Abstract
ADP-ribosylation factor 6 (Arf6) is a small GTPase that influences membrane receptor trafficking and the actin cytoskeleton. In adipocytes, Arf6 regulates the trafficking of the glucose transporter type 4 (GLUT4) and consequently insulin-stimulated glucose transport. Previous studies also indicated a role of Arf6 in adrenergic receptor trafficking, but whether this contributes to the control of lipolysis in adipocytes remains unknown. This was examined in the present study by using RNA interference (RNAi) and pharmaceutical inhibition in murine cultured 3T3-L1 adipocytes. Downregulation of Arf6 by RNAi impairs isoproterenol-stimulated lipolysis specifically but does not alter triacylglycerol (TAG) synthesis or the insulin signaling pathway. Neither total TAG amounts nor TAG fatty acid compositions are altered. The inhibitory effect on lipolysis is mimicked by dynasore, a specific inhibitor for dynamin, which is required for endocytosis. In contrast, lipolysis triggered by reagents that bypass events at the plasma membrane (e.g., forskolin, isobutylmethylxanthine or 8-bromo-cAMP) is not affected. Moreover, Arf6 protein levels in white adipose tissues are markedly increased in ob/ob mice, whereas they are decreased in obesity-resistant CD36 null mice. These changes reflect at least in part alterations in Arf6 mRNA levels. Collectively, these results suggest a role of the endocytic pathway and its regulation by Arf6 in adrenergic stimulation of lipolysis in adipocytes and potentially in the development of obesity.
Keywords: β-adrenergic receptor, trafficking
elevated plasma free fatty acids (FFA) concentrations cause insulin resistance by inhibiting insulin-mediated glucose uptake by skeletal muscle and insulin-mediated suppression of glucose production by the liver (4, 18, 57). Therefore, alterations in adipose tissue lipolytic activity and the rate of FFA release into plasma are important factors in the pathogenesis of obesity-related metabolic abnormalities (36). Adipose tissue lipolytic activity is regulated by hormonal and biochemical signals (6, 16); catecholamines and insulin being the major physiological regulators that stimulate and inhibit adipose tissue lipolytic rates, respectively (6, 11). During energy restriction and endurance exercise, the two major physiological states that require the mobilization of endogenous triglycerides as fuel, lipolysis of adipose tissue triglycerides and release of FFA into plasma, are induced in part by catecholamine stimulated β-adrenergic activation (13, 20). β-Adrenergic receptors (βARs) are G protein-coupled receptors (GPCR) that transmit a stimulatory signal to adenylyl cyclase to increase intracellular cAMP levels. Subsequent activation of protein kinase A (PKA) by cAMP causes phosphorylation-induced activation of hormone-sensitive lipase and its translocation from cytosol to the surface of the adipocyte lipid droplet. PKA also phosphorylates perilipin on the droplet, resulting in biochemical changes that enhance substrate accessibility and lipolysis by hormone-sensitive lipase. Additional signaling pathways including mitogen-activated protein kinase (MAPK), protein kinase C, and AMP-activated protein kinase have been identified to regulate the stimulation of lipolysis in adipocytes (17, 40, 49, 56). In addition to adrenergic stimulation, leptin and tumor necrosis factor (TNF)-α also contribute to enhancing lipolysis in adipocytes (14, 44). Recently, several more lipases [e.g., adipose triacylgylcerol (TAG) lipase, TAG hydrolase, adiponutrin, and GS2] that cause lipolysis of TAG in adipocytes have been identified, suggesting that multiple regulatory pathways are involved in this process (11).
Membrane receptor internalization and trafficking might be important for determining some of the downstream events in the lipolytic pathway (39). After ligand binding, many GPCRs undergo internalization to early endosomes from which they are sorted into recycling or degradative pathways in a highly regulated manner (12, 35). The β1- and β2AR subtypes undergo ligand-induced endocytosis via clathrin-coated vesicles but are then rapidly recycled back to the plasma membrane, restoring surface receptor numbers and function (42, 47). With prolonged agonist exposure, some receptors are redirected to lysosomes for degradation (35), which can result in tachyphylaxis (48). β3AR is less sensitive than the β1 and β2 to catecholamine-stimulated lipolysis and less prone to agonist-promoted internalization (32). Some recent studies demonstrated that distinct signaling pathways of adrenergic stimulation are differentially mediated by receptor trafficking. For example, inhibition of the endocytic machinery blocks β1AR-induced cardiac hypertrophy (37), suggesting receptor internalization is required for access to selected downstream effectors, which might not reside at the plasma membrane/cytosol interface. In contrast, Centaurin-α1-mediated inhibition of β2AR internalization enhances isoproterenol-stimulated cAMP accumulation (27).
