Abstract
Introduction
Dissemination of oral bacteria into the bloodstream has been associated with eating, oral hygiene, and dental procedures; including tooth extraction, endodontic treatment, and periodontal surgery. Recently, studies identified Streptococcus mutans, the primary etiological agent of dental caries, as the most prevalent bacterial species found in clinical samples from patients who underwent heart valve and atheromatous plaque surgery.
Methods
By using antibiotic protection assays, we tested the capacity of 14 strains of S. mutans to invade primary human coronary artery endothelial cells (HCAEC).
Results
Serotype e strain B14 and serotype f strain OMZ175 of S. mutans were able to efficiently invade HCAEC. Among the tested strains, serotype f S. mutans OMZ175 was the most invasive, whereas strains of serotype c S. mutans, the most prevalent serotype in dental plaque, were not invasive. Based on its high invasion rate, we further investigated the invasive properties of serotype f OMZ175. Using transmission electron microscopy and antibiotic protection assays we demonstrate that S. mutans OMZ175 is capable of attaching to the HCAEC surface, entering the cells and surviving in HCAEC for at least 29 h.
Discussion
Our findings highlight a potential role for S. mutans in the pathogenesis of certain cardiovascular diseases.
Keywords: invasion, serotype f, Strepococcus mutans
Bacteria colonizing the teeth, the only non-shedding surfaces of the body, form dental plaque, a multi-species biofilm that is normally compatible with oral health. However, environmental perturbations can alter the composition and metabolic activities of oral biofilms, allowing an increase in the proportions of species that are associated with the development of oral diseases, including dental caries and periodontitis (13, 19). Streptococcus mutans is recognized as a primary etiological agent of dental caries and possesses multiple virulence attributes that allow the organism to colonize, form biofilms, produce acids that damage tooth mineral, and to grow and metabolize in acidic environments (15). It is also recognized that S. mutans is able to gain access to the bloodstream, leading to transient bacteremias that may result in the development of infective endocarditis (12, 21). S. mutans strains are classified into four serotypes (c, e, f, and K), and the serological specificity is defined by rhamnose-glucose polysaccharide (RGP) on the cell wall (19, 24). The RGP of S. mutans consists of a backbone structure of α1,2-linked and α1,3-linked rhamnan with glucose side chains linked to alternate rhamnose units. Each serotype-specific polysaccharide has unique linkages of its glucose side chains (serotype c, α1,2-linkage; serotype e, β1,2-linkage; and serotype f, α1,3-linkage) (18, 27). Serotype c strains are predominant in dental plaque and differences in the binding affinities of the RGP to human oral tissues might have led to this biased distribution (29).
The dissemination of oral bacteria into the bloodstream is common in patients subjected to dental procedures, such as tooth extraction, endodontic treatment, and periodontal surgery (17). After an oral procedure, it has been demonstrated that bacteria can reach the heart, lungs and peripheral blood capillary system in less than a minute (12). Infective endocarditis is a serious and often fatal systemic disease with over 1000 case reports directly associating dental procedures or disease with the onset of endocarditis (6, 17). Approximately 15,000 new cases of infective endocarditis occur in the United States each year and about 20% of all cases require surgical intervention (10). Despite advances in early diagnosis, antimicrobial treatment, and surgical techniques, the reported mortality from referral centers is essentially unchanged over the past several decades (26).
