Abstract
Extracellular purinergic agonists regulate a broad range of physiological functions via P1 and P2 receptors. Using the epididymis as a model system in which luminal acidification is essential for sperm maturation and storage, we show here that extracellular ATP and its hydrolysis product adenosine trigger the apical accumulation of vacuolar H+-ATPase (V-ATPase) in acidifying clear cells. We demonstrate that the epididymis can hydrolyze luminal ATP into other purinergic agonists such as ADP via the activity of nucleotidases located in the epididymal fluid and in the apical membrane of epithelial cells. Alkaline phosphatase activity and abundant ecto-5′-nucleotidase protein were detected in the apical pole of principal cells. In addition, we show that nine nucleotidase genes (Nt5e, Alpl, Alpp, Enpp1, 2, and 3, and Entpd 2, 4, and 5), seven ATP P2 receptor genes (P2X1, P2X2, P2X3, P2X4, P2X6, P2Y2, P2Y5), and three adenosine P1 receptor genes (A1, A2B, and A3) are expressed in epithelial cells isolated by laser cut microdissection (LCM). The calcium chelator BAPTA-AM abolished the apical V-ATPase accumulation induced by ATP, supporting the contribution of P2X or P2Y in this response. The PKA inhibitor myristoylated protein kinase inhibitor (mPKI) inhibited adenosine-dependent V-ATPase apical accumulation, indicating the participation of the P1 A2B receptor. Altogether, these results suggest that the activation of P1 and P2 purinergic receptors by ATP and adenosine might play a significant role in luminal acidification in the epididymis, a process that is crucial for the establishment of male fertility.
Keywords: calcium, cAMP, epididymis, H+-ATPase, luminal acidification
several specialized cells, including renal intercalated cells, epididymal clear cells, and osteoclasts, are involved in environmental pH regulation via proton transport. Renal intercalated cells help regulate systemic acid/base balance via active luminal acidification of the collecting duct (10, 12, 71). Osteoclasts are involved in bone resorption via the establishment of an acidic environment between themselves and bones (12, 28, 71). In the epididymis, luminal acidification helps keep spermatozoa in a dormant state during their maturation and storage (1, 17, 37, 42, 43, 46). Impairment of the acidification capacity of the epididymis by genetic approaches or exposure to environmental factors has negative effects on sperm maturation and has profound consequences on male fertility (4, 23, 49, 64, 69). We showed previously (6, 7, 9, 23, 52) that epididymal clear cells are analogous to other proton-secreting cells, such as renal intercalated cells, by the fact that they express high levels of the proton-pumping vacuolar H+-ATPase (V-ATPase) in their plasma membrane and intracellular recycling vesicles. Clear cells are responsible for net luminal acidification in the distal region of the epididymis (the cauda epididymidis) and vas deferens (VD) via V-ATPase-dependent proton secretion (7).
In the epididymis and other acidifying mammalian cells, V-ATPase is regulated by several mechanisms including recycling of V-ATPase-containing vesicles to and from the plasma membrane (5, 12, 64). The subcellular localization of the epididymal V-ATPase is markedly modulated by the luminal environment (2, 13, 32, 49, 50, 64, 65). By in vivo perfusion of the epididymis we have shown (2, 6, 47, 65) that alkalinization of the lumen, addition of bicarbonate or angiotensin II, or activation of the bicarbonate-regulated soluble adenylate cyclase (sAC) regulates V-ATPase recycling. At the physiological luminal pH of 6.6, the V-ATPase is distributed between small apical microvilli and subapical vesicles. At the alkaline pH of 7.8, it is mainly located in numerous extended microvilli, correlating with an increase in proton secretion by these cells (64, 65). The alkaline pH-induced V-ATPase apical accumulation is calcium dependent (2), but the link between the luminal pH stimulus and intracellular calcium has not yet been elucidated. ATP is a paracrine or autocrine mediator that induces a calcium increase in epididymal epithelial cells through activation of purinergic receptors (63). Some purinergic receptors are regulated by extracellular pH, providing a potential link between the effects of luminal pH and the role of calcium in V-ATPase recycling in clear cells. Interestingly, extracellular ATP stimulates bone resorption in osteoclasts (29, 36), a process that also requires V-ATPase-dependent proton secretion, and we explored here the potential participation of ATP in the regulation of V-ATPase in clear cells of the epididymis.
While the mechanisms responsible for ATP release remain largely unknown, two processes have been proposed. ATP could be transported through channels, including the voltage-dependent anion channel (VDAC)-like “maxi” anion channels, the volume-sensitive organic anion channel (VSOAC), CLC-3, CLC-5, and connexins (62). In addition, pannexin 1 has been described in several tissues as an ATP-conducting pore (53, 54). ATP can also be released from cells by exocytosis of ATP-containing vesicles (53, 62). This purinergic agonist is known to regulate several functions such as ion secretion in the epididymis (20, 21, 74), smooth muscle contraction in the VD (26, 45, 57), and regulation of water transport and homeostasis in the kidney (56). Cell surface ATP receptors are separated into two families: the P2X receptor family consists of ligand-gated ion channels, and the P2Y receptor family includes G protein-coupled receptors (GPCRs). Both receptor subtypes are expressed in the mouse epididymis (63). The role of P2X receptors in fertility was indicated by the findings that P2X1 receptor knockout mice are infertile (45).
The action of extracellular ATP is limited by its breakdown by several ectonucleotidases that keep levels of extracellular ATP very low while generating adenosine. This process occurs through a three-step dephosphorylation reaction with the sequential formation of ADP, AMP, and adenosine (76). Adenosine itself can exert a broad range of physiological responses by activating adenosine P1 receptors A1, A2A, A2B, or A3 (15, 34, 66). Ectonucleotidases are divided into four families according to their enzymatic function: ectonucleotide triphosphate diphosphohydrolases (E-NTPDases 1–8) hydrolyze extracellular nucleoside tri- and diphosphates such as ATP and ADP, ectonucleotide pyrophosphatases/phosphodiesterases (E-NPP1,2,3) hydrolyze extracellular ATP, ecto-5′-nucleotidase (5′-NT) hydrolyzes only nucleoside monophosphates such as AMP, and alkaline phosphatases (APs) catalyze the whole dephosphorylation process to generate adenosine from ATP. APs in turn comprise four isoforms (nonspecific, placental, intestinal, and germ cell) (70, 76).
In the present study, we investigated 1) the role of luminal ATP and its hydrolysis product adenosine in the regulation of V-ATPase recycling in the epididymis; 2) the participation of ectonucleotidases in this response; and 3) the characterization of the receptors and second messengers involved in the regulation of V-ATPase recycling in clear cells. We used a combination of techniques including luminal in vivo perfusion, confocal microscopy, laser cut microdissection (LCM), and molecular biology to unravel the complex purinergic pathway involved in V-ATPase regulation and in the maintenance of a low pH in the lumen of the epididymis.
MATERIALS AND METHODS
Chemicals
Adenosine 5′-triphosphate (ATP), adenosine, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (acetoxymethyl ester) (BAPTA-AM) and horseradish peroxidase (HRP) were purchased from Sigma-Aldrich (St. Louis, MO); adenosine-5′-(γ-thiotriphosphate), lithium salt (ATPγS) was purchased from Axxora (San Diego, CA); myristoylated protein kinase inhibitor (mPKI) was purchased from Enzo Life Sciences International (Plymouth Meeting, PA); the ELF 97 endogenous phosphatase detection kit was purchased from Invitrogen (Carlsbad, CA); PCR reagents were purchased from Applied Biosystems (Foster City, CA); the EnzyLight ATP bioluminescence assay kit was purchased from BioAssay Systems (Hayward, CA); and the ADP-Glo assay kit was purchased from Promega (Madison, WI).
Antibodies
For indirect immunofluorescence microscopy, epididymis sections were double-stained with different primary antibody combinations depending on the purpose of the study. All antibodies were diluted in Dako antibody diluent (Dako North America). For quantification of the extension of V-ATPase-labeled microvilli, we used our affinity-purified rabbit polyclonal antibody raised against a synthetic peptide corresponding to the COOH-terminal region of rat ATP6V1B1 (the V-ATPase “B1” subunit) (8) (diluted 1:1,000). A commercial goat antibody against HRP was used to examine endocytosis (diluted 1:2,500). For characterization of 5′-NT in the epididymis we used a monoclonal antibody against rat 5′-NT (gift from Brigitte Kaissling, Univ. of Zurich; diluted 1:200) (30) and our rabbit antibody against the B1 subunit of V-ATPase (8). Goat anti-rabbit IgG coupled to FITC, donkey anti-goat IgG coupled to Cy3, and donkey anti-mouse IgG coupled to Cy3 (Jackson Immunologicals, West Grove, PA) were used as secondary antibodies.
