Abstract
Arterial thrombosis is a common disease leading to severe ischemia beyond the obstructing thrombus. Additionally, endothelial dysfunction at the site of thrombosis can be rescued by l-arginine supplementation or arginase blockade in several animal models. Exposure of rat aortic endothelial cells (RAECs) to thrombin upregulates arginase I mRNA and protein levels. In this study, we further investigated the molecular mechanism of thrombin-induced arginase changes in endothelial cells. Thrombin strikingly increased arginase I promoter and enzyme activity in primary cultured RAECs. Using different deletion and point mutations of the promoter, we demonstrated that the activating protein-1 (AP-1) consensus site located at −3,157 bp in the arginase I promoter was a thrombin-responsive element. Electrophoretic mobility shift assay and chromatin immunoprecipitation assay further confirmed that upon thrombin stimulation, c-Jun and activating transcription factor-2 (ATF-2) bound to the AP-1 site, which initiated the transactivation. Moreover, loss-of-function studies using small interfering RNA confirmed that recruitment of these two transcription factors to the AP-1 site was required for thrombin-induced arginase upregulation. In the course of defining the signaling pathway leading to the activation of AP-1 by thrombin, we found thrombin-induced phosphorylation of stress-activated protein kinase/c-Jun-NH2-terminal kinase (SAPK/JNK or JNK1/2/3) and p38 mitogen-activated protein kinase, which were followed by the phosphorylation of both c-Jun and ATF-2. These findings reveal the basis for thrombin induction of endothelial arginase I and indicate that arginase inhibition may be an attractive therapeutic alternative in the setting of arterial thrombosis and its associated endothelial dysfunction.
Keywords: endothelium, activating protein-1
arterial thrombosis is a common vascular disease caused by occlusion of a vessel via an obstructing thrombus and is associated with tremendous morbidity and mortality. Although thrombi can be removed by mechanical means and regional thrombolytic infusion, endothelial dysfunction can persist. Thrombus-associated vasospasm and rethrombosis after successful removal of the original thrombus remain vexing clinical problems (37). In addition to regulating vascular tone and blood pressure, the vascular endothelium has antithrombotic properties and modulates interactions between the blood vessel wall, circulating leukocytes, and platelets. Nitric oxide is a powerful antithrombotic molecule and vasodilator produced by endothelial nitric oxide synthase (eNOS) through l-arginine (33, 31, 49). Endothelial dysfunction contributes significantly to vasospasm and rethrombosis (1, 4, 10). In addition to eNOS, l-arginine is also a substrate of arginase, the final enzyme of the hepatic urea cycle hydrolyzing l-arginine to urea and l-ornithine (21, 22). Arginase is constitutively expressed in endothelial cells of different vascular beds by two isoforms, arginase I and II. In rat endothelium, arginase I is the predominant isoform expressed, whereas arginase II is the major isoform present in mouse vascular endothelium, human aortic endothelial cells, and human umbilical vein endothelial cells (HUVEC) (6, 32, 41, 43, 44, 54). Increasing evidence has recognized arginase as a novel target for therapy in vascular disease. Upregulation of arginase activity and/or expression was reported in atherosclerosis-prone mice and endothelial cells of patients with pulmonary arterial hypertension; underlying oxidized low-density lipoproteins, ischemia-reperfusion, obesity, diabetes, and aging induced endothelial dysfunction (14, 15, 41, 43–45, 55). Thrombin-enhanced arginase enzyme activity in HUVEC was found to occur via a Rho pathway-dependent mechanism (32).
Using acetylcholine-stimulated endothelium-dependent relaxation (EDR), our initial studies in several animal models revealed that acute arterial thrombosis causes endothelial dysfunction. Lack of blood flow (ischemia) alone without intraluminal thrombus did not affect EDR. Nitric oxide levels were decreased accordingly, and l-arginine administration restored EDR, suggesting normal eNOS function and that impaired l-arginine availability underlies arterial thrombosis-induced endothelial dysfunction (12, 23, 39). We first investigated the mechanism of this pathology in HUVEC. eNOS activity and expression as well as l-arginine transport were not affected by thrombin, thrombin receptor agonist peptide (TRAP), or fibrin treatment. Interestingly, high-dose thrombin treatment or TRAP increased both arginase II activity and expression, while fibrin exposure had no appreciable effect (56). We continued to investigate the mechanism using cell culture-mediated studies and an animal model of thrombosis. Recently, we found that exposure of rat aortic endothelial cells (RAECs) to thrombin upregulated arginase I mRNA and protein expression. Furthermore, both specific and nonspecific arginase inhibitors ameliorated endothelial dysfunction after thrombosis in rats (27, 56). However, the molecular mechanisms of thrombin-induced upregulation of arginase I are unknown.
To understand how expression of the arginase I gene is induced in response to thrombin, we have examined the role of thrombin on arginase I promoter and enzyme activity using transfected RAECs in this study. Our objective was to identify the thrombin-responsive element in the arginase I promoter and to reveal the transcription factors and signaling pathways involved.
MATERIALS AND METHODS
Cell culture.
RAECs were primary cultured as described previously (27). Isolated RAECs were initially plated onto T75 flasks in a 37°C, humidified, 5% CO2 incubator. They were maintained in DMEM medium (Sigma, St. Louis, MO) containing 15% fetal bovine serum, 0.009% heparin, and 0.015% endothelial cell growth supplement. The cells were grown to confluence before initiation of experiments and were used between passages 3 and 5. Transfection experiments were performed using Targefect F-2 and peptide enhancer (Targeting System) as described previously (5). Adult male Sprague-Dawley rats weighing 350 to 500 mg were used for isolated RAECs. Animals were handled and cared for under Cleveland Clinic guidelines and in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996), and the Association for Assessment and Accreditation of Laboratory Animal Care International. The experimental protocols were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and were performed in accordance with the recommendations of Good Laboratory Practices.
