Abstract
Niemann-Pick C (NPC) disease is an autosomal recessive, lethal, neurodegenerative disorder caused by mutations in NPC1. Using the glial fibrillary acidic protein (GFAP) promoter, we demonstrated previously that astrocyte-specific expression of Npc1 decreased neuronal storage of cholesterol in Npc1−/− mice, reduced numbers of axonal spheroids, and produced less degeneration of neurons, reactive astrocytes, and loss of myelin tracts in the central nervous system. GFAP-Npc1, Npc1−/− mice exhibited markedly enhanced survival and death was not associated with the severe terminal weight loss observed in Npc1−/− mice. Intestinal transit is delayed in Npc1−/− mice, but is normal in GFAP-NPC1, Npc1−/− until late in the course of their disease. Since glia play an important role in the enteric nervous system, we studied morphology and cholesterol content of intestines from Npc1−/− mice and examined the effect of GFAP-promoted restoration of Npc1 in enteric glia. While the number of neurons was not altered, the total amount of cholesterol stored in the small intestine was decreased, as was the number of neurons with inclusions and the number of inclusions per neuron. We conclude that expression of Npc1 by enteric glial cells can ameliorate the enteric neuropathology and we speculate that dysfunction of the enteric nervous system contributes to the retarded intestinal transit, weight loss, and demise of Npc1−/− mice.
Keywords: Niemann-Pick C, neurodegeneration, fibrillary astrocytes, enteric glia, transgenics
INTRODUCTION
Niemann-Pick (type C) disease is a pan-ethnic autosomal recessive disorder of only partially known pathogenesis (Vincent et al., 2003). Npc1−/− mice are a near-perfect model of the human disease in that they exhibit similar biochemical deficits and develop identical visceral and central nervous system (CNS) pathology, including lipid inclusions in neurons and other cell types (Shio et al., 1982; Loftus et al., 1997). Npc1−/− mice die prematurely and exhibit degeneration of neurons in the brain and a marked terminal decline in body weight (Zhang et al., 2008). Although weight loss in Npc1−/− mice might be secondary to lack of eating and drinking as a result of poor somatic neuromuscular coordination, loss of Npc1 in the enteric nervous system (ENS) might contribute to the morbidity and/or mortality by impairing gastrointestinal physiology. Lipid storage in enteric neurons is a common finding in patients with NPC (Gilbert et al., 1981), many of whom have diarrhea, which could reflect ENS dysfunction (Dinari et al., 1980; Patterson et al., 2001).
In the present study, we investigate the enteric neuropathology of Npc1−/− mice and examine the effects of glial Npc1 expression on the survival and morphology of enteric neurons. The pathogenesis of CNS neuropathology has been studied in Npc1−/− mice and recent investigations suggest that astrocyte Npc1 activity may modulate CNS disease. It has been established that the neurological degeneration seen in Npc1−/− is intrinsic to the CNS, as it can be prevented by transgenic reintroduction of wild-type Npc1 in multiple CNS tissues (Loftus et al., 2002). We have also shown that CNS neurodegeneration in Npc1−/− mice can be ameliorated by selective restoration of wild-type Npc1 under control of the glial acidic fibrillary protein (GFAP) promoter, indicating that Npc1 expression in fibrillary astrocytes is sufficient to decrease neuronal lipid storage and prolong neuronal survival in the brain (Zhang et al., 2008). We hypothesized that similar neurodegenerative change might occur in the ENS of the Npc1−/− mouse and that this might also be ameliorated by GFAP-promoter driven expression of wild-type Npc1.
MATERIALS AND METHODS
Mice
GFAP-Npc1 transgenic mice have been described (Zhang et al., 2008). We exclusively used the GFAPENpc1 transgenic line for the work described in this report. The transgenic mice were crossed to Npc1+/− carriers maintained on a BALB/cJ background. GFAP-Npc1, Npc1+/− are crossed to the Npc1+/− mice maintained on a BALB/cJ background, and GFAP-Npc1-positive Ncp1−/− animals were studied (about one-eighth of offspring since the transgene and Ncp1 are not linked; the background is thus three-fourths BALB/cJ).
Materials
The NPC1 antibody, generated against amino acids 1254-1273 (NKAKSCATEERYGTERER) of the human NPC1 protein, was custom made and purchased from Invitrogen Corporation (Carlsbad, CA). The β-actin antibody was purchased from Sigma Chemical Company (St. Louis, MO). Peroxidase conjugated goat secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The West Pico SuperSignal Substrate for Western Blotting and Bicinchoninic Acid (BCA) protein assay kits were purchased from Pierce Chemical Company (Rockford, IL).