Tissue Fixation and Immunofluorescence
Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were acquired, retained, and used under protocols reviewed and approved by the Institutional Animal Care and Use Committees of Massachusetts General Hospital. Sexually mature male rats were anesthetized with Nembutal (65 mg/kg body wt ip) and perfused via the left ventricle with PBS followed by fixative containing 4% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate, and 5% sucrose in 0.1 M sodium phosphate buffer (PLP). Tissues were processed for immunofluorescence as described previously (6, 8, 47, 65). Briefly, the epididymis and VD were harvested and further fixed by immersion in PLP overnight, washed in PBS, and cryoprotected in PBS containing 30% sucrose for >4 h at room temperature. These tissues were embedded in optimum cutting temperature (OCT) compound (Tissue Tek, Sakura Finetek, Torrance, CA), mounted on a cutting block, and sliced with a Leica 3050 cryostat (Leica Microsystems, Bannockburn, IL). Five- to ten-micrometer sections were picked up onto Superfrost Plus slides (Fisher, Pittsburgh, PA) and stored at 4°C before immunostaining. Sections were rehydrated for 15 min in PBS and treated for 4 min either with 1% SDS to retrieve antigen (11) or with 1% SDS and 0.1% Triton X-100 to permeabilize endocytic vesicles filled with HRP (2, 47, 50, 65). Sections were then washed in PBS for 5 min and blocked in PBS containing 1% BSA for 15 min. The first primary antibody was applied in a moist chamber for 90 min at room temperature or overnight at 4°C. Sections were washed in high-salt PBS (2.7% NaCl) twice for 5 min and once in normal PBS. The secondary antibody was then applied for 1 h at room temperature, followed by washes as described above. Double staining was performed by repeating the steps described above with the second primary and secondary antibodies. Slides were mounted in Vectashield medium containing the DNA marker 4′,6-diamidino-2-phenylindole (DAPI) to stain the nucleus (Vector Laboratories, Burlingame, CA).
In Vivo Perfusion of Distal Cauda and Vas Deferens and Immunostaining
We showed previously (2, 7, 65) that activation of V-ATPase-dependent proton secretion in clear cells is correlated with apical membrane V-ATPase accumulation and a marked elongation of V-ATPase-labeled microvilli. In the present study we therefore assessed the role of luminal purinergic agonists on V-ATPase apical accumulation in clear cells by measuring the extension of V-ATPase-labeled microvilli as a readout assay after in vivo luminal perfusion of the epididymis.
Sprague-Dawley rats (Charles River Laboratories) were acquired, retained, and used in compliance with National Institutes of Health recommendations. Sexually mature male rats were anesthetized with 2.5% isoflurane. A perigenital incision was performed to expose the VD and epididymidis, and a small incision was made in a portion of tubule from the proximal cauda epididymidis to insert a catheter (made by warming and elongating a polyethylene tubing above a flame; ID 0.86 mm, OD 1.27 mm; Becton Dickinson, Franklin Lakes, NJ). Epididymis and VD were perfused through the lumen in an anterograde manner as previously described (65). A perfusion rate of 35 μl/min was regulated with a syringe pump (model 100, KD Scientific, Holliston, MA), and the perfusate flowing out of the tubule was collected with a microcannula inserted in the VD. Before the perfusion with purinergic agonists, the lumen of the epididymis was washed free of sperm with a phosphate-buffered saline adjusted to the resting pH of 6.6 (control condition). Epididymal tubules were then perfused for 15 min with purinergic agonists (ATP, ATPγS, or adenosine) in the presence or absence of inhibitors (BAPTA-AM, mPKI). HRP (0.25 mg/ml; Sigma) was added to the perfusate as a marker of endocytosis to localize the apical pole of the cells. The lumen was then flushed for 3 min with ice-cold perfusing solution in the continued presence of agonists or inhibitors, as applicable, to remove nonendocytosed HRP. After 10 min of final perfusion with PLP fixative, VD and cauda epididymidis were harvested and further fixed by immersion in PLP overnight at 4°C. Tissues were washed in PBS, cryoprotected, embedded. Cryosections were cut and double-stained for the B1 subunit of V-ATPase and HRP, as described above.
Image Acquisition
Some images were acquired with a Nikon Eclipse 800 epifluorescence microscope (Nikon Instruments, Melville, NY) and a Hamamatsu Orca 100 charge-coupled device (CCD) camera (Hamamatsu, Bridgewater, NJ), analyzed with IPLab scientific image processing software (Scanalytics, Fairfax, VA), and imported into Adobe Photoshop image editing software as TIFF files (Adobe Systems, San Jose, CA).
Single-cell pictures were acquired with a Zeiss Radiance 2000 confocal microscope (Zeiss Laboratories) using LaserSharp 2000 version 4.1 software. TIFF pictures were imported into IPLab to quantify the extension of V-ATPase-labeled microvilli, as described below. z Series (0.1-μm interval) were taken for some cells and imported into Volocity software (Improvision, version 4.1) for three-dimensional (3D) reconstruction. Final animations were exported as Quicktime movies.
Quantification of V-ATPase Apical Membrane Recruitment
The level of accumulation of V-ATPase in microvilli was quantified by immunofluorescence after in vivo luminal perfusion of the epididymis in the presence of purinergic agonists with and without inhibitors. The extension of V-ATPase-labeled microvilli in clear cells was quantified as we have previously described (2, 47, 49, 50, 65). Ten-micrometer cryostat sections were double-stained for V-ATPase and HRP. Single-cell confocal images were imported into IPLab software as TIFF files, and the segmentation procedure was used to measure the area occupied by V-ATPase-positive microvilli on each cell. This value was normalized for the width of the cell measured along the apical pole. At least three epididymides from different animals were perfused for each condition. The epididymis from the opposite side of the same animal was used as control. Quantification analysis was performed separately in proximal and distal cauda. Between 10 and 22 cells were examined for each perfused epididymal region. Two- and one-way ANOVA tests were performed, followed by a post hoc Student t-test or a Bonferroni test. Differences were considered significant at P < 0.05.
Fluorescence-Based Method for Detecting Endogenous Phosphatase Activity on Epididymal Sections
Endogenous AP activity was detected with the ELF 97 endogenous phosphatase detection kit (Molecular Probes) on fixed rat epididymal sections according to the manufacturer's recommendations. Briefly, 5-μm rat epididymal sections were rehydrated for 10 min in PBS adjusted to pH 7.4. They were then permeabilized in 0.2% Tween 20 in PBS for 10 min at room temperature and rinsed three times for 5 min in PBS. ELF 97 phosphatase substrate was diluted 20-fold in detection buffer, filtered through a 0.2-μm pore size, and applied to sections. Phosphatase reaction on ELF 97 substrate resulted in the fast production of an intensely fluorescent precipitate at the site of enzymatic phosphatase activity. The reaction was monitored through a long-pass barrier emission filter (B-2A, Nikon) on an epifluorescence microscope and stopped by submerging the sample in PBS containing 25 mM EDTA and 5 mM levamisol, pH 8.0.
Measurement of ATP Hydrolysis in Lumen of Epididymis Perfused in Vivo
The ectonucleotidase activity of the cauda region of the epididymis was assessed in vivo by determining the level of hydrolysis of ATP in the luminal perfusate. Rat epididymides were perfused in vivo as described above. After sperm was removed from the lumen, 100 μM ATP or the nonhydrolyzable ATP analog ATPγS (also at 100 μM) was perfused under control conditions for 15 min. The solution was collected, and the ATP or ATPγS concentration was assessed with the EnzyLight ATP bioluminescence assay (BioAssay Systems). As a negative control, 100 μM ATP and 100 μM ATPγS were kept for 25 min on the bench to determine their potential spontaneous hydrolysis. ATP and ATPγS concentrations were determined in perfusates collected from three paired experiments, following the protocol suggested by the manufacturer. Briefly, 100 μl of a fivefold dilution of each sample was transferred in triplicate into wells of a white opaque 96-well plate (Costar, Corning, NY) and incubated for 10 min with 90 μl of reconstituted reagent containing the assay buffer, d-luciferin, and luciferase (95:1:1). Luminescence was read on a Centro XS3 LB960 luminometer (Berthold Technologies, Oak Ridge, TN) with an integration time of 1 s per plate. ATP and ATPγS concentrations were extrapolated from the linear ATP and ATPγS standard curves.
Measurement of ATP Hydrolysis and ADP Production by Epididymal Luminal Fluid and Apical Membrane of Epididymal Epithelial Cells in Vitro
The nucleotidase activity of the epididymal fluid and apical membranes isolated from epididymal epithelial cells was assessed in vitro by measuring both ATP hydrolysis and ADP production with bioluminescence assays. As a negative control, the nonhydrolyzable ATPγS was also examined. The contribution of enzymes to these two processes was determined by preheating the epididymal fluid and apical membrane samples at 90°C for 10 min before the incubation with ATP or ATPγS.
Apical membrane preparation.
Epithelial cell apical membranes were isolated with the Mg2+ precipitation technique for purification of brush-border membranes (BBM) from kidney and intestinal cells, as previously described (3, 44, 48, 60). BBM were obtained from the corpus/cauda region of three rat epididymides. Briefly, the tissue was homogenized in 2 ml of buffer containing 250 mM sucrose, 18 mM Tris-HEPES, 1 mM EDTA, and Complete protease inhibitors, pH 7.4, with a glass homogenizer. The homogenate was incubated in the presence of 10 mM MgCl2 on ice for 20 min and then centrifuged at 7,700 g for 15 min. The pellet was discarded, and the supernatant was further centrifuged at 20,000 g for 15 min. The pellet was resuspended in a buffer containing 150 mM KCl, 5 mM Tris-HEPES, and Complete protease inhibitors, pH 7.4, by passing through a 25-gauge 5/8-in. needle and was then centrifuged at 1,900 g for 15 min. The pellet was discarded, and the supernatant was centrifuged at 30,900 g for 15 min. The supernatant was discarded, and the pellet was resuspended in a small volume of buffer by passing through a needle as described above. The protein concentration in the BBM preparation was measured with the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL).
