis.
Student's t-test, paired or unpaired as appropriate, was applied in comparing two sets of data, and one-way ANOVA was applied to compare three sets of data. Statistical analyses were performed with SigmaStat (Aspire Software International). Unless otherwise stated, the results are presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Dual-cell patch clamping.
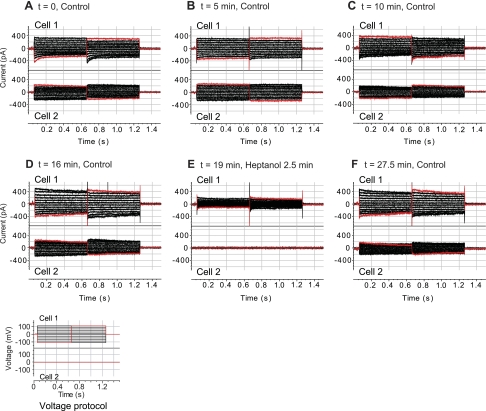
As illustrated by Fig. 2A, voltages applied to the stepped cell (cell 1) produced junctional currents in the recipient coupled cell (cell 2). Dual-patch clamping of PE-NPE cell couplets revealed a wide range of junctional conductances, with a mean ± SE of 6 ± 3 nS (n = 6, 20 mV). The fractional contribution of the junctional currents to the total currents was likewise variable, presumably reflecting variation in the numbers of gap junctions coupling the PE and NPE cells. For example, the baseline junctional currents contributed ∼50% to the total currents measured in the stepped cell of the couplet of Fig. 2A, and ∼80% of that measured in the couplet of Fig. 3A. The junctional currents shown in Fig. 2, A–D, were stable when measured under control conditions during a period of 14 min but were abolished after perfusion with 4.3 mM heptanol (Fig. 2E). The currents in the stepped cells were correspondingly reduced, with the residual currents proceeding across the plasma membrane. Heptanol is a commonly used blocker of connexin gap junctions (8, 44). The response to heptanol documented that the currents measured in the recipient cells passed through the gap junctions and not through cytoplasmic strands. The inhibition produced by heptanol was largely reversible (Fig. 2F).
Fig. 2.
Effect of heptanol on junctional currents of a PE-NPE cell couplet. Following the voltage protocol displayed at bottom, voltages applied to the stepped cell (cell 1) produced junctional currents recorded in the recipient cell (cell 2) (A). Currents in both cells were stable when measured under control conditions at points during the subsequent 16 min (B–D). Perfusion with 4.3 mM heptanol for 2.5 min abolished the junctional currents (E), but currents in both the stepped and recipient cells were restored after subsequent washout for 7.5 min (F). t, Time.
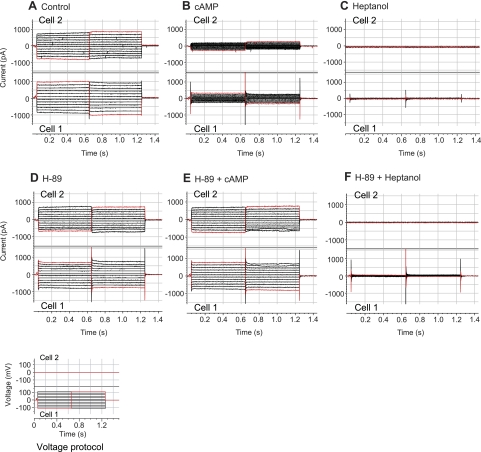
Fig. 3.
Effects of dibutyryl-cAMP, H-89, and heptanol on junctional currents of PE-NPE cell couplets. Currents in the stepped cell (cell 1; A–F, bottom) and in the recipient cell (cell 2; A–F, top) were recorded during the course of perfusing cAMP (500 μM dibutyryl-cAMP) and heptanol (4.3 mM) sequentially with or without H-89 (2 μM) in separate couplets. H-89 prevented the inhibitory effect of cAMP.
