Abstract
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies have revealed age-related under-activation, where older adults show less regional brain activation compared to younger adults, as well as age-related over-activation, where older adults show greater activation compared to younger adults. These differences have been found across multiple task domains, including verbal working memory (WM). Curiously, both under-activation and over-activation of dorsolateral prefrontal cortex (DLPFC) have been found for older adults in verbal WM tasks. Here, we use event-related fMRI to test the hypothesis that age-related differences in activation depend on memory load (the number of items that must be maintained). Our predictions about the recruitment of prefrontal executive processes are based on the Compensation Related Utilization of Neural Circuits Hypothesis (CRUNCH; Reuter-Lorenz and Cappell, 2008). According to this hypothesis, more neural resources are engaged by older brains to accomplish computational goals completed with fewer resources by younger brains. Therefore, seniors are more likely than young adults to show over-activations at lower memory loads, and under-activations at higher memory loads. Consistent with these predictions, in right DLPFC, we observed age-related over-activation with lower memory loads despite equivalent performance accuracy across age groups. In contrast, with the highest memory load, older adults were significantly less accurate and showed less DLPFC activation compared to their younger counterparts. These results are considered in relation to previous reports of activation-performance relations using similar tasks, and are found to support the viability of CRUNCH as an account of age-related compensation and its potential costs.
Although healthy aging brings declines across a broad spectrum of cognitive and behavioral meaures (Park and Reuter-Lorenz, 2009), some abilities show greater preservation than others. One important example is verbal working memory (WM). Craik and Rabinowitz (1984; see Craik and Jennings, 1992, for a review) recognized that the ability of older adults simply to maintain several items in memory over a brief interval is relatively spared in contrast to their ability to maintain and manipulate a small memory set concurrently. Rote verbal maintenance is presumed to be relatively preserved because these abilities place minimal demand on executive functions, which are known to be adversely affected by advancing age (Moscovitch and Winocur, 1992; West, 1996; Raz, 2000).
However, neuroimaging results from studies of age differences in verbal maintenance have suggested that this account is in need of revision. Two general findings are especially relevant. First, even when performance levels on simple WM tasks are matched, the patterns of activation in the brains of older and younger adults are different (e.g., Cabeza et al., 2004; Grady et al., 1998; Reuter-Lorenz et al., 2001). In other words, as we and others have noted, older adults may be able to achieve the same performance outcomes as younger adults using different neural circuity (Cabeza, 2002; Grady, 2008; Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Cappell, 2008). Such findings suggest that age-equivalent performance does not necessarily imply age-equivalent or age-preserved neural substrates. Second, dorsolateral regions of prefrontal cortex (DLPFC), especially Brodmann’s areas 9 and 46, are prominent sites of activation differences due to age (Cabeza et al., 2004; Rypma and D’Esposito, 2000, Rypma et al., 2001). These regions are widely recognized as being critical for executive functions, especially those involved in manipulation and monitoring operations that are thought to contribute minimally to WM tasks that require only short-term maintenance (Jonides et al., 1997; Reuter-Lorenz and Sylvester, 2005; Wager and Smith, 2003). Together these findings raise questions about the role of DLPFC in simple maintenance and whether it changes with age. These questions are the focus of the present report.
Aging, WM, and Dorsolateral Prefrontal Cortex
While recruitment of DLPFC has figured prominently in neuroimaging work examining the effects of age on verbal WM, the results are seemingly inconsistent. On the one hand, several studies have reported age-related over-activation in DLPFC during the performance of simple verbal maintenance tasks (Cabeza, et al., 2004; Reuter-Lorenz, et al., 2000; 2001)1. This suggests that older adults may rely on DLPFC-mediated executive processes to a greater extent than do younger adults for verbal maintenance, possibly due to declines in pure storage capacity (Dobbs and Rule, 1989; Babcock and Salthouse, 1990; Reuter-Lorenz, et al., 2001). On the other hand, age-related under-activation of DLPFC has also been reported in studies of verbal WM, which has been interpreted as an inability on the part of seniors to recruit the requisite executive processing operations (Rypma and D’Esposito, 2000; Rypma, et al., 2001). Moreover, both patterns of over- and under-activation have been most evident in regions of right DLPFC and far less pronounced in regions of left ventro- and dorso-lateral prefrontal cortex known to be involved in verbal maintenance (e.g., Narayanan, et al., 2005).