Arginase activity assay.
Arginase activity in cell lysates was determined according to the established protocol (24, 34). In this assay, arginase activity was determined as the conversion of l-[guanidine-14C] arginine to [14C]urea, which was converted to 14CO2 by urease and trapped as Na214CO3 for scintillation counting. Arginase activity was expressed as pmoles urea formed per hour per milligram of protein at 37°C.
Arginase I-luciferase reporter constructs.
5′-Deletion arginase I promoter-luciferase (Luc) constructs—−4.78, −3.29, −2.78, and −1.41 kb arginase I promoter-Luc—were used and have been described previously (17). The −3.11 kb arginase I promoter-Luc was generated by BamHI/NcoI digestion of the −3.29-kb arginase I promoter-Luc, followed by religation.
Internal deletion mutants were made using standard PCR techniques. The primers used to create mutant sites were as follows (mutated base pairs are in lower case): CCAAT transcription factor-nuclear factor 1 (CTF-NF1) mutant, forward 5′-AATTGGGATCCACAGGTTAAGccgacGT; CTF-NFI mutant, reverse 5′-ACATACCATGGCCCTGAGGAGGTTCTCC; AP4-1 mutant, forward 5′-AATTGGGATCCgatGGTTAAGAACCA; AP4-1 mutant, reverse same as CTF-NFI mutant reverse; AP4-2 mutant, forward 5′-AATTGGGATCCACAGGTTAAGAACCAGTGCCTTAGATGTTCTGAGGCCAtcaCTTTGGAG; AP4-2 mutant, reverse, same as CTF-NFI mutant reverse; AP-1 mutant, forward 5′-AATTGGGATCCACAGGTTAAGAACCAGT; AP-1 mutant, reverse 5′-ACATACCATGGCCCTGAGGAGGTTCTCCTTGCTGTATGGGTTCTGAGAGgac. All constructs generated by PCR were validated by DNA sequencing.
Luciferase and β-galactosidase assay.
Luciferase activity was measured for reporter expression in triplicate according to the instructions provided in the Luciferase Reporter Assay System (Promega, San Luis Obispo, CA). Transfection efficiency was corrected by cotransfection with a plasmid containing the β-galactosidase gene driven by the SV40 promoter (Promega). β-Galactosidase activity was measured with Galactolight Plus detection system (Tropix). All results were verified by multiple independent experiments using three different preparation of endotoxin-free plasmid DNA.
Electrophoretic mobility shift assay and supershift assay.
Nuclear extracts (20 μg) were prepared from vehicle versus thrombin-treated RAECs using CelLytic NuCLEAR Extraction Kit (Sigma). Electrophoretic mobility shift assay (EMSA) was performed with wild-type and mutant biotin-labeled double-stranded oligonucleotide probes designed to encompass the −3,157 bp AP-1 binding element. To distinguish specific from nonspecific protein-DNA complex formation, incubations were also performed in the presence of 20-fold excess unlabeled probe. An EMSA kit was used (Pierce). The sequences of arginase I promoter DNA probes used for EMSA were as follows: wild-type AP-1 probe, CCA GTC TGA CTC TCA GAA CC; mutant AP-1 probe, CCA GTC GTC CTC TCA GAA CC. For the supershift assay, the following antibodies were used: control IgG, c-Jun, JunB, JunD, c-fos, fosB, activating transcription factor-2 (ATF-2), Fra-1, and Fra-2 antibodies (2 μg, all from Santa Cruz Biotechnology) were incubated with nuclear extracts at 4°C overnight, then incubated with labeled probe for 30 min at room temperature. The DNA-protein complexes were resolved by electrophoresis on 6% polyacrylamide gels (Invitrogen, Carlsbad, CA).
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation (ChIP) assay was performed using a kit (Upstate Biotechnology) according to the manufacturer's instructions with some modifications. RAECs were treated with vehicle or thrombin. Cells were cross-linked, lysed, and sonicated at 4°C (Branson Sonifier 450). Approximately 1% of the resulting sample was kept to serve as an input genomic DNA control, and the rest was precleared with salmon sperm DNA-protein A. Cell lysates were incubated with candidate antibody overnight with rotation, followed by 1-h incubation with protein A-agarose for ChIP. Immunoblot staining was used to detect the specific candidate protein. The precipitated chromatin-protein complexes were washed and eluted, and cross-links were reversed. The genomic DNA fragments were isolated, purified, and amplified by PCR (5). For the sequence spanning from −3,296 bp to −3,111 bp, the primer sequences were 5′-AATTGGGATCCACAGGTTAAGAACCAGT-3′ and 5′-ACATACCATGGCCCTGAGGAGGTTCTCCTT-3′. For the sequence spanning from −2,340 bp to −2,157 bp, the primer sequences were 5′-CTCACTCTACATTTGCGAGAC-3′ and 5′-ACGATACGCTGCTCCATCTT-3′.
Immunoblot analysis.
Protein expression was determined by Western blot analysis. Cells were lysed in sample buffer, Laemmli (Sigma). Samples (25 μg) (Bradford Assay) were loaded and separated by 10% to 12% SDS-PAGE (Bio-Rad). Proteins were transferred to a polyvinylidene difluoride membrane (Millipore) by semidry transfer. Antibodies to phospho-SAPK/JNK, SAPK/JNK, phospho-p38 MAP kinase, p38 MAP kinase, phospho-c-Jun, and phospho-ATF-2 were from Cell Signaling; c-Jun, ATF-2, and arginase I were from Santa Cruz; tubulin was from Sigma. ECL reagent (Promega) was used for chemiluminescence.
