Abstract
The ATP-sensitive potassium (KATP) channel couples intracellular metabolic state to membrane excitability. Recently, we demonstrated that neuronal KATP channels are functionally enhanced by activation of a nitric oxide (NO)/cGMP/cGMP-dependent protein kinase (PKG) signaling cascade. In this study, we further investigated the intracellular mechanism underlying PKG stimulation of neuronal KATP channels. By performing single-channel recordings in transfected HEK293 and neuroblastoma SH-SY5Y cells, we found that the increase of Kir6.2/SUR1 (i.e., the neuronal-type KATP) channel currents by PKG activation in cell-attached patches was diminished by 5-hydroxydecanoate (5-HD), an inhibitor of the putative mitochondrial KATP channel; N-(2-mercaptopropionyl)glycine, a reactive oxygen species (ROS) scavenger, and catalase, a hydrogen peroxide (H2O2)-decomposing enzyme. These reagents also ablated NO-induced KATP channel stimulation and prevented the shifts in the single-channel open- and closed-time distributions resulting from PKG activation and NO induction. Bath application of H2O2 reproduced PKG stimulation of Kir6.2/SUR1 but did not activate tetrameric Kir6.2LRKR368/369/370/371AAAA channels. Moreover, neither the PKG activator nor exogenous H2O2 was able to enhance the function of KATP channels in the presence of Ca2+ chelators and calmodulin antagonists, whereas the stimulatory effect of H2O2 was unaffected by 5-HD. Altogether, in this report we provide novel evidence that activation of PKG stimulates neuronal KATP channels by modulating intrinsic channel gating via a 5-HD-sensitive factor(s)/ROS/Ca2+/calmodulin signaling pathway that requires the presence of the SUR1 subunit. This signaling pathway may contribute to neuroprotection against ischemic injury and regulation of neuronal excitability and neurotransmitter release by modulating the function of neuronal KATP channels.
Keywords: nitric oxide, hydrogen peroxide, single channel, patch clamp, adenosine 5′-triphosphate-sensitive potassium channel
the atp-sensitive potassium (KATP) channel serves as an important metabolic sensor by coupling intracellular metabolic status to changes in transmembrane potassium fluxes and cell excitability (4, 51, 54). Cellular functions regulated by these channels include neuronal excitability, neurotransmitter and hormone release, vascular tone, heart rate, and protection of neurons and cardiomyocytes under metabolic stress (66). The KATP channel is an octameric protein (9, 68) composed of four pore-forming subunits (Kir6.2 or Kir6.1) (34, 62), which are members of the inwardly rectifying potassium channel family, and four regulatory subunits (the sulfonylurea receptors SUR1, SUR2A, or SUR2B) (1, 35, 37), which are members of the ATP-binding cassette protein superfamily. KATP channels are widely distributed, and their molecular compositions exhibit tissue specificity. For instance, KATP channels in central neurons are composed of Kir6.2 and SUR1 subunits, whereas in cardiac and skeletal muscles they are composed of Kir6.2 and SUR2A subunits (35, 56). The Kir6.2 subunit possesses ATP binding sites responsible for channel inhibition (19, 54, 70), whereas the SUR subunit can further modulate ATP sensitivity and confers the channel sensitivities to sulfonylurea drugs (inhibition) and to MgADP and synthetic KATP channel openers (stimulation) (24, 38, 67).
Classically recognized for the ability to regulate smooth muscle and platelet functions, cGMP-dependent protein kinase (PKG) is increasingly becoming appreciated as an important component of many signal transduction processes in diverse cell types (for reviews, see Refs. 29 and 30). Functional modulation of KATP channels by cGMP, presumably through activation of PKG, has been demonstrated in follicle-enclosed Xenopus oocytes (63, 64), pancreatic β-cells (61), vascular smooth muscle cells (42), and cardiac myocytes (26, 27). Findings from our recent work further reveal that PKG exerts bidirectional functional regulation on neuronal-type KATP channels; this includes a predominating stimulatory action, which can be reproduced by nitric oxide (NO) via activation of a cGMP/soluble guanylyl cyclase (sGC)/PKG signaling cascade, and an inhibitory action, which may involve direct phosphorylation of the channel or some closely associated regulatory protein(s) (14). It has been suggested that NO induces reactive oxygen species (ROS) generation and renders cardioprotection by activation of PKG and mitochondrial ATP-sensitive potassium (i.e., mitoKATP) channels (75), the putative KATP channels present in the inner mitochondrial membrane that are sensitive to 5-hydroxydecanoate (5-HD). However, whether ROS or mitoKATP channels are involved in NO/PKG-induced stimulation of plasma membrane KATP channels is not known.
In the present study, we investigated the signaling mechanism underlying the stimulatory actions of PKG and NO on Kir6.2/SUR1 channels in transiently transfected human embryonic kidney (HEK)293 cells and human neuroblastoma SH-SY5Y cells. More specifically, roles of ROS, the mitoKATP channel, Ca2+, and calmodulin in PKG signaling were examined. With single-channel recordings performed in both cell-attached and inside-out patch configurations, our study provides four lines of novel findings. First, stimulation of neuronal KATP channels by NO and PKG results from intracellular signaling mediated by activation of the 5-HD-sensitive factor(s) (possibly mitoKATP channels) and subsequent generation of ROS, particularly hydrogen peroxide (H2O2). Second, H2O2 and related ROS stimulate neuronal KATP channels by indirect interaction with the SUR1 subunit. Third, activation of a Ca2+/calmodulin-dependent process is required to mediate the KATP channel stimulation downstream of ROS/H2O2. Last, NO/PKG/ROS signaling stimulates KATP channel by modifying channel gating, rather than altering cellular metabolism.
MATERIALS AND METHODS
Construction of cDNAs
Neuronal-type KATP channels were reconstituted using cDNAs encoding the sulfonylurea receptor SUR1 (hamster) and the pore-forming subunit Kir6.2 (mouse) as described previously (44, 45, 47). In addition, cDNAs encoding Kir6.2LRKR368/369/370/371AAAA (i.e., Kir6.2FL4A), a trafficking mutant that can be functionally expressed without the SUR subunit, were also prepared. All cDNA constructs were subcloned into mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA), except the wild-type Kir6.2, which was subcloned into pIRES-EGFP (Clontech, Mountain View, CA), and the flag-tagged wild-type and mutant SUR1 (i.e., fSUR1 and fSUR1G1479R; see Supplemental Material), which were subcloned in pECE. (Supplemental data for this article is available online at the American Journal of Physiology-Cell Physiology website.) The plasmids prepared with Qiagen maxipreps (Qiagen, Valencia, CA) that were to be used for transient transfection were verified by DNA sequencing.
Cell Culture and Transient Transfection
HEK293 cells and human neuroblastoma SH-SY5Y cells (ATCC, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM/F12; Mediatech, Herndon, VA), supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin, at 37°C in humidified 5% CO2. Cells were transiently transfected using the FuGENE 6 reagent (Roche, Indianapolis, IN) mixed with expression plasmids containing cDNAs of interest in serum-free medium. A marker gene encoding the green fluorescent protein (pEGFP-1; Clontech) was cotransfected with pcDNA3Kir6.2FL4A in a ratio of 1.5:10. No additional marker gene was included when expressing wild-type Kir6.2, because the vector pIRES-EGFP would provide cistronic EGFP expression to mark positive transfection. Transfection was carried out according to the manufacturer's protocols. The cells were replated the following day at a density of 5,000–20,000 cells/dish onto 12-mm glass coverslips precoated with 1.5 μg/ml fibronectin (Sigma-Aldrich, St. Louis, MO) to be recorded 48–72 h after transfection (45).
Electrodes, Recording Solutions, and Single-Channel Recordings
The recording electrodes were pulled from thin-walled borosilicate glass with an internal filament (MTW150F-3; World Precision Instruments, Sarasota, FL) using a P-97 Flaming Brown puller (Sutter Instrument, Novato, CA), and they were then fire-polished to a resistance of 5–10 MΩ. The intracellular (bath) solution consisted of (in mM) 110 KCl, 1.44 MgCl2, 30 KOH, 10 EGTA, and 10 HEPES, pH to 7.2. The extracellular (intrapipette) solution consisted of (in mM) 140 KCl, 1.2 MgCl2, 2.6 CaCl2, and 10 HEPES, pH to 7.4. The same combination of recording solutions was used for recordings performed in both HEK293 and SH-SY5Y cells. Inside-out and cell-attached single-channel recordings (25) were performed using a recording chamber (RC26; Warner Instruments, Hamden, CT) filled with the intracellular (bath) solution, and the recording pipette was filled with the extracellular solution. The use of symmetrical recording solutions (140 mM K+) resulted in an equilibrium potential for potassium (EK) and a resting membrane potential (Vm) of ∼0 mV in both cell types, as determined from the current-voltage (I-V) relationship of the KATP channel. All recordings were carried out at room temperature, and all patches were voltage-clamped at −60 mV (i.e., with −60 mV intrapipette potentials) unless specified otherwise. Single-channel currents were recorded with an Axopatch 200B patch-clamp amplifier (MDS Analytical Technologies-Axon Instruments, Sunnyvale, CA), low-pass filtered (3 dB, 2 kHz), and digitized at 20 kHz on-line using Clampex 9 software (Axon) via a 16-bit analog-to-digital converter (Digidata acquisition board 1322A; Axon).
Reagents
Working solutions of 1,4-dihydro-5-(2-propoxyphenyl)-7H-1,2,3-triazolo[4,5-d]pyrimidine-7-one (zaprinast), KT5823, KT5720, 5-HD, N-(2-mercaptopropionyl)glycine (MPG), adenosine 5′-triphosphate magnesium salt (MgATP), DETA NONOate (NOC-18), BAPTA-AM, and fluphenazine-N-2-chloroethane, dihydrochloride (SKF-7171A, HCl) were diluted from aliquots before use with bath recording solutions. Stock solutions were prepared as followed: zaprinast, KT5823, KT5720, BAPTA-AM, and SKF-7171A in DMSO, and 5-HD, MPG, MgATP, and NOC-18 in H2O; all were stored at −80°C in aliquots. Catalase (human erythrocyte) and H2O2 were prepared directly from original stocks. All chemicals were obtained from Calbiochem (EMD Biosciences; San Diego, CA) or Sigma-Aldrich. During inside-out patch recordings, MgATP (30 μM) was included in the bath and drug solutions to prevent channel rundown. All working drug solutions were put on ice and kept away from light. Drugs were applied through a pressure-driven perfusion system (BPS-8; ALA Scientific Instruments, Westbury, NY) to the recording chamber via a micromanifold positioned closely to cell-attached or inside-out patches.
Data Analysis
Single-channel currents.
Digitized single-channel records were detected with Fetchan 6.05 (events list) of pCLAMP (Axon) using a 50% threshold crossing criterion and were analyzed with Intrv5. Analysis was performed at the main conductance level (∼70 pS). Only patches with infrequent multiple-channel activity were used for single-channel analysis. Duration histograms were constructed as described previously (44), and estimates of exponential areas and time constants were obtained using the method of maximal likelihood estimation. The number of exponential functions required to fit the duration distribution was determined by fitting increasing numbers of functions until additional components could not significantly improve the fit. Mean durations were corrected for missed events by taking the sum of the relative area (a) of each exponential component in the duration frequency histogram multiplied by the time constant (τ) of the corresponding component. Each of the single-channel properties was then normalized to the corresponding controls obtained in individual patches (taken as 1).