The recruitment of right DLPFC for verbal maintenance presents a puzzle. We have proposed an hypothesis that we refer to as “CRUNCH” or “compensation-related utilization of neural circuits hypothesis” that we believe can accommodate these seemingly discrepant findings (Reuter-Lorenz and Cappell, 2008; Reuter-Lorenz and Mikels, 2006). According to CRUNCH, in order to maintain optimal performance in the face of age-related neural declines, older adults need to recruit more neural resources than their younger counterparts for any given level of task demand. Older adults’ additional resource recruitment is what we propose to be the source of at least some instances of age-related over-activation (see also, Davis, et al., 2008). Importantly, while this compensatory neural strategy has an upside, according to CRUNCH, it also has a cost. Because older adults tend to engage more neural resources than young adults at low levels of task demand, they have fewer resources available to meet the processing requirements of more demanding tasks; this is the “crunch”. Therefore, older adults are more likely than young adults to reach their resource limitations at lower levels of task demand, leading to performance declines for more demanding tasks.
In the studies that have reported age-related over-activation in DLPFC, participants were required to maintain four items, a memory load that is within the capacity of WM for young adults (Cowan, 2001; Miller, 1956; Luck and Vogel, 1997) and does not generally recruit DLPFC (e.g., Reuter-Lorenz, et al., 2001, Wager and Smith, 2003). However, because age is associated with declines in neural efficiency2, maintenance of only four items may require the allocation of additional neural resources, leading to age-related over-activation of DLPFC at this relatively low level of memory load. In contrast, in the studies that observed age related under-activation of DLPFC, the memory load, six items, was at the upper limit of WM capacity (Cowan, 2001; Miller, 1956; Luck and Vogel, 1997), and more likely to surpass the capacity of senior adults (Callicott, et al., 1999). At this high level of memory load younger adults recruit processing resources mediated by DLPFC, whereas older adults have reached their resource ceiling and are unable to meet this level of task demand. Thus, at higher loads seniors will show under-activation in association with markedly reduced performance (Reuter-Lorenz and Cappell, 2008). Therefore, we propose that due to age-related changes in neural efficiency and associated compensatory processes, the level of task demand will determine whether age-related over- or under-activation is observed in DLPFC.
There are several results in the literature that lend support to this hypothesis. Mattay et al. (2006) recently observed age-related over-activation of DLPFC at low memory loads and under-activation at a high memory load in a single group of young and older adults performing a verbal n-back task. The n-back task requires verbal maintenance and a variety of executive operations, including updating, task management, and selection (Smith and Jonides, 1999; Wager and Smith, 2003). Importantly, over-activation was associated with age-equivalent performance, whereas under-activation was obtained when seniors performed more poorly. A related result has also been reported by Rypma and his colleagues (Rypma, et al., 2007; Rypma and D’Esposito, 2001) who find that better performance in seniors is associated with greater activation.
A second ambiguity to arise from the set of studies described above relates to the stage(s) of processing at which age-related differences in DLPFC activation occur. At present, while some studies have revealed age-related differences in DLPFC during WM maintenance (Reuter-Lorenz, et al., 2000; 2001), others have isolated these differences to retrieval (Cabeza, et al., 2004; Rypma and D’Esposito, 2000), and yet others have not been designed to identify differences at specific stages of processing (Mattay, et al., 2006; Rypma, et al., 2001). By linking specific sites of age-related activation change to different epochs of a working memory task (target, delay, probe) we can make inferences about the neurocognitive processing resources (e.g, associated with encoding, retention/maintenance, retrieval) being drawn upon to compensate for declines in neural efficiency.