The ADP-ribosylation factor 6 (Arf6), a member of the family of Ras-related, low molecular mass (∼20 kDa), GTP-binding proteins, is required for internalization of many membrane receptors, including βARs (22, 27). There are six mammalian Arfs that are grouped into three classes. Arf6 is the sole class III member, which is of interest with regard to GPCR trafficking because it is localized to both plasma membrane and endosomes (10). In this study, we asked whether receptor trafficking influences adrenergic stimulation of lipolysis in adipocytes. We evaluated the importance of Arf6-mediated receptor endocytosis in regulating adipocyte β-adrenergic stimulation of lipolysis and examined whether Arf6 levels may be subject to metabolic regulation.
MATERIALS AND METHODS
Materials.
Fetal calf serum (FCS), calf serum (CS), Dulbecco's modified Eagle's medium (DMEM), and Lipofectamine RNAiMAX were from Invitrogen (Carlsbad, CA) and a small interfering RNA (siRNA) construction kit was from Ambion (Austin, TA). Mouse anti-Arf6 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-pAkt, anti-pMAPK, anti-total Akt and anti-total MAPK antibodies were from Cell Signaling Technology (Beverly, MA). Dynasore was from Scientific Exchange (Center Ossipee, NH). Other reagents were from Sigma (St. Louis, MO).
Cell culture of 3T3-L1 cells and differentiation into the adipocyte phenotype.
3T3-L1 cells were cultured to confluence in DMEM containing 20% calf serum with medium change every 2 days as previously described (52). Two days after cell confluence, differentiation was initiated by adding differentiation medium 1 (0.5 mM methylisobutylxanthine (IBMX), 0.25 μM dexamethasone, 1 μg/ml insulin in DMEM containing 10% fetal bovine serum). Two days later, methylisobutylxanthine and dexamethasone were removed and insulin (1 μ g/ml) was maintained for 2 more days. Thereafter, cells were grown in DMEM containing 10% fetal bovine serum in the absence of differentiating reagents with media replacement every 2 days.
Whole cell protein extraction and immunoblotting.
Whole cell lysates were prepared as previously described (50). Briefly, cell monolayers were washed twice with ice-cold phosphate-buffered saline and lysed at 4°C for 30 min with a lysis buffer containing 50 mM Tris · HCl, pH 7.6, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 1% protease inhibitor cocktail solution (Sigma, St. Louis, MO). The cell lysates were clarified by centrifugation at 10,000 g for 10 min at 4°C before separation by SDS-PAGE and immunoblotting as described (50).
siRNA construction and transfection.
The previously verified siRNA (30) directed against mouse Arf6 were constructed and purified employing the Silencer siRNA construction kit (Ambion) as described (50). Six to 8 days after initiation of differentiation, 3T3-L1 adipocytes were washed twice with PBS before treatment with trypsin-EDTA for 5 min at 37°C. Cells were collected into 10 ml collagenase (0.5 mg/ml) in PBS and spun at 400 g at 4°C in a tabletop centrifuge for 5 min. The collagenase solution was removed and the cells were resuspended into growth media. Cells were then plated in growth medium without antibiotics to obtain ∼70% confluence at the time of transfection. Transfection of siRNA (10 nM final concentration) was performed using Lipofectamine RNAiMAX according to the manufacturer's instructions. A scrambled siRNA (Ambion) was used as a negative control.
Glycerol release assay.
Stimulation of adipocytes and measurement of glycerol release were performed as described previously (15). Before assaying glycerol release, adipocytes were starved in DMEM with 0.5% fatty acid free bovine serum albumin (BSA) for 4 h. The cells were then incubated with 2% BSA in DMEM and treated as indicated before lipolytic stimulation. Lipolysis was assessed from the release of glycerol in the culture medium (56) using free glycerol reagent (Sigma).
Thin layer chromatography.
Adipocytes were incubated with DMEM containing 200 μM [3H]oleic acid/BSA (2:1, 1 μCi/ml) for 1 h. The cells were washed on ice three times with cold PBS containing 0.5% BSA and scraped in 500 μl of PBS. Lipids were extracted by the method of Bligh-Dyer (3) in presence of 50 μg diacylglycerol standard and separated on silica gel 60A plates (Whatman, Clifton, NJ) using a mobile phase of hexane:diethyl ether:acetic acid, 70:30:1. Spots corresponding to major lipid species, identified by standards (trioleoylglycerol and 1-palmitoyl-2-oleoyl-sn-glycerol) run simultaneously and visualized by iodine vapors, were scraped and the radioactivity in each fraction was quantified by liquid scintillation spectrometry.
Quantitative gas chromatography analysis of TAG.