Epididymal fluid collection.
Sperm was collected by luminal in vivo perfusion of the cauda region of six rat epididymides with a control perfusing solution as described above. Collection of sperm in the VD was stopped when perfusate became clear and free of spermatozoa. Sperm was centrifuged for 30 min at 4°C at 16,000 g, and the supernatant was further centrifuged for 30 min at 4°C at 16,000 g. Supernatant was labeled as epididymal fluid, and protein concentration was measured with the BCA assay (Pierce).
In vitro ATP bioluminescence assay.
For ATP assay, dilutions of epididymal fluid or BBM were incubated with 100 μM ATP or 100 μM ATPγS for 15 min at room temperature. As a negative control, 100 μM ATP and 100 μM ATPγS were incubated for 15 min at room temperature in the absence of epididymal fluid and BBM. The participation of enzymes in ATP hydrolysis was determined by preheating the epididymal fluid and BBM extracts at 90°C for 10 min before the 15-min incubation with ATP or ATPγS. ATP and ATPγS concentrations were measured in triplicate for each sample with the EnzyLight ATP bioluminescence assay as described above.
In vitro ADP bioluminescence assay.
For ADP assay, 5 μg of epididymal fluid or BBM was incubated with 50 μM ATP for 20 min at room temperature. As a negative control, 50 μM ATP was incubated for 20 min at room temperature in the absence of epididymal fluid and BBM. The participation of enzymes in ADP generation was determined by preheating the epididymal fluid and BBM extracts at 90°C for 10 min before the 20-min incubation with ATP. ADP concentration was measured in triplicate for each sample with the ADP-Glo assay (Promega), following the manufacturer's protocol. Briefly, incubations of ATP with epididymal fluid or BBM were performed in a 25-μl total volume in separate wells of a white opaque 96-well plate (Costar). Twenty-five microliters of ADP-Glo Reagent was added to each sample to deplete the unconsumed ATP and incubated for 40 min at room temperature. Fifty microliters of Kinase Detection Reagent was then added to convert ADP into ATP and introduce luciferase and luciferin to detect ATP. Luminescence was read on a Centro XS3 LB960 luminometer (Berthold Technologies) with an integration time of 0.25 s per well. ADP concentration was extrapolated from the linear standard curves.
Laser Cut Microdissection
LCM was performed on adult rat epididymal epithelium with a method adapted from Da Silva et al. (24). Briefly, after isolation of the epididymis in cold RNase-free PBS, tissues were embedded in OT compound (Tissue Tek, Sakura Finetek), fast frozen on dry ice, and sectioned with a Leica 3050 cryotome (Spencer Scientific, Derry, NH) at 10 μm. Sections were picked up onto metal-framed polyethylene naphthalate membrane slides (Molecular Devices, Sunnyvale, CA) and dehydrated in 70% ethanol for 30 s. After washing in RNase-free water, slides were stained with hematoxylin and eosin with the H&E Staining Kit for LCM (MMI Molecular Machines & Industries, Haslett, MI). Epithelial cells were harvested on CapSure HS LCM caps (Molecular Devices) with limited contamination from the surrounding smooth muscle cells and spermatozoa with the MMI CellCut system (MMI Molecular Machines & Industries). Three slides per epididymal region (caput, corpus, and cauda) were used to harvest epithelial cells.
Total RNA Extraction and Reverse Transcription
LCM samples were treated as we have described previously (24) by extracting total RNA with the PicoPure RNA Isolation Kit protocol (Molecular Devices). Epithelial cells isolated by LCM from different epididymal regions (caput, corpus, cauda) were lysed in PicoPure extraction buffer (Molecular Devices) and pooled. DNase treatment was performed in the purification column with the RNase-Free DNase set (Qiagen, Valencia, CA).
To isolate total RNA from whole adult epididymides, tissues were frozen in liquid nitrogen, then powdered in a pestle and mortar, and homogenized in RLT lysis buffer (Qiagen). RNA was isolated with the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol, and genomic DNA contamination was eliminated with the RNase-free DNase set (Qiagen). The quality of RNA both from epithelial cells isolated by LCM and from whole epididymis was assessed with Agilent RNA 6000 pico and Agilent RNA nano kits on the 2100 Bioanalyzer (Quantum Analytics, Foster City, CA). RNA samples were aliquoted and stored at −80°C before reverse transcription.
Four micrograms of RNA from whole epididymis and 2.5 μg of RNA from epithelial cells isolated by LCM were denatured for 10 min at 70°C in the presence of 1.25 U/μl random hexamers and 1.25 U/μl oligo(dT) and immediately kept on ice. RNA was then converted into first-strand cDNA for 1 h at 42°C in (mM) 10 Tris·HCl, pH 8.3, 50 KCl, 5 MgCl2, and 1 each dNTP, with 1 U/μl RNase inhibitor and 2.5 U/μl murine leukemia virus (MuLV) reverse transcriptase (all reagents from Applied Biosystems). GAPDH expression was assessed in each sample by performing a 35-cycle PCR.
Polymerase Chain Reaction
Polymerase chain reaction (PCR) was performed as we have described previously (24). Two nanograms of reverse transcription templates was mixed with 1.25 U of AmpliTaq Gold DNA polymerase (Applied Biosystems), 50 mM KCl, 10 mM Tris·HCl, pH 8.3, 2.0 mM MgCl2, 200 μM each dNTP, and 0.5 μM forward and reverse oligonucleotide primers. PCR was performed in a Flexigene thermal cycler (Techne, Princeton, NJ) with the following parameters: 8 min at 95°C to activate the polymerase, followed by 30–40 cycles of melting for 30 s at 95°C, annealing for 30 s at 60°C, extension for 45 s at 72°C, and a final extension for 10 min at 72°C. PCR products were resolved in a 2.5% agarose gel containing GelStar stain (Lonza, Rockland, ME). A no-template control was included in each PCR plate. Oligonucleotide primer pairs were designed by using OligoPerfect Designer software (Invitrogen) to amplify a short sequence (100–200 bp) of rat cDNAs. Primers were synthesized by Invitrogen. Sequences of primers used for the PCR are listed in Table 1.
Table 1.
Primer sequences used to detect purinergic receptor subtypes and ectonucleotidases by RT-PCR
| Primer ID | Species | Gene Name | Sequence ID | 5′ to 3′ | Product Length, bp | Sequence 5′ to 3′ |
|---|---|---|---|---|---|---|
| RP2X1 | Rat | P2rx1 | NM_012997 | FOR | 128 | AGG GAG TCA CAC CAG ACC AG |
| REV | GCT GGA CAG GCT ATG GAA AC | |||||
| RP2X2 | Rat | P2rx2 | NM_053656 | FOR | 114 | TAA GGC CTC TGG GGA GAA GT |
| REV | CTT GGG GTC CCT GAA TAC TG | |||||
| RP2X3 | Rat | P2rx3 | NM_031075.1 | FOR | 118 | GCT GAG TTC ACC AAG ATG CTC |
| REV | ACT GAA GGC CCA GAA GAG GT | |||||
| RP2X4 | Rat | P2rx4 | NM_031594 | FOR | 100 | GGA AGG AGG CTC TCT CAG GT |
| REV | AAA GCT AAC CCT GCC TTT GC | |||||
| RP2X5 | Rat | P2rx5 | NM_080780 | FOR | 115 | GGA GAG GGG AGG CAA GTA AG |
| REV | AGC AAG AGC TGA ACT GCA CA | |||||