Transit Time
Mice were transferred from standard chow pellets to powdered chow with 10% charcoal in the morning, with or without overnight fasting (separate experiments). They were observed approximately every 30 minutes and times were recorded when black stools appeared. Mice were timed twice with the average being used as one measurement.
Immunoblot analysis
The relative amounts of NPC1 protein in the large intestine was determined using immunoblot analysis. The large intestine was homogenized in RIPA buffer (20 mM Tris pH 8.0, 2 mM EDTA, 150 mM NaCl; 1% Triton X-100, 10 mM SDS) using glass-Teflon homogenizer. Protein samples were separated using 7% SDS-PAGE under reduced conditions and then transferred to a nitrocellulose membrane. In brief, blocking buffer (10 mM sodium phosphate pH 7.4, 150 mM NaCl, 0.05% Tween 20, and 5% non-fat dry milk) was used to block non-specific sites on the nitrocellulose membrane (2 h). The membranes were then incubated in blocking buffer containing the appropriate dilution of primary antibody (4°C for 16 hr). The membranes were rinsed with blocking buffer (3 × 10 min) to remove residual primary antibody and then incubated in blocking buffer containing the appropriate dilution of peroxidase-conjugated goat secondary antibody (90 min). The membranes were rinsed with blocking buffer (3 × 10 min) to remove residual secondary antibody and enhanced chemiluminescence was performed to obtain autoradiograms.
Light Microscopy
The entire gastrointestinal tract from the esophagus to the anus was removed by dissection and fixed in fresh 4% paraformaldehyde in phosphate buffered saline (PBS) for at least 4 hours. The tissues were washed several times with PBS and shifted to 70% ethanol (with intermediate changes in 30% and 50%) ethanol. 5-mm long segments of stomach, distal ileum, proximal colon and rectum were embedded in paraffin and used to prepare 4-μm-thick paraffin sections. Some of the latter were stained with hematoxylin and eosin. Others were immunostained with an antibody that recognizes the pan-neuronal marker, Hu (Molecular Probes, Eugene, OR) (Phillips et al., 2004). An automated immunostainer (Benchmark XT, Ventana Medical Systems) was used with the immunostainer’s “standard” conditions (60 minutes, cell conditioner 1) and a 32 minute incubation period with a 1:1250 dilution of the primary antibody. Slides were counterstained with hematoxylin.
Neuronal Counts
From each paraffin-embedded segment of distal ileum, proximal colon, or rectum, a total of 10 transverse, 4 μm-thick sections, separated by no less than 50 microns, were cut and immunostained with anti-Hu. For myenteric ganglia, the number of neuronal cell bodies (any immunoreactive perikaryon located between the two layers of the muscularis propria that measured larger than the nuclei of adjacent smooth muscle cells) in the myenteric plexus were counted around the entire bowel circumference. For submucosal ganglia in the proximal colon, neuronal cell bodies in the complete circumference of 10 random sections were counted. All sections were coded and evaluated without knowledge of the animal’s genotype.
Electron Microscopy
Several 1-mm3 pieces of large intestinal wall were fixed in 3% glutaraldehyde for several hours before transfer to 70% ethanol and embedding in plastic resin. One-micron-thick sections were prepared and stained with toluidine blue. Thin sections were stained with osmium tetroxide and uranylic acid and examined with a Zeiss 910 electron microscope. At least 10 myenteric ganglia and multiple randomly selected fields of muscularis propria, submucosa, and mucosa were examined and imaged digitally. Samples were coded such that genotypes were not known until after ultrastructural data had been collected.
Cholesterol Determinations
Mouse tissues were homogenized in hypotonic buffer (20mM Tris pH 8.0 2mM EDTA) using a plastic piston. Cholesterol was extracted using a modified method by Peter and Reynolds (1977). In brief, samples were mixed with 500 μl of extraction mixture (Isopropanol:Water:10M NaOH – 250:125:10) and 10 μl of internal standard α-cholestane (1 mg/ml) was added and vortexed for 1 minute. Then 200 μl of n-octane was added and mixed on rocking platform for 20 min followed by centrifugation for 5 min at 5,000 × g. 150 μl of the octane layer was collected in the GC glass vial and evaporated. Samples were then derivatized to trimethylsilyl ethers using 30 μl BSTFA with 1% TMCS (100°C, 30 minutes) (Pierce, Rockford, IL). All samples were processed in duplicates. Samples were analyzed using a HP5890/HP5970 (Hewlett-Packard Co., Avondale, PA) GC/MS equipped with an SPB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm) (Supelco, Bellefonte, PA, USA) as previously described by Ubhayasekera et al. (2004) Data was acquired and evaluated using an MSD Productivity ChemStation Software (Agillent Technologies, CA, USA).