Lipids were extracted by the method of Bligh-Dyer in presence of internal standard (T21:0 TAG, 10 nmol/mg protein) and separated on silica gel 60A plates. Spots corresponding to TAG were visualized with 0.01% rhodamine 6G and identified with TAG standard. The bands were scraped and extracted with chloroform:methanol 3:1. FA methyl esters of the TAG fractions were prepared by reaction with methanol:acetyl chloride 4:1 at 70°C for 1 h. Quantitative gas chromatography (GC) analysis was conducted (Hewlett-Packard 5890 GC, Palo Alto, CA) with a 30 m × 0.32 mm Omegawax 250 column (Sigma) and a flame ionization detector. The injector temperature was kept at 250°C. Helium was used as the carrier gas at constant flow rate of 1 ml/min. The initial temperature of the GC oven was 180°C, held for 2 min, ramped at 10 degree/min to 200°C, held for 2 min, ramped at 4 degree/min to 220°C, held for 15 min, and finally ramped at 70 degree/min to 240°C and kept for 2 min. Instrument response was calibrated using the Supelco 37 Component FAME Mix (Sigma) to relate relative peak areas of FAME peaks to molar ratios of a C21:0 internal standard.
Lipogenesis assay.
Lipogenesis was assayed as previously described (28). Briefly, cells after the indicated treatment were incubated with 5 mM d-[U-14C]glucose (1 μCi per well) for 60 min at 37°C. Cells were then washed on ice three times with cold PBS, scraped into 1 ml of PBS, and shaken vigorously with 5 ml of Betafluor scintillant (National Diagnostics, Manville, NJ). The samples were settled overnight, and radioactivity in the organic phase was determined by liquid scintillation counting.
Protein extraction from mouse white adipose tissues.
Mice were euthanized (CO2 overdose) and epididymal fat pads (WAT) were removed and rapidly frozen in liquid nitrogen. About 50 mg tissue were homogenized with 500 μl lysis buffer by using a tissue homogenizer at maximum speed for 20 s. The homogenates were incubated on ice for 30 min and spun at 10,000 g at 4°C in a tabletop centrifuge for 10 min. The supernatant was transferred to a new tube and stored at −70°C until used for Western blot analysis.
RNA extraction and real-time quantitative PCR.
RNA was extracted from adipose tissue samples by using TRIzol and reverse transcription was performed using the SuperScript III First-strand Synthesis System (Invitrogen). Real-time quantitative PCR assays were performed on an ABI 7500 Fast Real-time PCR System (Applied Biosystems) using SYBR Green PCR Master Mix. Primers used for mouse Arf6 analysis are 5′-GGAGCTGCACCGCATTATCA-3′ and 5′-CTCATGGGGTTTCATGGCATC-3′. The relative mRNA level of Arf6 was quantified and normalized to 36B4 mRNA.
Statistical analyses.
The data are presented as means ± SE and Student's t-test was used in the statistical analysis. A P value < 0.05 was considered statistically significant.
RESULTS
Depletion of Arf6 in 3T3-L1 adipocytes impairs isoproterenol-stimulated lipolysis.
Arf6 mediates agonist-stimulated internalization of a broad array of GPCR including βARs (21). To understand whether receptor internalization plays a role in adrenergic stimulation of lipolysis in adipocytes, 3T3-L1 adipocytes were treated with a previously validated siRNA against Arf6 (30). The efficiency of the siRNA in downregulating Arf6 in differentiated adipocytes is shown in Fig. 1A. Two days after siRNA treatment, the rate of lipolysis was assessed by measuring glycerol release. As shown in Fig. 1B, depletion of Arf6 significantly decreased basal lipolysis (P < 0.05) suggesting a role of Arf6 in regulating nonadrenergic stimulated lipolysis. The adrenergic signaling pathway acting via the βARs represents a prime regulator of triglyceride breakdown in adipocytes. Accordingly, cells were stimulated for 1 h with isoproterenol, a ligand for all three βARs, and a dramatic increase of lipolysis was observed as expected. In contrast, isoproterenol-stimulated lipolysis was inhibited in Arf6-depleted cells (Fig. 1B) (P < 0.05). Similar inhibitory effects were observed when the cells were stimulated with isoproterenol for 10 or 30 min (Fig. 1C). These results suggest that internalization and/or trafficking of βARs may be important for its regulatory function on lipolysis in adipocytes.
Fig. 1.
Depletion of ADP-ribosylation factor-6 (Arf6) inhibits isoproterenol (Iso)-stimulated lipolysis in adipocytes. 3T3-L1 adipocytes were treated with a negative control small interfering RNA (siRNA, NC) or an siRNA-recognizing Arf6. A: 2 days posttransfection, whole cell lysates were prepared and analyzed by immunoblotting (IB) using antibodies as indicated. B: cells were starved and stimulated with 10 μM Iso for 1 h, and glycerol release was assayed and normalized to total protein. *P < 0.05. The data represent means ± SE of four independent experiments. C: cells were starved and stimulated with 10 μM Iso for 10 or 30 min, and glycerol release was assayed and normalized to total protein. *P < 0.05.