| RP2X6 | Rat | P2rx6 | NM_012721.1 | FOR | 118 | GCC TGA AAA GGG CTA GAG TG |
| REV | TGG CAA GCT TTA CTT CAG CA | |||||
| RP2X7 | Rat | P2rx7 | NM_019256 | FOR | 118 | GAG GAC CTT TGT TGG GAT CA |
| REV | ATG GGG TTC AAG GTC ATC AG | |||||
| RP2Y1 | Rat | P2ry1 | NM_012800 | FOR | 171 | TAT GAT GCA GCT TCC ACT GC |
| REV | GCT TCA AGA TCT GGC AGA AAA | |||||
| RP2Y2 | Rat | P2ry2 | NM_017255 | FOR | 124 | CCG AGC TTC AGC CTC TTA AA |
| REV | CGC TCT ACC GCT GAG CTA AA | |||||
| RP2Y4 | Rat | P2ry4 | NM_031680 | FOR | 100 | CAT GAG GAA AGC ATC AGC AG |
| REV | CCC TTC ATA TCC AGC AGC AG | |||||
| RP2Y5 | Rat | P2ry5 | BC098703 | FOR | 105 | TGA CTA AAC CAC TGG GAC TGC |
| REV | AAC AGG TAA CCG TCC GTA GG | |||||
| RP2Y6 | Rat | P2ry6 | NM_057124 | FOR | 135 | GGC AGT GTC CTT TCT ATG AGG |
| REV | GGC AAT GAA CAA AGT CCA CA | |||||
| RP2Y10 | Rat | P2ry10 | BC168967 | FOR | 148 | GCA GGC AAG AAA AGG AAG TG |
| REV | TCA TGG TCA AAG AAA CCA ACA | |||||
| RP2Y12 | Rat | P2ry12 | NM_022800 | FOR | 165 | TTC AGA AAT TCC TTG ATG AGC A |
| REV | CCT TGG AGG AGT CTG GAC ACT A | |||||
| RP2Y13 | Rat | P2ry13 | AY639875 | FOR | 143 | CAA CTC TCT TCC TGG CAA CA |
| REV | CTG ACT GCT GTG GTG CTC AT | |||||
| RP2Y14 | Rat | P2ry14 | NM_133577 | FOR | 120 | TCT GCG AGT TAC CCC AAA AC |
| REV | GCA TGA AAC AGA GGT GAG CA | |||||
| RADORA1 | Rat | Adora1 | NM_047395.1 | FOR | 178 | TTC CAT TTC TGC TTG CCT CT |
| REV | CAT ATG GGT CCC AGG AAC TG | |||||
| RADORA2A | Rat | Adora2a | NM_053294 | FOR | 133 | AGG GAA GGA TTC CAG AGC AT |
| REV | AGT CCC TCT GGA AGG AAA GG | |||||
| RADORA2B | Rat | Adora2b | NM_017161 | FOR | 139 | GGT GGA AAC TTG GAG AGC TG |
| REV | CAG CTA GGG GAT TTT GCT CA | |||||
| RADORA3 | Rat | Adora3 | NM_012896 | FOR | 112 | CAG AAC CTG CGT TGA AGG AT |
| REV | GCG CCA TCT TTT ATT TGC TC | |||||
| ENPP1 | Rat | Enpp1 | NM_053535.1 | FOR | 130 | GGC CTG ATA ACA TCG AGA GC |
| REV | CGG TCC TGG TAG AAG CTG AG | |||||
| ENPP2 | Rat | Enpp2 | NM_057104.2 | FOR | 101 | CAC TGA GGC TCA CAA TCC AA |
| REV | GTG ACT GTG CCC TGG AAA AC | |||||
| ENPP3 | Rat | Enpp3 | NM_019370.2 | FOR | 116 | GCA GAA GAC CTT TGG GTT GA |
| REV | ATT TCA GAG ACG GGC TGT GT | |||||
| ENTPD1 | Rat | Entpd1 | NM_022587.1 | FOR | 180 | CCC AGC TGA ACA GCC ATT AT |
| REV | TCT CTC CAG CCA GCA AGT TT | |||||
| ENTPD2 | Rat | Entpd2 | NM_172030.1 | FOR | 102 | CCT GTG ACT CAG GGT GAG GT |
| REV | CTC TGG TGC CTG CCT TTC TA | |||||
| ENTPD3 | Rat | Entpd3 | NM_178106.2 | FOR | 140 | GTG CTT TCT TCC CAC TGC TC |
| REV | CCC ACT TTG GAA GCA AAC AT | |||||
| ENTPD4 | Rat | Entpd4 | NM_001108384.1 | FOR | 169 | GAG AGC GTG GAC CAG GAG TA |
| REV | CAA CAA GGA CTG TCG AGC AA | |||||
| ENTPD5 | Rat | Entpd5 | NM_199394.1 | FOR | 115 | AGG AGG GAG GGG TTT GTA GA |
| REV | ACC AGC TAT ATG CCC ACC AA | |||||
| ENTPD6 | Rat | Entpd6 | NM_053498.1 | FOR | 134 | CAG TGG GGC AGG TTA GAA AG |
| REV | GGT GGT GGC TCA GCT TAC TC | |||||
| ENTPD7 | Rat | Entpd7 | NM_001107595.1 | FOR | 113 | CAG TTG CCC GTT TTC TCT GT |
| REV | CGT TTT GGT TGC CTT GAA AT | |||||
| ENTPD8 | Rat | Entpd8 | NM_001033565.1 | FOR | 129 | TGA TGG CGA TGT CTA AAG CA |
| REV | GAC TGG AGC CCT CAA GTG TC | |||||
| NT5E | Rat | Nt5e | NM_021576 | FOR | 155 | GGT TAG TCC AGG GAA TGC AA |
| REV | CTA AAT CCC CAG CCC CTA TG | |||||
| NS-AP | Rat | Alpl | NM_013059 | FOR | 106 | CTG CTC AGG GTG AGA CTT CC |
| REV | GAG TGT GTG TGC GTC CTG TC | |||||
| I-AP | Rat | Alpi | NM_022680 | FOR | 153 | CCA GAA CAG CAC CAC TAC CA |
| REV | GCC CCC AGT AGT AGC ATC AG | |||||
| PLA-AP | Rat | Alpp | J02980 | FOR | 109 | ACG AGG TCA GGA GAT GGA GA |
| REV | GGG TTC AAG CGA TTA TCC TG | |||||
| G-AP | Rat | Alpp2 | XM_001064732 | FOR | 107 | CTG GAG CCC TAC ACC GAC T |
| REV | TCA GCT GAC ACC AAC AGC AT | |||||
| PAP | Rat | Acpp | M32397.1 | FOR | 124 | CTA ATG GGC AAA GGA CCT GA |
| REV | CGT TCT AGG TCG GGT ATG GA | |||||
| GAPDH | Rat | Gapdh | NM_017008.3 | FOR | 77 | AGA GAG AGG CCC TCA GTT GCT |
| REV | TGG AAT TGT GAG GGA GAT GCT |
FOR, forward; REV, reverse.
RESULTS
Effect of Luminal ATP and Adenosine on Apical Membrane V-ATPase Recruitment
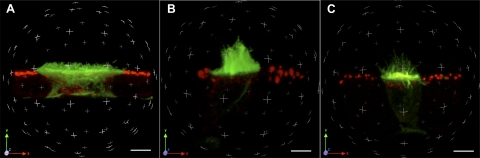
Cauda regions of rat epididymides were perfused in vivo in paired experiments, one epididymis being perfused with control solution and the other with the agonist. 3D reconstructions from a z series of confocal images taken from 10-μm-thick epididymis sections double-stained for V-ATPase (green) and HRP (red) showed that under control conditions V-ATPase is distributed between the intracellular compartment and short apical microvilli in clear cells (Fig. 1A). In the presence of luminal ATP (Fig. 1B) and adenosine (Fig. 1C), clear cells were narrower compared with control tissue, and the V-ATPase was located mainly in numerous and well-developed microvilli. The corresponding movies showing complete 3D reconstructions from which Fig. 1, A–C, were taken are provided as supplemental material (Supplemental Videos 1, 2, and 3).1
Fig. 1.
Three-dimensional (3D) reconstruction of clear cells under control conditions or luminally exposed to ATP or adenosine. Ten-micrometer sections of cauda epididymidis were double-stained for the vacuolar H+-ATPase (V-ATPase) B1 subunit (green) and horseradish peroxidase (HRP; red) after luminal perfusion with a control solution (A) or a solution containing 600 μM ATP (B) or 300 μM adenosine (C). 3D reconstructions were performed from a series of 0.1-μm optical sections taken in the z plane. Scale bars, 5 μm. Movies showing 3D rotations of these image stacks are available as supplemental material [Supplemental Videos 1 (A), 2 (B), and 3 (C)].
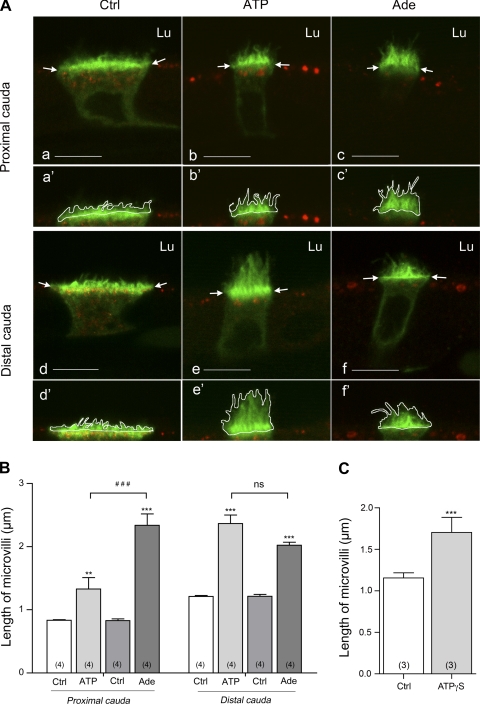
Quantification was performed on single confocal z sections, and the response elicited by luminal ATP and adenosine was compared in clear cells located in the proximal and the distal cauda of the epididymis (Fig. 2). In these single optical sections, the same patterns of V-ATPase staining were observed. As we have previously described, under control conditions the V-ATPase was present in short microvilli as well as in intracellular vesicles in both the proximal (Fig. 2A, a and a′) and distal (Fig. 2A, d and d′) cauda; luminal ATP (Fig. 2A, b and b′, e and e′) and adenosine (Fig. 2A, c and c′, f and f′) induced the recruitment of V-ATPase into elongated microvilli. ATP induced a significant extension of V-ATPase-labeled microvilli in both the proximal and distal cauda epididymidis (Fig. 2B). Interestingly, the mean microvillus length value was significantly higher in the distal cauda compared with the proximal cauda region (P < 0.001). This led to a 1.6-fold increase in microvillus length in the proximal cauda versus a higher 1.95-fold increase in the distal cauda compared with their respective controls. This result indicates a greater response of clear cells to luminal ATP in the most distal region of the epididymis.
Fig. 2.