Evidence to support a correlation between oral infections and the occurrence of advanced coronary atherosclerosis has been accumulating (20). Some periodontal pathogens as well as dental plaque inhabitants, such as viridans streptococci, have been reported to be associated with infective endocarditis and atherosclerotic/atheromatous plaque (5, 8, 16, 20, 31). Recently, Nakano and co-workers (22) reported that S. mutans was the most prevalent bacterial species detected in diseased heart valve tissues as well as in atheromatous plaques, with an incidence of 68.6% and 74.1%, respectively. In addition, Kozarov and colleagues (14) reported that DNA from S. mutans was recovered from atheromatous plaque in 22.5% of young patients (~27 years old) and 44.4% of older patients (~67 years old). When using DNA fingerprinting to compare S. mutans isolates from dental plaque and an infected heart valve from a patient who underwent heart surgery, Nomura and colleagues demonstrated that the oral isolates differed from those found in the heart valve (25). In a study with patients who underwent cardiovascular operations, it was demonstrated that S. mutans serotype e was prevalent in dental plaque and in cardiovascular samples from diseased patients, whereas serotype c strains, the most common serotype found in dental plaque, prevailed in the healthy control group (23). Collectively, these findings lend credence to the idea that there are subpopulations of S. mutans carried in humans that, while not necessarily associated with caries, may have an enhanced capacity to interact, and possibly invade, the cells of the cardiovascular system.
Invasion of host cells consists of an active, bacterially-driven process wherein signal transduction pathways of otherwise non-phagocytic cells are subverted to accommodate bacterial entry (11). In 2003, Stinson and colleagues (30) reported that viridans group streptococci were capable of invading human umbilical vein endothelial cells (HUVEC). Streptococcus gordonii was considered the most invasive species, but differences in invasion efficiency were observed among strains. In light of the new findings (24–27) reporting a high frequency of detection of certain S. mutans serotypes from patients with atherosclerosis and infective endocarditis, we investigated the invasive properties of 14 strains of S. mutans belonging to serotypes c, e, and f, and then further characterized the capacity of a highly invasive strain, serotype f strain, OMZ175, to enter and persist in endothelial cells.
Material and methods
Streptococcus mutans strains and serotype determination
Streptococcus pyogenes HSC5 is a known invasive streptococcal isolate (28) and was chosen as a positive control for this study. The serotype of 14 selected S. mutans strains (Table 1) was confirmed by multiplex-polymerase chain reaction using primers SC-F (5′-CGG AGT GCT TTT TAC AAG TGC TGG-3′) and SC-R (5′-AAC CAC GGC CAG CAA ACC CTT TAT-3′) for serotype c determination; SE-F (5′-CCT GCT TTT CAA GTA CCT TTC GCC-3′) and SE-R (5′-CTG CTT GCC AAG CCC TAC TAG AAA-3′) for serotype e determination; and SF-F (5′-CCC ACA ATT GGC TTC AAG AGG AGA-3′) and SF-R (5′-TGC GAA ACC ATA AGC ATA GCG AGG-3′) for serotype f determination, as described elsewhere (29).
Table 1.
Streptococcus mutans strains used in this study
| S. mutans strains | Serotype | Source or reference |
|---|---|---|
| UA159 | c | University of Alabama |
| GS5 | c | R. J. Gibbons |
| NG8 | c | A. Bleiweis collection |
| Ingbritt175 | c | A. Bleiweis collection |
| DP-5 | c | A. Bleiweis collection |
| Smith | c | R. Burne collection |
| C5(9)2 | c | M. Klein (13) |
| C2(5)3 | c | M. Klein (13) |
| B2 | e | A. Bleiweis collection |
| B14 | e | A. Bleiweis collection |
| V100 | e | A. Bleiweis collection |
| C(32)51 | e | M. Klein (13) |
| OMZ175 | f | B. Guggenheim |
| 1SA | f | R. Burne collection |
Preparation of confluent human coronary artery endothelial cells culture
Primary human coronary artery endothelial cells (HCAEC; Lonza, Allendale, NJ), which are non-phagocytic cells, were chosen for the invasion assays. The HCAEC were cultured in endothelial cell basal medium-2 (EBM-2; Lonza) supplemented with EGM-2MV single-use aliquots (Lonza), as described by the supplier. The HCAEC were maintained at 37°C in a humidified, 5% CO2 atmosphere. The cells were harvested by trypsinization and washed in EBM-2 medium. One milliliter of the suspension containing 105 endothelial cells was then seeded per well in 24-well flat-bottom tissue culture plates (Sarstedt, Newton, NC) followed by overnight incubation at 37°C in a 5% CO2 atmosphere (4).