RNA interference.
c-Jun and ATF-2 small interfering RNAs (siRNAs) targeting rat sequences were designed and synthesized by Santa Cruz. Target F-2 and peptide enhancer (Targeting System) were used to transfect RAECs to ablate protein levels. Twenty-four hours after transfection, cells were treated with vehicle or thrombin for 4 h. Immunoblot analysis was performed to detect protein levels.
Statistical analysis.
All results obtained were confirmed in at least three independent experiments. Statistical comparisons of single parameters between two groups were done by paired or unpaired Student's t-test. One-way analysis of variance with Newman-Keuls post hoc testing was used to compare means of three or more groups. Continuous variables are presented as means ± SD. Significance was accepted at P < 0.05.
RESULTS
Thrombin stimulates arginase I transcription activation and enzyme activity in RAECs.
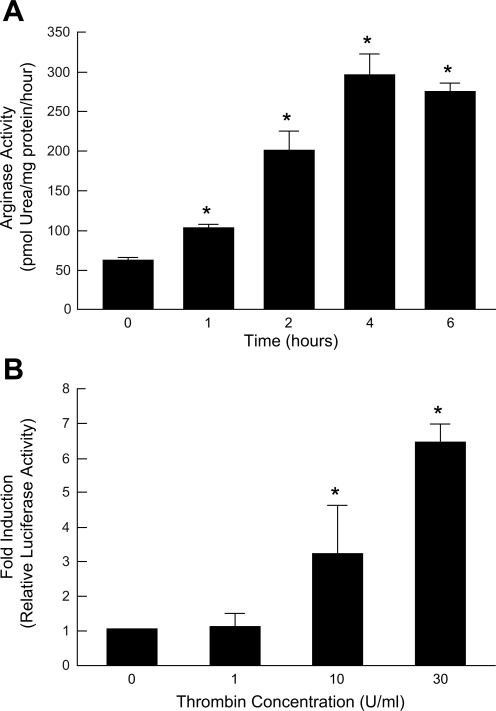
We have shown that exposure of RAECs to thrombin, an active component of a thrombus, upregulates arginase I mRNA and protein expression without affecting eNOS (27). To evaluate the function of the enzyme stimulated by thrombin, arginase activity was measured by the conversion of l-[guanidine-14C] arginine to [14C]urea (24, 34). RAECs were treated with thrombin (30 U/ml), and arginase activity over a time course was determined. Thrombin-induced arginase activity was observed as early as 1 h after exposure and reached maximum activity at 4 h, increasing 4.7-fold (Fig. 1A).
Fig. 1.
Thrombin induces arginase enzyme activity and arginase I promoter. A: arginase activity in rat aortic endothelial cells (RAECs) exposed to thrombin (30 U/ml) at different time points. Increased arginase enzyme activity was observed as early as 1 h after thrombin exposure and reached maximum activity at 4 h, increasing 4.7-fold. n = 3; *P < 0.05 vs. time 0. B: relative luciferase activity in 1 μg DNA-transfected RAECs incubated with vehicle or different concentrations of thrombin for 4 h. Thrombin (10 U/ml) induced a 3.2 ± 1.4-fold increase in luciferase activity compared with control, whereas 30 U/ml of thrombin induced a 6.4 ± 0.5-fold increase. n = 3; *P < 0.05 vs. vehicle (concentration 0).
To directly determine the effect of thrombin on arginase I transcription, RAECs were cotransfected with a ∼4.8 kb murine arginase I promoter-Luc construct and a plasmid containing β-galactosidase driven by the SV40 promoter to normalize for transfection efficiency (17). Forty-eight hours after transfection, cells were treated with different concentrations of thrombin for 4 h, and then luciferase and β-galactosidase activities were determined. Ten and 30 U/ml thrombin exposure induced a 3.2 ± 1.4- and 6.4 ± 0.5-fold increase in luciferase activity compared with controls, respectively (Fig. 1B). These data indicate that thrombin causes a dramatic increase in arginase activity, which is a result of an increase in arginase gene expression.
Analyses of the arginase I promoter in response to thrombin.
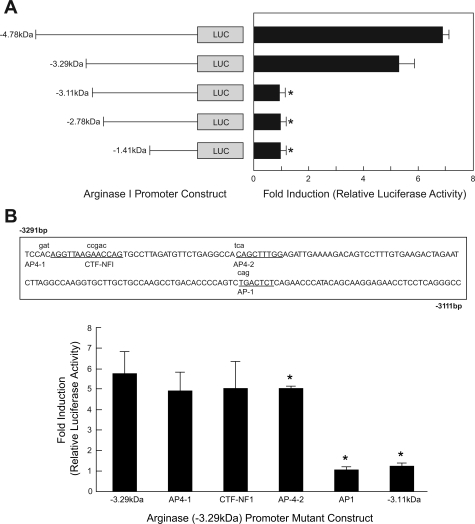
To further investigate the thrombin responsive element(s), a series of 5′-deletion arginase I promoter-Luc constructs were used. RAECs were cotransfected with five different 5′-deletion arginase I promoter-Luc constructs: −4.78, −3.29, −3.11, −2.78 and −1.41 kb arginase I promoter-Luc and SV40 promoter β-galactosidase plasmids (17). Forty-eight hours after transfection, cells were treated with thrombin (30 U/ml) for 4 h; luciferase and β-galactosidase activities were then determined. Luciferase activity fold induction showed that the thrombin-responsive element was located between −3.29 kb and −3.11 kb in the arginase I promoter (Fig. 2A).
Fig. 2.