The Current Study
An important question for CRUNCH and for the cogntive neuroscience of aging more generally is whether additional regions recruited by older adults during the performance of specific tasks are the same regions recruited by young adults in response to increases in task demand (Stern, 2002). If so, this would strongly suggest that while older adults may be more challenged at lower objective levels of task demand than are younger adults, the basic neural and cognitive processes that contribute to task performance are essentially age-invariant (see also Park and Reuter-Lorenz, 2009). Alternatively, age-related over-activations may represent the recruitment of cognitive or neural resources that young adults would not normally engage, even in the face of high task demand (Stern, 2002). This would suggest that aging results in a reorganization of basic neurocognitive architecture. Of course, it is also possible that some additional mechanisms are available and utilized for compensation (i.e., scaffolding; see Park and Reuter-Lorenz, 2009) across the lifespan, whereas others develop in response to the neural losses associated with aging.
The above discussion highlights the importance of simultaneously investigating the potentially interacting effects of both age and task demand on the neural correlates of WM maintenance. While CRUNCH offers a plausible explanation for the seemingly discrepant findings for DLPFC, under- and over-activations were previously observed across different groups of participants and different laboratories, and so it is possible that these inconsistencies resulted from systematic differences in participants, tasks, or data analysis procedures. Thus, in the present study, we investigated DLPFC activity in young and older adults at three levels of verbal WM load. By using loads of 4, 5, and 7 items, we covered a range of task demands that has been previously associated with both age-related over-activation and under-activation, thereby permitting the possibility of observing both effects in the same participant group. Based on CRUNCH, we predicted: (1) age-related over-activation of DLPFC at low verbal WM loads and (2) age-related under-activation of DLPFC at high verbal WM loads. Furthermore, the use of a delayed item-recognition task in concert with event-related fMRI design allowed us to compare age-related differences in DLPFC activation across encoding, maintenance, and retrieval stages.
Materials and Methods
Participants
Twenty-one young (11 females) and 23 senior (13 females) adults were paid to participate in the study. See Table 1 for detailed participant information. Young participants were recruited using advertisements posted on the University of Michigan campus. Older adults were recruited from Ann Arbor and surrounding communities through the University of Michigan Institute of Gerontology and newspaper and television advertisements. All participants were right-handed, native English-speakers with normal or corrected to normal vision and hearing and no history of head injury or neurological or psychiatric illness, and a minimum MMSE of 25. Informed consent was obtained from all participants, and all procedures were approved by the University of Michigan’s Institutional Review Board.
Table 1.
| Table 1a. Participant information. | ||
|---|---|---|
| Young Adults | Seniors | |
| N | 21 | 23 |
| N (females) | 11 | 13 |
| Mean Age (SD) | 20.8 (1.7) | 68.4 (6.5) |
| Minimum Age | 18 | 61 |
| Maximum Age | 24 | 82 |
| Years PSE (SD) | 2.5 (2.0) | 4.9 (3.2) |
| Table 1b. Neuropsychological Test Scores | |||||
|---|---|---|---|---|---|
| Youngs | Seniors | ||||
| Test | Mean | SD | Mean | SD | Age Difference |
| Spatial Span Forwards | 10.00 | 1.45 | 7.74 | 1.45 | *** |
| Spatial Span Backwards | 9.00 | 1.55 | 7.22 | 1.68 | ** |
| Digit Span Forwards | 11.71 | 2.35 | 10.00 | 1.78 | * |
| Digit Span Backwards | 8.29 | 2.10 | 6.48 | 2.25 | * |
| WCST Correct | 70.81 | 10.09 | 71.70 | 12.06 | n.s. |
| WCST Non-perseverative Errors | 7.71 | 6.31 | 18.43 | 15.06 | ** |
| WCST Perseverative Errors | 8.05 | 4.25 | 14.57 | 11.36 | * |
| Reading Span | 3.10 | 1.34 | 2.13 | 0.87 | * |
| APM-Short | 9.19 | 1.60 | 4.04 | 2.38 | *** |
| Morningness/Eveningness | 36.62 | 7.27 | 53.52 | 5.16 | *** |
| MMSE | n/a | n/a | 29.17 | 1.23 | n/a |
| Digit Symbol-Coding | n/a | n/a | 64.78 | 17.74 | n/a |
SD = Standard deviation; PSE = Post-secondary education
WCST= Wisconsin Card Sort Test; APM= Advanced Progressive Matrices; MMSE= Mini-mental State Exam; SD = Standard deviation;
= p < .05;
= p < .005;
= p < .0005; n.s. = not significant
WM Task and Procedure
Data were acquired during two experimental sessions that occurred on separate days. The minimum duration between sessions was one day and the maximum duration was 13 days (mean = 3.9 days). Seniors were tested in the morning and young adults were tested during the afternoon (May, et al., 1993). On testing day 1, participants were administered a series of neuropsychological tests in a session lasting 1–2 hours (see Table 1), and practiced the WM tasks they were to perform in the scanner. On testing day 2, participants completed four blocks of a verbal WM task while in the fMRI scanner.
