Abstract
Integrin-associated focal adhesion complex formation and turnover plays an essential role in directing interactions between epithelial cells and the extracellular matrix during organogenesis, leading to appropriate cell spreading, cell-matrix adhesion, and migration. Autosomal recessive polycystic kidney disease (ARPKD) is associated with loss of function of PKHD1-encoded protein fibrocystin-1 and is characterized by cystic dilation of renal collecting tubules (CT) in utero and loss of renal function in patients if they survive the perinatal period. Normal polycystin-1 (PC-1)/focal adhesion complex function is required for control of CT diameter during renal development, and abnormalities in these complexes have been demonstrated in human PC-1 mutant cystic cells. To determine whether loss of fibrocystin-1 was associated with focal adhesion abnormalities, ARPKD cells or normal age-matched human fetal (HF)CT cells in which fibrocystin-1 had been decreased by 85% by small interfering RNA inhibition were compared with normal HFCT. Accelerated attachment and spreading on collagen matrix and decreased motility of fibrocystin-1-deficient cells were associated with longer paxillin-containing focal adhesions, more complex actin-cytoskeletal rearrangements, and increased levels of total β1-integrin, c-Src, and paxillin. Immunoblot analysis of adhesive cells using site-specific phospho-antibodies demonstrated ARPKD-associated loss of activation of focal adhesion kinase (FAK) by phosphorylation at its autophosphorylation site (Y397); accelerated FAK inhibition by phosphorylation at Y407, S843, and S910; as well as increased activation of c-Src at pY418. Paxillin coimmunoprecipitation analyses suggested that fibrocystin-1 was a component of the normal focal adhesion complex and that actin and fibrocystin-1 were lost from ARPKD complexes.
Keywords: migration, extracellular matrix, fibrocystin
the focal adhesion complex plays a critical role in the regulation of epithelial cell-extracellular matrix (ECM) adhesion, migration, differentiation, and proliferation of anchorage-dependent epithelial cells. Cell-matrix attachment induces focal clustering of heterodimeric integrin receptors and initiates the recruitment and binding to the intracellular COOH-terminus of β1-integrin of the many structural and signaling proteins that comprise the multimolecular focal adhesion complex. These include the structural proteins talin, tensin, vinculin, and α-actinin as well as the signaling proteins focal adhesion kinase (FAK), c-Src, p130cas, and paxillin (17, 19, 32, 43, 58, 61, 62). Since proteins of the focal adhesion complex also associate with filamentous actin, this multimolecular complex provides a means for communication between the external ECM environment and the internal cellular cytoskeleton that provides tensile strength and exertion of bidirectional mechanical forces (13). Polycystin-1, the product of the PKD1 gene that is mutated in autosomal dominant polycystic kidney disease (ADPKD), is thought to exert mechanotransducer functions and is known to form complexes with β1-integrin and focal adhesion component proteins in renal epithelial cells (14, 33, 52, 54).
Focal adhesion number, size, distribution, and function are tightly regulated by site-specific phosphorylation of FAK, c-Src, paxillin, and p130cas. In response to integrin occupancy, FAK is autophosphorylated at tyrosine (Y) residue 397 in its kinase domain and the resultant conformational changes create binding sites for c-Src kinase at Y576/7 and Y861 (1, 5, 6, 18, 36, 58). When activated by integrin-mediated cell adhesion, cytoplasmic c-Src is translocated to cell membrane-associated focal contacts where conformational change facilitates autophosphorylation at Y418 as well as Src homology 2-mediated phosphorylation of FAK at Y576/7 and Y861 (5, 6, 60), thus enhancing FAK activity. Negative regulation of FAK and c-Src are also mediated by site-specific phosphorylation associated with conformational change including phosphorylation of c-Src at Y529 and of FAK at Y407, S843, and S910 (12, 21). FAK and c-Src bind and phosphorylate multiple substrates including paxillin, an adaptor protein that is associated with focal adhesion assembly and whose phosphorylations at Y31 and Y118 are negatively regulated by mechanical force (4, 15, 39, 61).
Focal adhesions are dynamic, adhesive structures whose coordinated turnover by assembly and disassembly at the leading and trailing edges of cells facilitates cell attachment and motility. Knockout of several focal adhesion associated proteins including β1-integrin FAK, c-Src, paxillin, p130Cas, and polycystin-1 have been shown to reduce cellular migration, to increase ECM adhesiveness, and to cause embryonic lethality in mice (17, 19, 20, 22, 27, 33, 44). Strictly regulated migration of specific cell types is essential for normal embryonic development and morphogenesis of many organs. In the kidney, coordinated migration of the ureteric bud epithelium through the blastemal matrix is essential for the appropriate induction and differentiation of normal nephrons (11, 25, 33). Insufficiency of the focal adhesion-associated proteins tensin, polycystin-1, and nephrocystin-1 are associated with renal cystic malformation in mice (26, 31, 41, 53, 56).
Autosomal recessive polycystic kidney disease (ARPKD) is a hereditary monogenic disorder caused by mutations in the polycystic kidney and hepatic disease gene-1 (PKHD1) gene that is often lethal in infancy and is a common cause of renal failure in children. ARPKD kidneys share several phenotypic characteristics with ADPKD including bilaterally enlarged, multicystic kidneys with proliferative, differentiation, cell-matrix, and epithelial polarization defects. They differ from ADPKD in that ARPKD cysts arise as ectatic expansions of ureteric bud-derived collecting tubules (37, 53). Both the PKD1-encoded polycystin-1 (PC-1) and the PKHD1-encoded fibrocystin-1 are developmentally regulated proteins that are expressed at high levels in the ureteric bud epithelium in the normal developing kidney where they have been localized to basal areas of the cell membrane that are in focal contact with the ECM (33, 50, 54).
Since previous studies from several groups had demonstrated abnormalities in focal adhesion turnover and mechanotransduction in ADPKD and shown that PC-1 can form complexes with β1-integrin and other focal adhesion proteins, that PC-1 can interact with PC-2, and that PC-2 can interact with fibrocystin-1 (14, 48), we undertook the present studies to examine the role of focal adhesion abnormalities in ARPKD. Alterations in adhesion-dependent focal adhesion protein content and distribution in human ARPKD vs. age-matched normal human kidneys were correlated with specific abnormalities in the site-specific phosphorylations of FAK, c-Src, and paxillin associated with activation and inhibition that regulate the kinetics of the focal adhesion-related functions of cell spreading, adhesion, and migration. Furthermore, endogenous PKHD1 knockdown studies using small interfering RNA (siRNA) implicated a direct role for PKHD1 in focal adhesion function in human renal epithelia during normal development.
MATERIALS AND METHODS
Tissue Source and Cell Culture
Normal and ARPKD kidneys were freshly collected at source in the operating room (National Disease Research Interchange, Philadelphia, PA and International Institute Advancement of Medicine, Philadelphia, PA) according to protocols approved by the Institutional Review Board and National Institutes of Health (NIH) and were deidentified prior to use. No tissues had been subjected to warm ischemia and all were validated by routine pathology.
Human renal epithelial cells.
Clonal cell lines of age-matched (19 wk gestation), microdissected, temperature-sensitive conditionally immortalized, normal human fetal collecting tubules (HFCT, clone 7F) and cyst-lining ARPKD (clone 5E) epithelia (34, 51, 55) were selected based on extensive marker analysis showing retention of in vivo cell characteristics and functions (14, 33, 38, 51, 52, 54, 57). Monolayer cultures were grown on collagen-coated (300 μg/ml, type I BD Biosciences, Bedford, MA) tissue culture plastic (Costar, Corning, NY) at the permissive temperature of 33°C in specific growth factor-supplemented medium: HEPES-buffered Click/RPMI (Quality Biologicals, Gaithersburg, MD) containing 5 μg/ml transferrin (Sigma, St. Louis, MO), 5 × 10−8 M dexamethasone (Sigma) and 1% fetal bovine serum (FBS, Crystalgen, Plainview, NY). At 80% confluence cells were transferred to the nonpermissive temperature of 37°C for 5–12 days to achieve maximal differentiation prior to experimentation.
mIMCD cells.
Mouse inner medullary collecting duct (mIMCD-3) cells (35) were cultured in DMEM/F12 containing 4.5 g/l d-glucose (GIBCO), supplemented with 10% FBS (GIBCO), 50 U/ml penicillin, and 50 mg/ml streptomycin.
Cell Spreading Assays
Two thousand HFCT and ARPKD cells were allowed to attach to type 1 collagen-coated glass coverslips (18 mm2) in six-well tissue culture plates (Corning Costar) by incubation in cell type-specific tissue culture medium at 37°C for 30 min, 4 h, and 24 h. After immunofluorescence labeling for paxillin and actin (see below Immunofluorescence for details), coverslips were mounted onto slides with Vectashield (Vector Laboratories, Burlingame, CA), and epifluorescence was viewed by deconvolution microscopy (Nikon Eclipse 800, Melville, NY, by kind courtesy of Dr. Y. Ioannou, Mount Sinai School Medicine). Collected images were analyzed with use of MetaVue software (Molecular Devices, Downington, PA). All experiments were repeated three times and multiple replicates examined at each time point.
Cell Adhesion Assays
Either 2,000 or 5,000 cells per well of 96-well collagen-coated plates were allowed to attach for 30 min, 4, 24, or 48 h (steady state) at 37°C. Unattached cells were removed by gentle aspiration prior to addition of Aqueous MTS reagent (Cell Titer Aq, Promega, Madison, WI). Color development was carried out for 4 h at 37°C and measured at 492 nm by using a plate reader (Bio-Rad 550, Bucks, UK). Values from parallel control plates, lacking cells were subtracted (33). All experiments were repeated three times and multiple replicates were examined at each time point.