Multiple-channel currents.
In patches where multiple-channel activities of KATP channels were observed for more than 10% of the recording time, the digitized current records were analyzed using Fetchan 6.05 (browse) of pCLAMP to integrate currents in 120-s segments. The current amplitude (I) values (current amplitude = integrated current/acquisition time) were then normalized to the corresponding controls obtained from the same patches to yield relative current amplitude (control as 1). The relative current amplitude obtained from current integration and the normalized open probability (NPo) obtained from single-channel analysis were then pooled and averaged to calculate relative channel activity presented in the bar graphs and data tables, because these two are equivalent when the single-channel conductance remains the same (47).
Statistics
Data were averaged and are presented as means ± SE. Statistical comparisons were made using Student's two-tailed one-sample, paired, or unpaired t-tests or one-way ANOVA followed by Dunnett's multiple comparison tests. Significance was assumed when P < 0.05. Statistical comparisons were performed using Prism (GraphPad Software, San Diego, CA).
RESULTS
In the present study, we investigated the potential signaling elements that mediate PKG stimulation of neuronal KATP channels. The activity of wild-type and mutant Kir6.2/SUR1 (i.e., the neuronal-type KATP) channels was monitored by single-channel recordings performed in either cell-attached or inside-out configurations in transfected HEK293 cells and human neuroblastoma SH-SY5Y cells. We first examined whether the stimulation of Kir6.2/SUR1 channels by PKG activation in intact cells is sensitive to treatment of 5-HD (an inhibitor of the putative mitoKATP channel), ROS scavenging, or enzymatic decomposition of H2O2. The roles of 5-HD-sensitive factor(s) and ROS in mediating Kir6.2/SUR1 channel stimulation induced by NOC-18, a NO donor, were also determined. We subsequently examined how the activity of Kir6.2/SUR1 channels is modulated by exogenous H2O2 in cell-attached and excised, inside-out membrane patches, respectively. Furthermore, we tested whether H2O2 modulates the activity of tetrameric Kir6.2LRKR368/369/370/371AAAA (i.e., Kir6.2FL4A) channels, a trafficking mutant of Kir6.2 (77) capable of functional expression in the absence of the SUR subunit. We also determined whether the activity of the 5-HD-sensitive factor(s) is required for H2O2 to enhance Kir6.2/SUR1 channel function. Finally, we examined whether PKG and ROS stimulation of neuronal KATP channels is mediated by intracellular Ca2+/calmodulin. In this report, “the 5-HD-sensitive factor” is used in place of “the mitoKATP channel,” considering that the evidence for an ion channel identity of mitoKATP is still inconclusive and that the proposed role of the 5-HD-sensitive factor(s) in mediating plasma membrane KATP channel activation by PKG may not depend on its being an “ion channel” in mitochondria.
Zaprinast Stimulated Kir6.2/SUR1 Channels in Intact HEK293 Cells by Activation of PKG
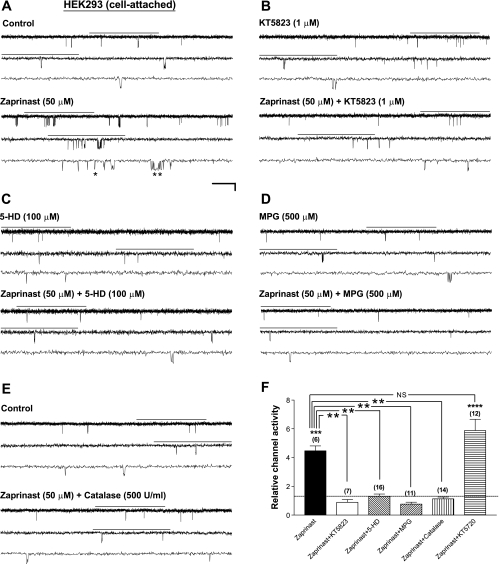
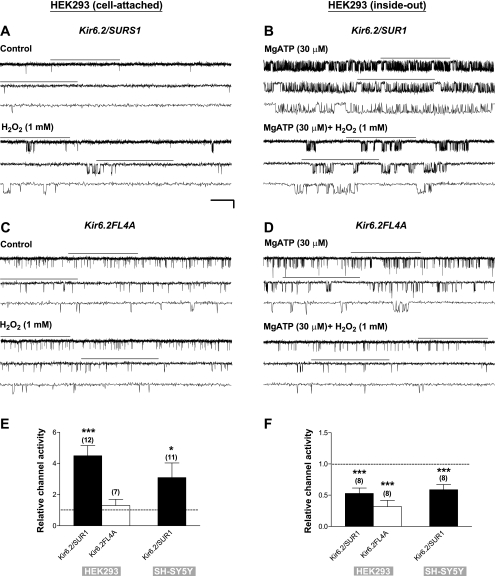
To induce PKG activation, we applied zaprinast, a selective, membrane-permeable inhibitor of cGMP-specific phosphodiesterase (PDE), by bath perfusion to intact HEK293 cells expressing Kir6.2/SUR1, the neuronal-type KATP channels. Single-channel currents were recorded in the cell-attached configuration to preserve the integrity of the intracellular milieu for potential signaling. EK and Vm were both around 0 mV, as determined from the I-V relationship of the single-channel currents of Kir6.2/SUR1 channels. Patches were voltage-clamped at a membrane potential of −60 mV throughout this study. In Fig. 1, as in all trace illustrations, the current traces marked with a horizontal bar on top are displayed at increasing temporal resolution in successive traces (arranged from top to bottom). Bath application of zaprinast (50 μM) increased the single-channel currents of Kir6.2/SUR1 channels (Fig. 1A) in a concentration-dependent manner (see Supplemental Fig. 1), whereas zaprinast exerted no effect when the membrane-permeable selective PKG inhibitor KT5823 (1 μM) was coapplied (Fig. 1B); the averaged normalized NPo (i.e., the relative channel activity) of Kir6.2/SUR1 channels was 4.47 ± 0.34 (control as 1) (Fig. 1F, zaprinast; P < 0.001, two-tailed one-sample t-test) and 0.89 ± 0.19 (Fig. 1F, zaprinast+KT5823; no significant change), respectively. The single-channel conductance remained the same. Thus the zaprinast-induced KATP channel stimulation was completely abolished by KT5823 (Fig. 1F, zaprinast vs. zaprinast+KT5823; P < 0.01, Dunnett's multiple comparison test following one-way ANOVA). In contrast, KT5720 (1 μM), a membrane-permeable inhibitor selective for cAMP-dependent protein kinase (PKA), did not affect PKG's stimulatory action (Fig. 1F, zaprinast vs. zaprinast+KT5720). These results indicate that zaprinast stimulated Kir6.2/SUR1 KATP channels specifically via activation of cGMP/PKG but not PKA signaling in intact HEK293 cells.
Fig. 1.
Enhancement of Kir6.2/SUR1 channel activity by PKG activation in transfected HEK293 cells requires the activities of the 5-hydroxydecanoate (5-HD)-sensitive factor(s) [possibly mitochondrial ATP-sensitive K+ (mitoKATP) channels] and ROS. Recombinant Kir6.2/SUR1 channels were expressed in HEK293 cells by transient transfection. Recordings were performed in symmetrical high-K+ (140 mM) solutions at room temperature in the cell-attached configuration, and the membrane potential was clamped at −60 mV. Drugs were applied by bath perfusion. A–E: single-channel current traces of the Kir6.2/SUR1 channel obtained from a cell-attached patch before (top traces) and during (bottom traces) application of the cGMP-specific phosphodiesterase V (PDE V) inhibitor zaprinast alone (A) or zaprinast together with the selective PKG inhibitor KT5823 (B), the selective mitoKATP channel inhibitor 5-HD (C), the membrane-permeable ROS scavenger N-(2-mercaptopropionyl)glycine (MPG; D), or the H2O2-decomposing enzyme catalase (E). Cells were incubated with respective inhibitors (except catalase) for at least 15 min before index drug perfusion. Downward deflections represent openings from closed states. For all current trace panels, marked segments of raw recordings (with horizontal lines above) are shown in successive traces at increasing temporal resolution, revealing singular openings (*) and bursts of openings (**). Horizontal scale bars represent 1 s, 300 ms, and 100 ms for traces from top to bottom in each set of 3 traces, and the vertical scale bar represents 4 pA. F: averaged relative channel activity (i.e., normalized open probability, NPo) of Kir6.2/SUR1 channels obtained during application of drugs in individual groups of cell-attached patches. In addition to the inhibitors/enzyme described in A–E, the selective PKA inhibitor KT5720 (1 μM) was also included to verify the specificity of zaprinast for PKG activation. NPo values of all groups were normalized to the corresponding control (taken as 1) obtained before drug application in individual patches to yield the relative channel activity. Dashed line indicates the control level. Data are means ± SE of 6–16 patches (no. of patches in individual groups is provided in parentheses). **P < 0.01; ***P < 0.001; ****P < 0.0001 (two-tailed, one-sample t-tests within individual groups or Dunnett's multiple comparison tests between groups).
The Increase in Single-Channel Activity of Kir6.2/SUR1 Channels by PKG Activation Was Abrogated by 5-HD, an Inhibitor of the MitoKATP Channel and Ischemic Preconditioning, in Intact HEK293 Cells
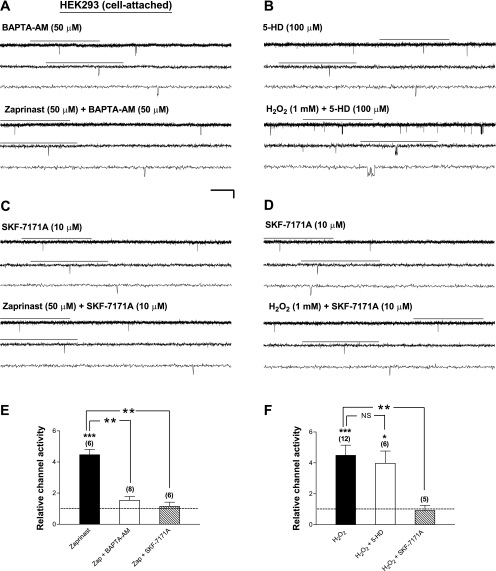
What signal might relay activation of PKG to opening of plasma membrane KATP channels? Several lines of evidence indicate that mitoKATP channels, the putative KATP channels present in the inner mitochondrial membrane, are positively modulated by PKG, possibly via Ca2+/phospholipid protein kinase (PKC) activation (3, 17, 18, 39). Could the mitoKATP channel serve as a downstream signal of PKG to mediate the stimulation of Kir6.2/SUR1 channels? To test this possibility, we pretreated transfected HEK293 cells with the mitoKATP channel inhibitor 5-HD (100 μM) for at least 15 min, followed by cell-attached patch recordings of Kir6.2/SUR1 channels before and during application of zaprinast (50 μM) in the continuous presence of 5-HD (100 μM). 5-HD, a natural lipid component of human milk, has been shown to disrupt ischemic tolerance conferred by ischemic and pharmacological preconditioning in heart and brain, which action is thought to result from inhibition of mitoKATP channels (31, 49). We found that zaprinast did not induce an increase in the single-channel activity of Kir6.2/SUR1 channels when 5-HD was coapplied (Fig. 1, C and F, zaprinasat+5-HD), revealing a significant abrogation of the zaprinast effect by 5-HD (Fig. 1F, zaprinast vs. zaprinast+5-HD; P < 0.01). 5-HD by itself did not suppress basal KATP channel activity in intact cells (data not shown). These results indicate that cGMP/PKG-induced stimulation of Kir6.2/SUR1 channels in intact HEK293 cells required the activity of the 5-HD-sensitive factor(s), possibly mitoKATP channels, for signal transduction.