A: Double-immunofluorescence staining for V-ATPase B1 subunit (green) and HRP (red) in cryostat sections from rat epididymis perfused in vivo with control solution (Ctrl; a, d), 600 μM ATP (b, e), and 300 μM adenosine (Ade; c, f). Bars, 8 μm. Lu, lumen. V-ATPase apical accumulation was assessed by measuring the mean length of B1-labeled microvilli of clear cells as shown in a′–f′ and described in materials and methods. B: quantification of the length of B1-labeled microvilli in clear cells of the proximal (left) and distal (right) cauda epididymidis under control conditions (Ctrl) and after ATP and adenosine perfusion. C: quantification of the length of B1-labeled microvilli in clear cells of the distal cauda epididymidis under control conditions and after adenosine-5′-(γ-thiotriphosphate) (ATPγS) perfusion. Data are means ± SE; 2-way ANOVA test was used to compare different treatments to the control condition in each cauda region (**P < 0.01, ***P < 0.001) and to compare the different treatments to each other (###P < 0.001; ns, nonsignificant) (B). A Student's t-test was used to compare paired experiments (C). Numbers of rats per group are indicated in parentheses.
Luminal adenosine also induced a significant extension of V-ATPase-labeled microvilli in the proximal and distal cauda epididymidis (Fig. 2B). The V-ATPase response to luminal adenosine was not significantly different between the proximal and distal cauda (P > 0.05). However, in the proximal cauda, the mean microvillus length measured in the presence of adenosine was significantly higher compared with the length measured in the presence of ATP (P < 0.001), whereas the difference between the lengths measured in the presence of ATP or adenosine was not significant in the distal cauda (Fig. 2B).
Altogether these data indicate that both luminal ATP and adenosine induce significant V-ATPase recruitment to the apical membrane of clear cells in the proximal and distal cauda. The participation of ATP in this process was more apparent in the distal cauda compared with the proximal cauda, whereas no variation in the efficacy of adenosine to recruit V-ATPase was observed between the two regions. Extracellular ATP can be hydrolyzed by ectonucleotidases present in the plasma membrane of epithelial cells with an extracellular oriented catalytic site. Therefore, the higher V-ATPase recruitment observed in the distal cauda after luminal ATP perfusion could be due to an ectonucleotidase-mediated hydrolysis of ATP into adenosine in the extracellular space.
Effect of Nonhydrolyzable Purinergic Agonist ATPγS on V-ATPase Recruitment
To assess the potential involvement of ectonucleotidases in ATP-mediated V-ATPase membrane recruitment, rat epididymides were perfused in vivo with ATPγS (600 μM), a nonhydrolyzable ATP analog. Results of quantification of the length of V-ATPase-labeled microvilli in the presence of luminal ATPγS compared with control are shown in Fig. 2C. Quantification was performed in the distal cauda, which corresponds to the region in which luminal ATP is more potent in inducing V-ATPase recruitment and microvillus extension. The mean V-ATPase-labeled microvillus length value increased from 1.15 ± 0.10 under control conditions to 1.70 ± 0.31 in the presence of luminal ATPγS. This increase was statistically significant by one-tailed Student t-test but was not by two-tailed t-test. Luminal ATPγS was therefore less potent than luminal ATP in inducing V-ATPase recruitment and microvillus elongation in the distal cauda. This result suggests that hydrolysis of extracellular ATP by ectonucleotidases contributes to generate agonists such as adenosine in order to “activate” V-ATPase-rich clear cells.
Expression of Ectonucleotidases in Rat Epididymal Epithelial Cells
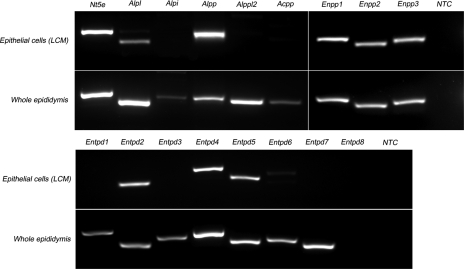
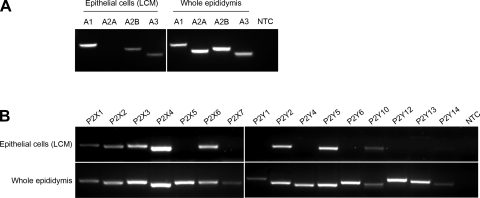
The expression of genes coding for ATP-hydrolyzing enzymes was investigated by RT-PCR in rat epididymal epithelial cells isolated by LCM as well as in whole epididymis (Fig. 3). Four isoforms of AP (nonspecific Alpl, intestinal Alpi, placental Alpp, and germ cell Alppl2), three E-NPPs (Enpp1, Enpp2 and Enpp3), eight E-NTPDases (Entpd 1 to 8), 5′-NT (Nt5e), and prostatic acid phosphatase (Acpp) were screened in both mRNA templates. Except for Entpd8, all 16 hydrolyzing enzymes were detected in the whole epididymis at the mRNA level. Among these ectonucleotidases, nine are expressed in epididymal epithelial cells (Nt5e, Alpl, Alpp, Enpp1, 2, and 3, and Entpd 2, 4, and 5), as determined in samples isolated by LCM.
Fig. 3.
RT-PCR analysis of ectonucleotidase mRNA expression in epididymal epithelial cells isolated by laser cut microdissection (LCM) and in whole epididymis. Top: primers were designed for rat ecto-5′-nucleotidase (Nt5e), 4 isoforms of alkaline phosphatase (Alpl, Alpi, Alpp, Alpp2), prostatic acid phosphatase (Acpp), and 3 isoforms of ectonucleotide pyrophosphatase/phosphodiesterase (Enpp1, Enpp2, Enpp3). Bottom: 8 isoforms of ectonucleotide triphosphate diphosphohydrolase (Entpd1, Entpd2, Entpd3, Entpd4, Entpd5, Entpd6, Entpd7, Entpd8) were analyzed. All enzymes except Entpd8 were detected in whole epididymis mRNA extracts. In epithelial cells, 9 enzymes were detected: Alpl, Alpp, Enpp1, Enpp2, Enpp3, Entpd2, Entpd4, and Entpd5. NTC, no-template control.
Immunolocalization of Ecto-5′-Nucleotidase in Caudal Region of Rat Epididymis
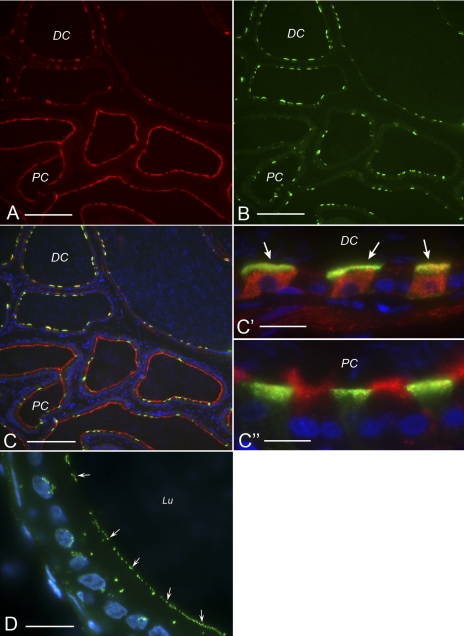
To further determine the involvement of ectonucleotidases in the generation of adenosine in the lumen of the epididymis, we investigated the expression of 5′-NT by immunofluorescence in the cauda region of rat epididymis (Fig. 4A). Double staining for the B1 subunit of V-ATPase (Fig. 4B) showed a different pattern of 5′-NT expression between the distal corpus/proximal cauda and the distal cauda (Fig. 4C). Whereas 5′-NT was highly expressed in the apical membrane of B1-negative principal cells in the proximal cauda (Fig. 4. C and C“), this apical staining completely disappeared in the distal cauda (Fig. 4, C and C′), where 5′-NT was mainly localized in the cytoplasm of B1-positive clear cells (Fig. 4, C and C′). These results indicate that 5′-NT is in direct contact with luminal contents in the proximal cauda region of the epididymis. The terminal step in extracellular adenosine formation from AMP that is achieved by this enzyme could therefore occur in this epididymal region.
Fig. 4.
Cellular localization of ecto-5′-nucleotidase and endogenous alkaline phosphatase activity in rat epididymis. Double-staining for ecto-5′-nucleotidase (A) and the V-ATPase B1 subunit (B) revealed a region-specific localization of ecto-5′-nucleotidase. C: merge picture; C′ and C″ show higher magnification of some tubules shown in C. In the proximal cauda (PC) ecto-5′-nucleotidase is highly expressed in the apical membrane of principal cells (negative for V-ATPase). In contrast, in the distal cauda (DC) it is localized in the cytoplasm of clear cells (positive for V-ATPase, arrows) and is absent from principal cells. D: alkaline phosphatase activity was detected in the apical membrane of epididymal epithelial cells (arrows) with the ELF 97 substrate. Lu, Lumen. Bars: 120 μm for A, B, and C; 14 μm for C′ and C″; 10 μm for D.
Detection of Endogenous Phosphatase Activity in Apical Membrane of Epithelial Cells in Rat Epididymis
Extracellular APs participate in the three-step process of ATP degradation into adenosine. We examined the endogenous phosphatase activity by histochemistry on rat epididymis sections, using a fluorescence-based method (Fig. 4D). Rapidly after incubation with the ELF 97 fluorescent substrate, a marked AP activity was detected in the apical membrane of epithelial cells from the cauda (Fig. 4D, arrows). Strong AP activity was also observed in smooth muscles surrounding the epididymal epithelium (data not shown). Moderate phosphatase activity was also detected in the cytoplasm of some epithelial cells.