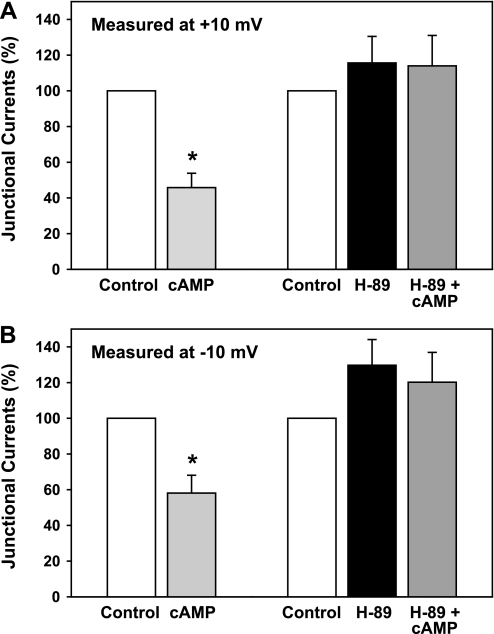
Under our experimental conditions, perfusion with a membrane-permeant form of cAMP (500 μM dibutyryl-cAMP) reduced junctional currents within approximately 5 min. After 10 min, these currents were reduced by 54 ± 8% (n = 6, P < 0.005, t-test), measured at a step potential of +10 mV, and by 42 ± 10% (n = 6, P < 0.01), measured at −10 mV (Figs. 3 and 4). In contrast, blocking cAMP-activated kinase (PKA) with H-89 (7) prevented the cAMP-triggered reduction in currents (n = 6). In the experiments of Figs. 3 and 4, couplets were perfused with 2 μM H-89 for 15 min before applying the cAMP in the continued presence of 2 μM H-89. Perfusion with 4.3 mM heptanol at the conclusion of the dual patch-clamp experiments abolished the junctional currents. Perfusion with H-89 alone did not significantly affect baseline junctional currents at either +10 or −10 mV (one-way ANOVA).
Fig. 4.
Summary of effects of dibutyryl-cAMP, with (n = 6) or without (n = 6) the PKA blocker H-89, on junctional currents of PE-NPE cell couplets. Values were calculated at voltages of +10 mV (A) and −10 mV (B). In the absence of H-89, dibutyryl-cAMP inhibited the junctional currents by ∼50% (*P < 0.01).
Lucifer Yellow dye transfer.
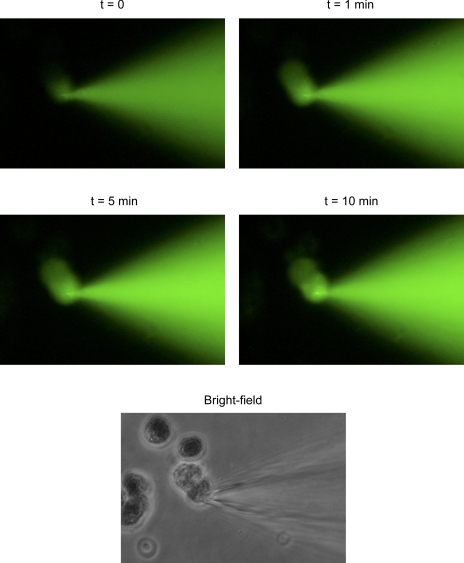
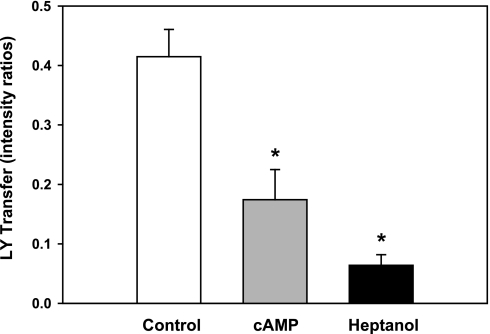
Parallel studies of gap junctional permeability were conducted by monitoring dye transfer. As illustrated by Fig. 5, Lucifer Yellow fluorescence was detected shortly after breaking into the donor cell. Transfer of dye proceeded more slowly over the succeeding 10 min. Figure 6 summarizes the relative intensities of fluorescence in recipient and donor cells measured 10 min after initiating measurement, and corrected for background fluorescence. As in the case of the junctional currents, 500 μM dibutyryl-cAMP reduced the rate of Lucifer Yellow dye transfer. Measured after 10 min, the cAMP lowered the fluorescence ratio from 0.41 ± 0.05 (n = 15) in control couplets to 0.17 ± 0.05 (n = 20), an inhibition of 59 ± 19%. In parallel experiments, perfusion with 4.3 mM heptanol reduced the fluorescence ratio to 0.06 ± 0.02 (n = 6). One-way ANOVA and pairwise comparisons with the Tukey test indicated that the inhibitions produced by both cAMP and heptanol were significant at the 0.002 probability level. The inhibitions produced by cAMP and heptanol were not significantly different from each other.