Analyses of the arginase I promoter in response to thrombin. A: RAECs were cotransfected with 5 different deletion arginase I promoter-luciferase (Luc) constructs and a SV40 promoter β-galactosidase plasmid to normalize for transfection efficiency. Luciferase activity fold induction showed that the thrombin-responsive element was located between −3.29 kb to −3.11 kb in the arginase I promoter. n = 3; *P < 0.05 vs. −4.78 kb. B: a bioinformatics search indicated that there were 4 different consensus sequences within this region (sequence with consensus sites labeled in capitals and mutants in lowercase). RAECs were transfected with 4 mutants of arginase I promoter-Luc constructs (−3.29 kb). Activating protein-1 (AP-1; −3,157 bp) was identified as the thrombin-responsive regulatory element. CTF-NF1, CCAAT transcription factor-nuclear factor 1. n = 4l; *P < 0.05 vs. −3.29 kb.
According to the prediction by a bioinformatics search (MacVector, Cary, NC), there are four different consensus sequences within this region: two AP-4, one AP-1, and one CTF-NF1. Mutants (Fig. 2B as shown in lowercase above the underlined sequences) for the consensus sequence sites predicted by the bioinformatics search were designed and created. RAECs were cotransfected with these four mutant arginase I promoter-Luc constructs, as well as β-galactosidase plasmids to normalize for the transfection efficiency. Relative luciferase activities were determined. The AP-1 sequence located at −3,157 bp was identified as the thrombin-responsive regulatory element (Fig. 2B).
Binding of c-Jun and ATF-2 to the AP-1 site.
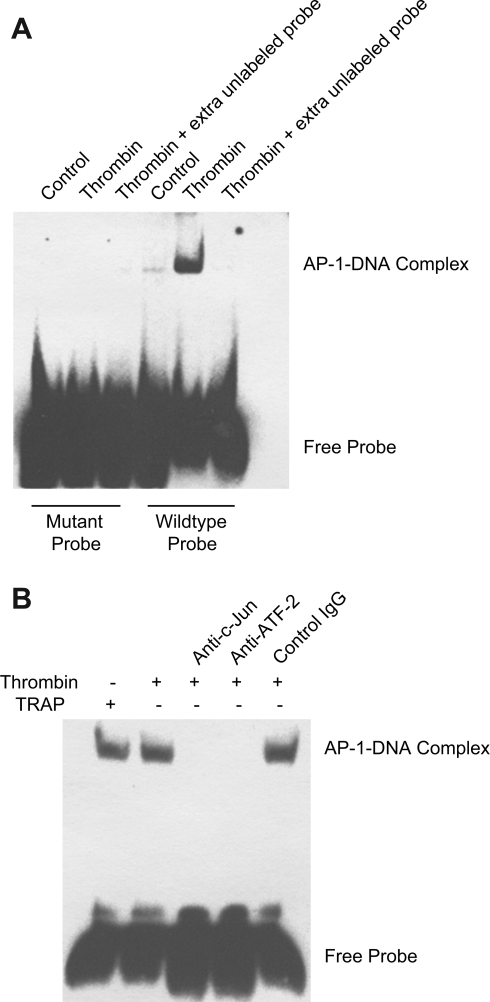
In addition to the luciferase assay using mutant arginase I promoter-Luc, we further performed EMSA to confirm that this AP-1 site was the thrombin-responsive regulatory element. Nuclear extracts were prepared from vehicle and thrombin-treated RAECs. Wild-type and mutant double-stranded oligonucleotide probes were designed to encompass the AP-1 binding element (−3,157 bp) and labeled with biotin. As shown in Fig. 3A, nuclear extracts from RAECs treated with thrombin (30 U/ml; 30 min) formed a specific complex with oligonucleotide containing the wild-type AP-1 site, whereas no specific complex formation was observed for nuclear extracts from untreated RAECs or with the oligonucleotide containing the mutant AP-1 sequence. To distinguish specific from nonspecific protein-DNA complex formation, incubations were performed in the presence of extra unlabeled probe (Fig. 3A).
Fig. 3.
Identification of transcription factors binding to AP-1 elements within the arginase I promoter. A: electrophoretic mobility shift assay (EMSA) analysis was performed with nuclear extracts from RAECs treated with control and thrombin (30 U/ml) for 30 min. A 20-fold excess unlabeled probe was used to distinguish specific from nonspecific protein-DNA complex formation. Nuclear extracts from RAECs treated with thrombin formed a specific complex with oligonucleotides containing the wild-type AP-1 site. B: supershift assay was performed with nuclear extracts from RAECs treated with thrombin in the absence and presence of c-Jun and ATF-2 specific antibodies. Control IgG was used as a negative control. Thrombin receptor agonist peptide (TRAP) was used as a positive control. ATF-2, activating transcription factor 2. Results shown are representative of three independent experiments.
To dissect the protein composition of this complex, supershift assays were performed with labeled wild-type AP-1 probe. Different AP-1 protein antibodies from Jun, Fos, ATF, and Jun dimerization partner (JDP) families and a control antibody were added to the nuclear extracts before electrophoresis to detect further decreases in electrophoretic mobility. The addition of c-Jun and ATF-2 antibodies to the EMSA reaction mixture resulted in the inhibition of the specific complex formation (Fig. 3B), indicating that c-Jun and ATF-2 are the proteins binding to this site in RAECs. However, there were no changes in DNA-protein binding with other AP-1 antibody-incubated nuclear extracts (data not shown). TRAP was used as a positive control. Because TRAP is a thrombin receptor-specific agonist peptide with the NH2 terminus of the tethered ligand, this implicated the role of the thrombin receptor on AP-1-mediated arginase I transcription.
The AP-1 site is responsible for endogenous c-Jun, ATF-2 binding in vivo.