RESULTS
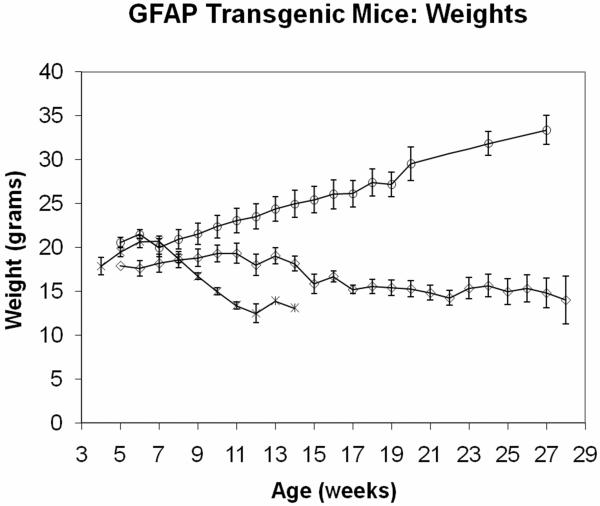
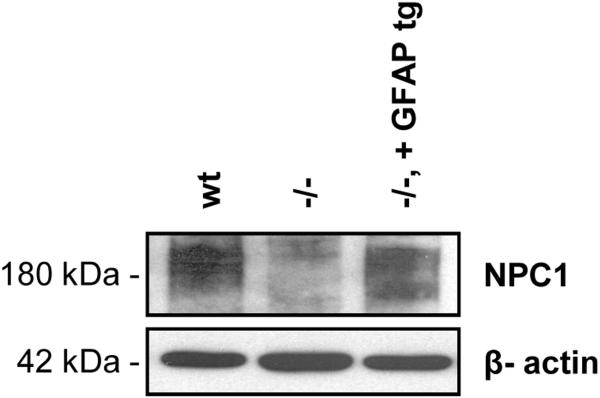
As depicted in Figure 1, GFAP-Npc1, Npc1−/− mice maintain their weight much closer to that of Npc1+/+ mice, in marked contrast to Npc1−/− mice, which loose nearly half of their maximal weight by their time of death (or euthanasia, performed when males are less than 11 grams and females are less than 10 grams). Western immunoblot analysis showed near normal restoration of Npc1 in the large intestine of GFAP-Npc1, Npc1−/− mice (Figure 2; perhaps because of the large amount of proteolytic activity present, we were not successful with Western immunoblots of small intestine samples). The transit time measured with charcoal added to the diet and with the frequent daytime “arousals” caused by q. 30 min. observations (mice are nocturnal) showed lengthened transit time in the Npc1−/− mice compared to controls, Table I. The GFAP-Npc1, Npc1−/− mice showed normal transit times until late in the course when their transit times lengthened to that of Npc1−/− mice, Table I. Although fasting increased transit times, the relative differences remained. Similar amounts of food, expressed as % body weight, were consumed by the three genotypes. However, most of the mice have “wet bottom,” a sign of diarrhea or continuous micturition, at the terminal stage.
Figure 1.
Weights of GFAP-Npc1, Npc1−/− (diamonds), Npc1−/− mice (asterisks), and Npc1+/+ (circles) mice. The error bars provide standard deviations. (Modified from Zhang et al., 2008 with additional data.)
Figure 2.
Western blot analysis of Npc1 expression in large intestine. The diffuse band at 180 kDa is caused by glycosylation of Npc1. β-actin is used as a loading control.
Table I.