Memory load varied between 4, 5 and 7 letters. These loads, which likely exceed pure WM capacity (e.g., Cowan 2001), were selected based on prior work from our lab and others indicating a verbal memory load of four items produced robust age-related over-activation in prefrontal regions together with minimal age differences in accuracy (Reuter-Lorenz et al., 2000; Cabeza et al., 2004). Testing CRUNCH also required the inclusion of higher loads to assess age-related under-activation with increased task demands.
Each trial of the WM task was composed of three phases. The first (1500 ms) was a target phase in which 4, 5, or 7 black, uppercase consonants appeared simultaneously on a grey background surrounding a centrally located, red fixation point (diameter = 1.5 mm). Letters (height = 11 mm) fell on an imaginary circle (radius = 35 mm) surrounding the fixation point. The second phase was an unfilled delay period of variable duration (4 s, proportion = 0.5; 6 s, proportion = 0.25; 8 s, proportion = 0.125; or 10 s, proportion = 0.125) during which participants fixated on the red fixation point. Finally, there was a probe phase (1500 ms) during which a single black, lowercase consonant was presented and participants indicated whether the identity of the probe item was a member of the most recently presented memory set. On one half of the trials, the probe identity matched one item in the memory set and on the remaining half of the trials the probe identity did not match any of the items in the memory set. “Match” responses were made with a right index finger button press, and “non-match” responses with a right middle finger button press. Response times (RT) were measured from probe onset until the button was pressed. Each trial was followed by a variable inter-trial interval (1.5 s, proportion = 0.5; 3 s, proportion = 0.25; 4.5 s, proportion= 0.125; or 6 s, proportion= 0.125). Note that variable delay periods were included to reduce collinearity between target and probe covariates (Ollinger, Corbetta, & Shulman, 2001), but delay interval does not figure prominently in our analyses due to the limited number of trials per cell. Similarly, variation in inter-trial interval minimized collinearity between probe and target covariates.
Each trial block consisted of eight trials of each memory load presented in quasi-random order, for a total of 24 trials per block. At the beginning of each block, and after the 8th and 16th trial of each block, a 20-s unfilled fixation period occurred during which participants fixated on the red dot presented in the center of the screen. Participants were instructed to respond as accurately as possible and to maintain eye fixation on the red dot for the duration of each block3.