Fig. 5.
Transfer of Lucifer Yellow dye through gap junctions of a PE-NPE cell couplet. Transfer of the fluorescent dye from the donor to the recipient cell was observed as early as 1 min after break-in. A bright-field photomicrograph of the PE-NPE couplet taken at the conclusion of the experiment is included at bottom.
Fig. 6.
Effects of dibutyryl-cAMP and heptanol on Lucifer Yellow (LY) transfer through PE-NPE gap junctions. In comparison to control couplets (n = 15), cAMP (n = 20) and heptanol (n = 6) reduced gap junctional permeability (*P = 0.002, ANOVA) in parallel experiments. In this figure and Fig. 10, the dye transfer has been quantified as the mean intensity ratio (± SE) in the recipient to the donor cell.
Expression of Cx43 and Cx40.
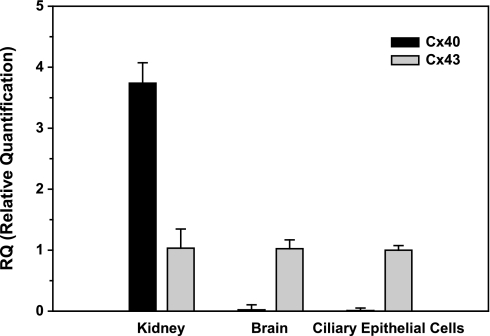
qRT-PCR indicated that bovine ciliary epithelium expressed Cx43 at least 100-fold more than Cx40 under baseline conditions (Fig. 7). Positive and negative controls for the Cx40 primers were provided by measurements conducted on RNA from kidney and brain, respectively (Fig. 7), conforming to the known organ distribution of Cx40 (e.g., Refs. 17 and 29).
Fig. 7.
Relative quantification of message for connexin 43 (Cx43) and Cx40 by quantitative RT-PCR (qRT-PCR). Values are presented as means ± SD. RNA was prepared from duplicate samples extracted from bovine kidney and brain and from a single harvest of bovine ciliary epithelial cells. Each value was averaged from four real-time qRT-PCR measurements. Message detected in ciliary epithelial cells for Cx43 was two orders of magnitude larger than that for Cx40. Message for the widely distributed Cx43 was also detected in bovine kidney and brain. However, as expected, Cx40 was substantially expressed in kidney, but not in brain.
Knockdown of Cx43.
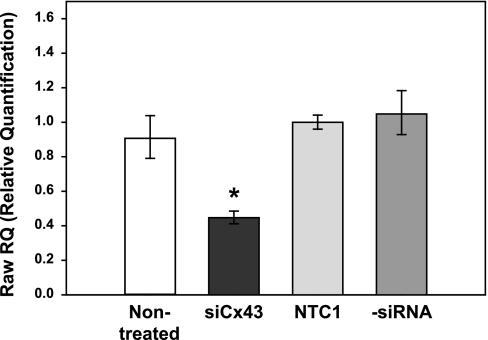
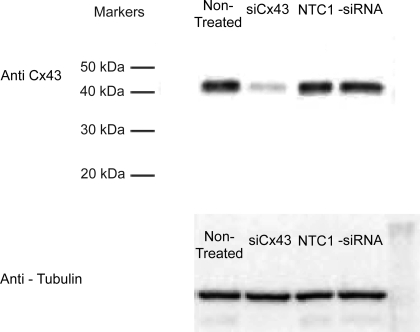
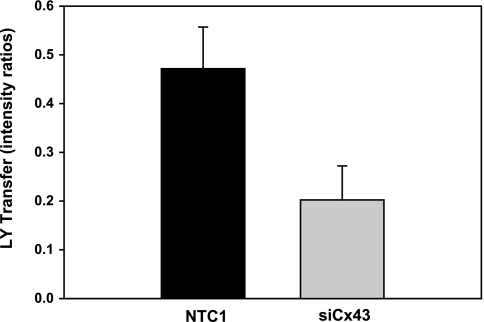
Transfection of harvested bovine ciliary epithelial cells with siCx43 inhibited Cx43 mRNA expression by 55 ± 7% after 24 h (n = 4, P < 0.001) relative to cells transfected with the NTC1 control siRNA (Fig. 8). As illustrated by Fig. 9, siCx43 also reduced expression of Cx43 protein in replicate experiments. Normalizing the intensities to those of the untreated controls, and calculating the uncertainty as one-half of the difference between the band intensities, the mean values were 18 ± 8% (siCx43), 105 ± 11% (NTC1 control), and 84 ± 16% (−siRNA control). Correspondingly, siCx43 knockdown reduced functional gap junctional communication, as determined by Lucifer Yellow dye transfer between PE and NPE cells 48 h after transfection (Fig. 10). The ratio of fluorescence intensity in recipient to donor cell was 0.47 ± 0.09 (n = 11) 10 min after whole cell patch formation in PE-NPE couplets transfected with the NTC1 control siRNA. In contrast, siCx43 siRNA decreased the fluorescence intensity ratio to 0.20 ± 0.07 (n = 13), an inhibition of ∼60% (P < 0.02) (Fig. 10). The similarity of the qRT-PCR (Fig. 9) and Western immunoblot results (Fig. 10) obtained with nontreated and nontargeted control cells suggests that the siRNA did not affect viability.