In Vitro and in Vivo Detection of Nucleotidase Activity in Rat Epididymis
To further investigate the presence of nucleotidase activity in the cauda region of the epididymis, we measured ATP hydrolysis and ADP production in vitro after incubation of epididymal fluid samples and BBM preparations with ATP and ATPγS (Fig. 5, A–C). The participation of enzymes in ATP hydrolysis and ADP production was evaluated by preheating the epididymal fluid and BBM to 90°C before incubation.
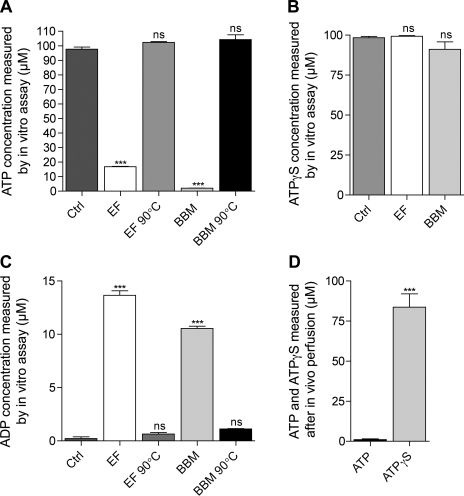
Fig. 5.
In vitro and in vivo detection of ATP hydrolysis in the epididymis. Nucleotidase activity was assessed in vitro in the epididymal fluid (EF) and in brush-border membranes (BBM) by measuring the hydrolysis of 100 μM ATP (A) or 100 μM ATPγS (B) after in vitro incubation with a control solution (Ctrl), EF, BBM, or EF and BBM that had been preheated to 90°C before the incubation (EF 90°C and BBM 90°C, respectively). C: production of ADP was measured after incubation of 50 μM ATP with a control solution (Ctrl), EF, BBM, or EF and BBM preheated to 90°. D: nucleotidase activity was also assessed in vivo by perfusing the epididymal lumen with 100 μM ATP or 100 μM ATPγS and measuring the concentration of ATP and ATPγS in the collected luminal fluid. Data are means ± SE. One-way ANOVA test was used to compare different conditions (A–C) to control. ***P < 0.001; ns, nonsignificant. A Student's t-test was used to compare paired experiments in D. (***P < 0.001).
After in vitro incubation of 100 μM ATP with epididymal fluid and BBM, only 17% and 2% of the initial ATP concentration were measured, respectively (Fig. 5A). These concentrations were significantly lower compared with a negative control performed without epididymal fluid and BBM, in which ∼98% of the initial ATP concentration was measured after incubation. In addition, no significant loss of ATP was measurable after incubation of 100 μM ATP with preheated epididymal fluid or BBM. These values were not significantly different from the control. These results indicate that both epididymal fluid and BBM contain nucleotidase activity that is responsible for the partial ATP hydrolysis in vitro, and that denaturation of the enzymes by preheating the samples at 90°C significantly inhibited this process. In addition, after in vitro incubation of 100 μM nonhydrolyzable ATPγS with epididymal fluid and BBM, we measured 99% and 91% of the initial ATPγS concentration, respectively (Fig. 5B). These values were not significantly different from the control condition and confirm that disappearance of ATP after incubation with BBM and epididymal fluid was due to ATP hydrolysis. To further confirm ATP hydrolysis after incubation with epididymal fluid and BBM, we measured the production of ADP in vitro (Fig. 5C). After incubation of 50 μM ATP with 5 μg of BBM or 5 μg of epididymal fluid, ADP concentrations were 13.66 ± 0.84 μM and 10.54 ± 0.43 μM, respectively. These maximum ADP concentrations were detected after 15 min, followed by a progressive decrease in ADP content, indicating further degradation into adenosine (data not shown). The maximum ADP concentration values were significantly higher compared with control, in which only 0.32 ± 0.22 μM ADP was detected. In addition, only 0.65 ± 0.26 and 1.12 ± 0.09 μM ADP were detected after incubation of ATP with preheated epididymal fluid and BBM, respectively. ADP concentrations in these samples were not significantly different compared with the controls, indicating the total inhibition by denaturation of the enzymes responsible for ADP production. These results indicate that after 15 min of incubation with epididymal fluid and BBM ATP is significantly hydrolyzed into ADP.
The nucleotidase activity of the cauda epididymidis was also evaluated in vivo by perfusing the epididymal lumen with 100 μM ATP or ATPγS (Fig. 5D). The concentration of ATP in the perfusate collected at the exit of the epididymis was 1% of the initial ATP concentration, indicating that most ATP had been hydrolyzed during its transit through the epididymal lumen. In addition, the concentration of ATPγS was 84% of the initial ATPγS concentration in the collected perfusate, indicating that most ATPγS remained intact during transit in the epididymis. Approximately 16% of ATPγS was ”lost“ during transit, presumably because of binding to and/or internalization by epididymal epithelial cells.
Altogether these results indicate that, once released into the lumen of the epididymis, ATP can be degraded very quickly by enzymes localized both in the apical membrane of epithelial cells and in the epididymal fluid.
Expression of P1 and P2 Purinergic Receptor mRNA in Epididymal Epithelial Cells
The expression of P1 and P2 receptors in adult rat epididymis was examined by RT-PCR on mRNA extracted from whole epididymis and from epithelial cells isolated by LCM (Fig. 6, A and B).
Fig. 6.
RT-PCR analysis of P1 and P2 receptors in epididymal epithelial cells isolated by LCM and in whole epididymis. A: whereas all P1 receptor subtypes were detected in the whole epididymis, only A1, A2B, and A3 transcripts were detected in epithelial cells. B: P2 receptor primers were designed to detect 7 members of the P2X family (P2x1, P2x2, P2x3, P2x4, P2x5, P2x6, P2x7) and 9 members of the P2Y family (P2y1, P2y2, P2y4, P2y5, P2y6, P2y10, P2y12, P2y13, P2y14). All P2 subtypes were detected in the whole epididymis, but only 8 subtypes (P2x1–4, P2x6, P2y2, 5, 10) were detected in epididymal epithelial cells.
The different subtypes of adenosine-P1 receptors were detected with primers designed against the rat A1, A2A, A2B, and A3 receptor sequences (Fig. 6A). Whereas the four adenosine receptor subtypes were highly expressed in the whole epididymis, only A1, A2B, and A3 were detected in epithelial cells.
To determine the mRNA expression profile of the 16 different P2 subtype receptors, primers were designed to detect seven P2X ligand-gated ion channels (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, P2X7) and nine P2Y GPCRs (P2Y1, P2Y2, P2Y4, P2Y5, P2Y6, P2Y10, P2Y12, P2Y13, P2Y14). As shown in Fig. 6B, all the P2X ligand-gated ion channels were detected in the whole epididymis, but only five subtypes were detected in epithelial cells isolated by LCM: P2X1, P2X2, P2X3, P2X6, and, at apparently greater levels, P2X4. Whereas all subtypes of P2Y receptors were expressed in the whole epididymis, only three subtypes were detected in epithelial cells: P2Y2, P2Y5, and P2Y10. These results demonstrate that epithelial cells of the epididymis express several subtypes of P1 and P2 purinergic receptors at the mRNA level.
ATP-Dependent V-ATPase Apical Membrane Accumulation is Calcium Dependent
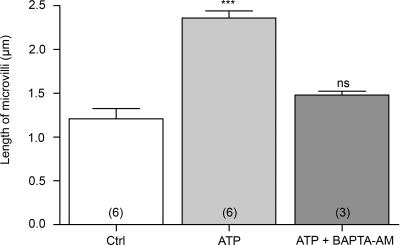
Both P2X and P2Y ATP receptors are known to induce an increase in intracellular calcium. We therefore investigated the participation of calcium as a second messenger in the ATP-dependent V-ATPase apical membrane accumulation in clear cells. Rat epididymides were perfused in vivo with luminal ATP, with or without the calcium-chelator BAPTA-AM, and the distribution of V-ATPase and length of B1-labeled microvilli in clear cells was quantified as described above (see Fig. 2). Luminal ATP significantly increased the length of clear cell V-ATPase-labeled microvilli by a factor of almost 2 (Fig. 7; P < 0.001 vs. control). Luminal perfusion with ATP in the presence of BAPTA-AM significantly inhibited the response elicited by ATP alone. In addition, the mean microvillus length value after perfusion with ATP and BAPTA-AM was not significantly different from the value measured under control conditions. The V-ATPase accumulation in the apical membrane of clear cells mediated by ATP is therefore calcium dependent.
Fig. 7.
Role of calcium in luminal ATP-dependent V-ATPase apical accumulation in clear cells. Rat epididymides were luminally perfused in vivo with a control solution (Ctrl), 600 μM ATP, or 600 μM ATP in the presence of the calcium chelator BAPTA-AM (10 μM) (ATP + BAPTA-AM). Quantification of the length of V-ATPase-labeled microvilli in clear cells from the distal cauda epididymidis showed a significant elongation by ATP compared with control and inhibition of the ATP-elicited response by BAPTA-AM. Data are means ± SE. One-way ANOVA test was used to compare different treatments to control. ***P < 0.001. Numbers of rats per group are indicated in parentheses.