The experimental tasks were programmed using e-Prime (version: 1.1.4.4; Schneider, et al., 2002) and IFIS (version: 1.0.14.20; Invivo, Orlando, FL). During the fMRI session, stimuli were presented on VisuaStim XGA head-mounted display goggles (Resonance Technologies, Inc.) and responses were recorded with a response box that rested in the participant’s right hand.
fMRI Data Acquisition
Images were acquired using a 3T whole-body MRI scanner (General Electric), equipped with the standard quadrature headcoil. Functional T2* blood oxygenation level dependent (BOLD) images were acquired using a spiral sequence with 43 contiguous axial 3-mm slices (TR = 2 s, TE = 30 ms, flip angle = 90°, FOV = 22 cm, in-plane matrix = 64 × 64 voxels). A T1-weighted gradient echo (GRE) anatomical volume was also acquired in the same FOV and slice orientation as the functional scans (43 3-mm slices, TR = 300 ms, TE = 3.7 ms, flip angle = 90°, in-plane matrix = 64 × 64 voxels). In addition, a 106-slice, high-resolution anatomical volume was acquired by using spoiled gradient-recalled acquisition in steady state (SPGR) imaging (1.4-mm slices, TR = 10 ms, TE = 3.4 msec, flip angle = 23°, FOV = 24 cm, in-plane matrix = 256 × 256 voxels). The T1 GRE images were acquired at the start of the scanning session, and the SPGR images were acquired at the end of the scanning session. Head movement was minimized with foam padding and a restraint strapped across the participant’s forehead.
Data Analysis
Behavioral Data Analysis
Accuracy and RT data were analyzed in separate 2 (age group: young, senior) × 3 (memory load: 4, 5, 7 letters) analyses of variance (ANOVAs). Planned, pairwise t-tests were also performed between the age groups at each level of memory load; a Bonferroni correction was applied to control for the inflation of false positives which results from performing multiple comparisons and all reported p-values reflect this correction. For t-tests, only p-values less than 0.2 are reported.
fMRI Data Analysis
Functional images were corrected for slice-acquisition timing differences using a local C++ program that performs sinc interpolation of the eight nearest neighbors in the time series. Head movement was corrected using the Automated Image Registration (AIR 3.08) package (Woods, Grafton, et al., 1998). Subsequent processing and analysis was done using SPM99 (Wellcome Department of Cognitive Neurology, London). SPGR images were corrected for signal inhomogeneity with a high-pass filter using the program developed by Glover and Christoff (Stanford University, Stanford, CA), and were then co-registered to the T1 GRE images. The skull was removed from the SPGR images by using the BET (brain extraction tool) method from FSL (Smith, 2002) and these images were then normalized to the SPM99 T1 template, which is in the Montreal Neurological Institute (MNI) space. The same normalization parameters were then applied to the functional images. After spatial normalization, functional images were smoothed with a 9-mm FWHM Gaussian filter. All of the analyses included a temporal high-pass filter, and each image was scaled to have a global mean intensity of 100. The functional images were also globally mean-normalized to equate overall image intensities over runs and between subjects.
Whole-brain and ROI general linear model analyses were conducted using SPM99, with separate regressors and intercepts created for each of the runs, for every combination of memory load (low, medium, or high), delay period (4, 6, 8, or 10s), and response type (correct or incorrect). The target, delay, and probe epochs were each modeled separately by convolving a boxcar function, time-locked to epoch onset and with a duration lasting the epoch of interest, with SPM99’s canonical hemodynamic response function.
For each participant, contrast coefficients were estimated for each of the target, delay period, and probe epochs between the different levels of WM load. Contrasts between each memory load and the baseline fixation condition were also conducted. Statistical parametric maps were then placed into second-level mixed-effects analyses, with subject identified as a random effect. Unless stated otherwise, the type I error rate was controlled by setting the false detection rate (FDR) threshold at p < .05 (Nichols and Hayasaka, 2003). All activations are reported in Talairach coordinates (Talairach and Tourneaux, 1988). Coordinates were converted from MNI to Talairach space using a transformation developed by Brett, Christoff, Cusack, and Lancaster (2001).