Preparation of the bacterial inoculum
S. mutans strains and S. pyogenes HSC5 were cultured overnight in Brain–Heart Infusion (BHI) broth. Bacterial cultures were transferred to a microcentrifuge tube and collected at 14,000 g for 5 min. Pellets were washed twice with sterile phosphate-buffered saline (pH 7.2) and resuspended in supplemented EBM-2 without antibiotics to obtain bacterial suspensions containing 1 × 107 and 5 × 105 colony-forming units (CFU)/ml of S. mutans and S. pyogenes, respectively. Before infecting HCAEC, bacterial species were sonicated twice for 15 s to disperse aggregates.
Infection of HCAEC monolayers and antibiotic protection assays
Medium from HCAEC cultures was aspirated and wells were washed three times with prewarmed EBM-2 medium without antibiotics. One milliliter of each strain of S. mutans (Table 1) and S. pyogenes HSC5 (positive control strain) containing 1 × 107 CFU/ml or 5 × 105 CFU/ml, respectively, was added to triplicate wells containing 1 × 105 HCAEC and the plates were incubated for 2 h in the absence of antibiotics. Of note, S. mutans was not able to replicate in EBM-2 during the infection period. To determine the number of bacterial cells that were able to reach the intracellular compartment, antibiotic protection assays were performed as described elsewhere (4, 30) with the following modifications. After 2 h of co-culturing HCAEC with S. mutans or S. pyogenes, the wells were washed three times with fresh prewarmed EBM-2 without antibiotics to remove planktonic and adventitiously-bound bacteria. One milliliter of EBM-2 containing 300 μg/ml gentamicin and 10 μg/ml penicillin G was added to the wells and incubated for an additional 3 h at 37°C in a 5% CO2 atmosphere to eliminate extracellular bacteria. Next, the wells were washed three times with prewarmed EBM-2 without antibiotics and the HCAEC were lysed by osmotic shock in 1 ml of sterile deionized water for 20 min. The mixture of lysed HCAEC and free bacteria was collected from the wells and serially diluted in phosphate-buffered saline, followed by plating onto BHI agar and incubation at 37°C in a 5% CO2 atmosphere. After 2 days of incubation, the CFUs were counted and the percentage of intracellular bacteria relative to the initial inoculum was calculated.
Adherence, invasion, and persistence assays
S. mutans strains UA159 and OMZ175 were co-cultured with HCAEC for 30 min, 5 and 29 h as described above. Briefly, HCAEC were infected with S. mutans (1 × 107 CFU) in triplicate for 30 min, 5 and 29 h. For the 30-min time-point, wells were washed, bacterial cells were recovered 30 min after infection (see below) without killing with antibiotics, and CFUs were determined by plating. For the 5- and 29-h time-points, HCAEC were infected with bacterial cells for 2 h (without antibiotic) and then incubated in the presence of antibiotics for 3 and 27 h, respectively. To recover viable bacteria protected by the host, HCAEC were washed and lysed, and recovered bacteria were plated onto BHI agar as described above.
Transmission electron microscopy
HCAEC and S. mutans strains were co-cultured as described above for 30 min, 5 and 29 h in 24-well plates and processed for electron microscopy as follows. The media were removed and the cells were fixed in 2% paraformaldehyde/2% glutaraldehyde in 0.1 m cacodylate buffer, pH 7.2, for 1 h at room temperature. The cells were then washed three times in 0.1 m cacodylate buffer with 7% sucrose and postfixed in 1.0% osmium tetroxide in 0.1 m cacodylate buffer for 1 h. After rinsing in Michaelis buffer and en bloc staining with Kellenberger buffer, the cells were dehydrated with a graded series of ethanol. At this point, the cells were either released from the plate with propylene oxide and centrifuged into a pellet or left in the wells in preparation for en face sectioning. In both cases, the cells were infiltrated with a formulation of Poly-Bed 812 resin. Thin sections were examined on a JEOL 100CX transmission electron microscope.