Fig. 8.
Effect of small interfering RNA (siRNA) knockdown on relative quantification of message for Cx43 by qRT-PCR. Values are presented as means ± SD. Control cells were nontreated (control), treated with nontargeting control siRNA (NTC1), or treated with lipofectant alone (−siRNA). Relative to NTC1, transfection with siRNA directed against Cx43 (siCx43) reduced the message after 24 h (n = 4, *P < 0.001).
Fig. 9.
Effect of siRNA knockdown on Western immunoblot of Cx43. Expression of the Cx43 protein product was substantially reduced by the Cx43 siRNA in comparison to the control lanes 48 h after transfection. Tubulin served as the housekeeping control. Similar results were obtained in a replicate immunoblot, and the mean band intensities are provided in the text.
Fig. 10.
Effect of siRNA knockdown of Cx43 on Lucifer Yellow dye transfer across PE-NPE gap junctions. Values are means ± SE. Incubation with Cx43 siRNA for 48 h (n = 13) inhibited dye transfer observed in control cells (n = 11) by ∼60% (P < 0.02).
DISCUSSION
The salient results of the present study are that 1) under certain conditions, dibutyryl cAMP can acutely reduce gap junctional communication between PE and NPE cells, 2) the inhibitory action of cAMP can be entirely prevented by blocking PKA activity, and 3) knockdown of endogenous Cx43 reduces gap junctional permeability.
Opposing actions of cAMP on aqueous humor inflow.
The data support the suggestion, based on transmural measurements, that cAMP partially uncouples gap junctions linking PE and NPE cells (23). In contrast to its direct activation of maxi-Cl− channels of PE cells (24, 27), cAMP can lower gap junctional communication indirectly by activating PKA, at least under our experimental conditions. This observation adds to the literature documenting that cAMP exerts actions expected both to stimulate and inhibit net aqueous humor formation (10, 21). Stimulation by cAMP of the Na+-K+-2Cl− symport at the stromal surface (18) and of Cl− channels (6, 25) and Na+-K+-activated ATPase (37) at the aqueous surface should enhance secretion. In contrast, cAMP-triggered activation of the maxi-Cl− channels at the stromal surface (23) and acute inhibition of PE-NPE gap junctions should reduce net secretion. Under certain conditions, cAMP has also been reported to inhibit Na+-K+-activated ATPase of NPE cells (19, 40), which would likewise reduce the rate of aqueous humor formation. The predominant integrated effect of cAMP may be determined by compartmentation (21). Such compartmentation has been documented by Huang et al. (32), who found that 1 μM adenosine increased local cAMP concentration sufficiently to activate CFTR Cl− channels of Calu-3 airway cells while elevating the total cAMP content very little.
Connexin composition of PE-NPE gap junctions.