PKG Activation Failed to Stimulate Kir6.2/SUR1 Channels in the Presence of ROS Scavengers in Intact HEK293 Cells
Opening of the mitoKATP channel increases ROS levels in isolated heart and liver mitochondria (3); moreover, PKG activation appears to be responsible for NO-induced ROS generation in rat cardiomyocytes and the anti-infarct effect of NO in intact, isolated heart (75). Are ROS also involved in mediating the acute, stimulatory action of PKG on neuronal-type KATP channels? To examine this possibility, we evaluated the effect of MPG (500 μM), an ROS scavenger, on the enhancement of Kir6.2/SUR1 channel activity caused by PKG activation in intact HEK293 cells. Coapplication of the selective cGMP-specific PDE inhibitor zaprinast (50 μM) and MPG (500 μM) (following a 15-min MPG pretreatment) failed to increase the single-channel currents of Kir6.2/SUR1 channels in cell-attached patches (Fig. 1D). The relative channel activity (Fig. 1F, zaprinast+MPG) was clearly reduced from that obtained from patches treated with zaprinast alone (Fig. 1F, zaprinast vs. zaprinast+MPG; P < 0.01). These data indicate that removal of ROS was sufficient to abolish the stimulatory action of PKG on Kir6.2/SUR1 channels.
Catalase Abolished Kir6.2/SUR1 Channel Stimulation Induced by PKG Activation in Intact HEK293 Cells
ROS include free radicals and peroxides, among which H2O2 is a relatively stable derivative. The potential role of H2O2 in mediating the stimulatory effect of PKG on neuronal-type KATP channels was examined by coapplying catalase, an enzyme that decomposes H2O2 to water and oxygen, together with zaprinast in intact HEK293 cells that expressed Kir6.2/SUR1 channels. We found that in the presence of catalase (500 U/ml), zaprinast (50 μM) was incapable of enhancing the single-channel activity of Kir6.2/SUR1 channels in individual cell-attached patches (Fig. 1, E and F, zaprinast+catalase), in sharp contrast to the significant increase seen in the zaprinast group (Fig. 1F, zaprinast vs. zaprinast+catalase; P < 0.01). These results indicate that removal of H2O2 and related ROS prevented zaprinast from exerting its stimulatory action on Kir6.2/SUR1 channels expressed in intact HEK293 cells. This finding lends support to our hypothesis that ROS are required for PKG to induce KATP channel stimulation. Acting as a physiological signal downstream of PKG, ROS are probably generated at a low amount, which may not be detectable in intact cells with the use of fluorescent ROS indicators (see Supplemental Fig. 3).
Stimulation of Kir6.2/SUR1 Channels by PKG Activation Was Abrogated by the MitoKATP Channel Inhibitor and ROS Scavengers in Human Neuroblastoma SH-SY5Y Cells
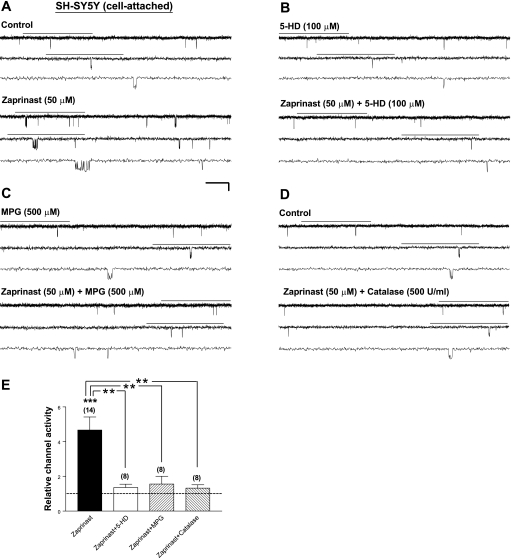
To determine whether the dependence of PKG-induced Kir6.2/SUR1 channel stimulation on the activities of 5-HD-sensitive factor(s) and ROS applies to KATP channels expressed in a neuronal background, we examined the effects of 5-HD and ROS scavengers, respectively, on the stimulatory effect of PKG on Kir6.2/SUR1 channels expressed in human neuroblastoma SH-SY5Y cells. Bath application of the cGMP-specific PDE inhibitor zaprinast (50 μM) enhanced the apparent opening and bursting frequencies of Kir6.2/SUR1 channels (Fig. 2A), resulting in a significant increase in the relative channel activity (4.67 ± 0.76; Fig. 2E, zaprinast, P < 0.001). In contrast, coapplication of zaprinast with 5-HD (100 μM; Fig. 2B), MPG (500 μM; Fig. 2C), or catalase (500 U/ml; Fig. 2D) did not increase the activity of Kir6.2/SUR1 channels. The stimulatory effect of zaprinast on the relative channel activity of Kir6.2/SUR1 channels was significantly abrogated (Fig. 2E; P < 0.01 for all 3 groups). These results agreed with the observations made in transfected HEK293 cells (see Fig. 1) and indicate that the activity of the 5-HD-sensitive factor(s) and generation of ROS, particularly H2O2, were required for cGMP/PKG signaling to elicit Kir6.2/SUR1 channel stimulation in a neuronal background.
Fig. 2.
Stimulation of Kir6.2/SUR1 channels by PKG activation in transfected human neuroblastoma SH-SY5Y cells exhibits dependence on the 5-HD-sensitive factor(s) and ROS generation. Recombinant Kir6.2/SUR1 channels were expressed in SH-SY5Y cells by transient transfection. Cell-attached patch-clamp recordings and drug application were administered as described in Fig. 1. A–D: single-channel current traces of Kir6.2/SUR1 channel obtained from a cell-attached patch before (top traces) and during (bottom traces) application of the cGMP-specific PDE inhibitor zaprinast alone (50 μM; A) or zaprinast (50 μM) together with the selective mitoKATP channel inhibitor 5-HD (100 μM; B), the membrane-permeable ROS scavenger MPG (500 μM; C), or the H2O2-decomposing enzyme catalase (500 U/ml; D). Scale bars are the same as in Fig. 1. E: averaged relative channel activity of Kir6.2/SUR1 channels obtained during application of drugs in individual groups. NPo values of all groups were normalized to the corresponding control (taken as 1) obtained before drug application in individual patches to yield the relative channel activity. Dashed line indicates the control level. Data are means ± SE of 8–14 patches (no. of patches in individual groups is provided in parentheses). **P < 0.01; ***P < 0.001 (two-tailed, one-sample t-tests within individual groups or Dunnett's multiple comparison tests between groups).
Functional Modulation of Kir6.2/SUR1 Channels by NO Donors Was Dependent on Activities of 5-HD-Sensitive Factors and ROS in Transfected HEK293 and SH-SY5Y Cells
NO is a highly diffusible gaseous neurotransmitter (11, 22), capable of influencing adjacent neurons without direct synaptic connections. We have previously demonstrated that NO enhances the activity of Kir6.2/SUR2B (i.e., the nonvascular smooth muscle-type KATP) (46) and Kir6.2/SUR1 (i.e., the neuronal-type KATP) (14) channels expressed in transfected mammalian cells. Notably, the stimulation of Kir6.2/SUR1 channels by NO is mediated through a sGC/PKG signaling cascade (14). NO donors also have been suggested to induce ROS generation in isolated cardiomyocytes, and this process depends on activation of PKG and opening of mitoKATP channels (75). To determine whether the functional modulation of neuronal KATP channels by NO is mediated through activation of the 5-HD-sensitive factor(s) and ROS, we evaluated the impact of the mitoKATP channel inhibitor 5-HD, the ROS scavenger MPG, and the H2O2-decomposing enzyme catalase, separately, on the stimulation of Kir6.2/SUR1 channels caused by the NO donor NOC-18 in intact HEK293 cells. NOC-18 (300 μM) increased the apparent opening and bursting frequency of Kir6.2/SUR1 channels in individual cell-attached patches; the averaged relative channel activity was significantly increased to 3.59 ± 0.78 (control as 1) (8 patches; P < 0.05). In contrast, application of NOC-18 (300 μM) in the presence of 5-HD (100 μM) or MPG (500 μM) did not enhance the single-channel activity of Kir6.2/SUR1 channels (9–11 patches). Moreover, NOC-18 only weakly stimulated Kir6.2/SUR1 channels when coapplied with catalase (500 U/ml; 13 patches; P < 0.05). Evidently, treatment of 5-HD, MPG, and catalase ablated or attenuated the stimulatory action of NOC-18 on Kir6.2/SUR1 channels expressed in these cells (P < 0.01 for all groups when respectively compared with the group treated with NOC-18 alone). Our findings thus indicate that in intact HEK93 cells, stimulation of Kir6.2/SUR1 channels by induction of NO, the upstream activator of PKG, depended on the activities of 5-HD-sensitive factor(s) and ROS.
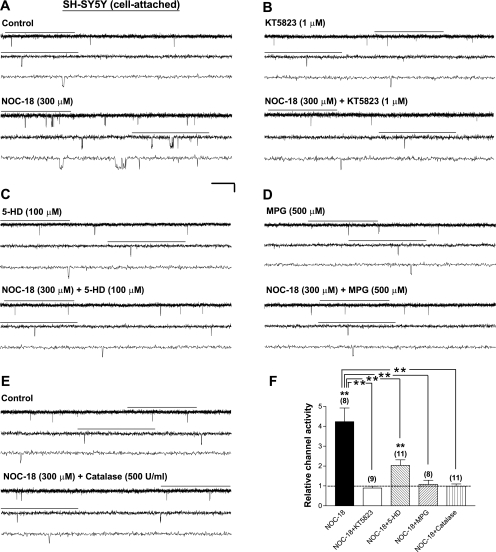
Similar approaches were performed on Kir6.2/SUR1 channels expressed in human neuroblastoma SH-SY5Y cells. NOC-18 (300 μM) significantly increased the single-channel activity of these channels in SH-SY5Y cells (Fig. 3, A and F, NOC-18; P < 0.01), whereas the NOC-18 effect was absent when the selective PKG inhibitor KT5823 was coapplied (Fig. 3, B and F, NOC-18+KT5823). The abrogation of the stimulatory effect of NOC-18 by inhibition of PKG (Fig. 3F, NOC-18+KT5823 vs. NOC-18; P < 0.01) indicates that NO stimulated KATP channels that were expressed in a neuronal background by inducing cGMP/PKG signaling. On the other hand, in the presence of the mitoKATP channel inhibitor 5-HD (100 μM), NOC-18 only slightly increased the Kir6.2/SUR1 single-channel activity (Fig. 3, C and F, NOC-18+5-HD; P < 0.01), which effect was significantly attenuated compared with data obtained from the NOC-18 group (Fig. 3F, NOC-18+5-HD vs. NOC-18; P < 0.01). These results indicate that the activity of the 5-HD-sensitive factor(s) or the mitoKATP channel was necessary for NO's stimulatory action. Moreover, as seen in HEK293 cells, NOC-18 failed to increase the single-channel activity of Kir6.2/SUR1 channels in the presence of MPG (500 μM) or catalase (500 U/ml) in intact SH-SY5Y cells (Fig. 3, D, E, and F, NOC-18+MPG and NOC-18+catalase); the stimulatory effect of NOC-18 was completely abolished (Fig. 3F; comparing NOC-18+MPG or NOC-18+catalase with NOC-18 alone; P < 0.01). Our findings thus indicate that NO-induced stimulation of Kir6.2/SUR1 channels expressed in a neuronal background required generation of ROS, particularly H2O2.