Inhibition of dynamin impairs isoproterenol-stimulated lipolysis.
To determine whether Arf6-mediated lipolysis is due to impaired internalization of βARs, independent pharmaceutical inhibition of receptor internalization was employed. Dynamin is required for membrane budding at a late stage during the transition from a fully formed pit to a pinched-off vesicle and may also fulfill other roles during earlier stages of vesicle formation. Activation of dynamin is necessary for AR internalization (23). Dynasore has been recently identified to interfere in vitro with the GTPase activity of dynamin1, dynamin2, and Drp1, the mitochondrial dynamin, but not of other small GTPases (34). Accordingly, this membrane-permeable inhibitor has been widely used to disrupt endocytosis (1, 33). Adipocytes were treated with or without 80 μM dynasore for 20 min before stimulation with isoproterenol for 1 h. Similar to depletion of Arf6, inhibition of dynamin impaired both basal and stimulated lipolysis (Fig. 2A) (P < 0.05). A time-course study showed that isoproterenol-stimulated lipolysis at 10 or 30 min was also inhibited by dynasore (Fig. 2B). These results suggest that receptor endocytosis following βAR activation is critical for its lipolytic effect. After agonist treatment, the βARs have been proposed to leave lipid rafts before internalization via clathrin-coated pits (43, 46). Since both clathrin-dependent and lipid rafts-dependent internalization pathways require dynamin activation, we specifically disrupted lipid rafts by cholesterol depletion as described (8). Adipocytes were treated with the cholesterol-depleting reagent methyl-β-cyclodextrin before stimulation. Disruption of lipid rafts enhanced basal lipolysis, which was suggested to be due to inhibitory effects on phosphodiesterase activity (41). Isoproterenol-stimulated lipolysis was not altered (Fig. 2C), suggesting lipid rafts are not involved in adrenergic-stimulated lipolysis. Collectively, the results using both genetic interference and pharmaceutical inhibition suggested a role of receptor endocytosis in maintaining basal lipolytic rates and in adrenergic stimulation of lipolysis in adipocytes.
Fig. 2.
Inhibition of dynamin, but not depletion of cholesterol, impairs Iso-stimulated lipolysis in adipocytes. A: 3T3-L1 adipocytes were starved and treated with DMSO (control, CTL) or dynasore (80 μM) for 20 min before stimulation with 10 μM Iso for 1 h, and the glycerol release was assayed and normalized to total protein. B: 3T3-L1 adipocytes were starved and treated with DMSO (CTL) or dynasore (80 μM) for 20 min before stimulation with 10 μM Iso for 10 or 30 min, and the glycerol release was assayed and normalized to total protein. C: 3T3-L1 adipocytes were starved and treated with 10 mM methyl-β-cyclodextrin (MCD) for 1 h before stimulation with 10 μM Iso for 1 h and the glycerol release was assayed. *P < 0.05. The data represent means ± SE of three independent experiments.
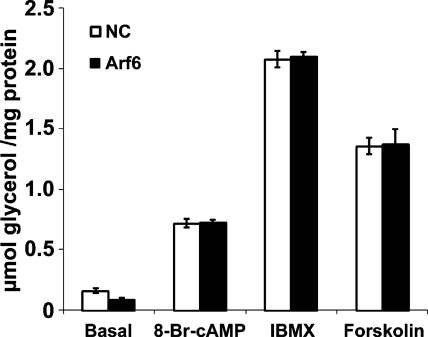
Depletion of Arf6 does not affect lipolysis stimulated by IBMX, forskolin, or bromo-cAMP.
To further confirm the important role of endocytosis in βAR-mediated lipolysis, lipolysis was stimulated by activating pathways downstream of receptor activation. Accordingly, 3T3-L1 adipocytes with or without depletion of Arf6 were stimulated with an adenylyl cyclase activator (forskolin, 10 μM), the phosphodiesterase inhibitor IBMX (0.5 mM), or a cell membrane-permeable cAMP analogue (8-bromo cAMP, 1 mM). Consistent with findings from a previous study, dramatic increases in lipolysis were observed in cells pretreated with negative control siRNA (Fig. 3) (38, 56). Significantly, lipolysis stimulated by forskolin, IBMX, or 8-bromo-cAMP was not affected by depletion of Arf6 (Fig. 3). This result confirms that Arf6 RNAi-mediated inhibition of lipolysis is due to impaired AR trafficking.
Fig. 3.
Arf6 depletion does not affect lipolysis stimulated by IBMX, forskolin, or cAMP analogue. 3T3-L1 adipocytes were treated with a negative control siRNA (NC) or an siRNA recognizing Arf6. Two days posttransfection, cells were starved and stimulated with 1 mM 8-bromo-cAMP (8-Br-cAMP), 0.5 mM IBMX, or 10 μM forskolin for 1 h, and glycerol release was assayed and normalized to total protein. *P < 0.05. The data represent means ± SE of three independent experiments.