Cx43 has been the only connexin consistently and rigorously identified in ciliary epithelium of multiple species and localized to PE-NPE junctions (4, 13, 14, 50). Despite early evidence implicating its presence in rabbit and rat preparations (50), later work indicated that Cx50 is not expressed in rat PE-NPE gap junctions (14). However, recent preliminary immunoblot and immunolocalization evidence has suggested that Cx50 is expressed on the basolateral surface of bovine NPE cells, contralateral to the PE-NPE gap junctions (43). Coffey et al. (14) also found evidence for Cx40 in the PE-NPE gap junctions of rat ciliary epithelium, using RT-PCR and antiserum developed by Dr. R. Gourdie (Medical University of South Carolina, Charleston, SC). In contrast, Calera et al. (4) were unable to detect Cx40 expression in mouse ciliary epithelium, using antibody purchased from Alpha Diagnostic International (San Antonio, TX) under conditions thought to minimize nonspecific staining. The present qRT-PCR measurements indicate that Cx40 expression of native bovine ciliary epithelial cells is no more than 1% of the Cx43 expression. These data are consistent with our functional observations that partial knockdown of message for Cx43 both reduced expression of Cx43 protein and produced large, comparable inhibitions of junctional currents (∼50%) and of Lucifer Yellow dye transfer (∼60%).
We conclude that Cx43 is a consistent major component of PE-NPE gap junctions and that Cx40 may have a more limited species distribution. This view is consistent with increasingly evident species variation in expression of other ciliary epithelial transport activities (22, 28).
Gap junctional target site of PKA.
The target site of phosphorylation by PKA is unclear. Cx43 is known to be phosphorylated by protein kinase C, MAP kinase, and the pp60src kinase (35). Although Cx43 might also be directly phosphorylated by PKA, most evidence argues against this view (20, 35). Furthermore, in contrast to the current observations, cAMP commonly increases Cx43 gap junctional permeability in other preparations (20, 35, 51), albeit reducing message for Cx43 in rat Leydig cells (53). In addition, intracellular cAMP had no acute effect on Cx43 and Cx40 gap junctions expressed in HeLa cells (33). One possible interpretation of the published data and our own results is that PKA might mediate rapid actions of cAMP, by targeting a regulatory protein not ubiquitously expressed in all gap junctions.
Potential gap junctional regulation by cAMP.
The present results suggest that cAMP may trigger inhibition of gap junctions in some species. It is unclear whether cAMP is delivered to the putative modulatory sites in sufficiently high concentrations to regulate PE-NPE gap junctional permeability and the rate of aqueous humor inflow in vivo. Intracellular cAMP is functionally compartmentalized (32), with cyclic nucleotide phosphodiesterases tending to limit the signal transduction through cAMP diffusion. We have been relating our findings to published studies of β-adrenergic agonists and antagonists and of forskolin, all of which alter cAMP production by membrane-bound adenylyl cyclase. However, the soluble bicarbonate- and Ca2+-dependent adenylyl cyclase (sAC) is present in bovine ciliary processes (39) and may also generate cAMP for delivery to the PE-NPE gap junctions. Recent work suggests that sAC is a physiological regulator of Na+ transport by renal cortical collecting duct cells (30). Whether sAC plays a comparable role in cAMP-dependent regulation of gap junctional permeability and of aqueous humor inflow remains to be determined.
GRANTS
This study was partly supported by National Institutes of Health Grant EY13624 (to M. M. Civan) and Core Grant EY01583, and a grant from Alcon Research, Ltd. Acknowledgement is made to the donors of the National Glaucoma Research Program, a program of the American Health Assistance Foundation, for support of this research.
DISCLOSURES
One or more authors is employed by and/or has a total financial interest worth more than US $10,000 (from consultancy honoraria/expert testimony/corporate grants/patents pending/royalties/other) and/or has a 5% equity in an entity related to the subject matter discussed in the article. Our article reports collaborative results, in part utilizing an siRNA knockdown strategy in bovine ciliary epithelial cells. That strategy is incorporated in a pending patent submitted by Alcon to lower intraocular pressure. Three of the coinventors listed on the patent application are J. E. Chatterton, A. F. Clark, and M. B. Wax while all three were employees of Alcon. J. E. Chatterton remains an employee of Alcon. A. F. Clark and M. B. Wax have since left Alcon. All three former or current employees (J. E. Chatterton, A. F. Clark, and M. B. Wax) retain stock options in Alcon. The decision to publish the results was entirely that of the authors, and not that of Alcon.
ACKNOWLEDGMENTS
We thank Drs. Peter Brink (Department of Physiology and Biophysics, State University of New York at Stony Brook, NY) and Michael Koval (Departments of Medicine and Cell Biology, Emory University, Atlanta, GA) for helpful discussions.