Fig. 3.
Kir6.2/SUR1 channels are stimulated by nitric oxide (NO) in a 5-HD-sensitive factor (possibly the mitoKATP)-, ROS-, and H2O2-dependent manner. Currents were obtained from transfected human neuroblastoma SH-SY5Y cells expressing Kir6.2/SUR1 channels in the cell-attached patch configuration. A–E: single-channel current traces obtained from individual cell-attached patches before (top traces) and during (bottom traces) application of DETA NONOate (NOC-18) alone (300 μM; A) or NOC-18 (300 μM) plus the selective PKG inhibitor KT5823 (1 μM; B), the selective mitoKATP channel inhibitor 5-HD (100 μM; C), the ROS scavenger MPG (500 μM; D), or the H2O2-decomposing enzyme catalase (500 U/ml; E). Cells were incubated with respective inhibitors (except catalase) for at least 15 min before drug perfusion. Scale bars are the same as in Fig. 1. F: averaged relative channel activity of Kir6.2/SUR1 channels obtained from cell-attached patches during drug treatment. NPo values were normalized to yield relative channel activity as described in Fig. 1F (control taken as 1, dashed line) and are means ± SE of 8–11 patches. **P < 0.01 (two-tailed, one-sample t-tests within groups or Dunnett's multiple comparison tests between groups).
Changes in the Single-Channel Properties of Kir6.2/SUR1 Channels by PKG Activation and NO Induction Exhibited Dependence on the Activities of 5-HD-Sensitive Factors and ROS
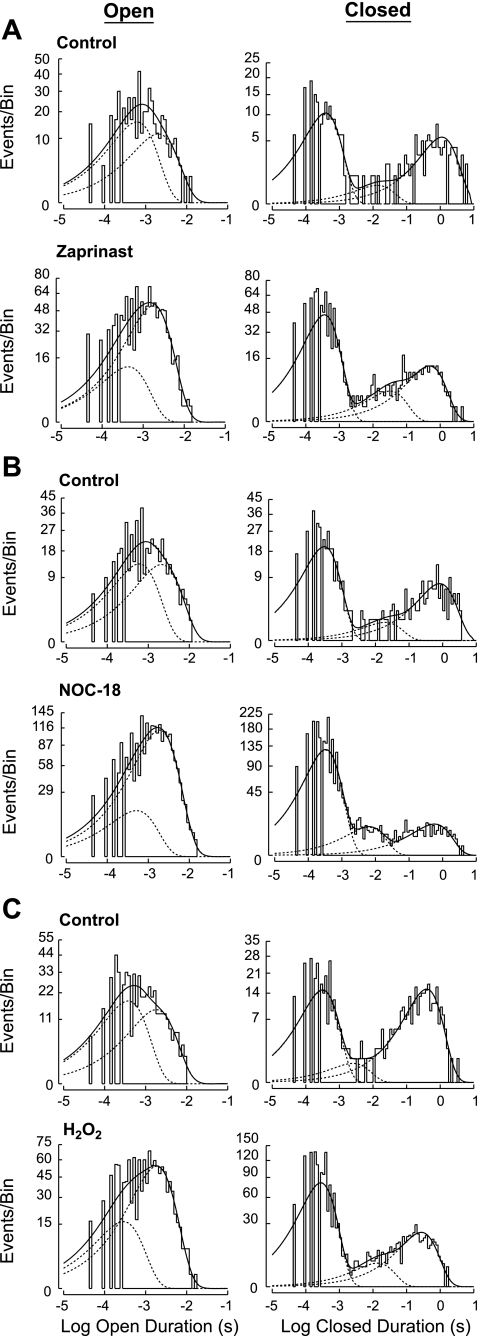
Channel function and its modulation is determined by the conformational changes that the channel undertakes to enable opening or closures of the pore for ion permeation, as reflected by the number of open and closed states it exhibits and the rates of transitions between different states. We analyzed the single-channel properties of Kir6.2/SUR1 channels, including NPo, opening frequency, corrected mean open duration, and mean closed duration, in patches suitable for single-channel analysis (see materials and methods for details). The open- and closed-duration distributions of the Kir6.2/SUR1 single-channel events in cell-attached patches could be best described by a sum of two open components and a sum of three closed components, respectively (Fig. 4A, control). Zaprinast, through cGMP/PKG activation, altered the gating (i.e., open and closed) properties of Kir6.2/SUR1 channels in intact cells, including more frequent entry into the open state (i.e., increased opening frequency), stabilization of the occurrence of the long open state, shortened dwelling time in the longest closed state (i.e., decreased duration/time constant of the longest closed state), and destabilization of the occurrence of the longest closed state (i.e., shifts of the relative distribution among closed states) (Fig. 4A, control vs. zaprinast; Table 1). Collectively, these changes led to increases in the normalized NPo, the opening frequency, and the corrected mean open duration plus a reduction in the mean closed duration (Table 1). We also evaluated how 5-HD and ROS scavengers influence the aforementioned changes in Kir6.2/SUR1 single-channel open and closed properties induced by zaprinast and found that the effects of zaprinast on the single-channel properties of Kir6.2/SUR1 channels, including open- and closed-duration distributions, were ablated by the mitoKATP channel inhibitor 5-HD, the ROS scavenger MPG, or the H2O2-decomposing enzyme catalase in both HEK293 and neuroblastoma SH-SY5Y cells (Table 1; Supplemental Fig. 2, representative patches). Our results thus indicate that PKG activation increased the activity of Kir6.2/SUR1 channels by modifying the gating properties of these channels in a 5-HD-sensitive factor (possibly mitoKATP channels)- and ROS-dependent manner.
Fig. 4.
Effects of zaprinast, H2O2, and NOC-18 on the open- and closed-duration distributions of the Kir6.2/SUR1 channel in representative cell-attached patches. These patches were obtained from transfected SH-SY5Y cells expressing Kir6.2/SUR1 channels. Frequency histograms of duration distributions fitted from events obtained before (top traces) and during (bottom traces) application of zaprinast (50 μM; A), NOC-18 (300 μM; B) or H2O2 (1 mM; C) in individual patches. Frequency histograms display open-duration distribution (left) and closed-duration distribution (right), respectively, for all patches. Duration histograms were constructed as described in materials and methods.
Table 1.
Roles of 5-HD-sensitive factors and ROS in mediating the stimulatory effects of PKG on normalized single-channel open and closed properties of recombinant Kir6.2/SUR1 channels expressed in intact HEK293 and SH-SY5Y cells
| Properties | Zaprinast | +5-HD | +MPG | +Catalase |
|---|---|---|---|---|
| HEK293 cells | ||||
| Open probability | 4.46 ± 0.42† | 1.36 ± 0.18 | 0.92 ± 0.18 | 1.10 ± 0.14 |
| Opening frequency | 3.92 ± 0.33‡ | 1.32 ± 0.17 | 0.77 ± 0.12 | 1.07 ± 0.05 |
| Mean open duration | 1.25 ± 0.08* | 1.06 ± 0.03 | 1.06 ± 0.11 | 1.04 ± 0.11 |
| Mean closed duration | 0.26 ± 0.02§ | 0.97 ± 0.15 | 1.54 ± 0.33 | 1.05 ± 0.09 |
| Number of patches | 5 | 14 | 7 | 12 |
| SH-SY5Y cells | ||||
| Open probability | 4.39 ± 0.76‡ | 1.36 ± 0.19 | 1.90 ± 0.69 | 1.53 ± 0.22 |
| Opening frequency | 3.74 ± 0.61‡ | 1.20 ± 0.14 | 1.44 ± 0.38 | 1.29 ± 0.21 |
| Mean open duration | 1.18 ± 0.07* | 1.12 ± 0.05* | 1.20 ± 0.20 | 1.22 ± 0.07* |
| Mean closed duration | 0.36 ± 0.06§ | 0.92 ± 0.11 | 1.16 ± 0.55 | 0.80 ± 0.16 |
| Number of patches | 13 | 8 | 5 | 5 |
Single-channel recordings of Kir6.2/SUR1 channels in cell-attached patches obtained from transfected HEK293 and SH-SY5Y cells were performed at −60 mV in symmetrical 140 mM K+ solutions. Zaprinast (50 μM) or zaprinast plus KT5823 (1 μM), 5-hydroxydecanoate (5-HD; 100 μM), N-(2-mercaptopropionyl)glycine (MPG; 500 μM), or catalase (500 U/ml) was applied by bath perfusion using a pressure-driven system. Single-channel properties were obtained as described in materials and methods. All values were normalized to the corresponding controls obtained in individual patches before index drug application (control taken as 1) and averaged. Data are means ± SE.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001 (two-tailed, one-sample t-tests).
Moreover, we found that the effects of NOC-18 on the normalized open and closed properties of Kir6.2/SUR1 channels in cell-attached patches obtained from transfected SH-SY5Y cells (Table 2) were reminiscent of those rendered by the PKG activator zaprinast (see Table 1), including increases in the NPo, opening frequency, and the corrected mean open duration as well as a reduction in the mean closed duration. Like zaprinast (Fig. 4A), NOC-18 caused shifts in the relative distributions of both open and closed states of Kir6.2/SUR1 channels in intact cells, by increasing the occurrence of the long open state, reducing the occurrence of the longest closed state, and exhibiting a tendency to shorten the dwelling time in the longest closed state (Fig. 4B, a representative patch); these changes underlay its effects on the corrected mean open duration and the mean closed duration (Table 2). Importantly, these NO donor effects on channel kinetics were largely prevented by 5-HD and totally eliminated by MPG and catalase (Table 2). Our results thus indicate that activation of the 5-HD-sensitive factor(s) (possibly mitoKATP channels) and generation of ROS (especially H2O2) were crucial for NO to modify the single-channel properties of Kir6.2/SUR1 channels.
Table 2.