Migration Assays
Differentiated HFCT and ARPKD cells were rendered fluorescent by preloading with calcein-AM (InVitrogen-Molecular Probes, Eugene, OR). Either 5,000 or 10,000 cells were seeded in 300 μl 1% FBS-containing medium in the upper chamber of 24-well plates containing Fluoroblock membrane assemblies (Falcon, HTS inserts, 8-μm pore size). After 4 h of equilibration the assemblies were transferred to wells containing 5% FBS and appearance of fluorescence in the lower chamber was measured at 2, 4, 6, 24, and 48 h using a microfluorimetric plate reader (excitation 485 nm, emission 535 nm: HTS 7000 Plus BioAssay Reader, Perkin Elmer, Waltham, MA). All experiments were repeated three times and multiple replicates were examined at each time point.
Transfection and siRNA Treatment
Five potential PKHD1 siRNA sequences with nontargeting controls were procured (On-Targetplus Duplex, Dharmacon, Lafayette, CO) and transfected into HFCT cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Real-time (RT) quantitative (q) PCR of cDNA (One Shot, BD Biosciences) was carried out after 48 h of incubation. The most effective sense strand was 5′-AUACAGACCUCUAUCUUAAUU and the next most effective was 5′-CGAGAUAGCUGUACUUUCAUU. The most effective siRNA sense sequence containing XhoI restriction sites was annealed to its antisense strand and subcloned into a short hairpin (sh) loop construct under control of a recombinant adeno-associated viral (rAAV) CMVe/CBA serotype-2 promoter [a generous gift from Dr. R. M. Linden, Mount Sinai School of Medicine (MSSM)]. Transduction efficiencies into HFCT cells of rAAV2-green fluorescent protein (GFP) empty vector [5.5 × 109 genome containing particles (gcp)/μl] and of rAAV2-GFP-PKHD1-shRNA (1.5 × 109 gcp/μl) in the presence or absence of helper wild-type adenovirus (8.2 × 107 plaque forming units/μl) were determined by fluorescence-activated cell sorting analysis of GFP positivity (courtesy of Dr. N. Clement, MSSM).
RNA Isolation, cDNA Synthesis, and RT-qPCR Analysis
Total RNA was isolated by using RNeasy (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA was synthesized from 1 μg RNA in 20 μl Sprint PowerScript PrePrimed Single Shots, containing reverse transcriptase with random hexamer primers (BD Biosciences and Clontech, San Jose, CA) by incubation for 1 h at 42°C. RT-qPCR analysis was carried out (Applied Biosystems Sequencer; Core Service, MSSM) by using the following sets of primer pairs: PKHD1 forward 5′-GGCTGCAGGCTGAGCTCTAA; PKHD1 reverse 5′-TGTGACTCAAGGGAGAAATGATCC. β-actin forward 5′-CCCACACTGTGCCCATCTAC; β-actin reverse 5′-GCTTCTCCTTAATGTCACGC. GAPDH forward 5′CAATGACCCCTTCATTGACC; GAPDH reverse 5′-GATCTCGCTCCTGGAAGATG. Data were analyzed with SDS 2.0 software. mRNA levels were measured in three replicates of 1 × 106 cells before and after treatment with PKHD1 siRNA or shRNA. Fold changes in PKHD1 mRNA were normalized to β-actin levels. GAPDH levels were also measured as an additional control to confirm the efficiency of siRNA or shRNA transfection.
Primary Antibodies
The following antibodies were used for Western immunoblot, immunoprecipitation, immunohistochemical, and/or immunofluorescence analyses: anti-β1-integrin, anti-paxillin, anti-FAK, anti-c-Src, and anti-p130cas (mouse monoclonals, BD Biosciences, San Jose, CA); anti-vinculin and anti-actin (mouse monoclonals, Sigma); anti-fibrocystin-1 (#3′5A, mouse monoclonal, generous gift of Dr. C. Ward, Mayo Clinic, MN); anti-phospho (p)Y397-, pY407-, pY861-, pS843-and pS910-FAK; anti-pY418- and pY529 c-Src; and anti-pY31- and pY118-paxillin (all rabbit polyclonals from Biosource, Camarillo, CA). Specific dilutions are noted in the figure legends. All commercial antibodies evaluated and selected for use were monospecific, showing a single band on Western blot.
Western Immunoblot Analysis
Cell proteins were extracted in lysis buffer containing 150 mM NaCl, 10 mM Tris pH 7.2, 1% Triton X-100, 0.5% Nonidet P-40, 1 mM EGTA, 1 mM EDTA, a comprehensive protease inhibitor cocktail, and the phosphatase inhibitors 2 mM sodium fluoride, 2 mM sodium vanadate, 2 mM tetramisole, and 2 mM sodium β-glycerophosphate (all from Sigma). Protein concentrations were quantified by the Bradford assay. Cell proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and analyzed by Western immunoblot analysis. Briefly, lysates were denatured by boiling in a final concentration of 2% SDS-2% β-mercaptoethanol at 95°C for 3 min. Then 20–50 μg of cell lysate was loaded per lane and separated proteins were transferred to nitrocellulose or polyvinylidene difluoride membranes, blocked in 5% nonfat milk in Tris-buffered saline-Tween (TBST) or 5% BSA for total and phosphorylated proteins, respectively. Membranes were incubated in primary antibody either for 1.5 h at room temperature or overnight at 4°C and washed in TBST. Conjugated horseradish peroxidase-coupled anti-mouse or anti-rabbit secondary antibody (Molecular Probes) was detected by enhanced chemiluminescence (ECL, Thermoscientific, Rockford, IL) and exposure on Hyblot-CL autoradiography Film (Denville Scientific, Metuchen, NJ). Equal loading and transfer of proteins were confirmed by Coomassie and Ponceau S staining of gels and membranes.
Immunoprecipitation
HFCT and ARPKD cells were lysed in the same buffer as that used for Western immunoblot analysis. Lysates were first precleared by incubation with protein A/G plus beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min at 4°C. Then 1 mg cell protein was immunoprecipitated overnight at 4°C with 1 μg/ml paxillin monoclonal antibody. Protein A/G plus beads (Santa Cruz Biotechnology) were added to the immunoprecipitated lysate and rotated for 2 h, overnight at 4°C. After 3 washes in PBS, the beads were boiled for 3 min in 2% SDS-2% β-mercaptoethanol Laemmli buffer. Then 200–600 μg of immunoprecipitated lysate was loaded per lane and subjected to SDS-PAGE and Western immunoblot analysis.
Immunohistochemistry
Human and mouse kidneys were sliced and fixed for 4 h at 4°C in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS containing calcium and magnesium. After three extensive washes in PBS at 4°C, fixed tissues were dehydrated in a series of graded ethanols and xylene and embedded in low-temperature paraffin; 5-μm sections were cut and dewaxed in xylene and hydrated in a graded series of ethanols prior to being washed in PBS. Endogenous peroxidase activity was inhibited by aqueous 7.5% hydrogen peroxide (Fisher, Fair Lawn, NJ) for 5 min and nonspecific binding to tissue sections or cells was blocked by 3% BSA or 10% horse or goat serum prior to incubation in the primary antibody for 45 min at room temperature in a humidified atmosphere. After three washes of 5 min each in PBS-0.02% Tween, avidin-biotin-enhanced anti-peroxidase immunohistochemistry was carried out (Vectastain Elite, Vector, Burlingame, CA), followed by color development in amino-ethyl-carbazole for 10–30 min. Light microscopic analysis was carried out under a Nikon Microphot FXA microscope using bright-field and Nomarski optics.
Immunofluorescence
Cells on collagen-coated glass coverslips were fixed for 5–10 min in 2% paraformaldehyde in PBS containing calcium and magnesium. After permeabilization in 0.01% Triton-PBS for 1 min, coverslips were incubated with a primary antibody diluted in PBS-0.2% BSA for 1 h at room temperature, washed in PBS, and then incubated in goat anti-mouse Alexa-594 or goat anti-rabbit Alexa-488 (1:1,000, Molecular Probes). Other cells were incubated with rhodamine phalloidin (33 nm in PBS, Molecular Probes, Eugene, OR). All coverslips were mounted onto slides by use of Vectashield and were viewed by deconvolution microscopy. All experiments were repeated three times with eight replicates.
Statistical Analysis
Where indicated, a P value of < 0.05 was chosen as the threshold for statistical significance in a two-tailed Student's t-test.
RESULTS
Aberrant renal morphogenesis and cystic dilation are characteristic of ADPKD and ARPKD. The proteins encoded by genes mutated in each of these diseases are thought to reside in a large multiprotein complex. Although ADPKD cells demonstrate abnormalities in ECM adhesion, epithelial cell migration, and branching morphogenesis, little is known about the focal adhesion complex in ARPKD. We thus set out to 1) characterize the focal adhesion complex in ARPKD and 2) determine the putative role of fibrocystin-1 in focal adhesion assembly and function. We compared focal adhesion complex composition, regulation by phosphorylation, and function with regard to cell spreading, cell-matrix adhesion, and migration. To directly evaluate the role of fibrocystin-1 in focal adhesion function, we manipulated endogenous levels in normal cells by siRNA knockdown and analyzed focal adhesion composition and function.