Intestinal Transit Times in Npc+/+ or +/−, Npc1−/− and GFAP-Npc1, Npc1−/− mice
| GFAP-Npc1, Npc1−/− |
||||
|---|---|---|---|---|
| Genotype | Npc1+/+ or +/− | Npc1−/− | □ 100 days | □ 100 days |
| Transit time without fasting | (4) 192.5 ± 27.3† | (3) 296.7 ± 37.9* | (3) 202.7 ± 13.7 | (3) 331.7 ± 47.3** |
| Transit time with fasting | (4) 111.9 ± 5.9 | (3) 144.2 ± 2.2* | (2) 111.8 ± 1.3 | ND |
| % body weight consumed during study |
(4) 4.3 ± 0.5 | (3) 6.05 ± 0.44†† | (2) 4.3 ± 0.75 | ND |
(n) ave. ± std. error
p □ 0.01 vs. Npc1+/+ or +/−
p □ 0.01 vs. □ 100 days
p □ 0.05 vs. Npc1+/+ or +/−
ND not determined
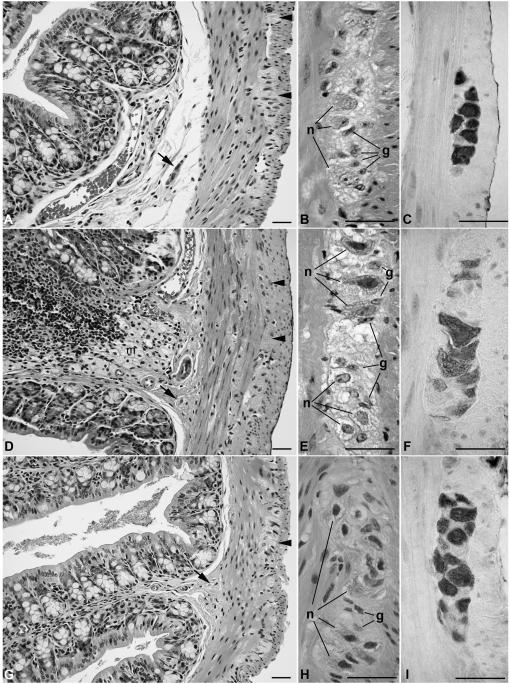
The gastrointestinal tracts of GFAP-Npc1, Npc1−/− and Npc−/− mice were indistinguishable grossly from wild-type animals and light microscopic examination of stomach, small intestine, and colon revealed intact bowel wall architecture with no inflammation or necrosis (Figure 3A-C). However, multifocal collections of “foamy” histiocytes with vacuolated cytoplasms were present in the submucosa of GFAP-Npc1, Npc1−/− and Npc−/− animals (Figure 3D). In addition, vacuolar inclusions were readily identified in the myenteric and submucosal ganglia and intermuscular nerves of Npc1−/− specimens, in contrast to Npc1+/− controls. In H&E-stained sections, inclusions appeared to be primarily within neurons, and to spare enteric glial cells (Figure 3E). The GFAP-Npc1 transgene ameliorated most of the neuronal pathology, although enlargement ganglion cell bodies and mild vacuolar change characteristic of Npc1−/− persisted in some neurons (Figure 3H). Apart from macrophages and neurons, no inclusions were observed in other cell types, including visceral smooth muscle, endothelial cells, or crypt or villus epithelium.
Figure 3.
Light microscopic appearance of H&E-stained and anti-Hu immunostained sections of colon from Npc1+/− (A-C), Npc1−/− (D-F), and GFAP-Npc1, Npc1−/− (G-I). Low magnification images (A, D, G) of the gut wall and higher magnification of myenteric ganglia illustrate a similar distribution of myenteric (arrowheads) and submucosal (arrows) ganglia in the three genotypes. Foamy macrophages were present focally in the submucosa of Npc1−/− (m in panel D) and GFAP-Npc1, Npc1−/− (not shown), as opposed to Npc1+/− controls (A). In Npc1−/− samples (D, E), and to a lesser degree in GFAP-NPC1, Npc1−/− mice (G, H), neuronal cell bodies (n) and nerves in both plexuses, as well as intermuscular nerves (i) appear vacuolated and enlarged. In contrast, glia (g) do not appear affected in any of the three genotypes. Hu immunoreactivity identifies the perikarya of myenteric neurons, which are enlarged and vacuolated in Npc1−/− samples, in contrast with the other genotypes. Scale bars = 20 μm.
Neuronal counts from myenteric ganglia were performed on sections of distal ileum, proximal colon and rectum and from submucosal ganglia in proximal colon from Npc1−/− and Npc1+/+ littermates at age 70 – 78 days but 117 day old GFAP-Npc1, Npc1−/− mice (the later time for the GFAP-Npc1, Npc1−/− mice chosen to be closer to their demise, i.e. at a more comparable stage of disease progression). Sections were immunostained with antibodies that recognize the pan-neuronal marker Hu, which highlighted perikarya of enteric neurons so that they were easily resolved from adjacent glial cells (Figure 3). Enlargement and vacuolization of ganglion cells in Npc1−/− samples was clearly evident in these immunostained sections (Figure 3F). Myenteric ganglion cell counts were normalized to circumference to compensate for potential effects of gut distension. No difference in the mean number of neuronal bodies per circumference was observed between the 3 genotypes (Table II), for each of the 3 intestinal regions in myenteric ganglia and in the proximal colon for submucosal ganglia.