ROI Analyses
The Wake Forest University PickAtlas software toolbox (version 1.03) was used to create ROIs based on Brodmann’s areas (BAs) and/or gyral anatomy (Maldjian, et al., 2003). Due to the discrepant findings of age-related differences in prefrontal activation reviewed in the introduction, and to test our predictions about the recruitment of executive processes as a function of memory load and age, the ROI analyses of critical importance focused on BA 46, 9 (middle frontal gyrus sub-region), and 45 (based on our prior imaging work, see Reuter-Lorenz et al., 2000). Left and right hemisphere ROIs were created for each of these regions and were dilated in three dimensions by a factor of one using PickAtlas. Within these six critical regions we tested for main effects and interactions involving age group, using a conservative alpha level of 0.008 when Bonferroni corrected for multiple comparisons. To test whether any effects we observed in these primary regions of interest were broadly characteristic of the working memory circuitry in general, we also examined whether the same patterns of activation were present across a range of other WM-related ROIs that included BA 32, 6, 47, 37, 39, 40, 47, the medial temporal lobes, and the superior parietal lobule. Due to its large size the BA 6 ROI was divided into four sub-regions on the basis of gyral anatomy: inferior, middle, and superior frontal gyri, and the portion of BA 6 on the medial surface of the frontal lobe.
For each subject, the SPM99 ROI toolbox was used to extract the mean event-related MR signal change of interest (in arbitrary units; Poldrack, 2007) from each ROI after removal of the modeled effects of all other conditions. Separately for each of the three task epochs, and for each ROI, mean MR signal change (henceforth, MR signal) was then entered into a 2 (age group) × 3 (memory load) ANOVA. For the subset of regions that showed a significant interaction of age-group and memory load, pairwise t-tests were performed between the age groups at each level of memory load, and Bonferroni correction was applied to control for false-positive inflation.
Finally, in order to assess the relationship between performance and PFC activity in each age group, Pearson’s r correlation coefficients were computed between MR signal in each ROI and participants’ mean accuracy and RT at each memory load. The Bonferroni-corrected alpha level for these correlations was 0.002 (Curtin and Schulz, 1998).
Results
Behavioral Data
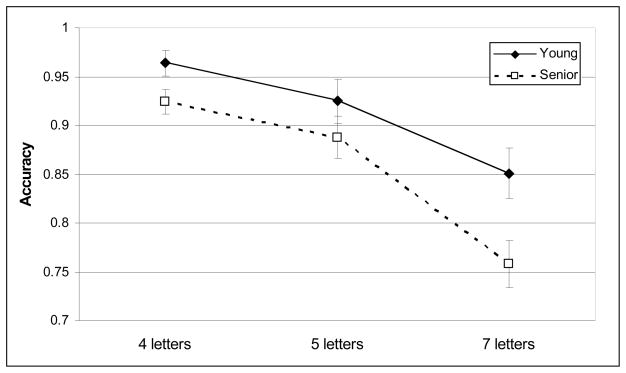
Young and seniors’ mean accuracy and RT are depicted in Figures 1 and 2. Seniors performed less accurately than young adults [F(1,42) = 5.6, p =.023]. Accuracy decreased with increasing memory load [F(2, 42) = 48.6, p < .001]. Age-group did not interact with memory load [F(2, 42) = 2.3, p = 0.11]. However, seniors were less accurate than young adults at a memory load of seven letters [t(42) = 2.61, p = 0.036]; whereas accuracy was age-equivalent when remembering four [t(42) = 2.17, p = 0.11] or five letters [t(42) = 1.21, n.s.].
Figure 1.
Mean accuracy.