Results
Serotype f S. mutans OMZ175 is highly invasive
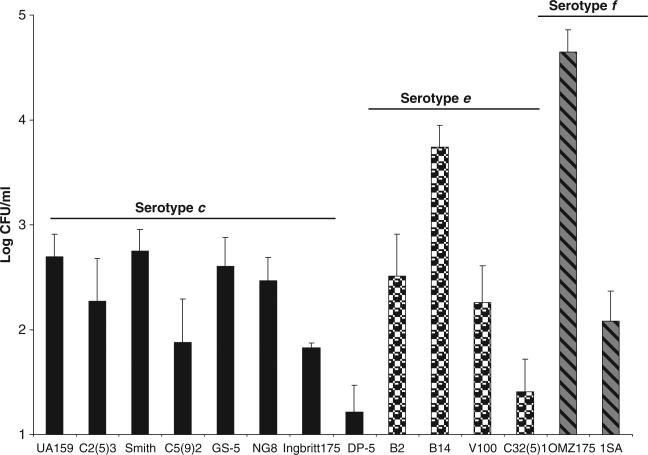
The invasive properties of 14 isolates of S. mutans belonging to different serotypes were investigated. Under the conditions tested, a serotype e strain, B14, and a serotype f strain, OMZ175, were able to efficiently reach the cytoplasm of HCAEC after 5 h of infection. However, OMZ175 was considerably more invasive than B14 with 0.22% (~4.4 × 104 CFU) and 0.05% (~5 × 103 CFU), respectively, of the initial inoculum localized within the intracellular compartment (Fig. 1). The serotype c strains displayed a very low invasive behavior (Fig. 1). Notably, 1.3% (9.7 × 103 CFU) of the initial inoculum of S. pyogenes HSC5, the positive control strain, was able to reach the HCAEC cytoplasm (data not shown). S. pyogenes is known to invade epithelial cells, here we demonstrate that endothelial cells can also be invaded very efficiently by this organism.
Fig. 1.
Invasive properties of Streptococcus mutans strains. The number of S. mutans colony-forming units (CFUs) recovered from the intracellular compartment of human coronary artery endothelial cells after 5 h of infection is shown. The data represent the log of the average ± SD of at least three independent experiments.
Adherence, invasion and persistence of S. mutans in HCAEC
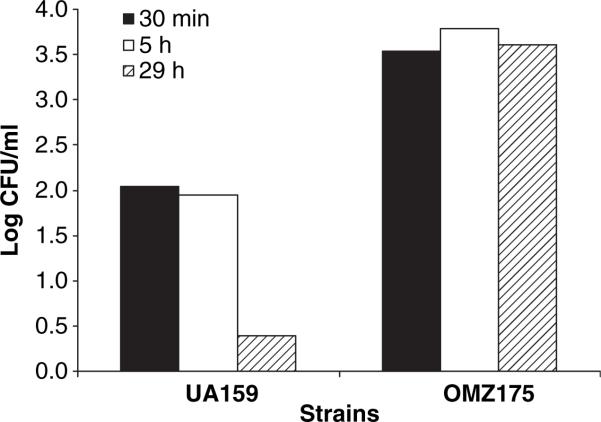
To assess the capacity of S. mutans to attach to HCAEC and to survive in the cytoplasm of HCAEC, the CFU of S. mutans OMZ175 and UA159 recovered after 30 min, 5 and 29 h of co-culture with HCAEC were compared. As shown in Fig. 2, no significant change in the number of S. mutans OMZ175 recovered from the intracellular compartment was observed over the experimental period. However, the number of UA159 cells significantly decreased after 29 h of invasion.
Fig. 2.
Persistence of Streptococcus mutans in human coronary artery endothelial cells (HCAEC). The data represent the percentage of the initial inoculum of S. mutans that was able to reach the intracellular compartment of HCAEC and remained viable at different times. Values shown are log of means ± SD from at least three independent experiments.