Expression of Full-Length Fibrocystin-1 Protein Is Decreased in Human ARPKD Cystic Epithelia Compared With Normal Age-Matched Renal Epithelial Cells
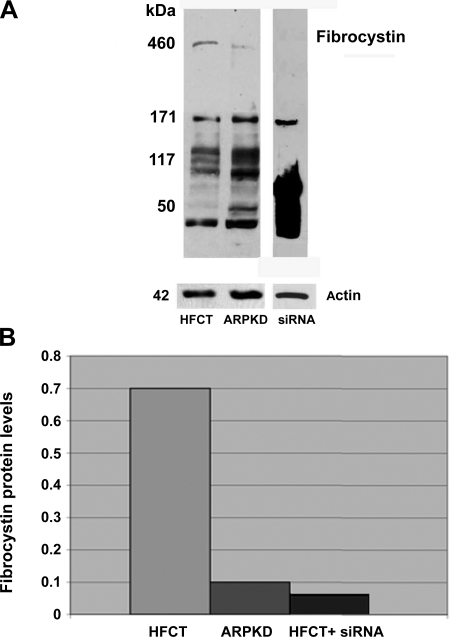
Fibrocystin-1 levels in ARPKD and age-matched HFCT cells were determined by Western immunoblot analysis (Fig. 1). Although significant levels of full-length (≥460 kDa) fibrocystin-1 protein were detected in normal HFCT cells, low or negligible levels were seen in ARPKD cells (Fig. 1A, lane 1 compared with lane 2). Western blot and densitometric analysis also showed that treatment of normal HFCT cells with siRNA for PKHD1 reduced endogenous levels of fibrocystin-1 to <10% of normal, attaining similar levels as those measured endogenously in this age-matched human ARPKD cell line (Fig. 1A, lane 3, and B).
Fig. 1.
Endogenous fibrocystin-1 in human autosomal recessive polycystic kidney disease (ARPKD) cells by comparison with normal human fetal collecting tubule (HFCT) cells. A: Western immunoblot analysis of fibrocystin-1 in age-matched normal HFCT and ARPKD epithelial cells. Confluent steady-state cells, 60 μg of cell lysate per lane. Full-length fibrocystin-1 is clearly detectable at >460 kDa in HFCT but barely detectable neither in ARPKD nor in PKHD1 siRNA-treated HFCT cells. Fibrocystin-1 1:500, actin 1:10,000. B: densitometry analysis of levels in HFCT and ARPKD after normalization to actin. Fibrocystin-1 expression ratio: HFCT/ARPKD 7:1. siRNA, small interfering RNA.
Expression of Focal Adhesion Complex-Associated Proteins Is Altered in Human ARPKD Cystic Epithelia Compared With Normal Age-Matched Renal Epithelial Cells
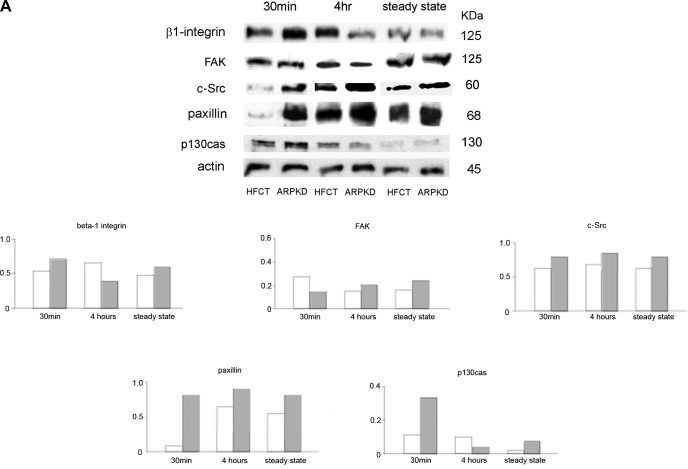
Anchorage-dependent focal adhesion assembly is a time-dependent process. In epithelial cell adhesion to ECM 30 min marks the beginning of cell attachment and spreading, 4 h marks a standard intermediate time point, and confluent cells represent the steady state. Therefore a comparative analysis of focal adhesion protein content in ARPKD vs. normal HFCT cells was carried out in adherent cells collected at three time points after adhesion to type 1 collagen. Western immunoblot analysis was carried out for the matrix collagen receptor β1-integrin, and the focal adhesion signaling and adaptor proteins FAK, c-Src, paxillin, and p130cas. Figure 2A shows that, after 30 min of attachment, several focal adhesion proteins were expressed at higher levels in ARPKD cells than in HFCT cells, including β1-integrin, c-Src, paxillin, and p130cas. By contrast, total FAK protein levels were similar in attached HFCT and ARPKD cells. After 4 h of attachment, β1-integrin, c-Src, and paxillin levels in HFCT cells increased, whereas in ARPKD cells β1-integrin and p130cas levels decreased. Overall, the maximal differences in the total levels of expression of focal adhesion component proteins in ARPKD vs. normal HFCT cells were observed in the early stages after ECM attachment, suggesting accelerated increased availability of receptor and adaptor proteins required for nascent focal contact formation in attaching ARPKD cells.
Fig. 2.
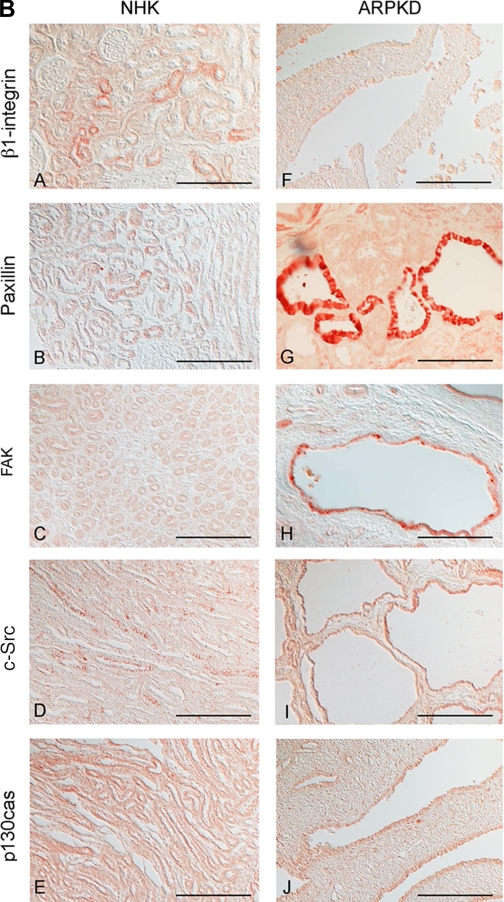
Time- and attachment-dependent expression of total levels of focal adhesion complex proteins in ARPKD and HFCT cells. A: Western immunoblot analysis of focal adhesion proteins in age-matched normal HFCT and ARPKD epithelial cell lines. Equal numbers of cells in suspension were plated on collagen I for 30 min, 4 h, or 7–11 days (confluent steady state); attached cells were analyzed for content of total β1-integrin, focal adhesion kinase (FAK), c-Src, paxillin, and p130cas; and levels were normalized to actin content. Cell lysate, 30 μg per lane. Anti-β1-integrin, 1:500; anti-FAK, 1:500; anti-c-Src, 1:150; anti-paxillin, 1:2,000; anti-p130cas, 1:500; anti-actin, 1:10,000. Densitometry analysis was carried out for focal adhesion proteins at each time point. B: immunohistochemistry of focal adhesion complex proteins in age-matched pediatric (4–5 yr) normal human kidney (NHK; A–E) and end-stage ARPKD (F–J). Anti-β1-integrin, 1:50; anti-paxillin, 1:200; anti-FAK, 1:50; anti-c-Src, 1:150; anti-p130cas, 1:50. Aminoethylcarbazole (red) reaction product. Bright-field and Nomarski optics. Scale bar represents 100 μm.
Focal adhesion protein localization in vivo was also compared by immunohistochemistry of age-matched normal human pediatric and ARPKD kidneys. β1-Integrin, paxillin, FAK, c-Src, and p130cas were all localized to human ARPKD cystic epithelia, with the strong cyst-lining epithelial staining seen for paxillin, FAK, and c-Src (Fig. 2, B and G–I). These quantitative profiles resemble those seen in actively adherent ARPKD cells, modeled by 4 h of ECM attachment in culture. Whereas c-Src expression was seen predominantly in the apical regions of normal collecting tubules, both apical and diffuse intracellular staining was seen in ARPKD cystic epithelia (Fig. 2, B and D vs. I).
Adhesion-Dependent, Site-Specific Phosphorylation of Focal Adhesion Complex Proteins Is Abnormal in ARPKD Compared With Normal Age-Matched Renal Epithelia
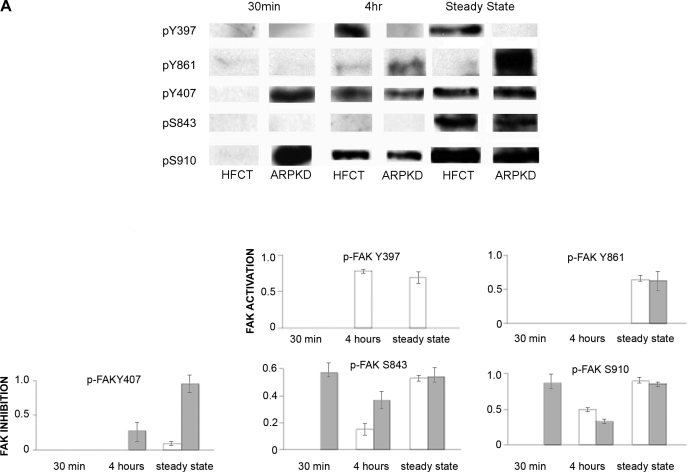
The assembly and turnover of focal contacts is tightly regulated by phosphorylation on specific amino acid residues conferring activation or inhibition. Western immunoblot analysis using phospho-specific antibodies was used to compare phosphorylation of key regulatory sites in HFCT and ARPKD cells at 30 min, 4 h, and steady state after attachment to ECM collagen (Fig. 3A). After 30 min of attachment, ARPKD cells exhibited high levels of FAK phosphorylation on serines (S) 843 and S910 that inhibit kinase activity, whereas HFCT cells showed none. FAK inhibition in ARPKD cells at this early adhesion time point suggested that activation of FAK by tyrosine 397 phosphorylation might be delayed or completely abolished. After 4 h of attachment, FAK-Y397 was phosphorylated in HFCT, but not in ARPKD cells, nor was this site phosphorylated in steady-state ARPKD cells. After 4 h of attachment and at steady state, phosphorylation of another inhibitory site, FAK-Y407, by c-Src was increased in ARPKD cells by comparison to HFCT, further suggesting that FAK activity in ARPKD was abolished. Adhesion-dependent phosphorylation of FAK Y407, S483, and S910 occurred in HFCT in steady state. No differences were observed between HFCT and ARPKD in the time-dependent profile of phosphorylation of FAK on Y861. Overall, these results not only showed an absence of activation of FAK on its autophosphorylation (Y397) site but also suggested premature inactivation by phosphorylation of multiple FAK inhibitory sites in ARPKD epithelial cells.