Depletion of Arf6 does not affect TAG formation.
We further studied whether TAG formation is affected by Arf6 depletion thus altering the intracellular pool available for lipolysis. Two days after siRNA transfection, cells were incubated with 200 μM [3H]oleic acid, and the partitioning of oleic acid into polar lipid, diacylglycerol, and TAG was studied. The lipid synthesis from fatty acids was not altered by depletion of Arf6 (Fig. 4A). A previous study demonstrated the role of Arf6 on insulin-stimulated trafficking of glucose transporter type 4 (GLUT4) transporter to the plasma membrane and glucose uptake in 3T3-L1 adipocytes (30). We examined whether de novo lipogenesis was altered by Arf6. Cells treated with siRNAs were incubated with [14C]glucose, and lipid radioactivity was measured. Under basal condition, lipogenesis was not inhibited by treatment with Arf6 siRNA (Fig. 4B). However, insulin-stimulated lipogenesis was decreased around 30% (Fig. 4B) (P < 0.05) likely due to the decreased insulin-stimulated glucose uptake as previously demonstrated in the same cell system (30). To further validate that the effect of Arf6 depletion did not reflect a change in the TAG pool, we directly measured the contents and fatty acid compositions of TAG in adipocytes by quantitative GC. Total TAG amounts were not altered by Arf6 depletion (Fig. 4C). Moreover, compositions of the major fatty acids in TAG were also not significantly changed (supplemental Table S.1). These data indicate that the availability of TAG for lipolysis is not altered after Arf6 knockdown in our current experimental system, which is consistent with the observations that lipolysis stimulated by IBMX, forskolin, or bromo-cAMP was not altered in Arf6-depleted cells.
Fig. 4.
Effects of Arf6 depletion on fatty acid (FA) partitioning, lipogenesis, and triacylglycerol (TAG) accumulation. 3T3-L1 adipocytes were treated with a negative control siRNA (NC) or an siRNA recognizing Arf6. A: 2 days posttransfection, cells were incubated with DMEM containing 200 μM [3H]oleic acid/BSA (2:1, 1 μCi/ml) for 1 h. FA partitioning into TAG, diacylglycerol (DAG), and polar lipids (PL) was measured and normalized to total protein. B: cells were starved and stimulated with 10 nM insulin for 15 min. Lipogenesis were assayed using 5 mM d-[U-14C]glucose (1 μCi per well) and normalized to total protein. *P < 0.05. Results are the means ± SE of three independent experiments. C: total lipids were extracted and TAG were measured by quantitative gas chromatography and normalized to total protein. Results are means ± SE of three independent experiments.
Depletion of Arf6 does not affect insulin signaling.
Insulin signaling is a major physiological regulatory pathway that inhibits lipolysis in adipocytes. During fasting, lipolysis is initiated mainly by diminution of insulin signaling (24). Since both Arf6 depletion and insulin stimulation lead to inhibition of adrenergic-stimulated lipolysis, we explored whether these two pathways overlap. First, we confirmed the antilipolytic effect of insulin in our model system. As anticipated, insulin inhibits isoproterenol-stimulated lipolysis (Fig. 5A). We next studied the effects of Arf6 depletion on insulin signaling. 3T3-L1 adipocytes treated with negative control siRNA or siRNA-recognizing Arf6 were serum starved before stimulation with 0, 1, or 10 nM insulin for 10 min. Total cell lysates were prepared in the presence of protease and phosphatase inhibitors and analyzed by Western blot analysis. Knockdown of Arf6 had no significant effect on insulin-stimulated protein kinase B (PKB)/Akt activation (Fig. 5B). Moreover, insulin-stimulated MAPK activation was also not affected by siRNA against Arf6 (Fig. 5B). This result is consistent with our previous finding that inhibition of insulin receptor internalization by depletion of dynamin has no effect on insulin-stimulated PKB/Akt and MAPK activation (51). Since the inhibitory effect of insulin on lipolysis in adipocytes is mainly due to the activation of phosphodiesterase by PKB/Akt (25), this result suggests that Arf6 depletion-mediated inhibition of adrenergic-stimulated lipolysis is independent of that of insulin signaling.
Fig. 5.
Arf6 depletion does not alter insulin signaling. A: 3T3-L1 adipocytes were starved and treated with 10 nM insulin (Ins) for 15 min before stimulation with 10 μM Iso for 1 h, and the glycerol release was assayed and normalized to total protein. *P < 0.05. The data represent means ± SE of three independent experiments. B: 3T3-L1 adipocytes were treated with negative control siRNA (NC) or an siRNA recognizing Arf6. Cells were starved and stimulated with Ins at indicated concentrations for 10 min. The whole cell lysates were prepared and subjected to immunoblot (IB) analysis utilizing antibodies recognizing pAkt (Ser473), total Akt (tAkt), pMAPK, and total MAPK (tMAPK). Results are representative of three independent experiments.