Table II.
Number of Ganglion Cells in the Myenteric and Submucosal Plexus of Npc1+/+, Npc1−/−, and GFAP-Npc1, Npc1−/− mice
| GANGLION CELLS PER CIRCUMFERENCE | ||||
|---|---|---|---|---|
| Myenteric Plexus |
Submucosal Plexus | |||
| Distal Ileum | Proximal Colon | Rectum | Proximal Codon | |
| Npc1 +/+ | 55 ± 24† | 106 ± 21 | 53 ± 27 | 23.5 ± 7.2 |
| Npc1 −/− | 55 ±24′ | 103 ± 3 | 47 ± 7 | 18.6 ± 7.0 |
| GFAP-Npc1, Npc1−/− | 50 ± 29 | 100 ± 30 | 34 ±11 | 15.5 ± 7.9 |
mean ± std. dev.; none of the differences between genotypes were significant (Student T Test; p>0.05); 3 animals per group. Calculations based on the observed standard deviations and sample sizes indicate that differences in mean values greater than 38% must be present for a 90% likelihood of statistical significance (power = 90%).
The average numbers per circumference of bowel (used as the denominator to control for distension) are expressed in the table.
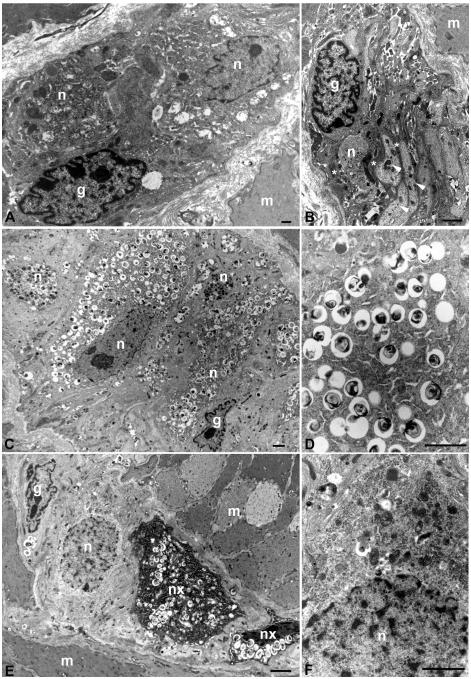
Electron microscopic evaluation of samples from the same set of mice used in the light microscopic studies showed extensive neuronal inclusions in enteric ganglion cells of Npc1−/− animals, which were not present in GFAP-Npc1, Npc1−/− mice (Figure 4). The perikarya of virtually myenteric and submucosal ganglion cell in Npc1−/− mice were markedly enlarged due to the accumulation of abundant membrane-bound cytoplasmic vacuoles, which contained a mixture of membranous and amorphous material. Inclusions were not identified in non-neuronal cell types such as smooth muscle, endothelium, intestinal epithelium, or enteric glial cells. Ultrastructural differences between GFAP-Npc1, Npc1−/− and Npc1−/− mice were dramatic. In the former, far fewer neurons had any inclusions and the abundance of inclusions per neuron was much less overall, although even in GFAP-Npc1, Npc1−/− mice, rare neurons could be found with fairly numerous inclusions. This heterogeneity differed markedly from the pan-neuronal changes seen in the Npc1−/− mice.
Figure 4.
Ultrastructural appearance of myenteric ganglia in Npc1+/− (A, B), Npc1−/− (C, D), and GFAP-Npc1, Npc1−/− (E, F) mice. (A) Normal features of myenteric ganglia include intimately associated neurons (n) and glial cells (g) with mitochondrial, endoplasmic reticulum, cytoskeletal filaments, and other organelles, but no pathological inclusions. (B) The filament-rich cytoplasm of an enteric glial cell (g) appears more electron dense than a neuronal cell body (n) or neuronal processes (arrowheads). The glial cell extends multiple processes (asterisks), which surround portions of the neuronal cell body and interdigitate between axons and dendrites. (C) The majority of neuronal perkarya (n) in Npc1−/− mice are swollen with cytoplasmic membranous inclusions, but glial cells (g) are not affected. (D) Higher magnification of cytoplasmic inclusions demonstrates their lamellar appearance. (E) Enteric glia (g) and many neurons (n) in GFAP-Npc1, Npc1−/− mice do not contain inclusions. Those neurons in which inclusions persist (nx) have fewer vacuoles than in Npc1−/− mice. In this example, their perikarya appear “shrunken” and electron dense with condensed nuclei, but this was not a consistent observation. (F) Higher magnification of perikaryal cytoplasm from a neuron with no inclusions. (m) = smooth muscle. Scale bars = 2 microns.