Fig. 3.
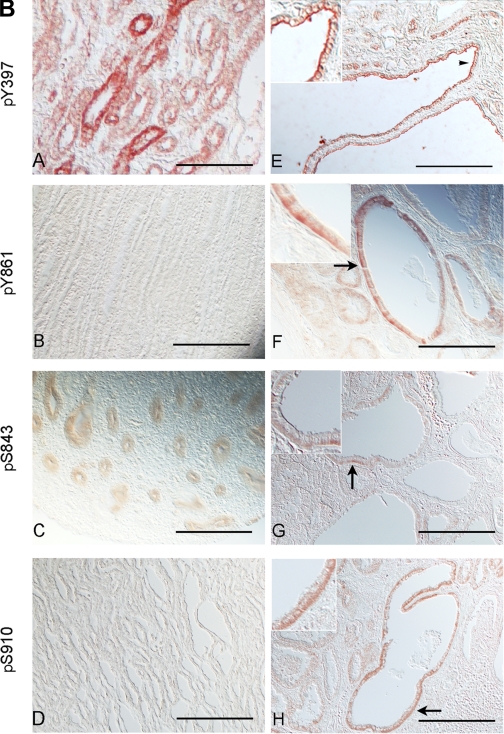
Time- and attachment-dependent site-specific phosphorylation of FAK in ARPKD cells by comparison to normal HFCT epithelia. A: Western immunoblot analysis of site-specific phosphorylation of FAK. Equal numbers of cells were plated on collagen I for 30 min, 4 h, or 7–11 days (confluent steady state) and attached cells were analyzed for pY397-FAK, pY407-FAK, pY861-FAK, pS843-FAK, and pS910-FAK. Cell lysate, 45 μg per lane. Anti-pY397-FAK, 1:1,000; anti-PY407-FAK, 1:1,000; anti-pY861-FAK, 1:1,000; anti-pS843-FAK, 1:2,000; anti-pS910-FAK, 1:2,000. After initial immunoblotting, membranes were stripped and reimmunoblotted for total FAK. Densitometry analysis was carried out and the fraction of phosphorylated FAK to total FAK levels were calculated at each time point. B: immunohistochemistry of phospho-FAK in age-matched pediatric (4–5 yr) normal human kidneys (A–D) and end-stage ARPKD (E–H) tissue sections. Anti-pY397-FAK, 1:100; anti-pY861-FAK, 1:100; anti-pS843-FAK, 1:200; anti-pS910-FAK 1:200. Aminoethylcarbazole (red) reaction product. Most pY861-, pS843- and pS910-FAK staining was seen in basal cell membranes in ARPKD (F–H, arrows and insets). By contrast, pY397-FAK was localized to apical membranes of ARPKD cyst epithelia (E, arrowhead and inset). Bright-field and Nomarski optics. Scale bar represents 100 μm.
Persistent fetal gene expression and mispolarization of transporters, channels, and receptors are common in ARPKD cystic epithelia. Also FAK is highly phosphorylated at its activating autophosphorylation Y397 site during normal development. Immunohistochemistry was therefore used to compare localization of phospho-FAK species in age-matched normal pediatric and ARPKD kidneys (Fig. 3B). In normal postnatal kidneys, immunoreactivity for pY397-FAK was seen at basolateral cell surfaces of collecting tubule epithelia, consistent with a role in mediation of communication with the extracellular environment (Fig. 3B, A). Somewhat surprisingly, reaction product for pY397-FAK was seen in ARPKD cyst-lining epithelia from end-stage pediatric ARPKD kidneys, but it was localized to apical cell surfaces (Fig. 3B, E), where it would not be able to play a role in ECM responses. As also noted in cells in culture, inhibitory pY861-FAK, pS843-FAK, and pS910-FAK showed apparent increased levels of expression in ARPKD cystic epithelia and were localized to the basal membranes (Fig. 3B, F–H).
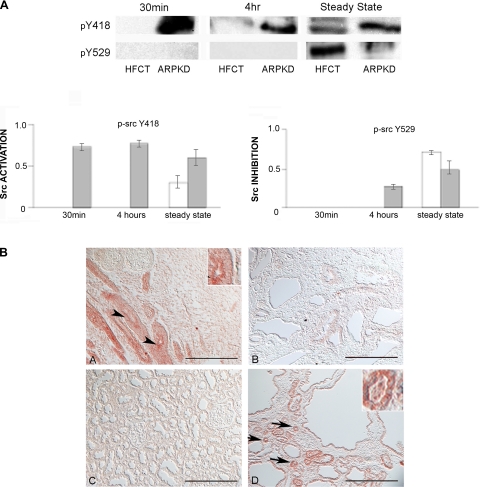
During focal adhesion recruitment, FAK switches from an autoinhibitory conformation to an open state, which unmasks a c-Src binding site. In turn, focal adhesion recruitment activates Src on Y418 and then an inhibitory site at Y529 is dephosphorylated. Thus altered FAK regulation in ARPKD led us to examine the degree of c-Src activation. Figure 4A shows a marked increase in adhesion-dependent phosphorylation of Src-Y418 in ARPKD cells after 30 min and 4 h of attachment and at steady state by comparison to HFCT cells where pY418-src was only detected in steady state. Consistent with the mechanism of c-Src regulation, phosphorylation of the negative regulatory site Y529 was higher in HFCT cells, although only seen after steady state had been reached. It is possible that increased c-Src activation in ARPKD may directly inhibit FAK by hyperphosphorylation on the inhibitory site FAK-Y407, as observed in ARPKD cells in Fig. 3A. Although no pY418-Src was detected in normal postnatal kidneys (Fig. 4BC) it was observed in some normal human fetal kidneys where it was restricted to ureteric bud epithelia (Fig. 4BA), and localized predominantly to apical membranes. Similar apical staining of pY418- Src was also seen in some small cysts from pediatric end-stage ARPKD kidneys (Fig. 4, B and D), suggesting this as an example of persistent fetal protein expression patterns in ARPKD.
Fig. 4.
Time- and attachment-dependent site-specific phosphorylation of c-Src in ARPKD by comparison to normal HFCT cells. A: Western immunoblot analysis of site-specific phosphorylation of c-Src. Equal numbers of cells plated onto type I collagen for 30 min, 4 h, or 7–11 days (confluent steady state) and attached cells analyzed for pY418-Src and pY529-Src. Cell lysate, 30 μg per lane. Anti-pY418-Src, 1:1,000; anti-pY529-Src, 1:1,000. After initial immunoblotting, membranes were stripped and reimmunoblotted for total c-Src. Densitometry analysis was carried out and the fraction of phosphorylated c-Src to total c-Src was calculated. The experiment was repeated 3 times and showed similar results. B: immunohistochemistry of anti-pY418-Src in normal and ARPKD tissue sections. A: 16-wk normal human fetal kidney. B: 19-wk fetal early-stage ARPKD. C: 4-yr normal human kidney. D: 4-yr end-stage ARPKD. Aminoethylcarbazole (red) reaction product is seen at the apical membranes of the developing ureteric bud epithelium in normal fetal kidneys (A, arrowhead and inset) and in small cysts of ARPKD kidneys (D, arrows and inset). No staining is seen in normal pediatric kidneys. Nomarski optics. Scale bar represents 100 μm.
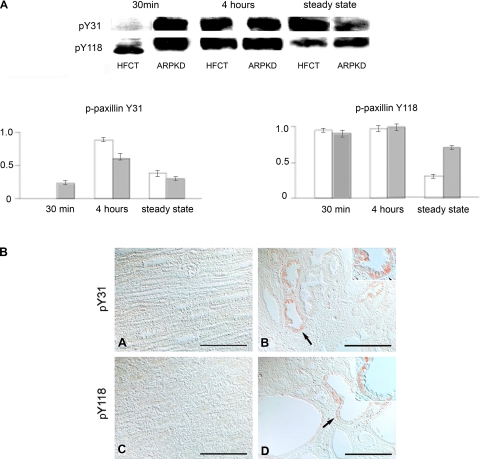
Increased levels of pY418-Src but the absence of pY397-FAK in ARPKD cells suggested that phosphorylation of their common substrate, paxillin, might be altered. Using site-specific anti-phospho-paxillin antibodies in Western immunoblot analysis, phosphorylation of paxillin at Y31 and at Y118 was observed in ARPKD cells (Fig. 5A). Although phosphorylation of paxillin on Y31 and Y118 was seen in both HFCT and ARPKD cells after 4 h of attachment, striking differences between the cell types were seen after 30 min of attachment including the accelerated phosphorylation of Y31-paxillin in ARPKD cells. By contrast, in HFCT cells, only a molecular mass (59-kDa) form of pY118-paxillin was seen after 30 min of attachment. Immunohistochemistry did not detect pY31- or pY118-paxillin immunoreactivity in normal pediatric kidneys, but both were detected in cyst-lining epithelia of ARPKD kidneys at basal cell membranes and diffusely in the cytoplasm (Fig. 5B).