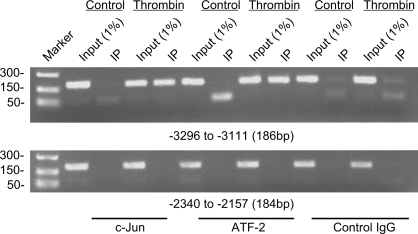
To determine whether c-Jun and ATF-2 are associated with the arginase I promoter upon stimulation by thrombin in vivo, ChIP assays were performed with specific c-Jun and ATF-2 antibodies by using chromatin from untreated and thrombin-treated RAECs. With primers amplifying sequences spanning from position −3,296 bp to −3,111 bp of the arginase I promoter, which includes the AP-1 site, an 186-bp DNA fragment was amplified from anti-c-Jun and anti-ATF-2 chromatin precipitates in thrombin-treated cells (Fig. 4, top). However, anti-c-Jun and anti-ATF-2 precipitates from untreated control RAECs resulted in little or no DNA amplification. Appropriate primers for the region spanning from positions 2,340 bp to −2,157 bp (contains one AP-4 and one CTF-NFI site) were also used to amplify chromatin immunoprecipitated with anti-c-Jun and anti-ATF-2. In contrast to −3,296 bp to −3,111 bp of the arginase I promoter, no DNA fragment was detected from either control or thrombin-treated samples (Fig. 4, bottom). These results demonstrate that thrombin induced specific binding of c-Jun and ATF-2 to the −3,157 bp AP-1 site in RAECs.
Fig. 4.
Chromatin immunoprecipitation (ChIP) assays demonstrate specific binding of c-Jun and ATF-2 to the −3,157 bp AP-1 site in response to thrombin. Assays were performed using anti-c-Jun, anti-ATF-2, and control antibodies, with RAECs treated with control or thrombin for 30 min. Two distinct sets of primers spanning different regions of arginase promoter were used to amplify the immunoprecipitated (IP) DNA. Top: amplified DNA, using primers spanning from −3,296 to −3,111 bp, containing the AP-1 site. Bottom: amplified DNA, using primers spanning from −2,340 to −2,157 bp as a negative control. The PCR products of both input and ChIP samples were loaded as indicated and analyzed using a 2% agarose gel. Results shown are representative of three independent experiments.
The necessary role of c-Jun and ATF-2 on thrombin-induced arginase upregulation.
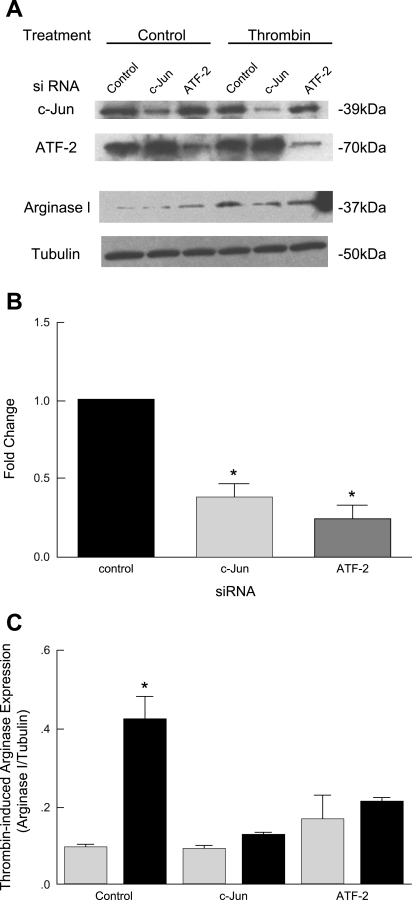
To understand the specific requirement for c-Jun and ATF-2 on thrombin-induced arginase upregulation, we performed loss-of-function studies with specific siRNA. RAECs were transfected with control, c-Jun, and ATF-2 siRNA (designed and produced by Santa Cruz) 48 h before incubation with thrombin. Protein was then harvested for immunoblotting. We first examined the specificity of our siRNAs for selective knockdown of the target c-Jun and ATF-2 proteins. We found that each siRNA was specific for its target. c-Jun siRNA knocked down the c-Jun protein without affecting ATF-2. A similar result was obtained with ATF-2 siRNA (Fig. 5, A and B). To examine the role of c-Jun and ATF-2 on thrombin-induced arginase I induction, we did the immunoblot for arginase I in the same cell lysates. In control siRNA-transfected cells, thrombin induced arginase I upregulation. In contrast, in cells transfected with c-Jun or ATF-2 siRNA, thrombin did not lead to arginase I upregulation (Fig. 5, A and C). These data demonstrate the central roles of c-Jun and ATF-2 in the upregulation of arginase I in RAECs in response to thrombin exposure.
Fig. 5.
c-Jun and ATF-2 are required for thrombin-induced arginase upregulation. A: immunoblot analyses. RAECs were transfected with control, c-Jun, or ATF-2 small interfering RNA (siRNA). Twenty-four hours later, transfected cells were exposed to thrombin for 4 h. c-Jun, ATF-2, arginase, and tubulin protein abundance was assessed. Each siRNA was specific for knockdown of its target. B and C: densitometric analyses. Transfection of cells with c-Jun or ATF-2 siRNA did not lead to arginase I upregulation. Results shown are representative of three independent experiments. *P < 0.05 vs. control siRNA.
MAP kinase mediates the effect of thrombin on AP-1 activation.