Adenosine-Dependent V-ATPase Apical Membrane Accumulation Involves cAMP-Protein Kinase A Pathway
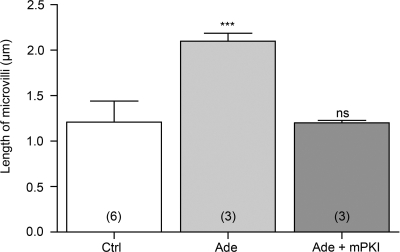
We showed that three subtypes of G protein-coupled adenosine receptors (A1, A2B, and A3) are expressed in rat epididymal epithelial cells. Among these receptors, A1 and A3 are known to signal through Gi/o and to inhibit adenylyl cyclase (AC) and cAMP accumulation, whereas A2B signals through Gs to stimulate AC and cAMP accumulation (38). We investigated the participation of protein kinase A (PKA), the downstream effector of cAMP, in the adenosine-dependent V-ATPase apical membrane accumulation. Rat epididymides were perfused in vivo with luminal adenosine, with or without the PKA inhibitor mPKI, and the distribution of V-ATPase and length of B1-labeled microvilli in clear cells was quantified as described above (see Fig. 2). Luminal adenosine induced an almost twofold increase in the length of clear cell V-ATPase-labeled microvilli (Fig. 8; P < 0.001 vs. control). Luminal perfusion with adenosine in the presence of mPKI significantly inhibited the response elicited by adenosine alone. In addition, the mean microvillus length value after perfusion with adenosine and mPKI was not significantly different from the value measured in control conditions. The adenosine-dependent V-ATPase apical membrane accumulation therefore involves PKA. These results indicate the participation of the receptor A2B, which is the only subtype expressed in epithelial cells that triggers an increase of cAMP.
Fig. 8.
Role of protein kinase A (PKA) in luminal adenosine-dependent V-ATPase apical accumulation in clear cells. Rat epididymides were luminally perfused in vivo with a control solution (Ctrl), 300 μM adenosine (Ade), or 300 μM adenosine in the presence of 10 μM of the PKA inhibitor myristoylated protein kinase inhibitor (Ade + mPKI). Quantification of the length of V-ATPase-labeled microvilli in clear cells from the distal cauda epididymidis showed a significant elongation by adenosine compared with control and a complete inhibition of the adenosine response by mPKI. Data are means ± SE. One-way ANOVA test was used to compare different treatments to control. ***P < 0.001. Numbers of rats per group are indicated in parentheses.
DISCUSSION
The epididymis was the first intact epithelial tissue in which the role of luminal ATP and apical P2 receptors was described (39, 74). The present study shows that not only extracellular ATP but also its hydrolysis product adenosine play a role in the regulation of epithelial cell function in the epididymis. Luminal ATP and adenosine induce the accumulation of V-ATPase in the plasma membrane of acidifying clear cells, which is accompanied by a marked elongation of clear cell microvilli. We previously showed (2, 65) that apical V-ATPase accumulation correlates with an increase in proton secretion by clear cells. Thus both extracellular ATP and adenosine might participate in the maintenance of an acidic pH in the lumen of the epididymis.
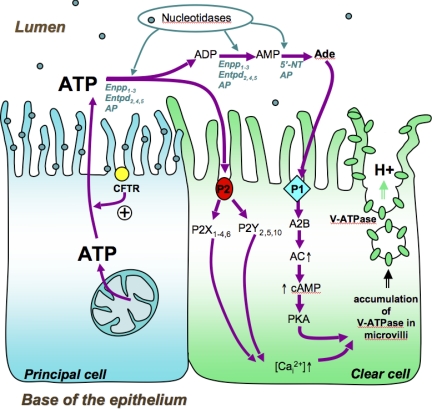
ATP could be released either from sperm or from epididymal epithelial cells (72) via exocytosis of ATP-containing vesicles or via the conducting pore of channels, including CLC-3 and CLC-5 (53, 54, 62). We previously showed (33) that CLC-3 is highly expressed in epididymal principal cells, while CLC-5 colocalizes with the V-ATPase in the apical membrane and intracellular vesicles of clear cells. Future studies will be required to determine their potential participation in ATP release into the lumen of the epididymis. The contribution of the cystic fibrosis transmembrane conductance regulator (CFTR) either in the direct transport of ATP or as a regulatory element for ATP release has been proposed (14, 27, 35, 55, 61, 62). In the epididymis, CFTR is expressed in the apical membrane of epididymal principal cells (51, 58, 64), and, interestingly, several mutations of the CFTR gene are related to male infertility (68, 73). CFTR could therefore play a crucial role in the modulation of V-ATPase-dependent luminal acidification via its potential participation in ATP release from principal cells. This series of events would orchestrate a luminal cell-cell cross talk between principal cells and clear cells via purinergic signaling. Our proposed model is illustrated in Fig. 9.
Fig. 9.
Proposed model of purinergic-dependent V-ATPase apical accumulation in clear cells. The P2 purinergic receptor subtypes P2X1,4,6 and P2Y2,5,10 are expressed in clear cells. Extracellular luminal ATP induces a calcium-dependent V-ATPase accumulation in well-developed microvilli in clear cells. Extracellular ATP is also rapidly degraded into adenosine by ecto-nucleotidases that are expressed in the apical membrane of principal cells or by nucleotidases located in the epididymal fluid. AP, alkaline phosphatase; 5′-NT, 5′-nucleotidase. Extracellular adenosine activates the P1 receptor subtype A2B, which is also expressed in clear cells. Luminal adenosine triggers a PKA-dependent V-ATPase microvillus accumulation in clear cells. Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in the apical membrane of principal cells and has been proposed to regulate ATP secretion in several cell types. We propose that principal cells release ATP into the epididymal lumen via a mechanism that requires the involvement of CFTR. Future studies will be required to examine this hypothesis.
In addition to the role of ATP in inducing apical membrane accumulation of V-ATPase in clear cells, we also revealed the contribution of adenosine to this process. A lower response was observed with ATPγS compared with the response elicited by ATP in the distal cauda epididymidis, indicating that ATP hydrolysis into adenosine contributed to the V-ATPase apical accumulation in this segment. A previous study showed a regional difference in extracellular purine degradation in different regions of the VD, with a higher capacity of the proximal VD (next to the epididymis) to form adenosine (25). In the present study, the rapid degradation of ATP that we observed with both in vivo and in vitro assays confirmed the existence of a high nucleotidase activity in the lumen of the epididymis. A concomitant increase in ADP concentration was detected in epididymal fluid and epithelial cell apical membranes incubated with ATP, confirming the enzymatic hydrolysis of ATP. We specifically localized this enzymatic activity on the luminal side of epithelial cells by immunofluorescence and functional immunocytochemical assays. ATP is sequentially degraded into adenosine through a three-step process mediated by several specialized enzymes. Our screening by RT-PCR for nucleotidases indicated that nine members are expressed in epithelial cells of the epididymis and are potentially involved in luminal ATP degradation. Among these enzymes, the protein 5′-NT was broadly expressed in the apical membrane of principal cells in the proximal cauda region and was found intracellularly in clear cells in the distal cauda region. 5′-NT is a glycosyl phosphatidylinositol (GPI)-anchored protein that can be released from the membrane via digestion by exogenous phosphatidylinositol-specific phospholipase C (PI-PLC) (67), and it can be physiologically shed in response to activators of endogenous phospholipases. The pattern of distribution of 5′-NT we describe here in the cauda epididymidis indicates that the enzyme might be partially shed from the brush border of principal cells between the proximal and distal cauda region, and is then ingested by clear cells via endocytosis in the distal cauda. A luminal factor present only in the distal cauda might therefore be responsible for the shedding of 5′-NT into the luminal fluid. Its internalization by clear cells would then contribute to limiting luminal adenosine formation.
Functional purinergic receptors have been reported previously on primary monolayer cultures of rat epididymal cells (74), and several purinergic receptors (P2X4, P2Y1, and P2Y2) have been characterized in mouse epididymis (63). In our study we detected eight subtypes of purinergic ATP-receptors by RT-PCR in rat epididymal epithelial cells (P2X1, P2X2, P2X3, P2X4, P2X6, P2Y2, P2Y5, and P2Y10), indicating a major role of the purinergic system in these cells. Among the P2X ATP-gated calcium-permeable receptors found, it is interesting to note that the intracellular calcium response elicited by ATP on P2X4 receptor was potentiated at alkaline pH (77), a condition that also induces the recruitment of V-ATPase into the apical membrane of clear cells in the epididymis (2, 47, 50). Interestingly, we showed that V-ATPase recruitment stimulated by luminal ATP was inhibited in the presence of the calcium chelator BAPTA-AM. However, while our data indicate a requirement for calcium in this response, possibly related to its effect on vesicle fusion and/or cytoskeletal reorganization, they do not demonstrate that ATP triggered an increase in intracellular calcium.
Adenosine is known to play an important role in the contraction of the VD (31) and in the regulation of anion secretion in this organ (15). Our study demonstrates the expression of A1, A2B, and A3 adenosine P1 receptors in epididymal epithelial cells. In addition, we show here that the adenosine-dependent V-ATPase apical membrane accumulation involves the cAMP/PKA pathway. Of the adenosine receptors that are expressed in epididymal epithelial cells, only A2B induces an intracellular cAMP increase after adenosine stimulation (38). We therefore hypothesize that the adenosine-dependent V-ATPase response is mediated by the receptor A2B.