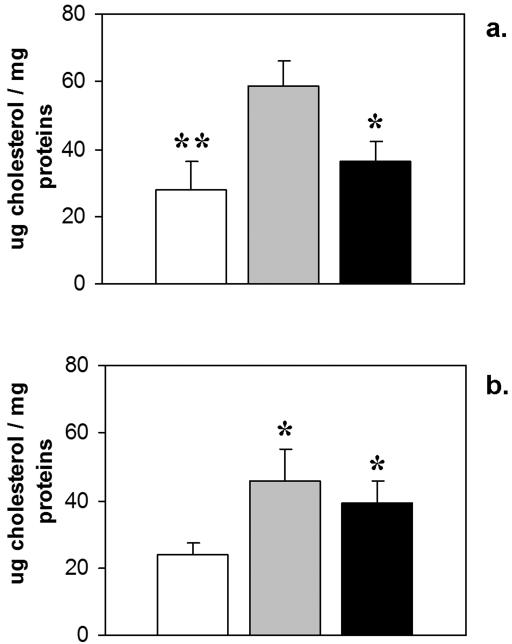
The ultrastructural findings correlated with the cholesterol content of the small and large intestine (Figure 5). The cholesterol content of both tissues was increased approximately 2-fold in Npc1−/− compared to Npc1+/+ mice. The presence of the GFAP-Npc1 transgene lowered the cholesterol to normal levels in small intestine and showed a trend to decreasing it in large intestine.
Figure 5.
Micrograms of cholesterol per mg protein in Npc1+/+ (white); Npc1−/− (grey); and GFAP-Npc1, Npc1−/− (black) mice. Error bars are std. dev. (a) small intestine (*p ≤ 0.05, **p ≤ 0.01 to Npc1−/−; Student T Test). (b) large intestine (*p ≤ 0.05 to Npc1+/+; Student T Test).
DISCUSSION
Glial cells in the peripheral and central nervous systems have diverse functions, including metabolic activities, which are necessary for the health and well-being of neighboring neurons. Studies of Npc1−/− mice highlight the complex relationship between neurons and glia, and suggest that loss of Npc1 activity in the latter may be a critical factor in the neural pathology of NPC disease. Loftus et al. (2002) re-introduced the wild-type Npc1 gene into Npc1−/− mice by targeting its expression to neurons and glia of the CNS through the use of the prion protein promoter (Borchelt et al., 1996). Neurodegeneration was prevented, life span was normalized, and the sterility of Npc1−/− females was corrected. The rescue did not completely rectify the accumulation of GM2 or GM3 gangliosides in some neurons and glia (Loftus et al., 2002). The prion promoter directs expression in non-neuronal cells as well, e.g. T and B lymphocytes, and liver and spleen storage were corrected in two out of three transgenic lines. As the prion promoter is also active in the enteric nervous system (Clarke et al., 2007), it is possible that enteric neural storage was also corrected, but this was not studied. Previously, we narrowed replacement to glial cells by using the GFAP promoter to replace Npc1 protein, and found that expression in fibrillary astrocytes was sufficient to ameliorate degeneration, cytoskeletal abnormalities, and pathological lipid accumulation in CNS neurons (Zhang et al., 2008).
A large body of data suggests that abnormal NPC1 function in glia may impact lipid trafficking between neurons and glial cells (Ong et al., 2001). Our previous work on the central nervous system of GFAP-Npc1, Npc1−/− mice, strongly suggested improvement via a trophic effect of astrocytes on neurons (Zhang et al., 2008). These results strengthened earlier data that indicate an important role of the Npc1 protein in glia. NPC1 protein was detected predominantly in peri-synaptic astrocytic processes surrounding axon terminals and dendrites (Patel et al., 1999; Hu et al., 2000) in primates, but less so in rodents (Falk et al., 1999; Prasad et al., 2000), although sterol synthesis is most abundant in glia in both classes. Xie et al. (2003) found that most cholesterol turnover in Npc1−/− mice was not due to a 24-hydroxylase activity as it is in normal mice, and they concluded that the cholesterol turnover in Npc1−/− mice might primarily reflect glial cell and myelin turnover. Oligodendrocytes have been found to be altered in the Npc1−/− brain: Takikita et al. (2004) found that pre-myelinating oligodendrocytes were abundant in the Npc1−/− brain but pi-glutathione-S-transferase positive mature oligodendrocytes were decreased and polysialylated-NCAM, which is a negative regulator of myelinization, persisted in the Npc1−/− as compared to normals. In addition, Apolipoprotein D, an abundant astrocyte product (Patel et al., 1995) is up-regulated in Npc1−/− mice (Yoshida et al., 1996).