Effects of NO on normalized single-channel open and closed properties of recombinant Kir6.2/SUR1 channels in intact SH-SY5Y cells in the presence and absence of 5-HD and ROS scavengers
| Properties | NOC-18 | +5-HD | +MPG | +Catalase |
|---|---|---|---|---|
| Open probability | 4.23 ± 0.79† | 1.89 ± 0.26† | 1.08 ± 0.21 | 1.00 ± 0.15 |
| Opening frequency | 3.52 ± 0.59† | 1.62 ± 0.22* | 0.97 ± 0.16 | 0.96 ± 0.11 |
| Mean open duration | 1.18 ± 0.05† | 1.13 ± 0.09 | 1.05 ± 0.10 | 1.03 ± 0.07 |
| Mean closed duration | 0.37 ± 0.05‡ | 0.94 ± 0.45 | 1.65 ± 0.68 | 1.21 ± 0.19 |
| Number of patches | 7 | 10 | 8 | 9 |
Single-channel recordings of Kir6.2/SUR1 channels expressed in transfected SH-SY5Y cells were performed in the cell-attached patch configuration at −60 mV in symmetrical 140 mM K+ solutions. DETA NONOate (NOC-18) alone (300 μM) or NOC-18 (300 μM) in the presence of 5-HD (100 μM), MPG (500 μM), or catalase (500 U/ml) was applied by bath perfusion. Single-channel properties were obtained and normalized as described in Table 1 (control taken as 1). Data are means ± SE.
P < 0.05;
P < 0.01;
P < 0.0001 (two-tailed, one-sample t-tests).
Exogenous H2O2 Increased the Single-Channel Activity of Kir6.2/SUR1 Channels in Cell-Attached Patches but Suppressed These Channels in Excised Membrane Patches
ROS play an important role in cell signaling. Almost all of the classic oxidases generate superoxide anions, but most aspects of (oxidant) signaling have been linked to the more stable derivative, H2O2 (20). Our findings described above imply a permissive role of ROS, especially H2O2, in mediating Kir6.2/SUR1 channel stimulation induced by PKG activation (see Figs. 1, D–F, and 2, C–E). To determine directly how H2O2 modulates the activity of neuronal-type KATP channels, we monitored single-channel currents of Kir6.2/SUR1 channels expressed in transfected HEK293 cells in the cell-attached patch configuration before and during bath application of exogenous H2O2. H2O2 (1 mM) elevated the single-channel activity of Kir6.2/SUR1 channels (Fig. 5A); the apparent opening frequency was increased, whereas the single-channel conductance did not change. The significant increase in the relative channel activity by H2O2 (Fig. 5E, Kir6.2/SUR1 for HEK293; P < 0.001) indicates that H2O2 was able to stimulate neuronal-type KATP channels in intact HEK293 cells. Furthermore, H2O2 also stimulated Kir6.2/SUR1 channels in intact neuroblastoma SH-SY5Y cells (Fig. 5E, Kir6.2/SUR1 for SH-SY5Y; P < 0.05). Evidently, H2O2 could act as a positive modulator of KATP channels in a neuronal background.
Fig. 5.
Exogenous H2O2 activates Kir6.2/SUR1 channels in a SUR subunit-dependent manner in intact cells but suppresses both Kir6.2/SUR1 and Kir6.2FL4A channel activities in excised inside-out membrane patches. Currents were obtained from transiently transfected HEK293 cells expressing Kir6.2/SUR1 (A, B, E, F) or tetrameric Kir6.2FL4A channels (C, D, E, F). In addition, data obtained from neuroblastoma SH-SY5Y cells are included in E and F. A and B: single-channel current traces of Kir6.2/SUR1 channels in a cell-attached patch (A) or inside-out patch (B) before (top traces) and during (bottom traces) bath perfusion of H2O2 (1 mM). C and D: single-channel current traces of Kir6.2FL4A channel recorded from a representative cell-attached patch (C) or inside-out patch (D) before (top traces) and during (bottom traces) bath perfusion of exogenous H2O2 (1 mM). For inside-out recordings, MgATP at a concentration of 30 μM was included in the bath and drug solutions to prevent current rundown. Scale bars are the same as in Fig. 1. E and F: averaged relative channel activity (i.e., normalized NPo) of Kir6.2/SUR1 and Kir6.2FL4A channels recorded in cell-attached (E) or inside-out (F) patch configurations in individual experimental groups obtained from transfected HEK293 or SH-SY5Y cells. Drug effect on the relative channel activity was compared with the corresponding control obtained from the same patch and normalized as described in Fig. 1F (control as 1, dashed line). Data are means ± SE of 7–12 patches. *P < 0.05; ***P < 0.001 (two-tailed, one-sample t-tests within individual groups).
The stimulatory effect of H2O2 described above may result from direct or indirect modification of Kir6.2/SUR1 channel proteins. To distinguish between these two possibilities, we administered exogenous H2O2 by bath perfusion to inside-out membrane patches excised respectively from transfected HEK293 and SH-SY5Y cells that expressed Kir6.2/SUR1 channels. MgATP (30 μM) was included in bath and drug solutions during inside-out recordings to prevent current rundown in the cell-free condition (14, 44). The activity of KATP channels in inside-out patches was in general higher than in cell-attached patches (Fig. 5, B vs. A), due to partial relief of intracellular ATP inhibition after patch excision. Interestingly, H2O2 decreased rather than increased the Kir6.2/SUR1 channel activity in inside-out patches obtained from both HEK293 cells (Fig. 5, B and F, Kir6.2/SUR1 for HEK293; P < 0.001) and neuroblastoma SH-SY5Y cells (Fig. 5F, Kir6.2/SUR1 for SH-SY5Y; P < 0.001). These results indicate that in addition to the stimulatory effect observed in intact cells (see Fig. 5, A and E), H2O2 suppressed Kir6.2/SUR1 channel function, which became evident in the cell-free condition (Fig. 5, B and F), possibly by direct modification of the channel or some closely associated regulatory protein(s). Because H2O2 enhanced the activity of Kir6.2/SUR1 channels only in intact cells, the stimulatory action of H2O2 appears to be indirect. Moreover, the indirect, stimulatory effect of H2O2 appeared to predominate over its inhibitory effect, and therefore the summated effect for neuronal-type KATP channel modulation was stimulatory in intact cells (Fig. 5, A and E).
Tetrameric Kir6.2FL4A Channels Were Unresponsive to H2O2 in Intact Cells, Whereas the Channel Activity Was Attenuated by H2O2 in Cell-Free Membrane Patches
To determine which subunit of the Kir6.2/SUR1 channel is responsible for H2O2-mediated functional modulation, we examined the effects of H2O2 on KATP channels that were composed of the pore-forming Kir6.2 subunit alone. Functional expression of tetrameric Kir6.2 channels on the cell surface was made possible by removing the ER retention signal located at the COOH terminus of Kir6.2 using alanine substitutions (i.e., LRKR368/369/370/371AAAA mutation) (77). We monitored the single-channel currents of Kir6.2LRKR368/369/370/371AAAA (i.e., Kir6.2FL4A) channels expressed in transfected HEK293 cells in both cell-attached and inside-out patches. A higher activity of Kir6.2FL4A channels in inside-out than in cell-attached patches (Fig. 5, D vs. C) was likely due to partial relief of ATP-mediated inhibition upon patch excision. Interestingly, different from octameric Kir6.2/SUR1 channels (see Fig. 5A), tetrameric Kir6.2FL4A channels in cell-attached patches were not stimulated by H2O2 (1 mM) (Fig. 5, C and E, Kir6.2FL4A for HEK293), indicating that the Kir6.2 subunit was not, or not primarily, responsible for the positive response of Kir6.2/SUR1 channels to H2O2 in intact cells. On the other hand, H2O2 (1 mM) reduced the single-channel activity of Kir6.2FL4A channels when applied directly to inside-out patches (Fig. 5, D and F, Kir6.2FL4A for HEK293; P < 0.001). This inhibitory effect of H2O2 was similar to that exerted on octameric Kir6.2/SUR1 channels in the same patch configuration (see Fig. 5B). Our data thus indicate that the Kir6.2 subunit or some regulatory component closely associated with this subunit might be directly modified by H2O2, leading to functional suppression of the channel in cell-free patches. By contrast, the SUR1 subunit of the channel was indispensable for the indirect, stimulatory effect of H2O2 on Kir6.2/SUR1 channels.
Effects of H2O2 on the Single-Channel Properties of Kir6.2/SUR1 and Kir6.2FL4A Channels in Intact Cells
The effects of exogenous H2O2 on the single-channel properties of octameric Kir6.2/SUR1 channels were analyzed (see materials and methods for details). The normalized values of NPo, opening frequency, corrected mean open duration, and mean closed duration (control as 1) obtained in intact HEK293 cells during H2O2 treatment were significantly altered (Table 3). We found that H2O2 increased the corrected mean open duration and reduced the mean closed duration by altering the relative distribution of open and closed states of Kir6.2/SUR1 channels (Fig. 4C, H2O2 vs. control, a representative cell-attached patch from SH-SY5Y cells). The increases in the opening frequency and the mean open duration, and consequently the NPo, plus the reduction in the mean closed time, formed the basis of KATP channel activation induced by H2O2 in intact cells (Table 3), which was reminiscent of the changes induced by zaprinast (Fig. 4A; Table 1). However, contrary to the stimulatory action elicited on octameric Kir6.2/SUR1 channels in cell-attached patches, exogenous H2O2 exerted no impact on the open and closed properties of Kir6.2FL4A channels in the same patch configuration (Table 3). These finding support the notion that the stimulatory effect of H2O2 on Kir6.2/SUR1 channels resulted from an indirect interaction of H2O2 with the SUR1 but not the Kir6.2 subunit (see Fig. 5, A, C, and E).
Table 3.
Effects of exogenous H2O2 on normalized single-channel open and closed properties of recombinant Kir6.2/SUR1 and Kir6.2FL4A channels in intact HEK293 cells
| Kir6.2/SUR1 | Kir6.2FL4A |
|||
|---|---|---|---|---|
| Properties | H2O2 | +5-HD | +SKF-7171A | H2O2 |
| Open probability | 4.30 ± 0.69† | 3.47 ± 0.74* | 0.91 ± 0.34 | 1.22 ± 0.45 |
| Opening frequency | 3.71 ± 0.53† | 2.89 ± 0.58* | 0.82 ± 0.36 | 1.09 ± 0.38 |
| Mean open duration | 1.13 ± 0.05* | 1.25 ± 0.12 | 0.90 ± 0.16 | 1.09 ± 0.04 |
| Mean closed duration | 0.33 ± 0.04‡ | 0.35 ± 0.06† | 1.71 ± 0.34 | 2.79 ± 1.84 |
| Number of patches | 11 | 5 | 5 | 6 |
Single-channel recordings of octameric Kir6.2/SUR1 and tetrameric Kir6.2FL4A channels expressed in transfected HEK293 cells were performed at −60 mV in symmetrical 140 mM K+ solutions in the cell-attached patch configuration. Application of H2O2 (1 mM) alone or coapplication of H2O2 together with the mitoKATP blocker 5-HD (100 μM) or the irreversible calmodulin antagonist SKF-7171A (10 μM) was achieved using a pressure-driven perfusion system. Single-channel properties were obtained as described in materials and methods. All values were normalized as described in Table 1 (control taken as 1) and averaged. Data are means ± SE.