Fig. 5.
Time- and attachment-dependent site-specific phosphorylation of paxillin in ARPKD cells by comparison to normal HFCT cells. A: Western immunoblot analysis using site-specific anti-phospho antibodies. Equal numbers of cells were plated onto type I collagen for 30 min, 4 h, or 7–11 days (confluent steady state) and attached cells analyzed for pY31-paxillin and pY118-paxillin. Cell lysate, 30 μg per lane. Anti-pY31-paxillin, 1:1,000; anti-pY118-paxillin, 1:1,000. After initial immunoblotting, membranes were stripped and reimmunoblotted for total paxillin, 1:1,000. Densitometry analysis was carried out and the fraction of phosphorylated paxillin to total paxillin levels was calculated at each time point. B: immunohistochemistry of phospho-paxillin in age-matched pediatric (4–5 yr) normal human kidneys (A and C) and end-stage ARPKD (B and D) tissue sections. Anti-pY31-paxillin, 1:100; anti-pY118-paxillin, 1:100. Aminoethylcarbazole (red) reaction product. Most pY31- and pY118-paxillin staining was seen in basal cell membranes in ARPKD (B and D, arrows and insets). By contrast, pY31 and pY118-paxillin were absent in normal epithelia (A and C). Bright-field and Nomarski optics. Scale bar represents 100 μm.
Focal Adhesion-Associated Cell Spreading, Adhesion, and Migration Are Abnormal in ARPKD Epithelial Cells
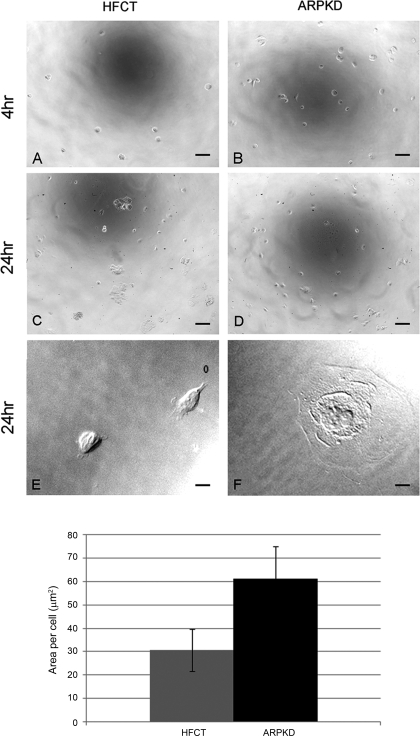
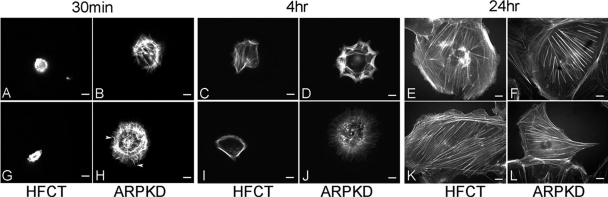
To compare the focal adhesion-associated functions in ARPKD and normal cells, we observed cells during attachment, spreading, and migration after plating onto type I collagen matrix (Fig. 6). When equal numbers of cells were seeded at sparse, subconfluent densities under identical conditions, more ARPKD cells attached than HFCT cells after 4 h (B, ARPKD, 413 ± 60 compared with A, HFCT, 68 ± 30; n = 3; 7 replicates per experiment) as well as after 24 h of attachment (D, ARPKD, 1,010 ± 130 compared with C, HFCT, 98 ± 20; and bar graph, n = 3, 7 replicates per experiment). Higher power images also showed increased spreading of ARPKD cells compared with HFCT cells (F, compared with E).
Fig. 6.
Time-dependent attachment and spreading of ARPKD cells by comparison to normal HFCT cells. A–F: phase contrast microscopy 4 h (A and B) and 24 h (C–F) after attachment; 2,000 cells per collagen-coated well. More ARPKD than HFCT cells attached. An ARPKD cell (F) was more spread than 2 HFCT cells (E; ×63 objective). Scale bar represents 6 μm. Bar graph: quantitation of area values in HFCT and ARPKD cells after 24 h adhesion to collagen I. Image J analysis software; n = 3; 4 replicates per condition; 20 cells.
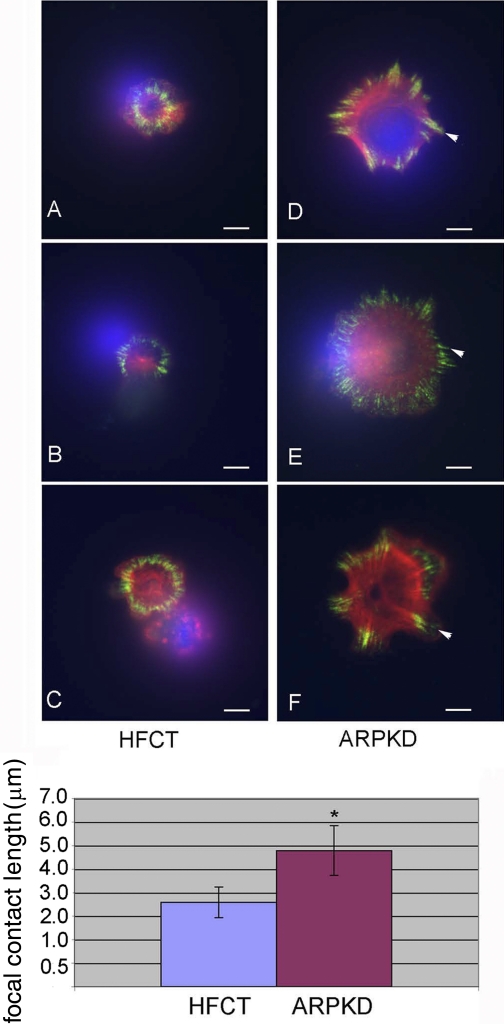
To determine whether increased cell spreading was associated with changes in cytoskeletal organization, cells were stained with rhodamine-phalloidin and examined by deconvolution microscopy (Fig. 7). Striking differences in the organization of filamentous actin were associated with increased spreading of ARPKD vs. HFCT cells after 30 min and 4 h of matrix adhesion. Whereas attached, single, spread HFCT cells had a cortical rim of actin (Fig. 7, C and I), ARPKD cells displayed a more complex pattern of interwoven stress fibers (Fig. 7, B, H, D, and J) associated with focal contacts as well as occasional filopodial extensions (Fig. 7, H, arrowheads). To further examine and quantitate these differences in focal contact formation, double labeling for actin and paxillin was carried out (Fig. 8). Marked differences were observed in ARPKD cells compared with HFCT cells after 30 min of ECM attachment. Paxillin-containing focal adhesions (green) in ARPKD cells were significantly longer than those in HFCT cells (Fig. 8, D–F and bar graph; P < 0.005). Taken together, these results suggest that the loss of normal membrane-associated fibrocystin-1 in ARPKD epithelia is associated with alterations in focal adhesion protein expression and leads to modifications in focal contact formation, cell attachment, and cell spreading.
Fig. 7.
Time- and attachment dependent distribution of actin cytoskeleton in ARPKD cells by comparison to normal HFCT cells. Rhodamine-phalloidin fluorescent labeling of filamentous actin 30 min (A, B, G, H), 4 h (C, D, I, J), and 24 h (E, F, K, L) after attachment; 2,000 cells were plated per collagen I-coated well. Note the thin cortical actin staining in HFCT cells after 4 h of attachment (C and I); complex actin organization in ARPKD cells after 30 min (B and H) and 4 h of attachment (D and J). Filopodial projections are also seen in ARPKD epithelia (H, arrowheads); n = 10.
Fig. 8.
Double fluorescent labeling of paxillin and actin in ARPKD cells by comparison to normal HFCT cells. A–C: HFCT. D–F: ARPKD 30 min attachment; 2,000 cells were plated per collagen-coated well. Rhodamine-phalloidin, 30 nm, red; paxillin, 1:200, goat anti-mouse Alexa-594, 1:1,000, green, n = 10. Deconvolution microscopy. Scale bar represents 6 μm. Bottom: statistically significant increase in the length of paxillin-positive focal contacts in ARPKD cells compared with HFCT cells, n = 30. Student's t-test, *P < 0.005.
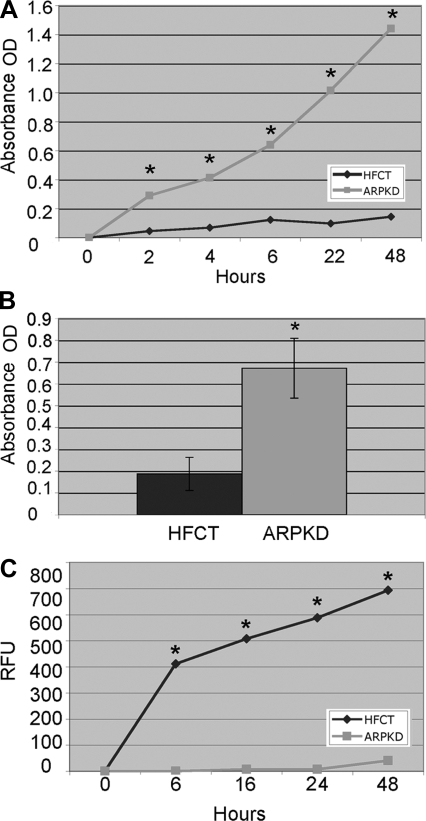
The observation that ARPKD cells exhibited premature spreading suggested that ECM adhesiveness and cell motility might be altered. The adhesion and migration functions of HFCT and ARPKD cells were compared longitudinally over a 48-h time course by quantitative analyses (Fig. 9). Significantly more ARPKD cells adhered to ECM collagen at 2, 4, 6, 22, and 48 h (Fig. 9A, a representative experiment, 7 replicates at each time point, P < 0.001). The consistency of this finding was confirmed in 3 separate experiments (Fig. 9B, 4-h adhesion, 10 replicates each experiment, P < 0.001). In addition to increased adhesive properties, ARPKD cells also exhibited decreased polarized migration in response to a growth factor concentration gradient (Fig. 9C, n = 3, 3 replicates per time point). A decrease in ARPKD migration was seen at every time point (6, 16, 24, and 48 h) compared with HFCT cells (P < 0.0001).