Transmission electron microscopy
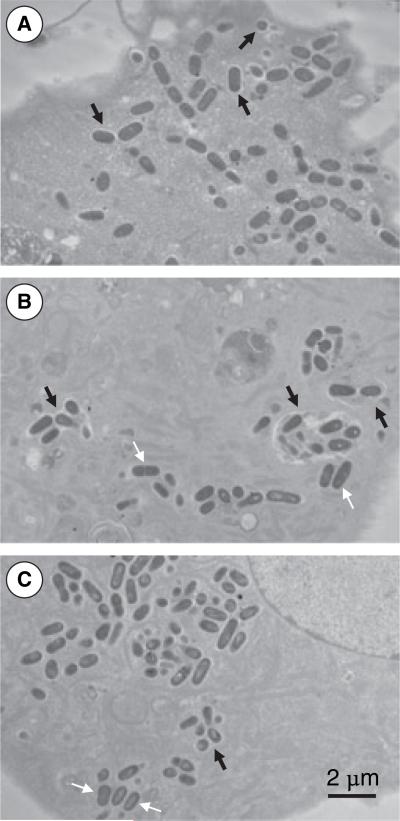
To confirm that S. mutans was indeed able to enter the cells and to begin to define its intracellular location, HCAEC monolayers were exposed to S. mutans OMZ175 for different time periods (30 min, 5 and 29 h) before being examined by transmission electron microscopy. As shown in Fig. 3, upon entry, the bacteria could be observed in vacuoles. However, after 5 and 29 h of infection, S. mutans was both localized in the vacuoles and free in the cytoplasm.
Fig. 3.
Electron micrographs of human coronary artery endothelial cells (HCAEC) invasion by Streptococcus mutans OMZ175 after 30 min (A), 5 h (B), and 29 h (C) of infection. The HCAEC monolayers co-cultured with S. mutans OMZ175 were fixed and prepared for transmission electron microscopy before being examined on a JEOL 100CX electron microscope. Upon entry, the bacteria were found in vacuoles (black arrows). At 5 and 29 h of infection, S. mutans was found to be either in vacuoles (black arrows) or free in the cytoplasm (white arrows).
Discussion
Epidemiological studies with Japanese children revealed that S. mutans serotype c is the most common serotype isolated from dental plaque (84.8%), followed by serotype e (13.3%). In contrast, serotype f was rarely isolated from dental plaque of the subjects of this study (1.9%) (29). Recently, a new S. mutans serotype was isolated from the blood of patients with infective endocarditis and from the oral cavity (serotype k) (24). Nakano and colleagues (23) reported that strains belonging to serotype e were frequently detected in dental plaque and in the heart valves of patients with cardiovascular diseases (CVD). However, serotype c strains prevailed in dental plaque of healthy subjects (no CVD). Of the 14 strains of S. mutans originally isolated from dental plaque that were tested here, only two, OMZ175 and B14, belonging to serotypes f and e, respectively (Fig. 1), were considered invasive. None of the serotype c strains of S. mutans, including two fresh clinical isolates from children with dental caries (13), displayed invasive behavior, which suggests that there may be important differences in strains belonging to different serotypes. Much of what is presently known concerning virulence attributes of S. mutans has been derived from studies with serotype c strains, including the common laboratory strains UA159, NG8, GS-5, and Ingbritt. The best characterized difference between serotypes of S. mutans is the cell-wall-associated polysaccharide RGP. It has been suggested that serotypes e and f originated from serotype c strains through the introduction of point mutations or deletions in the rgp loci (29). RGP acts as a putative adhesin for the binding of S. mutans to human monocytic and fibroblastic cells, as well as to platelets (2, 7). However, it is not known if the RGPs associated with sero-types e or f are able to mediate or enhance invasion or persistence within human cells. Given recent studies demonstrating the tremendous heterogeneity of S. mutans strains belonging to serotype c and the finding that there are strains carrying genes not present in the sequenced isolate UA159 (1, 9), it is reasonable to speculate that some serotype e and f strains could possess multiple factors that are different from, or not present in, serotype c strains that markedly enhance invasion and persistence in endothelial cells. Likewise, the fact that serotype f strain 1SA did not invade as well as OMZ175 is indicative of a multifactorial process for uptake into HCAEC. Dissecting the physiological and genetic traits of different serotypes of S. mutans will provide a better understanding of the spectrum of pathogenic mechanisms of this organism.