P < 0.05;
P < 0.001;
P < 0.0001 (two-tailed, one-sample t-tests).
The Stimulatory Effect of H2O2 Was Unaffected by the MitoKATP Channel Inhibitor 5-HD
An important question to address next was whether ROS are generated before or after activation of the 5-HD-sensitive factor(s) to mediate PKG-induced stimulation of Kir6.2/SUR1 channels. It has been suggested that mitoKATP channel opening leads to a moderate production of ROS in cardiomyocytes (3, 69) and vascular smooth muscle cells (40). If H2O2 is generated along the PKG-induced signaling pathway after, but not before, the activation of mitoKATP channels (or 5-HD-sensitive factors), the effect of “exogenous” H2O2 on plasma membrane KATP channels should not be affected by functional disruption of mitoKATP channels. Indeed, we found that in the presence of the mitoKATP channel inhibitor 5-HD (100 μM), H2O2 (1 mM) still effectively enhanced the activity of Kir6.2/SUR1 channels in cell-attached patches obtained from both HEK293 cells (Fig. 6, B and F, H2O2; Table 3) and neuroblastoma SH-SY5Y cells (data not shown). The increases in the relative channel activity during coapplication of H2O2 and 5-HD were not different from those obtained from patches treated with H2O2 alone (Fig. 6F, H2O2+5-HD vs. H2O2). Thus a possibility that exogenous H2O2 stimulates Kir6.2/SUR1 channels by activation of the 5-HD-sensitive factor(s) (or the mitoKATP channel) was not supported. As expected, exogenous H2O2 elicited similar changes in the open and closed single-channel properties of Kir6.2/SUR1 channels irrespective of the presence or absence of 5-HD (Table 3), which could account for the observations described above (Fig. 6, B and F, H2O2+5-HD). These results indicate that H2O2 stimulated Kir6.2/SUR1 channels in intact cells by altering their open and closed properties, and these effects were not mediated through activation of the 5-HD-sensitive factor(s). We thus propose that activation of PKG induces activation of the 5-HD-sensitive factor(s) before, or in parallel with, the generation of H2O2 and that H2O2 subsequently stimulates Kir6.2/SUR1 channels. Because the PKG-induced stimulation of Kir6.2/SUR1 channels was completely abolished by either the mitoKATP channel inhibitor 5-HD or ROS scavengers (see Figs. 1 and 2; Table 1; Supplemental Fig. 2), it is conceivable that activation of the 5-HD-sensitive factor(s), possibly the mitoKATP channel, and ROS generation are sequential rather than parallel events induced by PKG activation.
Fig. 6.
Stimulation of Kir6.2/SUR1 channels by PKG activators and exogenous H2O2 in intact cells is sensitive to suppression of intracellular Ca2+/calmodulin activities, whereas the stimulatory effect of exogenous H2O2 on the channel is not affected by the mitoKATP channel inhibitor 5-HD. Currents were obtained in the cell-attached patch configuration from transiently transfected HEK293 cells expressing Kir6.2/SUR1 channels. A–D: single-channel current traces of Kir6.2/SUR1 channels in a cell-attached patch before (top traces) and during (bottom traces) bath perfusion of zaprinast (50 μM; A and C) or H2O2 (1 mM; B and D) in the presence of the membrane-permeable Ca2+ chelator BAPTA-AM (50 μM; A), the mitoKATP channel inhibitor 5-HD (100 μM; B), or the membrane-permeable calmodulin antagonist SKF-7171A (10 μM; C and D). Scale bars are the same as in Fig. 1. E and F: averaged relative single-channel activity (i.e., normalized NPo) of Kir6.2/SUR1 channels obtained during drug application in individual groups. Drug effect on the relative channel activity was compared with the corresponding control obtained from the same patch and normalized as described in Fig. 1F (control as 1, dashed line). Data are means ± SE of 6–12 patches. *P < 0.05; **P < 0.01; ***P < 0.001 (two-tailed, one-sample t-tests within individual groups or unpaired t-tests between groups).
Stimulation of Kir6.2/SUR1 Channels by PKG Activation or Exogenous H2O2 in Intact Cells Was Abolished by Ca2+ Chelators and Calmodulin Antagonists
Because ROS did not stimulate Kir6.2/SUR1 channels directly in intact cells (see Fig. 5, A, B, E, and F), some additional signaling components are required to mediate the action of PKG/ROS on the channel. H2O2 may increase the Ca2+ permeability of the thapsigargin-sensitive intracellular stores and of the plasma membrane in pancreatic β-cells (53). To delineate the role of intracellular Ca2+ in PKG signaling, we examined the effects of BAPTA-AM (an intracellular Ca2+ chelator) and SKF-7171A (a cell-permeable calmodulin antagonist) on the stimulatory action of PKG activators, respectively. We found that in the presence of BAPTA-AM (50 μM; Fig. 6, A and E, zaprinast+BAPTA-AM) or SKF-7171A (10 μM; Fig. 6, C and E, zaprinast+SKF-7171A), zaprinast (50 μM) was unable to increase the relative activity of Kir6.2/SUR1 channels in intact HEK293 cells. PKG-induced changes in the single-channel open and closed properties of these channels were also prevented by BAPTA-AM and SKF-7171A (Table 4). These results indicate that Ca2+ and calmodulin were required to mediate PKG stimulation of Kir6.2/SUR1 channels. Moreover, SKF-7171A completely abolished the enhancement of Kir6.2/SUR1 channel activity caused by exogenous H2O2 (1 mM; Fig. 6, D and F, H2O2+SKF-7171A); it also ablated H2O2-induced changes in the open and closed properties of Kir6.2/SUR1 channels caused (Table 3) in intact cells. These results thus indicate that PKG and H2O2 stimulated Kir6.2/SUR1 channels by altering the gating properties of the channel via a Ca2+/calmodulin- but not the 5-HD-sensitive factor-dependent mechanism.
Table 4.
Roles of Ca2+ and calmodulin in mediating the stimulatory effects of PKG activation on normalized single-channel open and closed properties of recombinant Kir6.2/SUR1 channels in intact HEK293 cells
| Properties | Zaprinast | +BAPTA-AM | +SKF-7171A |
|---|---|---|---|
| Open probability | 4.46 ± 0.42† | 1.51 ± 0.33 | 1.08 ± 0.21 |
| Opening frequency | 3.92 ± 0.33‡ | 1.28 ± 0.26 | 0.97 ± 0.16 |
| Mean open duration | 1.25 ± 0.08* | 1.24 ± 0.21 | 1.05 ± 0.10 |
| Mean closed duration | 0.26 ± 0.02§ | 1.00 ± 0.27 | 1.65 ± 0.68 |
| Number of patches | 5 | 6 | 8 |
Single-channel recordings of Kir6.2/SUR1 and Kir6.2FL4A channels in HEK293 cells and Kir6.2/SUR1 channels in SH-SY5Y cells in cell-attached patches were performed at −60 mV in symmetrical 140 mM K+ solutions. Bath application of zaprinast (50 μM) alone or coappliation of zaprinast together with the Ca2+ chelator BAPTA-AM (50 μM) or the irreversible calmodulin antagonist SKF-7171A (10 μM) was administered using a pressure-driven perfusion system. Single-channel properties were obtained as described in materials and methods. All values were normalized as described in Table 1 (control taken as 1) and averaged. Data are means ± SE.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001 (two-tailed, one-sample t-tests).
DISCUSSION
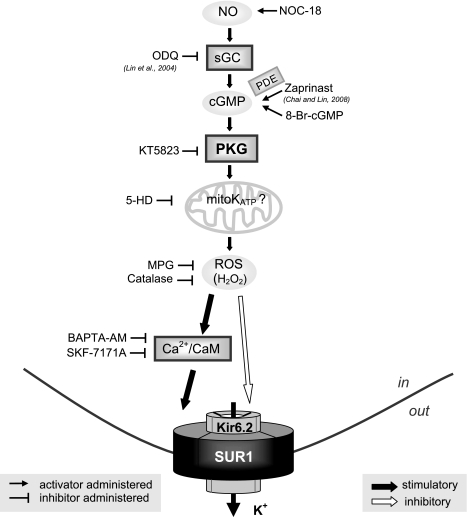
Neuronal KATP channels are involved in maintaining glucose homeostasis by glucose-sensing cells of the hypothalamus (50), regulating neuronal excitability (7, 59), and modulating neurotransmitter release (8). KATP channels also participate in neuroprotection under metabolic stress (60, 73, 76). In the present study, we demonstrate how PKG stimulates neuronal-type KATP channels via intracellular signaling. We provide novel evidence that the functional modulation of neuronal-type KATP channels by PKG and NO depends on activation of the 5-HD-sensitive factor(s), ROS generation, and subsequently, a Ca2+/calmodulin-dependent process (Fig. 7, a working model). Importantly, our findings imply that the plasma membrane KATP channel and the 5-HD-sensitive factor(s), possibly the mitoKATP channel, are functionally coupled during activation of PKG.
Fig. 7.
A cartoon representation of the proposed PKG-induced signaling pathway that modulates neuronal KATP channel function. Reagents used in our studies to manipulate individual signaling components are depicted, with inhibitors positioned at left and activators at right. Our findings suggest that PKG enhances the function of neuronal KATP channels in intact cells by activating the 5-HD-sensitive factor(s) and subsequent generation of ROS in a SUR1 subunit-dependent manner; moreover, the stimulatory effect of PKG can be reproduced by NO induction or exogenous H2O2. Furthermore, Ca2+/calmodulin activities are required for PKG and H2O2 to stimulate neuronal-type KATP channels. In addition to the stimulatory effect, H2O2 also exerts an (milder) inhibitory action on the KATP channel by direct modification of the Kir6.2 subunit or some closely associated protein(s). Like PKG (14), the predominating effect of ROS/H2O2 on neuronal KATP channels in intact cells is stimulatory. This novel NO/cGMP/PKG/5-HD-sensitive factor/ROS/Ca2+/calmodulin signaling pathway may contribute to the regulation of neuronal excitability, neurotransmitter release, and neuroprotection under ischemic or metabolic stress, by functionally modulating neuronal KATP channels.
Activation of PKG Induces Stimulation of Neuronal-Type KATP Channels
The cGMP/PKG signaling mechanism regulates smooth muscle relaxation (e.g., the vasculature, gastrointestinal tract, bladder, and penile), learning and memory, cell division, platelet aggregation, renin release, intestinal secretion, and cardioprotection (57, 65, 72). Zaprinast, a membrane-permeable, cGMP-specific PDE inhibitor, enhanced the single-channel activity of Kir6.2/SUR1 KATP channels in both transfected HEK293 (Fig. 1, A and F; Table 1) and human neuroblastoma SH-SY5Y cells (Figs. 2, A and E, and 4A; Table 1); the effect of zaprinast was concentration dependent (Supplemental Fig. 1). Similar stimulation of neuronal-type KATP channels is also induced by 8-bromo-cGMP, a membrane-permeable analog of cGMP (14). The stimulatory effects of zaprinast on Kir6.2/SUR1 channels resulted from specific PKG activation, because the effects were significantly abolished by KT5823, a selective PKG inhibitor (Fig. 1, B and F), but not by PKA inhibitors (Fig. 1F). These results lend strong support for a positive role of PKG in modulating the function of neuronal KATP channels in intact cells.