Fig. 9.
Adhesion and migration of ARPKD cells by comparison to normal HFCT cells. A: time course of collagen I adhesion of HFCT and ARPKD cells 2, 4, 6, 22, and 48 h after seeding of 5,000 cells per well. Colorimetric MTS assay (4-h), absorbance units [optical density (OD)] at 492 nm. Note statistically significantly increased adhesion of ARPKD cells compared with HFCT. Student's t-test, *P < 0.05, n = 8. B: 4-h adhesion of HFCT and ARPKD cells (n = 3, 10 replicates per experiment). Student's t-test, *P < 0.05. C: migration of HFCT and ARPKD cells; 10,000 calcein-AM preloaded cells were seeded per well and migration determined in response to a 1% to 5% FBS gradient. Relative fluorescence units (RFU) at 535 nm (n = 4 experiments, 3 replicates per time point). Student's t-test, *P < 0.0001.
Focal Adhesion Complex Composition Is Altered in ARPKD Cells
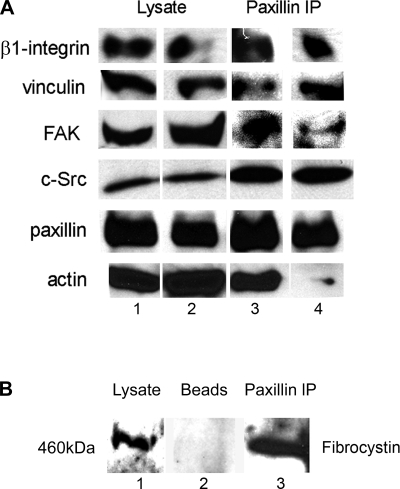
The observations that focal adhesion complex protein content, their regulation by phosphorylation, and their functions related to matrix attachment, cell spreading, adhesion, and migration were abnormal in ARPKD led us to suspect that assembly of the multimolecular focal adhesion complex assembly might be compromised in ARPKD epithelial cells. Since paxillin is a central scaffold component of the focal adhesion, it was selected to immunoprecipitate the focal adhesion complex in ARPKD and HFCT cells and to then determine binding interactors by Western immunoblot analysis (Fig. 10). Paxillin interacts with β1-integrin, FAK, c-Src, and vinculin in both HFCT and ARPKD cells (Fig. 10A). However, in marked contrast to HFCT cells, virtually no interaction of paxillin with actin was seen in ARPKD cells, suggesting that focal adhesion complex function is abnormal in ARPKD.
Fig. 10.
Composition of paxillin-containing focal adhesion complex in ARPKD cells compared with normal HFCT cells. A: Western immunoblot of HFCT (lane 1) and ARPKD (lane 2) cells at steady state. Cell lysate, 30 μg per lane. Anti β1-integrin, 1:500; anti-vinculin, 1:1,000; anti-FAK, 1:500; anti-c-Src 1:1,000; anti-paxillin, 1:2,000; anti-actin, 1:10,000. Identical cells: HFCT (lane 3) and ARPKD (lane 4) cells were immunoprecipitated with anti-paxillin 1 μg/1 mg of lysate and immunoprecipitates (IP) were subjected to immunoblot analysis. Note that very little actin was detected in the paxillin immunoprecipitates from ARPKD cells. B: association between paxillin and fibrocystin-1. Western immunoblot analysis of fibrocystin-1 in 30 μg mouse inner medullary collecting duct (mIMCD) cell lysate (lane 1) and paxillin immunoprecipitate from 1 mg of lysate (lane 3). Lane 2 is bead-only negative control. Anti-paxillin 1:2,000; anti-fibrocystin-1, 1:500.
Since PC-1 and nephrocystin-1 are known to be components of the focal adhesion complex, and since PC-2 is known to bind to PC-1 and fibrocystin-1, we next sought to determine whether fibrocystin-1 was a component of the normal focal adhesion. Figure 10B shows that fibrocystin-1 was detected in the paxillin immunoprecipitate from normal collecting duct cells. This suggested that loss of fibrocystin-1 in ARPKD epithelia might cause focal adhesion functional defects by virtue of disruption of the integrity of the ARPKD focal adhesion at the level of its interaction with paxillin.
siRNA Knockdown of PKHD1 mRNA in Normal HFCT Cells Increases Cell-Matrix Adhesion
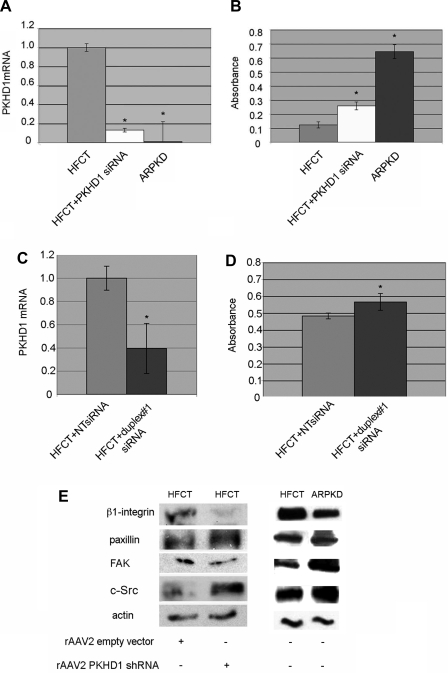
To determine whether loss of fibrocystin-1 alone is sufficient to alter cell-matrix adhesion avidity and focal adhesion protein expression levels in normal cells, PKHD1 mRNA was knocked down in normal HFCT cells. Transfection of our most effective PKHD1 siRNA into HFCT cells decreased PKHD1 mRNA by 85% (Fig. 11A; P < 0.05) whereas transfection of an unrelated, nontargeting siRNA had no effect (Fig. 11C). In a 4-h adhesion assay, PKHD1 siRNA-treated HFCT cells were significantly more adherent to type I collagen matrix compared with untreated cells (Fig. 11B, P < 0.001), showing that reduction of PKHD1 altered the phenotype of HFCT cells, closely resembling that of ARPKD cells. This appeared to be related to the degree of PKHD1 knockdown since a less efficient sequence that resulted in 60% knockdown of PKHD1 (Fig. 11C) caused a lesser degree, but still significant, increase in HFCT cell-matrix adhesion (Fig. 11D, P < 0.001). Furthermore, a >85% decrease in PKHD1 mRNA and protein (Fig. 1) levels in HFCT cells was associated with decreased levels of β1-integrin and increased total c-Src and paxillin levels as determined by Western immunoblot analysis (Fig. 11E). This result paralleled the patterns observed in ARPKD epithelial cells. Taken together, these results suggest that loss of fibrocystin-1 leads to focal adhesion-associated alterations in cell function in renal epithelial cells.
Fig. 11.
Effects of siRNA or short hairpin RNA (shRNA) knockdown of PKHD1 in normal HFCT cells. A: real-time quantitative PCR (qPCR) of PKHD1 mRNA content of HFCT cells transfected with PKHD1 siRNA, normalized to levels of actin mRNA. Note that PKHD1 siRNA reduced PKHD1 mRNA by 85% (white vs. gray bar). Negligible levels of PKHD1 mRNA were seen in ARPKD cells (black bar); n = 3; Student's t-test, *P < 0.05, compared with HFCT. B: MTS adhesion assay 4 h after plating 2,000 cells on collagen I. HFCT cells transfected with PKHDI siRNA (white bar) showed increased levels of adhesion compared with untreated HFCT (gray bar). Student's t-test, *P < 0.0001, n = 10, compared with HFCT. C: real-time qPCR of PKHD1 mRNA content of HFCT cells transfected with PKHD1 siRNA, duplex#1 compared with nontargeting (NT) sequence. Levels normalized to actin mRNA (n = 3). Student's t-test, *P < 0.05, compared with HFCT + NT. Note that PKHD1 mRNA was reduced 60%. D: MTS adhesion assay 4 h after plating 2,000 cells on collagen I. HFCT cells transfected with PKHDI siRNA duplex#1 showed increased levels of adhesion compared with HFCT cells transfected with NT sequence. Student's t-test, *P < 0.001, n = 10, compared with HFCT +NT. E: Western immunoblot analysis of attachment-dependent expression of focal adhesion complex proteins after rAAV2GFP-PKHD1 shRNA knockdown (>90% reduction PKHD1 mRNA and protein) in HFCT cells. After 24 h transduction with rAAV2GFP-PKHD1shRNA or empty vector (rAAV2-GFP), 5 × 106 cells were plated for 4-h on type I collagen and attached cells collected. Lysate, 15 μg per lane. Anti-β1-integrin, 1:500; anti-paxillin, 1:2,000; anti-FAK, 1:500; anti-c-Src, 1:1,000; anti-actin, 1:10,000.
DISCUSSION
Focal adhesion complexes are multimolecular assemblies linking the extracellular matrix to the intracellular actin cytoskeleton via integrin receptors at the primary sites of cell-matrix adhesion. These studies examined the role of the focal adhesion complex in ARPKD pathogenesis. Focal adhesions regulate cell adhesion, spreading, and migration. They act as traction points that enable specific cell shape changes and facilitate cell migration by means of remodeling processes. Cell migration is an integrated process that requires coordination of signaling molecules such as kinases, ubiquitin ligases, proteases, and phosphatases. Furthermore, focal adhesions establish signaling interfaces through which cells sense and respond to changes in their external environment.