In many respects, enteric glia and their relationship with enteric neurons are more like that of the central nervous system than autonomic or sensory ganglia (Gershon and Rothman, 1991; Savidge et al., 2007). Like CNS astroglia, enteric glial cells express GFAP, do not form myelin, are not associated with intra-ganglionic collagen, and each encircle multiple, as opposed to single, neurites. Enteric glia sometimes show terminal swellings resembling the end-feet of CNS astroctyes (Gershon and Rothman, 1991), which raises the possibility of an effect on endothelia such as induction of blood-brain properties (Janzer and Raff, 1987). Given these and many other similarities between the ENS and CNS (Gershon, 1999), analogous effects of constitutive Npc1 loss and selective replacement of Npc1 in glia might be predicted.
The results reported here suggest some similar, but not identical, effects of GFAP promoter-mediated Npc1 replacement in the enteric nervous system. Specifically, glial expression of Npc1 prevents most of the lipid accumulation evident in enteric neurons of Npc1−/− animals. As in the CNS, this suggests an important role for enteric glia in the regulation of lipid metabolism by neighboring ganglion cells. However, the cellular mechanism by which glial cell Npc1 activity prevents neuronal storage is unclear. Moreover, it seems unlikely that glial Npc1 activity is required to prevent neuronal lipid storage in wild-type mice, given that experimental ablation of enteric glia leads to intestinal inflammation and reduced numbers of myenteric neurons, but no neuronal vacuolization (Bush et al., 1998; Sofroniew et al., 1999; Cornet et al., 2001). Nonetheless, enteric glial expression of Npc1 does not appear to be sufficient to prevent lipid accumulation in neurons that lack the trafficking protein.
Rescue of the storage phenotype of enteric neurons correlates with increased lifespan, reduced weight loss, and initially, normalized intestinal transit times, consistent with the hypothesis that enteric neuropathology may contribute to the morbidity and/or mortality of Npc1−/− mice. However, in contrast with the CNS, loss of enteric neurons is not observed in Npc1−/− mice, so that any pathophysiological effects likely reflect sublethal injury to ganglion cells, as opposed to their death. Given that enteric neurons regulate peristalsis, mucosal absorptive-secretory activity, intestinal immune system, and many other vital activities, it is possible that perturbations of neural function led to the altered intestinal transit times which could contribute to weight loss and death. Physiological studies of other intestinal functions in Npc1−/− and GFAP-Npc1, Npc1−/− mice are needed to further investigate this possibility.
ACKNOWLEDGEMENTS
We thank Jim Hagenzieker for technical assistance, William S. Garver for antibody, and Melanie Esher and Jessica McVey for administrative support.
Grant Information: This work was supported by NIH 5RO1EB000343-05 and the Holsclaw Family Professorship of Human Genetics and Inherited Disease (RPE).