Regulation of white adipose tissue Arf6 levels in obesity-prone and -resistant mice.
To gain insight into the potential physiological role of Arf6 in regulating the mobilization of endogenous adipose tissue triglycerides and release of FFA into plasma, we investigated regulation of white adipose tissue (WAT) Arf6 in several obesity-prone or -resistant mouse models. Arf6 levels were found to be significantly increased in ob/ob mice (Fig. 6A). Since aging is correlated with increased adipocity, we compared levels of Arf6 in WAT from 30- and 10-wk-old mice. Interestingly, Arf6 levels dramatically rose in the older mice (Fig. 6B). CD36 null mice have smaller adipose tissue mass and are protected from high-fat diet-induced obesity (19). In contrast to obese mice, Arf6 levels were dramatically decreased in CD36 null mice (Fig. 6C). Consistent with this, Arf6 mRNA levels were significantly increased in WAT from ob/ob mice and decreased in CD36 null mice, suggesting transcriptional regulation of Arf6 in mouse WAT (Fig. 6D). Collectively, these results demonstrate positive correlation of Arf6 protein and mRNA levels with adiposity. Considering the inhibitory effects of Arf6 depletion on lipolysis, regulation of Arf6 levels may represent a novel feedback mechanism to mobilize TAG in adipose tissues.
Fig. 6.
Regulation of Arf6 levels in mouse white adipose tissues (WAT). Total proteins from WAT from mice as indicated in the figure were extracted and analyzed using antibody-recognizing Arf6 with tubulin used as a loading control. A: wild-type (WT) and ob/ob mice. B: 10-wk and 30-wk-old mice. C: WT and CD36 null mice. D: RNA of WAT from WT, ob/ob and CD36 null (Null) mice was extracted and Arf6 levels were measured by real-time quantitative PCR. *P < 0.05 (compared with WT). The data represent means ± SE (n = 4).
DISCUSSION
The data from this study provide evidence that the ADP-ribosylation factor 6, Arf6, plays a role in regulating basal lipolysis and its β-adrenergic activation in adipocytes. First, depletion of Arf6 by RNAi-mediated gene knockdown in 3T3-L1 adipocytes decreased both basal and isoproterenol-stimulated lipolysis. Second, pharmaceutical inhibition of dynamin, which blocks receptor internalization, had similar effects. Third, the increase in lipolytic activity stimulated by reagents that bypass receptor activation was not affected by depletion of Arf6. Fourth, Arf6 depletion and insulin signaling are independent pathways leading to inhibition of β-adrenergic-stimulated lipolysis in adipocytes. Fifth, Arf6 levels are dramatically altered in adipose tissues from obese or obesity-resistant mice. Collectively, these data demonstrate that Arf6 regulation of βAR trafficking is a novel mechanism that modulates basal and stimulated adipocyte lipolytic activity.
Arf6 depletion or pharmaceutical inhibition of dynamin impairs β-adrenergic-stimulated lipolysis in adipocytes (Fig. 1 and 2), suggesting that the effect of Arf6 depletion on adrenergic-stimulated lipolysis is due to impaired receptor internalization, which is an early event preceding receptor recycling. This is supported by our results that depletion of Arf6 or inhibition of dynamin decreases lipolysis after shorter term stimulation of isoproterenol (10 and 30 min) (Figs. 1C and 2B). Interestingly, depletion of Arf6 or inhibition of dynamin also inhibits basal lipolysis. Under basal condition, βARs can be constitutively internalized (45), and this could also be necessary for full lipolytic activity of the receptor. Alternatively, Arf6 and dynamin may mediate other βAR independent pathways that could play roles in lipolysis under basal condition without adrenergic stimulation.
Isoproterenol binds to all three βAR subtypes in adipocytes, and it is likely that the functional readout reflects their combined effects. However, the events that follow ligand receptor interaction have been best studied in the case of the β2AR. Ligand binding induces receptor internalization, which is followed by attenuation of signaling (desensitization) and receptor resensitization. (29). Desensitization is initiated at the plasma membrane with receptor phosphorylation by multiple protein kinases, including PKA and members of the GPCR kinase family, which ultimately results in receptor uncoupling from the stimulatory G protein (55) and in its trafficking to early endosomes via clathrin-coated vesicles (26). After dephosphorylation and resensitization on early endosomes, the receptor is then recycled back to the plasma membrane for renewed activation via Rab4-mediated rapid recycling (47) or Rab11-mediated slow recycling pathways (42). Internalization of the β2AR is dependent on activities of both Arf6 and dynamin. Further studies that disrupt different components of the endocytic and recycling pathways will be needed to determine the specific trafficking event(s) needed for β2AR lipolytic function.