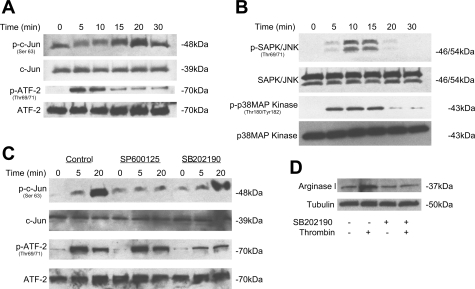
Phosphorylation by MAP kinase is thought to be a common event in the activation of AP-1 proteins. To determine whether thrombin induces phosphorylation of c-Jun and ATF-2, we treated RAECs with thrombin for 0, 5, 10, 15, 20, and 30 min and then immunoblotted for phospho-c-Jun and phospho-ATF-2. Thrombin induced a transient phosphorylation of these two transcription factors. Maximal phosphorylation of c-Jun was observed at 20 min and returned to basal level at 30 min. Phosphorylation of ATF-2 occurred after 5 min, and declined after 10 min. No change in total expression of JNK, c-Jun, and ATF-2 was observed at any time point (Fig. 6A).
Fig. 6.
MAPKs mediate the effect of thrombin on AP-1 activation in RAECs. A: RAECs were treated with thrombin (30 U/ml) for 0–30 min, and the abundance of phosphorylated (p) c-Jun and ATF-2 to total c-Jun and ATF-2 was evaluated by immunoblot analyses. Thrombin induced a transient phosphorylation of these two transcription factors. B: RAECs were treated with thrombin (30 U/ml) for 0–30 min, and the abundance of phosphorylated SAPK/JNK and p38 MAP kinase to total SAPK/JNK and p38 MAP kinase was evaluated by immunoblot analysis. C: RAECs were preincubated with DMSO, JNK inhibitor SP600125, or p38 MAPK inhibitor SB202190 for 0, 5, or 20 min. The abundance of phosphorylated c-Jun and ATF-2 to total c-Jun and ATF-2 was evaluated by immunoblot analysis. D: RAECs were incubated with thrombin (30 U/ml) and SB202190 for 4 h. The abundance of arginase I protein was assessed. Tubulin protein level was evaluated to serve as a loading control. Results shown are representative of three independent experiments.
In mammals, it was reported that two major MAP kinases can phosphorylate and activate c-Jun and ATF-2. They are JNK and the p38 MAP kinase (26). We assessed JNK and p38 MAP kinase phosphorylation in response to thrombin in RAECs treated with thrombin (30 U/ml) at different time points. Immunoblot analyses were used to determine protein abundance. Thrombin induced a transient phosphorylation of SAPK/JNK and p38 MAP kinase. Maximal activation of SAPK/JNK was observed after 10 min and declined to basal level within 30 min. p38 MAP kinase phosphorylation started at 5 min, maximized at 15 min, and declined after 15 min (Fig. 6B).
We then hypothesized that JNK phosphorylation induced c-Jun phosphorylation while p38 MAP kinase was responsible for ATF-2 phosphorylation. To test this hypothesis, we examined the effect of specific JNK inhibitor SP600125 and p38 MAP kinase inhibitor SB202190 on c-Jun and ATF-2 phosphorylation. Preincubation with SP6000125 (10−6 mol/l) inhibited thrombin-induced c-Jun phosphorylation, whereas SB202190 (10−6 mol/l) inhibited ATF-2 phosphorylation (Fig. 6C). These data indicated JNK and p38 MAP kinase phosphorylated c-Jun and ATF-2, respectively, which induced the activation of AP-1. To further determine the effect of p38 MAP kinase inhibition on thrombin-induced arginase I transcription, RAECs were incubated with both thrombin and SB202190. Immmunoblot analysis was then performed. In control cells, thrombin induced arginase I upregulation, which was prevented by SB202190 exposure (Fig. 6D).
DISCUSSION
We have previously shown that arterial thrombosis leads to endothelial dysfunction secondary to decreased nitric oxide bioactivity, and that this can be ameliorated via l-arginine supplementation or arginase blockade (12, 23, 27, 39, 56). Of note, rat endothelium exposed to thrombin for 6 h led to increased arginase I mRNA (6.8-fold) and arginase I protein levels (2.1-fold) (27). We believe that thrombin plays a central role in thrombus-induced endothelial dysfunction and investigated the molecular mechanisms of arginase changes after thrombin exposure. Thrombin is a coagulation system protease present at the sites of vascular injury. In addition to catalyzing the conversion of soluble fibrinogen into an insoluble fibrin clot, thrombin is a potent endothelial cell agonist inducing several genes in endothelial cells (47, 52). Previously, a 9-bp thrombin-responsive element was identified in the platelet-derived growth factor B-chain gene promoter and its binding protein, thrombin-inducible nuclear factor, which belongs to the Y box protein family (46, 48). Our recent studies demonstrated that the MAP kinase phosphatase-1 gene is thrombin responsive in HUVEC (9, 25). Here we report on the molecular mechanisms of thrombin-induced arginase upregulation. In particular, 1) the effect of thrombin on arginase I promoter activity and enzyme activity was assessed, 2) a thrombin-responsive element in RAECs was identified, 3) the transcription factors involved in thrombin-induced arginase I upregulation were determined, and 4) the signaling pathway mediating thrombin-induced arginase I upregulation in endothelial cells was defined. Our study reveals that after endothelial exposure to thrombin, c-Jun and ATF-2 are recruited to the AP-1 consensus sequence in the arginase I promoter and that arginase I transcription is upregulated.
Generation of a hemostatic clot requires conversation of fibrinogen to fibrin, which involves local pH, ionic strength, calcium concentration, fibrinogen, and other variables (8, 16, 36, 42). However, in situ thrombin concentration has the most profound physiological influence on fibrin clot formation (53). During a coagulation process, the concentration of free thrombin present can range from less than 0.1 U/ml to greater than 50 U/ml (2, 3, 11). In this study, we found that thrombin, at concentrations that mimic the circumstances of acute arterial thrombosis, induced arginase transcription. Thrombin signaling in endothelium is mediated by a family of 7-transmembrane G protein-coupled receptors called protease-activated receptors (PARs). This can result in multiple phenotype changes, including cell shape, permeability, vasomotor tone, migration, and angiogenesis (30). Thrombin-induced transcription changes have been well studied in low concentration, typically less than 1 to 2 U/ml. However, our finding suggests that there may be another group of genes that are specifically induced by higher, yet still pathologically relevant levels of thrombin, and arginase may represent one of those genes in endothelial cells. Alternatively, thrombin receptor 4 (PAR 4) requires high concentrations of thrombin if its coreceptor PAR 3 is absent or dysfunctional.