In our model (Fig. 9), both ATP and adenosine induce V-ATPase apical accumulation in clear cells. The balance between luminal ATP and adenosine might provide a fine mechanism for the regulation of V-ATPase-dependent proton secretion. First, APs are more active at alkaline pH, and alkalinization of the lumen of the epididymis might regulate the activity of these enzymes. Principal cells of the epididymis respond to a variety of basolateral agonists such as neurotransmitters and steroid hormones by secreting bicarbonate (18, 41, 75). Transient bicarbonate secretion was proposed to occur during sexual arousal to prime spermatozoa before ejaculation (15), and activation of CFTR was shown to play a role in this process (16, 19, 22, 40, 41, 59, 73). Activation of APs by the increase in luminal pH secondary to bicarbonate secretion would provide a negative feedback by which adenosine would be produced to activate V-ATPase proton secretion in clear cells via P1 receptors. It will be interesting to determine whether ATP secretion by principal cells is also increased after adrenergic and hormonal stimulation. At acidic pH, APs might be less active and promote the presence of luminal ATP that triggers a significant but lower recruitment of V-ATPase to the membrane via P2 receptors.
Summary
We describe here a complex ecto-purinergic regulation of the proton pump V-ATPase via activation of P2 and P1 receptors mediated by luminal ATP and adenosine in clear cells of the epididymis. These findings provide new insights on the regulation of the epididymal acidification process, a fundamental function for the establishment of male fertility.
GRANTS
This work was supported by National Institutes of Health Grants HD-40793 (S. Breton), DK-38452 (S. Breton and D. Brown), and DK-42956 (D. Brown). The Microscopy Core Facility of the Massachusetts General Hospital Program in Membrane Biology receives support from the Boston Area Diabetes and Endocrinology Research Center (DK-57521) and the Center for the Study of Inflammatory Bowel Disease (DK-43341).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid. II. Interaction of pH and a quiescence factor. Biol Reprod 30: 926–935, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochim Biophys Acta 647: 169–176, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 25: 4131–4141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Breton S, Hammar K, Smith PJ, Brown D. Proton secretion in the male reproductive tract: involvement of Cl−-independent HCO3− transport. Am J Physiol Cell Physiol 275: C1134–C1142, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 2: 470–472, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D. The B1 subunit of the H+ATPase is a PDZ domain-binding protein. Colocalization with NHE-RF in renal B-intercalated cells. J Biol Chem 275: 18219–18224, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Brown D, Breton S. H+V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J Exp Biol 203: 137–145, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Brown D, Breton S, Ausiello DA, Marshansky V. Sensing, signaling and sorting events in kidney epithelial cell physiology. Traffic 10: 275–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown D, Smith PJ, Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol 200: 257–262, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Cantiello HF. Electrodiffusional ATP movement through CFTR and other ABC transporters. Pflügers Arch 443, Suppl 1: S22–S27, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Carlin RW, Lee JH, Marcus DC, Schultz BD. Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol Reprod 68: 1027–1034, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3− transporters. Am J Physiol Cell Physiol 290: C1560–C1571, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Carr DW, Usselman MC, Acott TS. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol Reprod 33: 588–595, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Chan HC, Fu WO, Chung YW, Zhou TS, Wong PY. Adrenergic receptors on cultured rat epididymal cells: regulation of Cl− conductances. Biol Reprod 51: 1040–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Chan HC, Ruan YC, He Q, Chen MH, Chen H, Xu WM, Chen WY, Xie C, Zhang XH, Zhou Z. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol 587: 2187–2195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan HC, Zhou WL, Fu WO, Ko WH, Wong PY. Different regulatory pathways involved in ATP-stimulated chloride secretion in rat epididymal epithelium. J Cell Physiol 164: 271–276, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Chan HC, Zhou WL, Wong PY. Extracellular ATP activates both Ca2+- and cAMP-dependent Cl− conductances in rat epididymal cells. J Membr Biol 147: 185–193, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, Chan HC, Shi QX. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol Reprod 80: 115–123, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Da Silva N, Shum WW, Breton S. Regulation of vacuolar proton pumping ATPase-dependent luminal acidification in the epididymis. Asian J Androl 9: 476–482, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Da Silva N, Silberstein C, Beaulieu V, Pietrement C, Van Hoek AN, Brown D, Breton S. Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod 74: 427–438, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Diniz C, Fresco P, Goncalves J. Regional differences in extracellular purine degradation in the prostatic and epididymal portions of the rat vas deferens. Clin Exp Pharmacol Physiol 32: 721–727, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Fedan JS, Hogaboom GK, Westfall DP, O'Donnell JP. Comparison of contractions of the smooth muscle of the guinea-pig vas deferens induced by ATP and related nucleotides. Eur J Pharmacol 81: 193–204, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118: 199–208, 2007 [PMC free article] [PubMed] [Google Scholar]
- 28.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Gallagher JA. ATP P2 receptors and regulation of bone effector cells. J Musculoskelet Neuronal Interact 4: 125–127, 2004 [PubMed] [Google Scholar]
- 30.Gandhi R, Le Hir M, Kaissling B. Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry 95: 165–174, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Haynes JM. A2A adenosine receptor mediated potassium channel activation in rat epididymal smooth muscle. Br J Pharmacol 130: 685–691, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herak-Kramberger CM, Breton S, Brown D, Kraus O, Sabolic I. Distribution of the vacuolar H+ ATPase along the rat and human male reproductive tract. Biol Reprod 64: 1699–1707, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Isnard-Bagnis C, Da Silva N, Beaulieu V, Yu AS, Brown D, Breton S. Detection of ClC-3 and ClC-5 in epididymal epithelium: immunofluorescence and RT-PCR after LCM. Am J Physiol Cell Physiol 284: C220–C232, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol 281: F597–F612, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach WR, Guggino WB, Foskett JK, Engelhardt JF. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J Cell Biol 143: 645–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaunitz JD, Yamaguchi DT. TNAP, TrAP, ecto-purinergic signaling, and bone remodeling. J Cell Biochem 105: 655–662, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439: 737–740, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal 14: 99–108, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Leung GP, Gong XD, Cheung KH, Cheng-Chew SB, Wong PY. Expression of cystic fibrosis transmembrane conductance regulator in rat efferent duct epithelium. Biol Reprod 64: 1509–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Leung GP, Wong PY. Activation of cystic fibrosis transmembrane conductance regulator in rat epididymal epithelium by genistein. Biol Reprod 62: 143–149, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Levine N, Kelly H. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil 52: 333–335, 1978 [DOI] [PubMed] [Google Scholar]
- 43.Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol 213: 557–570, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maenz DD, Chenu C, Breton S, Berteloot A. pH-dependent heterogeneity of acidic amino acid transport in rabbit jejunal brush border membrane vesicles. J Biol Chem 267: 1510–1516, 1992 [PubMed] [Google Scholar]
- 45.Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403: 86–89, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Orgebin-Crist MC. Studies on the function of the epididymis. Biol Reprod 1, Suppl 1: 155–175, 1969 [DOI] [PubMed] [Google Scholar]
- 47.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van Hoek A, Brown D, Breton S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod 66: 1716–1722, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 20: 417–428, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietrement C, Da Silva N, Silberstein C, James M, Marsolais M, Van Hoek A, Brown D, Pastor-Soler N, Ameen N, Laprade R, Ramesh V, Breton S. Role of NHERF1, cystic fibrosis transmembrane conductance regulator, and cAMP in the regulation of aquaporin 9. J Biol Chem 283: 2986–2996, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod 74: 185–194, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reigada D, Mitchell CH. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol 288: C132–C140, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruan YC, Wang Z, Du JY, Zuo WL, Guo JH, Zhang J, Wu ZL, Wong HY, Chung YW, Chan HC, Zhou WL. Regulation of smooth muscle contractility by the epithelium in rat vas deferens: role of ATP-induced release of PGE2. J Physiol 586: 4843–4857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruz R, Andonian S, Hermo L. Immunolocalization and regulation of cystic fibrosis transmembrane conductance regulator in the adult rat epididymis. J Androl 25: 265–273, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev 79: S109–S144, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Schweickhardt C, Sabolic I, Brown D, Burckhardt G. Ecto-adenosinetriphosphatase in rat small intestinal brush-border membranes. Am J Physiol Gastrointest Liver Physiol 268: G663–G672, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81: 1063–1073, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615: 7–32, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Shariatmadari R, Sipila P, Vierula M, Tornquist K, Huhtaniemi I, Poutanen M. Adenosine triphosphate induces Ca2+ signal in epithelial cells of the mouse caput epididymis through activation of P2X and P2Y purinergic receptors. Biol Reprod 68: 1185–1192, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 212: 1753–1761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 135: 1108–1117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol 533: 77–88, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest 99: 2588–2601, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Ven K, Messer L, van der Ven H, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod 11: 513–517, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One 4: e4471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volonte C, D'Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J 276: 318–329, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Wong P. Electrolyte and fluid transport in the epididymis. In: Epithelial Secretion of Water and Electrolytes Heidelberg, Germany: Springer, 1990, p. 333–347 [Google Scholar]
- 73.Wong PY. CFTR gene and male fertility. Mol Hum Reprod 4: 107–110, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Wong PY. Control of anion and fluid secretion by apical P2-purinoceptors in the rat epididymis. Br J Pharmacol 95: 1315–1321, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong PY. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. Am J Physiol Renal Fluid Electrolyte Physiol 254: F121–F133, 1988 [DOI] [PubMed] [Google Scholar]
- 76.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem 278: 13398–13408, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.