The molecular details for the trafficking, signaling attenuation, and resensitization of β1AR and β3AR are less clear. Similar to β2AR, β1AR is rapidly internalized upon ligand binding, and its signaling capacity is attenuated (31). In contrast, β3AR seems less prone to these regulatory processes (32). The coexpression of all three subtypes of βAR in adipocytes suggests that coordination of the different receptors is required to finely regulate and achieve the full lipolytic action of catecholamine in adipocytes. This is supported by the presence of a hetero-oligomerizaion complex of β2AR and β3AR with unique signaling property (5). Signaling and lipolytic activity of the three βAR subtypes may be differentially regulated by Arf6 and dynamin, which will have to be determined. There are multiple signaling pathways leading to lipolysis in adipocytes, and it is possible that internalization and intracellular trafficking of the βARs will provide pathway selectivity. Therefore, the regulation of distinct signaling pathways resulting in the activation of different lipases (e.g., adipose TAG lipase, TAG hydrolase, Adiponutrin, and GS2) could involve vesicular trafficking.
The capacity of whole adipose tissues to release FFA after adrenergic stimulation integrates multiple factors, which include: 1) numbers of fat cells and total TAG accumulation in individual cells; 2) expression and functional activity of adrenergic receptors; and 3) other signaling pathways (e.g., insulin signaling). Ob/ob mice exhibit greater rates of basal and adrenergic-stimulated lipolysis in whole adipose tissues via an increase in fat cell number and intracellular TAG content and impaired insulin signaling (2, 53, 54). In contrast, adipocytes from other obesity models have impaired expression and activities of βARs (9) and higher levels of antilipolytic α2 adrenoceptors (7), indicating that net lipolytic capacity of adipose tissues is balanced by both stimulatory and inhibitory regulating factors. Our results suggest a role of Arf6 in basal and adrenergic-stimulated lipolysis in adipocytes. Moreover, WAT Arf6 levels are increased in ob/ob mice and aged mice but are decreased in lean CD36 null mice documenting a positive correlation with body fat mass (Fig. 6). These data suggest that Arf6 is likely to play an important role in chronic maintenance of lipolytic rates as well as in the acute regulation of these rates by adrenergic stimulation. In addition to its effects in the endocytic pathway, Arf6 also regulates a clathrin-coated complex for insulin-stimulated recycling of GLUT4 (30). Depletion of Arf6 or clathrin heavy chain impairs insulin-stimulated glucose uptake in adipocytes, suggesting a role of Arf6 in glucose homeostasis (30). Therefore, regulation of Arf6 could be a key factor balancing glucose and fatty acid metabolism.
Collectively, the results from the present study document that Arf6 is a novel regulator of adrenergic-stimulated and basal lipolysis via its influence on receptor trafficking. The marked change of Arf6 levels as a function of adiposity suggests that it has an important function in regulating lipolysis in vivo. Additional studies are needed to determine whether targeting Arf6-mediated lipolysis can alter the rate of FFA release from adipocytes as a therapeutic approach to modulate lipolytic rates and manage obesity.
GRANTS
This work was supported by a Pilot and Feasibility award and by the Adipocyte Biology Core from the Washington University Clinical Nutrition Research Unit (DK-56341 to X. Su), by National Institutes of Health Grants R21DK-082951 (to X. Su) and R01DK-033301 (to N. Abumrad), and by a Scientist Development Grant from the American Heart Association (835140N to X. Su).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Sam Klein and Pin Yue for critical reviews of the manuscript and for helpful suggestions.