By using different mutant constructs, we identified an AP-1 site located 3,157 bp upstream of the transcription start site of arginase I promoter. The sequence of this AP-1 site matches the AP-1 consensus sequence GAGTCA. AP-1 is a group of structurally and functionally related members of the Jun, Fos protein family and some members of ATF and JDP subfamilies, which form dimeric complexes (51). A broad range of physiological and pathological stimuli, including cytokines, growth factors, stress, and oncogenic signals, could activate AP-1. In human vascular endothelial cells, peroxisome proliferator-activated receptor activators were reported to inhibit thrombin-induced endothelin-1 production through AP-1 signaling pathway (13). Suppression of transcription factors Egr-1, AP-1, and NF-κB was reported to inhibit thrombin-induced tissue factor gene activation (38). Thrombin and tumor necrosis factor-α synergistically stimulate tissue factor expression in endothelial cells through c-Fos and c-Jun (28). In this study, EMSA and supershift assay revealed that this AP-1-DNA complex is composed of c-Jun and ATF-2. We also demonstrated that suppression of c-Jun and ATF-2 by specific siRNA results in a significant inhibition of arginase protein expression in thrombin treated RAECs. Thus, the novel finding that thrombin modulates endothelial arginase gene expression through AP-1 binding via transcription factors c-Jun and ATF-2 indicates that AP-1 may play a central role in endothelial dysfunction after thrombosis.
Endothelial cells are the first cells in an artery to encounter circulating thrombin, one of the key stimuli leading to the activation of multiple signaling pathways. In the current study, we characterized the role of the stress-activated family of MAP kinases in thrombin-induced arginase expression via AP-1 activation. Previously, JNK-induced phosphorylation of Thr69 and Thr71 was found essential for the transcription activation of ATF-2 in response to proinflammatory cytokines or ultraviolet radiation (18, 29). In wild-type embryonic fibroblasts, it was reported that p38 MAP kinase was not rate limiting for phosphorylation of ATF-2. However, in JNK-deficient cells, p38 MAP kinase substituted for JNK in the phosphorylation of ATF-2 (35). In our study, we found thrombin-induced phosphorylation of ATF-2 is earlier than JNK phosphorylation. Incubation with SP600125 did not prevent the phosphorylation of ATF-2 Thr69/71, but incubation of SB202190 was sufficient to prevent ATF-2 phosphorylation by thrombin. These observations indicate that in RAECs, p38 MAP kinase, but not JNK, phosphorylates ATF-2 in response to thrombin. It remains to be determined whether this is a thrombin-specific mechanism in endothelial cell signaling.
ATF-2 and c-Jun are key components of AP-1 and function as homodimers or heterodimers. c-Jun-ATF-2 heterodimers activate the expression of many target genes in response to a variety of cellular and environmental signals (7, 18, 22, 50). By ultraviolet cross-linking and immunoprecipitation, we demonstrated that a heterodimer of transcription factors c-Jun and ATF-2 is bound to the AP-1 site in the arginase I promoter following stimulation by thrombin. Thrombin stimulation of endothelial cells induced marked activation of SAPK/JNK and p38 MAP kinase, which was followed by transient phosphorylation of both c-Jun and ATF-2. Regulation of AP-1 activity can be achieved by changes in the transcription level of AP-1 subunits, control of the stability of their mRNAs, posttranslational processing, turnover of preexisting or newly synthesized AP-1 subunits, and specific interactions between AP-1 proteins and other transcription factors and cofactors (19). A previous report has shown that a low concentration of thrombin (1 U/ml) stimulates HUVEC arginase enzyme activity via the RhoA/ROCK pathway, without affecting the arginase protein level (32). Our current study also showed an increased activity of arginase in endothelial cells exposed to a higher dose of thrombin (30 U/ml). However, this was via MAP kinase-mediated phosphorylation, AP-1 activation, and arginase upregulation.
There are limitations to the current study. Additional studies in animal/preclinical models may be needed to confirm our observations and extend our understanding of this process in the in vivo environment. But, we believe the results presented here combined with previous animal studies provide compelling evidence for arginase's role in thrombosis-induced endothelial dysfunction (27). The predominant isoform of arginase in rats is arginase 1 and thus, this work may not translate to mechanistic relevance in humans where arginase 2 predominates. Further work in human endothelial cells and harvested arterial tissue is warranted. Lastly, arginase activation via nontranscriptional pathways should be investigated. Thus, alternative molecular pathways may play a role in thrombin's effects and are worthy of future study.
Thrombolytic treatments are able to restore blood flow in occluded peripheral arteries. However, vasospasm and rethrombosis complicate a large fraction of treated individuals which may reflect endothelial dysfunction after thrombosis (37, 40). The present findings have important clinical relevance. Our understanding of endothelial dysfunction may lead to adjuncts that prevent suboptimal clinical outcomes. Currently, we are evaluating l-arginine supplementation in human arteries (unpublished observations). The results of the present study provide the first direct evidence supporting a key role for the transcription factor AP-1 in mediating endothelial arginase, an enzyme that can modulate l-arginine levels. Our findings highlight the arginase pathway as a new therapeutic target in limiting endothelial dysfunction.