Activation of the 5-HD-Sensitive Factor is Required for PKG-Induced Stimulation of Neuronal-Type KATP Channels
Opening of mitoKATP channels has been suggested as a downstream event of PKG activation (75). Pharmacological tools such as diazoxide (an activator) and 5-HD (an inhibitor) have been extensively used to study the function of the mitoKATP channel, because the molecular identity of mitoKATP channels is not yet resolved. In isolated heart mitochondria, the mitoKATP channel inhibitor 5-HD may be converted to 5-HD-CoA by mitochondrial fatty acid CoA synthetase (28). However, 5-HD at 100 or 300 μM has little effect on the oxidation of any substrate (43). In this study we used 5-HD at 100 μM and found that zaprinast-induced stimulation of Kir6.2/SUR1 KATP channels was completely prevented by 5-HD in both transfected HEK293 (Fig. 1, A, C, and F; Table 1; Supplemental Fig. 2, A and B) and neuroblastoma SH-SY5Y cells (Fig. 2, A, B, and E; Table 1). Intriguingly, our findings implicate a positive functional coupling between plasma membrane KATP channels and the 5-HD-sensitive factor(s), which seems to take place, at least when PKG is activated (see Figs. 1, 2, and 3; Supplemental Fig. 2). Indeed, although 5-HD may directly block sarcolemmal (i.e., plasma membrane) KATP channels in cardiomyocytes under ischemic conditions (55), we found no evidence for an inhibitory action of 5-HD (100 μM) on the basal activity of neuronal-type KATP channels in intact cells (data not shown). Our data thus suggest that activation of the 5-HD-sensitive factor(s) (possibly mitoKATP channels) is required for PKG to stimulate neuronal KATP channels present on the plasma membrane. Nevertheless, we are mindful that to date, the putative mitoKATP channel still lacks a physical entity (especially as a “KATP channel”). Hence, in this report, the “5-HD-sensitive factor” was used to describe this intermediate signaling element positioned between PKG and ROS that mediates PKG stimulation of Kir6.2/SUR1 channels.
ROS Mediate PKG-Induced Stimulation of Neuronal KATP Channels in Intact Cells
ROS are generated by all aerobic cells, and most endogenously produced ROS are derived from mitochondrial respiration, with 1–2% of consumed oxygen converted to superoxide radical and subsequently to H2O2 (12, 16). ROS modulate synaptic transmission and plasticity (5) and contribute to the cardioprotection afforded by ischemic preconditioning (10, 71). H2O2 is an attractive candidate for cell signaling because, compared with other ROS, it is relatively stable and long-lived, and its neutral ionic state allows it to exit the mitochondria easily. In this study, the stimulation of Kir6.2/SUR1 channels by PKG activation was abolished by ROS scavenging in both transfected HEK293 cells (Fig. 1, A, D, and F; Table 1) and SH-SY5Y cells (Fig. 2, A, C, and E; Table 1; Supplemental Fig. 2, A and C). Moreover, the stimulatory effects of PKG activation were sensitive to and ablated by catalase, an enzyme that decomposes H2O2 (Figs. 1, A, E, and F, and 2, A, D, and E; Table 1; Supplemental Fig. 2, A and D). Our findings strongly suggest that the PKG-induced stimulation of neuronal KATP channels is mediated by ROS/H2O2 signaling.
NO Activates Neuronal KATP Channels in a 5-HD-Sensitive Factor- and ROS-Dependent Manner
NO produces a variety of actions in the nervous system by altering neuronal excitability and synaptic transmission through activation of sGC, which leads to cGMP accumulation, or through direct S-nitrosylation (2). PKGs are key enzymes of NO-cGMP and natriuretic peptide signaling cascades. Upregulating the NO/cGMP pathway by PDE-5 inhibition during in vivo chronic hypoxia may reduce neuron apoptosis, through an interaction involving several kinases (13). NO also is involved in preconditioning-mediated neuroprotection; its antiapoptotic effects are mediated, in part, by cGMP and PKG (for a review, see Ref. 21). The NO/cGMP/PKG pathway may mediate phosphorylation and activation of sarcolemmal (i.e., plasma membrane) KATP channels in rabbit ventricular myocytes (27), although it was later reported by the same group that the NO/cGMP/PKG signaling pathway contributes to ischemic preconditioning in rat heart by activating mitochondrial rather than sarcolemmal KATP channels (18). The novel evidence provided in the present study indicates that induction of NO stimulated neuronal-type KATP channels expressed in intact HEK293 and SH-SY5Y cells in a PKG-, 5-HD-sensitive factor-, and ROS-dependent manner (Figs. 3 and 4B; Table 2). These findings are reminiscent of those observed during activation of PKG (Figs. 1 and 2; Table 1), suggesting that stimulation of neuronal KATP channels by NO/cGMP/PKG signaling is mediated by activation of the 5-HD-sensitive factor(s), possibly the mitoKATP channel, and subsequent generation of ROS (in particular, H2O2). MitoKATP channels and ROS are implicated in the cardioprotective effect of ischemic preconditioning (58, 71) and the anti-infarct effect of NO in intact, isolated heart (75). It is possible that in the brain NO and PKG may exert neuroprotection by activating neuronal KATP channels via a 5-HD-sensitive factor/ROS signaling mechanism.
H2O2 Stimulates Neuronal KATP Channels Indirectly, and the SUR Subunit is Responsible for the Positive Response of the Channel to PKG/ROS Signaling
H2O2 has been suggested to regulate KATP channel activity in several cell types. For example, H2O2 causes sulfonylurea-sensitive hyperpolarization and suppression of insulin release in pancreatic β-cells (41) and mediates glutamate-dependent inhibition of dopamine release from striatum by activating KATP channels (6, 8). Importantly, in the current study we have provided direct evidence that H2O2 augments the single-channel activity of neuronal-type KATP channels expressed in intact HEK293 and neuroblastoma SH-SY5Y cells (Figs. 4C and 5, A and E; Table 3), whereas it had no effect on the activity of tetrameric Kir6.2FL4A channels (Fig. 5, C and E; Table 3) in the cell-attached patch configuration, suggesting that H2O2 elicits Kir6.2/SUR1 channel stimulation in a SUR1 subunit-dependent manner. H2O2 produced by glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation has been suggested to suppress central nervous system (CNS) neurotransmitter release by activating neuronal KATP channels (8). H2O2 may modulate neuronal KATP channels to control normal neural function.
Does H2O2 stimulate neuronal-type KATP channels directly or indirectly? Several studies have suggested a direct, concentration-dependent effect of H2O2 on KATP channel opening in inside-out membrane patches excised from cardiac myocytes (33). However, strong oxidants or sulfhydryl oxidizing agents can cause KATP channel closure in skeletal muscle cells (74), pancreatic β-cells (36), and cardiac cells (15). In the present study, the recombinant Kir6.2/SUR1 channel activity was suppressed rather than stimulated by H2O2 in inside-out patches (Fig. 5, B and F), ruling out the possibility that the stimulatory effect of ROS/H2O2 on neuronal KATP channels involves direct oxidation of redox-sensitive sites on the channel. We also found that H2O2 suppressed Kir6.2FL4A channels in inside-out patches (Fig. 5, D and F), which suggests that ROS/H2O2 can directly modify the pore-forming subunit Kir6.2 or some closely associated protein(s) to reduce channel activity. Nevertheless, the inhibitory effect of H2O2 on the Kir6.2/SUR1 channel is conceivably smaller than, and therefore masked by, its stimulatory effect in intact cells. Collectively, our findings suggest that H2O2 mediates neuronal KATP channel modulation triggered by PKG or primary signals such as NO in a KATP channel subunit-dependent manner: the SUR1 subunit is required for indirect stimulation of the channel by ROS, whereas the Kir6.2 subunit or some closely associated regulatory protein(s) may be directly modified by H2O2 to render KATP channel suppression.
Through what mechanism do H2O2 and related ROS achieve neuronal KATP channel stimulation indirectly? H2O2 can inhibit the glycolytic pathway and oxidative phosphorylation, causing a reduction in intracellular ATP (by reducing ADP phosphorylation; Ref. 32). However, it is not likely that H2O2 stimulates neuronal KATP channels in intact cells by reducing intracellular ATP/ADP levels (see Refs. 23 and 52) or the sensitivity of the channel to adenosine nucleotides. The reasons are fourfold. First, the tetrameric channel made solely of Kir6.2 (i.e., Kir6.2FL4A), the subunit that confers the channel ATP sensitivity, was not stimulated by PKG activation in intact cells (Fig. 5, C and E; Table 3), implying that PKG does not stimulate KATP channels by reducing the ATP sensitivity of the channel. These findings also argue against an involvement of intracellular ATP reduction in eliciting stimulation of Kir6.2/SUR1 channel downstream of PKG activation. Second, Kir6.2/SUR2A channels, a KATP channel isoform that exhibits roughly 10-fold lower ATP sensitivity (similar to the tetrameric Kir6.2 channels) than Kir6.2/SUR1 channels, were significantly stimulated by PKG activation and H2O2 (unpublished data); these results support our findings that the SUR but not the Kir6.2 subunit is responsible for PKG's stimulatory effect and that a change in the intracellular ATP level or the ATP sensitivity of the channel could not account for the stimulatory effect of PKG. Third, PKG activation can increase Kir6.2/SUR1 channel activity in intact HEK293 cells subjected to metabolic inhibition with sodium azide (4 patches; data not shown), indicating that the PKG effect is not likely caused by changes in the intracellular ATP level. Fourth, the stimulatory action of PKG activators was uncompromised in intact cells expressing the mutant channel Kir6.2/fSUR1G1479R, whose MgADP sensitivity was lost due to G1479R mutation (67) in the second nucleotide-binding domain of the SUR1 subunit (see Supplemental Table 1), implying that PKG stimulation of neuronal-type KATP channels does not involve alterations in the intracellular MgADP level or the MgADP sensitivity of the channel. Together, our findings suggest that PKG stimulation of neuronal KATP channels does not result from changes in the metabolic state or products, but rather may be accounted for by modulation of channel gating through intracellular signaling.
Stimulation of Neuronal KATP Channels by H2O2 Likely Occurs Downstream of the 5-HD-Sensitive Factor
In the present study, inhibition of the 5-HD-sensitive factor(s) was sufficient to abolish the stimulatory effect of PKG activation (Figs. 1, C and F, and 2, B and E; Table 1; Supplemental Fig. 2B) or NO induction (Fig. 3, C and F; Table 2), but not that of exogenous H2O2 (Fig. 6, B and F; Table 3), on Kir6.2/SUR1 channels, which excludes the possibility that H2O2 serves as a signal upstream of the 5-HD-sensitive factor(s) for Kir6.2/SUR1 channel stimulation. Interestingly, it has been suggested that opening of mitoKATP channels in cardiac muscle triggers preconditioning through free radical production (58, 71) and that activation of mitoKATP channels in vascular smooth muscle also causes generation of free radicals (40). Our data thus suggest that PKG activation stimulates plasma membrane KATP channels in neurons by inducing production of ROS through activation of the 5-HD-sensitive factor(s), possibly the mitoKATP channel.