REFERENCES
- 1.Abban CY, Bradbury NA, Meneses PI. HPV16 and BPV1 infection can be blocked by the dynamin inhibitor dynasore. Am J Ther 15: 304–311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bederman IR, Previs SF. Hormonal regulation of intracellular lipolysis in C57BL/6J mice: effect of diet-induced adiposity and data normalization. Metabolism 57: 1405–1413, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 4.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest 101: 1094–1101, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breit A, Lagace M, Bouvier M. Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J Biol Chem 279: 28756–28765, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal 18: 401–408, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Carpene C, Rebourcet MC, Guichard C, Lafontan M, Lavau M. Increased alpha 2-adrenergic binding sites and antilipolytic effect in adipocytes from genetically obese rats. J Lipid Res 31: 811–819, 1990 [PubMed] [Google Scholar]
- 8.Chamberlain LH, Gould GW. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem 277: 49750–49754, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol 8: 518–527, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem 278: 41573–41576, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr 27: 79–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24, 2001 [PubMed] [Google Scholar]
- 13.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition 22: 830–844, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Fruhbeck G, Aguado M, Gomez-Ambrosi J, Martinez JA. Lipolytic effect of in vivo leptin administration on adipocytes of lean and ob/ob mice, but not db/db mice. Biochem Biophys Res Commun 250: 99–102, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab 19: 3–9, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem 276: 45456–45461, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr 72: 558S––563S., 2000 [DOI] [PubMed] [Google Scholar]
- 21.Houndolo T, Boulay PL, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem 280: 5598–5604, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hunzicker-Dunn M, Gurevich VV, Casanova JE, Mukherjee S. ARF6: a newly appreciated player in G protein-coupled receptor desensitization. FEBS Lett 521: 3–8, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Iyer V, Tran TM, Foster E, Dai W, Clark RB, Knoll BJ. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol 147: 249–259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein S, Peters EJ, Holland OB, Wolfe RR. Effect of short- and long-term β-adrenergic blockade on lipolysis during fasting in humans. Am J Physiol Endocrinol Metab 257: E65–E73, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. CR Biol 329: 598–607, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA 96: 3712–3717, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence J, Mundell SJ, Yun H, Kelly E, Venkateswarlu K. Centaurin-alpha 1, an ADP-ribosylation factor 6 GTPase activating protein, inhibits beta 2-adrenoceptor internalization. Mol Pharmacol 67: 1822–1828, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem 270: 20801–20807, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV, Hsu VW. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol 178: 453–464, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang W, Curran PK, Hoang Q, Moreland RT, Fishman PH. Differences in endosomal targeting of human (beta)1- and (beta)2-adrenergic receptors following clathrin-mediated endocytosis. J Cell Sci 117: 723–734, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ. Structural basis for receptor subtype-specific regulation revealed by a chimeric beta 3/beta 2-adrenergic receptor. Proc Natl Acad Sci USA 90: 3665–3669, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu W, Ma H, Sheng ZH, Mochida S. Dynamin and activity regulate synaptic vesicle recycling in sympathetic neurons. J Biol Chem 284: 1930–1937, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci 28: 369–376, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Martins IJ, Redgrave TG. Obesity and post-prandial lipid metabolism. Feast or famine? J Nutr Biochem 15: 130–141, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Morisco C, Marrone C, Galeotti J, Shao D, Vatner DE, Vatner SF, Sadoshima J. Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes. Cardiovasc Res 78: 36–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett 439: 287–290, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res 98: 743–756, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Nakamura J. Protein kinase C attenuates beta-adrenergic receptor-mediated lipolysis, probably through inhibition of the beta1-adrenergic receptor system. Arch Biochem Biophys 447: 1–10, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Nilsson R, Ahmad F, Sward K, Andersson U, Weston M, Manganiello V, Degerman E. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal 18: 1713–1721, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the beta2-adrenergic receptor through a direct interaction. Biochem J 418: 163–172, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem 275: 41447–41457, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, Lonnqvist F, Arner P. Mapping of early signaling events in tumor necrosis factor-alpha -mediated lipolysis in human fat cells. J Biol Chem 277: 1085–1091, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Scarselli M, Donaldson JG. Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J Biol Chem 284: 3577–3585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwencke C, Okumura S, Yamamoto M, Geng YJ, Ishikawa Y. Colocalization of beta-adrenergic receptors and caveolin within the plasma membrane. J Cell Biochem 75: 64–72, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem 275: 27221–27228, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Small KM, Brown KM, Forbes SL, Liggett SB. Modification of the beta 2-adrenergic receptor to engineer a receptor-effector complex for gene therapy. J Biol Chem 276: 31596–31601, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Soeder KJ, Snedden SK, Cao W, Della Rocca GJ, Daniel KW, Luttrell LM, Collins S. The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J Biol Chem 274: 12017–12022, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Su X, Kong C, Stahl PD. GAPex-5 mediates ubiquitination, trafficking, and degradation of epidermal growth factor receptor. J Biol Chem 282: 21278–21284, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Su X, Lodhi IJ, Saltiel AR, Stahl PD. Insulin-stimulated Interaction between insulin receptor substrate 1 and p85alpha and activation of protein kinase B/Akt require Rab5. J Biol Chem 281: 27982–27990, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Su X, Mancuso DJ, Bickel PE, Jenkins CM, Gross RW. Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3–L1 preadipocytes. J Biol Chem 279: 21740–21748, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Turner SM, Roy S, Sul HS, Neese RA, Murphy EJ, Samandi W, Roohk DJ, Hellerstein MK. Dissociation between adipose tissue fluxes and lipogenic gene expression in ob/ob mice. Am J Physiol Endocrinol Metab 292: E1101–E1109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 141: 3388–3396, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Vaughan DJ, Millman EE, Godines V, Friedman J, Tran TM, Dai W, Knoll BJ, Clark RB, Moore RH. Role of the G protein-coupled receptor kinase site serine cluster in beta2-adrenergic receptor internalization, desensitization, and beta-arrestin translocation. J Biol Chem 281: 7684–7692, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3–L1 adipocytes. J Biol Chem 278: 43074–43080, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93: 870–876, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.