REFERENCES
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Clarke CM, Plata C, Cole B, Tsuchiya K, La Spada AR, Kapur RP. Visceral neuropathy and intestinal pseudo-obstruction in a murine model of a nuclear inclusion disease. Gastroenterology. 2007;133:1971–1978. doi: 10.1053/j.gastro.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet A, Savidge TC, Cabarrocas J, Deng W-L, Colombel J-F, Lassmann H, Desreumauz P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci USA. 2001;98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinari G, Rosenbach Y, Grunebaum M, Zahavi I, Alpert G, Nitzan M. Gastrointestinal manifestations of Niemann-Pick disease. Enzyme. 1980;25:407–412. doi: 10.1159/000459289. [DOI] [PubMed] [Google Scholar]
- Falk T, Garver WS, Erickson RP, Wilson JM, Yool AJ. Expression of Niemann-Pick type C transcript in rodent cerebellum in vivo and in vitro. Brain Res. 1999;839:49–57. doi: 10.1016/s0006-8993(99)01678-9. [DOI] [PubMed] [Google Scholar]
- Gershon MD. The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine. Harper Collins Publishers; New York: 1998. p. 320. [Google Scholar]
- Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- Gilbert EF, Callahan J, Viseskul C, Opitz JM. Niemann-Pick disease type C. Eur J Pediatr. 1981;136:263–274. doi: 10.1007/BF00442993. [DOI] [PubMed] [Google Scholar]
- Hu CY, Ong WY, Patel SC. Regional distribution of NPC1 protein in monkey brain. J Neurocytol. 2000;29:765–773. doi: 10.1023/a:1010942521671. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–356. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Erickson RP, Walkley SU, Bryant MA, Incao A, Heidenreich RA, Pavan WJ. Rescue of neurodegeneratin in Niemann-Pick C mice by a prion-promoter-driven Npc1 cDNA transgene. Hum Mol Genet. 2002;11:3107–3114. doi: 10.1093/hmg/11.24.3107. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- Ong WY, Kumar U, Switzer RC, Sidhu A, Suresh G, Hu CY, Patel SC. Neurodegeneration in Niemann-Pick type C disease mice. Exp Brain Res. 2001;141:218–231. doi: 10.1007/s002210100870. [DOI] [PubMed] [Google Scholar]
- Patel SC, Asotra K, Patel YC, McConathy WJ, Patel RC, Suresh S. Astrocytes synthesize and secrete the lipophilic ligand carrier apolipoprotein D. Neuroreport. 1995;6:653–657. doi: 10.1097/00001756-199503000-00017. [DOI] [PubMed] [Google Scholar]
- Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, Neufeld EB, Patel RC, Brady RO, Patel YC, Pentchev PG, Ong WY. Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann- Pick type C disease. Proc Natl Acad Sci USA. 1999;96:1657–1662. doi: 10.1073/pnas.96.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MC, Vanier MT, Suzuki K, Morris JA, Carstea ED, Neufeld EB, Blanchette-Mackie JE, Pentchev PG. Niemann-Pick disease type C: a lipid trafficking disorder. In: Scriver SR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw-Hill; New York: 2001. pp. 3611–3634. [Google Scholar]
- Peter F, Reynolds RG. Quantitative analysis of human serum cholesterol by thin-layer chromatographic spot test. Journal of Chromatography. 1977;143:153–160. [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Meth. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Prasad A, Fischer WA, Maue RA, Henderson LP. Regional and developemtnal expression of the Npc1 mRnA in the mouse brain. J Neurochem. 2000;75:1250–1257. doi: 10.1046/j.1471-4159.2000.0751250.x. [DOI] [PubMed] [Google Scholar]
- Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest. 2007;87:731–736. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- Shio H, Fowler S, Bhuvaneswaran C, Morris MD. Lysosome lipd sotrage disorder in NCTR-BALB/c mice. II. Morphologic and cytochemical studies. Am J Pathol. 1982;108:150–159. [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Bush TG, Blumauer N, Kruger Lawrence, Mucke L, Johnson MH. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res. 1999;835:91–95. doi: 10.1016/s0006-8993(99)01639-x. [DOI] [PubMed] [Google Scholar]
- Takikita S, Fukuda T, Mohri I, Yagi T, Suzuki K. Perturbed myelination process of premyelinating oligodendrocyte in Niemann-Pick type C mouse. J Neuropathol Exp Neurol. 2004;63:660–673. doi: 10.1093/jnen/63.6.660. [DOI] [PubMed] [Google Scholar]
- Ubhayasekera SJKA, Verleyen T, Dutta PC. Evaluation of GC and GC-MS method for the analysis of cholesterol oxidation products. Food chemistry. 2004;84:149–157. [Google Scholar]
- Vincent I, Bu B, Erickson RP. Understanding Niemann-pick type C disease: a fat problem. Curr Opin Neurol. 2003;16:155–161. doi: 10.1097/01.wco.0000063764.15877.1c. [DOI] [PubMed] [Google Scholar]
- Xie C, Lund EG, Turley SD, Russell DW, Dietschy JM. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Cleaveland ES, Nagle JW, French S, Yaswen L, Oshima T, Brady RO, Pentchev PG, Kulkarni AB. Molecular cloning of the mouse Apolipoprotein D gene and Its upregulating expression in Niemann-Pick disease type C mouse model. DNA Cell Biol. 1996;15:873–882. doi: 10.1089/dna.1996.15.873. [DOI] [PubMed] [Google Scholar]
- Zhang M, Strnatka D, Donohue C, Hallows JL, Vincent I, Erickson RP. Astrocyte-only Npc1 reduces neuronal cholesterol and triples life span of Npc1(−/−) mice. J Neurosci Res. 2008;86:2848–2856. doi: 10.1002/jnr.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]