Ureteric bud epithelial differentiation and the subsequently defined lumen size of collecting tubules are aberrant in ARPKD. Focal adhesion complex number, size, regulation, and function are abnormal in the similar but not identical genetic cystic disease of the kidney, ADPKD (14, 33), suggesting that focal adhesion properties might also be aberrant in ARPKD epithelia. We used human age-matched, conditionally immortalized, differentiated ARPKD and normal HFCT kidney epithelial cells to characterize focal adhesion complexes in this disease.
The timeline of attachment-dependent regulation of focal adhesion proteins was starkly different in ARPKD compared with normal epithelia. In normal HFCT cells β1-integrin and several focal adhesion protein expression levels peaked after 4 h of adhesion to type I (interstitial) collagen. By contrast, focal adhesion proteins in ARPKD epithelia, including β1-integrin, paxillin, c-Src, and p130-cas, were maximally increased after just 30 min of attachment, whereas FAK levels were equivalent. Hence, the initial events of focal contact assembly occurred at an accelerated rate in ARPKD cells.
FAK autophosphorylation at Y397 is essential for proper control of focal adhesion turnover and cell migration (7, 40). The absence of FAK activation by autophosphorylation at Y397 in ARPKD cells, even in steady state, was striking and markedly different from HFCT cells. This closely parallels previous observations made in ADPKD cells, which also lack FAK-Y397 phosphorylation and fail to recruit FAK to focal adhesion complexes (52, 56). Although FAK does not require c-Src for Y397 phosphorylation, binding to c-Src maximizes pY397 and allows c-Src to phosphorylate FAK on Y576/577, Y407, Y861, and Y925, which modulate FAK activity. Hence we examined FAK phosphorylation at additional sites. The Y861 site was only phosphorylated in the steady state in both HFCT and ARPKD epithelia, suggesting this was not a significant early activation event during focal contact assembly although we cannot rule out a compensatory activation of FAK by phosphorylation at the c-Src Y576/577 activation site.
In addition to decreased FAK activation in ARPKD cells there was evidence of increased inactivation of FAK in ARPKD cells by premature and increased levels of phosphorylation of its inhibitory sites: Y407, S843, and S910 (23). Phosphorylation of S843 and S910 was seen as early as 30 min after attachment and the c-Src-mediated Y407 site became phosphorylated 4 h after attachment. Abnormally increased FAK S910 phosphorylation by ERK2 in ARPKD epithelia is consistent with increased ERK1/2 activity found in ARPKD mouse models and ADPKD epithelia (8, 47, 49, 59).
Although pY397-FAK was not detected by Western blot in human fetal ARPKD cell lines, immunoreactivity was demonstrable by histochemistry in tissue sections of ARPKD kidneys from pediatric patients. This difference could have been due to the difference in age of the donor cells and tissues or due to increased sensitivity to immunodetection by amplified histochemistry achieved with this antibody. It should be noted, however, that although pY397-FAK was localized to end-stage pediatric ARPKD kidneys it was mispolarized to the apical cell membranes of cyst lining epithelia, where it would presumably be unable to participate in cell-matrix interactive responses. By contrast, normal collecting ducts exhibited the expected localization of pY397-FAK to basolateral cell membranes at the interface between epithelial cells and the ECM. This inversed polarity in ARPKD epithelia is strikingly similar to that seen for EGFR and Na+-K+-ATPases in both dominant and recessive polycystic kidney disease. Overall, the results define decreased activation and increased inhibition of FAK as a hallmark of ARPKD. Inactive FAK may contribute to aberrant focal adhesion turnover and thus lead to increased adhesion and decreased migration in ARPKD epithelial cells.
In contrast to abnormally inhibited FAK, parallel time course adhesion studies in ARPKD epithelia showed accelerated activation of c-Src by autophosphorylation at Y418 after just 30 min and also after 4 h of attachment. This is consistent with c-Src being in an open molecular configuration in ARPKD. c-Src is also excessively phosphorylated in the steady state. The loss of inactivation is manifested by lower levels of pY529 c-Src in ARPKD compared with HFCT cells (60). Increased Src activity was concomitant with FAK-Y407 phosphorylation, which resulted in premature inhibition of FAK. This abnormality may retard focal adhesion turnover in ARPKD cells. Taken together, these results are consistent with a fundamental alteration in FAK/Src regulation that contributes to aberrant ARPKD epithelial cell adhesion and suggest that c-Src rather than FAK-mediated pathways dominate in ARPKD.
Although normal pediatric and early-stage ARPKD collecting ducts both demonstrate barely detectable pY418 c-Src expression, normal fetal and end-stage pediatric ARPKD collecting ducts show very similar increased levels and apical localization. This may be attributed to either a delay of c-SrcY418 phosphorylation or persistent fetal regulation of c-Src in ARPKD, which would be similar to persistent fetal ErbB2 subunit expression and apical localization of EGFR/ErbB2 in polycystic kidney disease. It is known that normal outside-in signaling events via the integrin receptor and focal adhesion complex are required for initiation of epithelial polarization events. Therefore, abnormal FAK and c-Src localization in ARPKD suggests that loss of fibrocystin-1 may contribute to arrested maturation or differentiation via the focal adhesion complex, particularly since an association between paxillin and fibrocystin-1 has been demonstrated.
Paxillin is a 68-kDa adaptor protein that integrates focal adhesion responses to ECM attachment and growth factor-related signals and thus provides the potential for rapid responses by the cell to environmental changes (46). Paxillin is a downstream target of both FAK and c-Src (15) and phosphorylation at Y31 and Y118 regulate the assembly and turnover of focal adhesions (61). Similar to the observed accelerated phosphorylation of c-Src in ARPKD epithelia, paxillin phosphorylation at Y31 was also premature, seen after just 30 min of attachment of ARPKD epithelia but not in normal HFCT cells. Accelerated paxillin phosphorylation may contribute to increased ARPKD cell adhesion. Our results are consistent with studies using paxillin phosphomimetic mutants, which localize to and augment focal contacts (61). We also unexpectedly observed a lower molecular weight band (∼60 kDa) for pY118 paxillin in HFCT cells after 30 min of attachment, raising the possibility that the normally occurring 68-kDa phospho-paxillin band might correspond to a monoubiquitinated species. Ubiquitination of paxillin sequesters it from the focal adhesion and attenuates cell motility (10). Hence, it is plausible that this mechanism is active in ARPKD epithelia sooner after attachment than in HFCT epithelia. Accelerated phosphorylation of nascent focal contact-associated paxillin at Y31 and Y118 supports a more adhesive phenotype in ARPKD.
Polycystins-1 and 2 can reside in the same complex and individually interact with focal adhesion and cytoskeletal proteins. Loss of FAK from PC-1 complexes in ADPKD causes a major disruption in the focal adhesion complex. This complex has not been characterized in ARPKD. Since our observations show a preserved PC1/FAK association in ARPKD (data not shown), this suggests that although ARPKD and ADPKD share an accelerated adhesion phenotype, this is not related to abnormal PC1/FAK interactions in ARPKD. Isolation of the ARPKD focal adhesion complex by paxillin immunoprecipitation revealed loss of actin recruitment even though paxillin-vinculin associations were unperturbed in ARPKD. The observed loss of paxillin-actin interaction may inhibit proper focal adhesion turnover, leading to decreased migration of ARPKD epithelia. Thus far, PC-2 and the calcium-modulating ligand are known interactors with fibrocystin-1 (29, 48). We now show by coimmunoprecipitation that fibrocystin-1 also interacts with paxillin, implicating focal adhesion dysregulation in ARPKD-related fibrocystin-1 deficiency.
ARPKD epithelial cells demonstrate accelerated spreading, increased matrix adhesion, and decreased growth factor-mediated migration. At early time points of adhesion and spreading, actin cytoskeletal organization in ARPKD shows accelerated stress fiber formation leading into longer, peripheral, paxillin-containing focal adhesion complexes by comparison to HFCT. Larger focal adhesions have also been observed in ADPKD epithelia (52) and FAK null mouse embryonic fibroblasts (22). FAK phosphorylation at Y397 is absent in ADPKD and ARPKD cells, which may explain the increased numbers of longer focal adhesions and associated decreased migration, increased adhesion, impaired focal adhesion turnover, and slowed disassembly at the trailing edge. Focal adhesion size controls tension (16). Alterations in focal adhesion size are associated with a strikingly decreased migratory activity in response to a growth factor gradient. This is of interest in the context of ARPKD, which is a proliferative disease with demonstrated abnormalities in the EGF signaling pathway.
Like ADPKD epithelia, ARPKD epithelia are more adhesive to the extracellular matrix, and our observations suggest that several focal adhesion components contribute to this phenotype. Knockdown of fibrocystin-1 by use of siRNA in normal HFCT cells resulted in increased cell-matrix adhesion, supporting the hypothesis that loss of function of the PKHD1 gene is responsible for the focal adhesion-associated abnormalities in ARPKD cells. Conversely, stable overexpression of PKHD1/EGFP leads to alterations in ARPKD cell phenotypes such as reduced cell spreading and attachment to collagen matrix (data not shown). Interestingly, after 30 min of adhesion to collagen, endogenous levels of β1-integrin in ARPKD cells exceeded those of HFCT cells, similar to observations in ADPKD epithelia (52). Integrin β-subunit levels are also increased in independently derived ARPKD clonal cells (9). Since EGFR stimulation has been shown to activate β1-integrin transcription in 3T3 fibroblasts (3), the higher β1-integrin levels observed in ARPKD cells may be partly attributed to increased EGFR activity in ARPKD.