REFERENCES
- 1.Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol 65: 251–264, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Anguíta J, Chalfant ML, Civan MM, Coca-Prados M. Molecular cloning of the human volume-sensitive chloride conductance regulatory protein, pICln, from ocular ciliary epithelium. Biochem Biophys Res Commun 208: 89–95, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Brubaker RF. Clinical measurement of aqueous dynamics: implications for addressing glaucoma. In: Eye's Aqueous Humor: From Secretion to Glaucoma, edited by Civan MM. San Diego, CA: Academic, 1998, p. 234–284 [Google Scholar]
- 4.Calera MR, Wang Z, Sanchez-Olea R, Paul DL, Civan MM, Goodenough DA. Depression of intraocular pressure following inactivation of connexin43 in the nonpigmented epithelium of the ciliary body. Invest Ophthalmol Vis Sci 50: 2185–2193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caprioli J, Sears M, Bausher L, Gregory D, Mead A. Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci 25: 268–277, 1984 [PubMed] [Google Scholar]
- 6.Chen S, Inoue R, Inomata H, Ito Y. Role of cyclic AMP-induced Cl conductance in aqueous humour formation by the dog ciliary epithelium. Br J Pharmacol 112: 1137–1145, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990 [PubMed] [Google Scholar]
- 8.Christ GJ, Spektor M, Brink PR, Barr L. Further evidence for the selective disruption of intercellular communication by heptanol. Am J Physiol Heart Circ Physiol 276: H1911–H1917, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Chu TC, Candia OA. Effects of adrenergic agonists and cyclic AMP on the short-circuit current across the isolated rabbit iris-ciliary body. Curr Eye Res 4: 523–529, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Civan MM. Formation of the aqueous humor: transport components and their integration. In: The Eye's Aqueous Humor (2nd ed.), edited by Civan MM. San Diego, CA: Elsevier, 2008, p. 2–45 [Google Scholar]
- 11.Civan MM. Transporters beyond transport. Focus on “Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels.” Am J Physiol Cell Physiol 298: C11–C13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civan MM, Coca-Prados M, Peterson-Yantorno K. Pathways signaling the regulatory volume decrease of cultured nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 35: 2876–2886, 1994 [PubMed] [Google Scholar]
- 13.Coca-Prados M, Ghosh S, Gilula NB, Kumar NM. Expression and cellular distribution of the alpha 1 gap junction gene product in the ocular pigmented ciliary epithelium. Curr Eye Res 11: 113–122, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Coffey KL, Krushinsky A, Green CR, Donaldson PJ. Molecular profiling and cellular localization of connexin isoforms in the rat ciliary epithelium. Exp Eye Res 75: 9–21, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126: 487–497, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 126: 498–505, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Condorelli DF, Trovato-Salinaro A, Mudo G, Mirone MB, Belluardo N. Cellular expression of connexins in the rat brain: neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur J Neurosci 18: 1807–1827, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Crook RB, Takahashi K, Mead A, Dunn JJ, Sears ML. The role of NaKCl cotransport in blood-to-aqueous chloride fluxes across rabbit ciliary epithelium. Invest Ophthalmol Vis Sci 41: 2574–2583, 2000 [PubMed] [Google Scholar]
- 19.Delamere NA, King KL. The influence of cyclic AMP upon Na,K-ATPase activity in rabbit ciliary epithelium. Invest Ophthalmol Vis Sci 33: 430–435, 1992 [PubMed] [Google Scholar]
- 20.Dhein S, Polontchouk L, Salameh A, Haefliger JA. Pharmacological modulation and differential regulation of the cardiac gap junction proteins connexin 43 and connexin 40. Biol Cell 94: 409–422, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Do CW, Civan MM. Basis of chloride transport in ciliary epithelium. J Membr Biol 200: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Do CW, Civan MM. Species variation in biology and physiology of the ciliary epithelium: similarities and differences. Exp Eye Res 88: 631–640, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Do CW, Kong CW, To CH. Cyclic AMP inhibits transepithelial chloride secretion across bovine ciliary body/epithelium. Invest Ophthalmol Vis Sci 45: 3638–3643, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Do CW, Peterson-Yantorno K, Mitchell CH, Civan MM. cAMP-activated maxi-Cl− channels in native bovine pigmented ciliary epithelial cells. Am J Physiol Cell Physiol 287: C1003–C1011, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Edelman JL, Loo DD, Sachs G. Characterization of potassium and chloride channels in the basolateral membrane of bovine nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 36: 2706–2716, 1995 [PubMed] [Google Scholar]