Role of Ca2+ and Calmodulin in Mediating PKG/ROS-Induced Activation of Neuronal KATP Channels
In the present study, we have shown that both BAPT-AM (a chelator of intracellular Ca2+) and SKF-7171A (a selective calmodulin antagonist) effectively ablated PKG stimulation of the Kir6.2/SUR1 channel (Fig. 6, A, C, and E; Table 4). The stimulatory effect of H2O2 in intact cells was also prevented by suppression of calmodulin activity (Fig. 6, D and F; Table 3). These results suggest that intracellular Ca2+ and the Ca2+-binding protein calmodulin mediate the stimulatory effect of PKG/ROS signaling on neuronal KATP channels. The sensitivity of KATP channels to Ca2+ signaling was supported by our recent findings that both caffeine (47) and Ca2+ ionophores (48) activated neuronal-type KATP channels in intact cells (at least partly) by an increase in intracellular Ca2+. On the other hand, although H2O2 may activate Ca2+/calmodulin-dependent kinase II (CaMKII) by increasing the Ca2+ permeability from intracellular stores in rat pancreatic β-cells (53), our data do not support a role of CaMKII in the PKG signaling process (data not shown). Further investigation is required to delineate the nature of calmodulin-dependent KATP channel modulation.
In conclusion, in this article we report for the first time that NO and PKG stimulate neuronal-type KATP channels in a SUR1 subunit-dependent manner via activation of an intracellular signaling mechanism consisting of the 5-HD-sensitive factor(s) (possibly the mitoKATP channel), ROS/H2O2, Ca2+, and calmodulin (Fig. 7). Moreover, our findings implicate functional coupling, at least under conditions when NO/PKG signaling is activated, between plasma membrane KATP channels and 5-HD-sensitive factor(s). In light of the abundant evidence in the literature for a crucial role of 5-HD-sensitive factor(s) in the development of ischemic tolerance in heart and brain, functional coupling between plasma membrane KATP channels and 5-HD-sensitive factor(s) may facilitate coordinated operation of endogenous cytoprotective mechanisms to more effectively protect cells from ischemic injury. The novel NO/cGMP/PKG/5-HD-sensitive factor/ROS/Ca2+/calmodulin signaling pathway unveiled in this study may contribute to the regulation of neuronal excitability, neurotransmitter release, and neuroprotection under ischemic or metabolic stress by functionally modulating neuronal KATP channels. This modulatory mechanism also may have broad implication on the cellular functions regulated by KATP channels in different tissues.
GRANTS
This study was supported by a grant from the American Heart Association National Center (to Y.-F. Lin) and was conducted in a facility constructed with support from the National Center for Research Resources Research Facilities Improvement Program Grant C06 RR-12088-01.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Susumu Seino (Kobe University, Chuo-ku, Japan), Joseph Bryan (Baylor College of Medicine, Houston, TX), and Blanche Schwappach (University of Manchester, Manchester, UK) for the kind gifts of cDNA clones for Kir6.2, SUR1, and Kir6.2FL4A subunits. Flag-tagged SUR1 and fSUR1G1479R were kind gifts from Dr. Show-Ling Shyng (Oregon Health and Science University, Portland, OR). The single-channel analysis program Intrv5 was generously provided by Drs. Barry Pallotta (University of North Carolina, Chapel Hill, NC) and Janet Fisher (University of South Carolina, Columbia, SC).
REFERENCES
- 1.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, 4th, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β-cell high-affinity sulphonylurea receptor: a regulator of insulin secretion. Science 268: 423–426, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25: 510–517, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291: H2067–H2074, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11: 97–118, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci 17: 8695–8701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci 23: 2744–2750, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25: 4222–4231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci USA 100: 11729–11734, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/Kir6.X, KATP channels. Annu Rev Physiol 60: 667–687, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29: 207–216, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci 26: 105–131, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caretti A, Bianciardi P, Ronchi R, Fantacci M, Guazzi M, Samaja M. Phosphodiesterase-5 inhibition abolishes neuron apoptosis induced by chronic hypoxia independently of hypoxia-inducible factor-1alpha signaling. Exp Biol Med (Maywood) 233: 1222–1230, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Chai Y, Lin YF. Dual regulation of the ATP-sensitive potassium channel by activation of cyclic GMP-dependent protein kinase. Pflügers Arch 456: 897–915, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Coetzee WA, Nakamura TY, Faivre JF. Effects of thiol-modifying agents on KATP channels in guinea pig ventricular cells. Am J Physiol Heart Circ Physiol 269: H1625–H1633, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann NY Acad Sci 738: 8–14, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Cuong DV, Kim N, Youm JB, Joo H, Warda M, Lee JW, Park WS, Kim T, Kang S, Kim H, Han J. Nitric oxide-cGMP-protein kinase G signaling pathway induces anoxic preconditioning through activation of ATP-sensitive K+ channels in rat hearts. Am J Physiol Heart Circ Physiol 290: H1808–H1817, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci USA 95: 13953–13958, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol 15: 247–254, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Fiscus RR. Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals 11: 175–190, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336: 385–388, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Goldhaber JI, JI S, Lamp ST, Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricles. Implications for ischemia reperfusion injury. J Clin Invest 83:1800–1809, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J 16: 1145–1152, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 26.Han J, Kim N, Kim E, Ho WK, Earm YE. Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276: 22140–22147, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K+ channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 283: H1545–H1554, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol 542: 735–741, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 113: 1671–1676, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (cGK). In: Handbook of Exp Pharmacol 191. cGMP: Generators, Effectors and Therapeutic Implications, edited by Schmidt HH, Hofmann F, Stasch JP. Berlin: Springer, 2009, p. 137–162 [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi T, Kis B, Rajapakse N, Shimizu K, Busija DW. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke 34: 1015–1020, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hyslop PA, Hinshaw DB, Halsey WA, Jr, Schraufstätter IU, Sauerheber RD, Spragg RG, Jackson JH, Cochrane CG. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem 263: 1665–1675, 1988 [PubMed] [Google Scholar]
- 33.Ichinari K, Kakei M, Matsuoka T, Nakashima H, Tanaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. J Mol Cell Cardiol 28: 1867–1877, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science 270: 1166–1169, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Inagaki N, Gonoi T, Clement JP, IV, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16: 1011–1017, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Islam MS, Berggren PO, Larsson O. Sulfhydryl oxidation induces rapid and reversible closure of the ATP-regulated K+ channel in the pancreatic β-cell. FEBS Lett 319: 128–132, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem 271: 24321–24324, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol 47: 11–39, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Jang Y, Wang H, Xi J, Mueller RA, Norfleet EA, Xu Z. NO mobilizes intracellular Zn2+ via cGMP/PKG signaling pathway and prevents mitochondrial oxidant damage in cardiomyocytes. Cardiovasc Res 75: 426–433, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol 97: 365–373, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic β-cells. J Physiol 514: 471–481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res 74: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol 545: 961–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin YF, Chai Y. Modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience 152: 371–380, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cell. EMBO J 19: 942–955, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YF, Raab-Graham K, Jan YN, Jan LY. Nitric oxide stimulation of ATP-sensitive potassium channels: involvement of Ras/MAPK kinase pathway and contribution to neuroprotection. Proc Natl Acad Sci USA 101: 7799–7804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao X, Chai Y, Lin YF. Dual regulation of ATP-sensitive potassium channels by caffeine. Am J Physiol Cell Physiol 292: C2239–C2258, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Mao X, Lin YF. Calcium-dependent functional modulation of the ATP-sensitive potassium channel. Soc Neurosci Abstr 33: 43.–8., 2007 [Google Scholar]
- 49.Matejíková J, Kucharská J, Pintérová M, Pancza D, Ravingerová T. Protection against ischaemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial KATP channels and reactive oxygen species. Physiol Res 58: 9–19, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38: 917–925, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Nakazaki M, Kakei M, Koriyama N, Tanaka H. Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic β-cells. Diabetes 44: 878–883, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Nakazaki M, Kakei M, Yaekura K, Koriyama N, Morimitsu S, Ichinari K, Yada T, Tei C. Diverse effects of hydrogen peroxide on cytosolic Ca2+ homeostasis in rat pancreatic β-cells. Cell Struct Funct 25: 187–193, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Notsu T, Tanaka I, Takano M, Noma A. Blockade of the ATP-sensitive K+ channel by 5-hydroxydecanoate in guinea pig ventricular myocytes. J Pharmacol Exp Ther 260: 702–708, 1992 [PubMed] [Google Scholar]
- 56.Okuyama Y, Yamada M, Kondo C, Satoh E, Isomoto S, Shindo T, Horio Y, Kitakaze M, Hori M, Kurachi Y. The effects of nucleotides and potassium channel openers on SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pflügers Arch 435: 595–603, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286: H468–H476, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 87: 460–466, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. J Physiol 494: 399–409, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roper J, Ashcroft FM. Metabolic inhibition and low central ATP activate K-ATP channels in rat dopaminergic substantia nigra neurons. Pflügers Arch 430: 44–54, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A. Non-genomic actions of 17β-oestradiol in mouse pancreatic β-cells are mediated by a cGMP-dependent protein kinase. J Physiol 521.2: 397–407, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakura H, ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Lett 377: 338–344, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Sakuta H, Okamoto K, Watanabe Y. Modification by cGMP of glibenclamide-sensitive K+ currents in Xenopus oocytes. Jpn J Pharmacol 61: 259–262, 1993 [DOI] [PubMed] [Google Scholar]
- 64.Sakuta H, Okamoto K, Tandai M. Atrial natriuretic factor potentiates glibenclamide-sensitive K+ currents via the activation of receptor guanylate cyclase in follicle-enclosed Xenopus oocytes. Eur J Pharmacol 267: 281–287, 1994 [DOI] [PubMed] [Google Scholar]
- 65.Schlossmann J, Hofmann F. cGMP-dependent protein kinases in drug discovery. Drug Discov Today 10: 627–634, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Shyng SL, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP: distinct functions of the two nucleotide binding folds of the sulphonylurea receptor. J Gen Physiol 110: 643–654, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shyng SL, Nichols CG. Octameric stoichiometry of KATP channel complex. J Gen Physiol 110: 655–664, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial KATP channels. Mol Cell Biochem 242: 181–187, 2003 [PubMed] [Google Scholar]
- 70.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K-channels in the absence of the sulphonylurea receptor. Nature 387: 179–183, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273: 18092–18098, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Robinson PJ. Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem 68: 443–456, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Watts A, Hicks G, Henderson G. Putative postnatal pre- and postsynaptic ATP-sensitive potassium channels in the rat substantia nigra in vitro. J Neurosci 15: 3065–3074, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weik R, Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: characterization of the ATP-binding site. J Membr Biol 110: 217–226, 1989 [DOI] [PubMed] [Google Scholar]
- 75.Xu Z, Ji X, Boysen PG. Exogenous nitric oxide generates ROS and induces cardioprotection: involvement of PKG, mitochondrial KATP channels, and ERK. Am J Physiol Heart Circ Physiol 286: H1433–H1440, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol 38: 945–949, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537–548, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.