Cell adhesion and migration require normal extracellular matrix and focal adhesion protein regulation. During development of the mammalian metanephric kidney, ureteric bud branching is dependent on regulated cell-matrix adhesion and migration and requires normal focal adhesion dynamics. Several lines of evidence implicate an important role for cystic proteins in renal branching morphogenesis. Dominant active constructs or overexpression of PC-1 and PC-2 stimulate tubulogenesis whereas dominant negative constructs or siRNA inhibition of PC-1 inhibit tubulogenesis and cause lumen distension in vitro in three-dimensional collagen gels and in ureteric buds in embryonic mouse kidney organ culture (2, 24, 30, 33, 42). Furthermore siRNA inhibition of PKHD1 in mIMCD3 cells has been shown to inhibit tubulogenesis in collagen gels (28). This role is further suggested by the developmentally regulated high level of expression of PC-1 and fibrocystin-1 and their restricted localization to the ureteric bud-derived collecting duct structures of the fetal kidneys (33, 42, 50). During early development, cystic proteins are localized predominantly to cellular basal membranes, whereas localization at lateral cell-cell adherens junctions and apical cilia is seen at later stages of differentiation. Focal adhesions play a role in mechanotransduction by transmitting traction forces from the actin cytoskeleton to integrin receptors. Cell-cell junctions and primary cilia are also sites of mechanotransduction. Proper mechanotransduction from the apical to the basal cell surface is necessary to maintain an appropriately differentiated state (45). Thus fibrocystin-1, together with PC-1 and PC-2, may be involved in integration of mechanosensory signals from the basal, lateral, and apical surfaces of renal collecting tubule cells and loss of this function may lead to loss of control of collecting tubule lumen diameter (53).
Overall, our studies clearly implicate fibrocystin-1 in focal adhesion complex dysregulation, altering crucial cell-matrix adhesive and migratory functions that contribute to ARPKD pathogenesis.
GRANTS
The work was supported by NIH Grant P01DK62345 (P. Wilson and K. Amsler) and by the Graduate School of Mount Sinai School of Medicine.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Dr. Nathalie Clement, Barbara Bloswick, and Anastasia Liapis for expert technical assistance and to Drs. R. Michael Linden, Carlo Iomini, Irina Barash, and Justine Elliott for helpful discussion. Dr. Y. Ioannou kindly made available his deconvolution microscope for invaluable use in these studies, and Dr. C. Ward (Mayo Clinic) provided us with invaluable antibodies.
Present address for P. Wilson: Centre for Nephrology, University College London, Royal Free, Hampstead Campus, Rowland Hill St., London NW3 2PF, UK.
REFERENCES
- 1.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J 360: 255–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battini L, Fedorova E, Macip S, Li X, Wilson PD, Gusella GL. Stable knockdown of polycystin-1 confers integrin-alpha2beta1-mediated anoikis resistance. J Am Soc Nephrol 17: 3049–3058, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bellas RE, Bendori R, Farmer SR. Epidermal growth factor activation of vinculin and beta 1-integrin gene transcription in quiescent Swiss 3T3 cells. Regulation through a protein kinase C-independent pathway. J Biol Chem 266: 12008–12014, 1991 [PubMed] [Google Scholar]
- 4.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev 84: 1315–1339, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15: 954–963, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun 228: 662–668, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell 18: 253–264, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S, Shi WY, Wilson P, Mazumdar A. Role of lactosylceramide and MAP kinase in the proliferation of proximal tubular cells in human polycystic kidney disease. J Lipid Res 37: 1334–1344, 1996 [PubMed] [Google Scholar]
- 9.Daikha-Dahmane F, Narcy F, Dommergues M, Lacoste M, Beziau A, Gubler MC. Distribution of alpha-integrin subunits in fetal polycystic kidney diseases. Pediatr Nephrol 11: 267–273, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Didier C, Broday L, Bhoumik A, Israeli S, Takahashi S, Nakayama K, Thomas SM, Turner CE, Henderson S, Sabe H, Ronai Z. RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol Cell Biol 23: 5331–5345, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fan RS, Jacamo RO, Jiang X, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at Ser-843. Mediation by Ca2+, calmodulin, and Ca2+/calmodulin-dependent kinase II. J Biol Chem 280: 24212–24220, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell 16: 4329–4340, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng L, Burrow CR, Li HP, Wilson PD. Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochim Biophys Acta 1535: 21–35, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Glenney JR, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol 108: 2401–2408, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol 22: 901–915, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA 89: 8487–8491, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet 19: 361–365, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Hsia DA, Lim ST, Bernard-Trifilo JA, Mitra SK, Tanaka S, den Hertog J, Streblow DN, Ilic D, Ginsberg MH, Schlaepfer DD. Integrin alpha4beta1 promotes focal adhesion kinase-independent cell motility via alpha4 cytoplasmic domain-specific activation of c-Src. Mol Cell Biol 25: 9700–9712, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunger-Glaser I, Salazar EP, Sinnett-Smith J, Rozengurt E. Bombesin, lysophosphatidic acid, and epidermal growth factor rapidly stimulate focal adhesion kinase phosphorylation at Ser-910: requirement for ERK activation. J Biol Chem 278: 22631–22643, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377: 539–544, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Jacamo R, Jiang X, Lunn JA, Rozengurt E. FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. J Cell Physiol 210: 436–444, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Joly D, Ishibe S, Nickel C, Yu Z, Somlo S, Cantley LG. The polycystin 1-C-terminal fragment stimulates ERK-dependent spreading of renal epithelial cells. J Biol Chem 281: 26329–26339, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol 100: e40–e45, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol 136: 1349–1361, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted PKD1 mutation. Nat Genet 17: 179–181, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Mai W, Chen D, Ding T, Kim I, Park S, Cho SY, Chu JS, Liang D, Wang N, Wu D, Li S, Zhao P, Zent R, Wu G. Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol Biol Cell 16: 4398–4409, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagano J, Kitamura K, Hujer KM, Ward CJ, Bram RJ, Hopfer U, Tomita K, Huang C, Miller RT. Fibrocystin interacts with CAML, a protein involved in Ca2+ signaling. Biochem Biophys Res Commun 338: 880–889, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Nickel C, Benzing T, Sellin L, Gerke P, Karihaloo A, Liu ZX, Cantley LG, Walz G. The polycystin-1 C-terminal fragment triggers branching morphogenesis and migration of tubular kidney epithelial cells. J Clin Invest 109: 481–489, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, Hennies HC, Nurnberg P, Hildebrandt F. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71: 1161–1167, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene 23: 7928–7946, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Polgar K, Burrow CR, Hyink DP, Fernandez H, Thornton K, Li X, Gusella GL, Wilson PD. Disruption of polycystin-1 function interferes with branching morphogenesis of the ureteric bud in developing mouse kidneys. Dev Biol 286: 16–30, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Racusen LC, Wilson PD, Hartz PA, Fivush BA, Burrow CR. Renal proximal tubular epithelium from patients with nephropathic cystinosis: immortalized cell lines as in vitro model systems. Kidney Int 48: 536–543, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Fluid Electrolyte Physiol 265: F416–F424, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature 380: 538–540, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Risdon RA, Woolf AS. Developmental defects and cystic diseases of the kidney In: Heptinstall's Pathology of the Kidney, edited by Jennette JC, Olson JL, Schwartz MM, Silva FG. Philadelphia, PA: Lippincott-Raven, 1998, p. 1149–1206 [Google Scholar]
- 38.Rohatgi R, Greenberg A, Burrow CR, Wilson PD, Satlin LM. Na transport in autosomal recessive polycystic kidney disease (ARPKD) cyst lining epithelial cells. J Am Soc Nephrol 14: 827–836, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Schaller MD, Schaefer EM. Multiple stimuli induce tyrosine phosphorylation of the Crk-binding sites of paxillin. Biochem J 360: 57–66, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14: 92–101, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Schuermann MJ, Otto E, Becker A, Saar K, Ruschendorf F, Polak BC, Ala-Mello S, Hoefele J, Wiedensohler A, Haller M, Omran H, Nurnberg P, Hildebrandt F. Mapping of gene loci for nephronophthisis type 4 and Senior-Loken syndrome, to chromosome 1p36. Am J Hum Genet 70: 1240–1246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of PKD1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorenson CM, Sheibani N. Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn 215: 371–382, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 9: 1883–1895, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol 2: E231–E236, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Veizis IE, Cotton CU. Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am J Physiol Renal Physiol 288: F474–F482, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Gattone V, 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Wilson PD. In vitro methods in renal research In: Pediatric Nephrology, edited by Avner E, Harmon W, Niaudet P. Philadelphia, PA: Lippincott, Williams, Wilkins, 2004, p. 269–281 [Google Scholar]
- 52.Wilson PD, Geng L, Li X, Burrow CR. The PKD1 gene product, “polycystin-1,” is a tyrosine phosphorylated protein that colocalizes with alpha2/beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest 79: 1311–1323, 1999 [PubMed] [Google Scholar]
- 53.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wilson PD, Burrow CR. Cystic diseases of the kidney: role of adhesion molecules in normal and abnormal tubulogenesis. Exp Nephrol 7: 114–124, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Wilson PD, Dillingham MA, Breckon R, Anderson RJ. Defined human renal tubular epithelia in culture: growth, characterization, and hormonal response. Am J Physiol Renal Fluid Electrolyte Physiol 248: F436–F443, 1985 [DOI] [PubMed] [Google Scholar]
- 56.Wilson PD, Goilav B. Cystic disease of the kidney. Annu Rev Pathol 2: 341–368, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD. Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim Biophys Acta 1762: 647–655, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol 7: 681–689, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer 4: 470–480, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci 120: 137–148, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zimerman B, Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil Cytoskeleton 58: 143–159, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.