The ability of Porphyromonas gingivalis and Porphyromonas endodontalis to invade endothelial and epithelial cells also exhibits considerable strain-to-strain variability, with some strains classified as non-invasive (3). We also observed that invasion by S. mutans is strain dependent. Interestingly, our data clearly show that attachment and invasion of HCAEC by OMZ175 occurs very rapidly, because similar numbers of bacteria were recovered at 30 min and 5 h of invasion. The data presented here suggest that an extensive examination of the invasive behavior of different serotypes and strains isolated from the blood of patients with CVD would be fruitful.
Our results revealed that OMZ175 persists throughout the 29-h period of the infection and the rate of recovery of organisms does not change significantly during this time. Therefore, either the bacteria remain viable but do not multiply inside the host cells, or the population is maintained through a balance between cell division and death of the organisms in the host intracellular environment. The fact that other S. mutans strains yielded much lower recovery rates throughout the infection process indicates that the ‘non-invasive’ S. mutans strains have defects in either attachment to cells, in stimulating uptake, or both.
The presence of S. mutans OMZ175 within HCAEC upon infection was also confirmed by transmission electron microscopy. Examination of electron micrographs revealed that some intracellular S. mutans OMZ175 were found within vesicles whereas others appeared to be free in the cytoplasm. It will be critical to determine whether harboring of S. mutans in the cytoplasm or specific intracellular compartments by host cells contributes to the pathogenesis of CVD. The fact that S. mutans OMZ175 can remain viable in endothelial cells for prolonged periods indicates that invasive organisms may not be readily cleared by the immune system or antibiotic treatment, potentially contributing to the establishment of chronic disease and the triggering of other pathologies associated with CVD. Our findings further support the view that, in some cases, CVD may also have an infectious etiology. Studying the invasive properties of S. mutans isolated from patients with CVD and identifying the mechanisms involved in S. mutans invasion may lead to innovative approaches for the prevention and treatment of diseases elicited by invasive S. mutans.
Acknowledgments
We would like to thank Dr J. A. C. Lemos for critically reviewing this manuscript. This work was supported in part by NIH grant DE007202 to J.A., DE013545 to A.P.F., and AHA grant 0655897T to P.J.S.H.
References
- 1.Caufield PW, Saxena D, Fitch D, Li Y. Population structure of plasmid-containing strains of Streptococcus mutans, a member of the human indigenous biota. J Bacteriol. 2007;189:1238–1243. doi: 10.1128/JB.01183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia JS, Lin YL, Lien HT, Chen JY. Platelet aggregation induced by serotype polysaccharides from Streptococcus mutans. Infect Immun. 2004;72:2605–2617. doi: 10.1128/IAI.72.5.2605-2617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;187:139–144. doi: 10.1111/j.1574-6968.2000.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 4.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–5798. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39:179–182. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 6.Drangsholt MT. A new causal model of dental diseases associated with endocarditis. Ann Periodontol. 1998;3:184–196. doi: 10.1902/annals.1998.3.1.184. [DOI] [PubMed] [Google Scholar]
- 7.Engels-Deutsch M, Pini A, Yamashita Y, et al. Insertional inactivation of pac and rmlB genes reduces the release of tumor necrosis factor alpha, interleukin-6, and interleukin-8 induced by Streptococcus mutans in monocytic, dental pulp, and periodontal ligament cells. Infect Immun. 2003;71:5169–5177. doi: 10.1128/IAI.71.9.5169-5177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 9.Huang WC, Chen YY, Teng LJ, Lien HT, Chen JY, Chia JS. Chromosomal inversion between rrn operons among Streptococcus mutans serotype c oral and blood isolates. J Med Microbiol. 2008;57:198–206. doi: 10.1099/jmm.0.47428-0. [DOI] [PubMed] [Google Scholar]