GRANTS
This work was supported by the American Vascular Association (to V. S. Kashyap) and the National Institutes of Health Grants HL-080247 (to V. S. Kashyap), HL-29582 (to P. E. DiCorleto), and GM-57384 (to S. M. Morris).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully thank Dr. Jianzhong Shen (Auburn University, Auburn, AL) for continuous scientific advice and critical reviews of the manuscript.
REFERENCES
- 1.Ajani AE, Yan BP. The mystery of coronary artery spasm. Heart Lung Circ 16: 10–15, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, Béguin S, Hemker HC. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost 88: 576–582, 2002 [PubMed] [Google Scholar]
- 3.Allen GA, Wolberg AS, Oliver JA, Hoffman M, Roberts HR, Monroe DM. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost 2: 402–413, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol 34: 631–638, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol Cell Biol 27: 4207–4216, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bhoumik A, Takahashi S, Breitweiser W, Shiloh Y, Jones N, Ronai Z. ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol Cell 18: 577–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr ME, Gabriel DA, McDonagh J. Influence of Ca2+ on the structure of reptilase-derived and thrombin-derived fibrin gels. Biochem J 239: 513–516, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. J Biol Chem 279: 46678–46685, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cooke JP. The endothelium: a new target for therapy. Vasc Med 5: 49–53, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker HC, Negrier C. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost 93: 475–480, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Davis MR, Ortegon DP, Kerby JD, Ignarro LJ, Kashyap VS. Endothelial dysfunction after arterial thrombosis is ameliorated by l-arginine in combination with thrombolysis. J Vasc Interv Radiol 14: 233–239, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P, Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res 85: 394–402, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM, Zhang C. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 27: 1269–1275, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Glover CJ, McIntire LV, Brown CH, 3rd, Natelson EA. Rheological properties of fibrin clots. Effects of fibrinogen concentration, Factor XIII deficiency, and Factor XIII inhibition. J Lab Clin Med 86: 644–656, 1975 [PubMed] [Google Scholar]
- 17.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SJ. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene 353: 98–106, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Campbell D, Dérijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267: 389–393, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Herbein G, Varin A, Fulop T. NF-kappaB, AP-1, zinc-deficiency and aging. Biogerontology 7: 409–419, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol 114: 107–132, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Kanyo ZF, Scolnick LR, Ash DE, Christianson DW. Structure of a unique binuclear manganese cluster in arginase. Nature 383: 554–557, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Kashyap VS, Reil TD, Moore WS, Hoang TX, Gelabert HA, Byrns RE, Ignarro LJ, Freischlag JA. Acute arterial thrombosis causes endothelial dysfunction: a new paradigm for thrombolytic therapy. J Vasc Surg 34: 323–329, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kepka-Lenhart D, Ash DE, Morris SMJ. Determination of mammalian arginase activity. Methods Enzymol 440: 221–230, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. Am J Physiol Cell Physiol 294: C241–C250, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lewis C, Zhu W, Pavkov ML, Kinney CM, Dicorleto PE, Kashyap VS. Arginase blockade lessens endothelial dysfunction after thrombosis. J Vasc Surg 48: 441–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor alpha synergistically stimulate tissue factor expression in human endothelial cells: regulation through c-Fos and c-Jun. J Biol Chem 279: 36142–36147, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J 14: 1785–1797, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol 24: 41–53, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res 98: 1352–1364, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via the RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation 110: 3708–3714, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Morris SMJ, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol Endocrinol Metab 275: E740–E747, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Morton S, Davis RJ, Cohen P. Signalling pathways involved in multisite phosphorylation of the transcription factor ATF-2. FEBS Lett 572: 177–183, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nair CH, Shah GA, Dhall DP. Effect of temperature, pH and ionic strength and composition on fibrin network structure and its development. Thromb Res 42: 809–816, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med 338: 1105–1111, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol 17: 3406–3413, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Reil TD, Moore WS, Kashyap VS, Nene SS, Gelabert HA, Quinones-Baldrich WJ. The effects of thrombus, thrombectomy and thrombolysis on endothelial function. Eur J Vasc Endovasc Surg 19: 162–168, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg 220: 251–266, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J 77: 2813–2826, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res 99: 951–960, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res 102: 923–932, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res 101: 692–702, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Scarpati EM, DiCorleto PE. Identification of a thrombin response element in the human platelet-derived growth factor B-chain (c-sis) promoter. J Biol Chem 271: 3025–3032, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Shankar R, de la Motte CA, DiCorleto PE. Thrombin stimulates PDGF production and monocyte adhesion through distinct intracellular pathways in human endothelial cells. Am J Physiol Cell Physiol 262: C199–C206, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Stenina OI, Poptic EJ, DiCorleto PE. Thrombin activates a Y box-binding protein (DNA-binding protein B) in endothelial cells. J Clin Invest 106: 579–587, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med 3: 879–886, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Van Dam H, Duyndam M, Rottier R, Bosch A, de Vries-Smits L, Herrlich P, Zantema A, Angel P, van der Eb AJ. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J 12: 479–487, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner EF Introductory remarks. Oncogene 20: 2334–2335, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Waterfield MD, Scrace GT, Whittle N, Stroobant P, Johnsson A, Wasteson A, Westermark B, Heldin CH, Huang JS, Deuel TF. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature 304: 35–39, 1983 [DOI] [PubMed] [Google Scholar]
- 53.Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci 38: 15–23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 47: 245–251, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Lewis CM, Chandrasekharan UM, Kinney CM, Dicorleto PE, Kashyap VS. Arginase activity is increased by thrombin: a mechanism for endothelial dysfunction in arterial thrombosis. J Am Coll Surg 203: 817–826, 2006 [DOI] [PubMed] [Google Scholar]