- 26.Fan JS, Palade P. Perforated patch recording with β-escin. Pflügers Arch 436: 1021–1023, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Fleischhauer JC, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. PGE2, Ca2+, and cAMP mediate ATP activation of Cl− channels in pigmented ciliary epithelial cells. Am J Physiol Cell Physiol 281: C1614–C1623, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Gerometta RM, Malgor LA, Vilalta E, Leiva J, Candia OA. Cl− concentrations of bovine, porcine and ovine aqueous humor are higher than in plasma. Exp Eye Res 80: 307–312, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int 60: 190–201, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn R, Korn SJ. Prevention of rundown in electrophysiological recording. Methods Enzymol 207: 149–155, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA 98: 14120–14125, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 131: 293–305, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120: 701–713, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384: 205–215, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Lee PY, Podos SM, Mittag T, Severin C. Effect of topically applied forskolin on aqueous humor dynamics in cynomolgus monkey. Invest Ophthalmol Vis Sci 25: 1206–1209, 1984 [PubMed] [Google Scholar]
- 37.Liu R, Hintermann E, Erb C, Tanner H, Flammer J, Eberle AN, Haefliger IO. Modulation of Na/K-ATPase activity by isoproterenol and propranolol in human non-pigmented ciliary epithelial cells. Klin Monatsbl Augenheilkd 218: 363–365, 2001 [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin CW, Peart D, Purves RD, Carré DA, Peterson-Yantorno K, Mitchell CH, Macknight AD, Civan MM. Timolol may inhibit aqueous humor secretion by cAMP-independent action on ciliary epithelial cells. Am J Physiol Cell Physiol 281: C865–C875, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Mittag TW, Guo WB, Kobayashi K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am J Physiol Renal Fluid Electrolyte Physiol 264: F1060–F1064, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Nakai Y, Dean WL, Hou Y, Delamere NA. Genistein inhibits the regulation of active sodium-potassium transport by dopaminergic agonists in nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci 40: 1460–1466, 1999 [PubMed] [Google Scholar]
- 41.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 37: 15–26, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Sears ML. Autonomic nervous system: adrenergic agonists. In: Pharmacology of the Eye, edited by Sears ML. New York: Springer-Verlag, 1984, p. 193–248 [Google Scholar]
- 43.Shahidullah M, Delamere NA. Studies on connexin hemichannels in the porcine nonpigmented ciliary epithelium. Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting Abstract no. 1451, 2009 [Google Scholar]
- 44.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Stamer WD, Baetz NW, Yool AJ. Ocular aquaporins and aqueous humor dynamics. In: The Eye's Aqueous Humor (2nd ed.), edited by Civan MM. San Diego, CA: Elsevier, 2008, p. 47–70 [Google Scholar]
- 46.The AGIS investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 130: 429–440, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Toris CB, Camras CB. Aqueous humor dynamics II: clinical studies. In: The Eye's Aqueous Humor (2nd ed.), edited by Civan MM. San Diego, CA: Elsevier, 2008, p. 231–272 [Google Scholar]
- 48.Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91: 104–111, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 281: H1675–H1689, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Wolosin JM, Schütte M. Gap junctions and interlayer communication in the heterocellular epithelium of the ciliary body. In: The Eye's Aqueous Humor: From Secretion to Glaucoma, edited by Civan MM. New York: Academic, 1998, p. 135–162 [Google Scholar]
- 51.Yogo K, Ogawa T, Akiyama M, Ishida-Kitagawa N, Sasada H, Sato E, Takeya T. PKA implicated in the phosphorylation of Cx43 induced by stimulation with FSH in rat granulosa cells. J Reprod Dev 52: 321–328, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Yorio T. Cellular mechanisms in the actions of antiglaucoma drugs. J Ocul Pharmacol 1: 397–422, 1985 [DOI] [PubMed] [Google Scholar]
- 53.You S, Li W, Lin T. Expression and regulation of connexin43 in rat Leydig cells. J Endocrinol 166: 447–453, 2000 [DOI] [PubMed] [Google Scholar]