- 10.Jassal DS, Neilan TG, Pradnan AD, et al. Surgical management of infective endocarditis: early predictors of short term morbidity and mortality. Ann Thorac Surg. 2006;82:524–529. doi: 10.1016/j.athoracsur.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Kilian M. Systemic disease: manifestations of oral bacteria. In: MeGlice JR, Michalek SM, Cassell GH, editors. Dental microbiology. Harpers & Row; Philadelphia: 1982. pp. 832–838. [Google Scholar]
- 13.Klein MI, Bang S, Florio FM, et al. Genetic diversity of competence gene loci in clinical genotypes of Streptococcus mutans. J Clin Microbiol. 2006;44:3015–3020. doi: 10.1128/JCM.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 15.Lemos JA, Abranches J, Burne RA. Responses of cariogenic streptococci to environmental stresses. Curr Issues Mol Biol. 2005;7:95–107. [PubMed] [Google Scholar]
- 16.Li L, Messas E, Batista EL, Jr, Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linzer R, Reddy MS, Levine MJ. Immunichemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada S, Michalek SM, Kiyono H, Menaker L, McGhee JR, editors. Molecular microbiology and immunology of Streptococcus mutans. Elsevier Science Publishers; Amsterdam: 1986. pp. 29–38. [Google Scholar]
- 19.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15:403–413. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- 21.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 22.Nakano K, Inaba H, Nomura R, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. 2006;44:3313–3317. doi: 10.1128/JCM.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano K, Nemoto H, Nomura R, et al. Serotype distribution of Streptococcus mutans a pathogen of dental caries in cardiovascular specimens from Japanese patients. J Med Microbiol. 2007;56:551–556. doi: 10.1099/jmm.0.47051-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakano K, Nomura R, Nakagawa I, Hamada S, Ooshima T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol. 2004;42:198–202. doi: 10.1128/JCM.42.1.198-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura R, Nakano K, Nemoto H, et al. Isolation and characterization of Streptococcus mutans in heart valve and dental plaque specimens from a patient with infective endocarditis. J Med Microbiol. 2006;55:1135–1140. doi: 10.1099/jmm.0.46609-0. [DOI] [PubMed] [Google Scholar]
- 26.Paterick TE, Paterick TJ, Nishimura RA, Steckelberg JM. Complexity and subtlety of infective endocardites. Mayo Clin Proc. 2007;82:615–623. doi: 10.4065/82.5.615. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard DG, Gregory RL, Michalek SM, McGee JR. Biochemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada S, Michalek SM, Kiyono H, Menaker L, McGhee JR, editors. Molecular microbiology and immunology of Streptococcus mutans. Elsevier Science Publishers; Amsterdam: 1986. pp. 39–49. [Google Scholar]
- 28.Rosch J, Caparon M. A microdomain for protein secretion in Gram-positive bacteria. Science. 2004;304:1513–1515. doi: 10.1126/science.1097404. [DOI] [PubMed] [Google Scholar]
- 29.Shibata Y, Ozaki K, Seki M, et al. Analysis of loci required for determination of serotype antigenicity in Streptococcus mutans and its clinical utilization. J Clin Microbiol. 2003;41:4107–4112. doi: 10.1128/JCM.41.9.4107-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stinson MW, Alder S, Kumar S. Invasion and killing of human endothelial cells by viridans group streptococci. Infect Immun. 2003;71:2365–2372. doi: 10.1128/IAI.71.5.2365-2372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Meer JT, Van Wijk W, Thompson J, Vandenbroucke JP, Valkenburg HA, Michel MF. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet. 1992;339:135–139. doi: 10.1016/0140-6736(92)90207-j. [DOI] [PubMed] [Google Scholar]