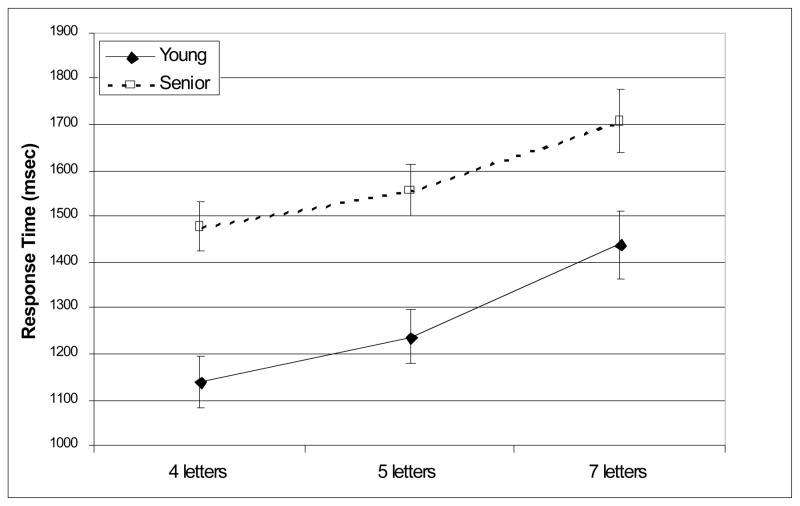
Figure 2.
Mean response time.
Seniors responded more slowly than young adults [F(1, 42) = 13.7, p < 0.001]. RT increased with increasing memory load [F(2, 42) = 72.2, p < 0.001]; however, age-group did not interact with memory load [F(2, 42) = 1.21, p = 0.298]. Seniors were slower than young adults at each memory load [4 letters: t(42) = 4.32, p < 0.001; 5 letters: t(42) = 3.96, p < .001; 7 letters: t(42) = 2.66, p = 0.033]4. Especially for loads 5 and 7, average response times indicate that seniors were most likely to have responded after the offset of the probe, which may have contributed to their lower accuracy. This difference in response time could also contribute to activation differences associated with the probe epoch; however, no such differences were observed (see below).
fMRI Data
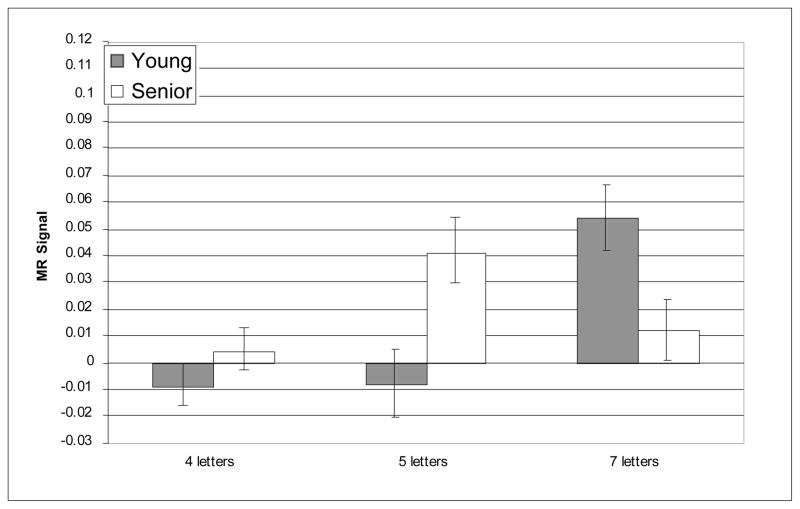
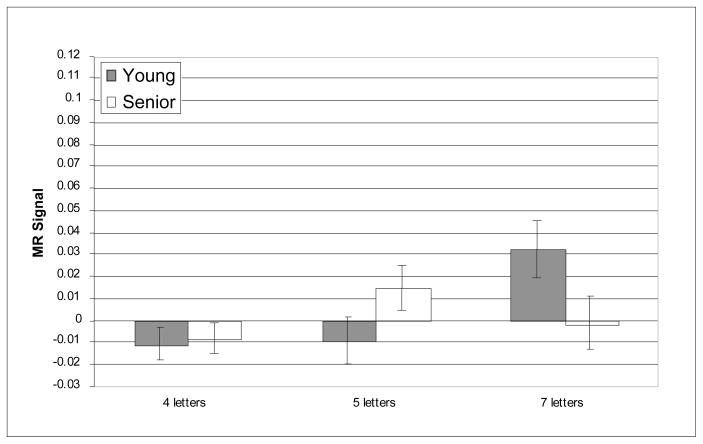
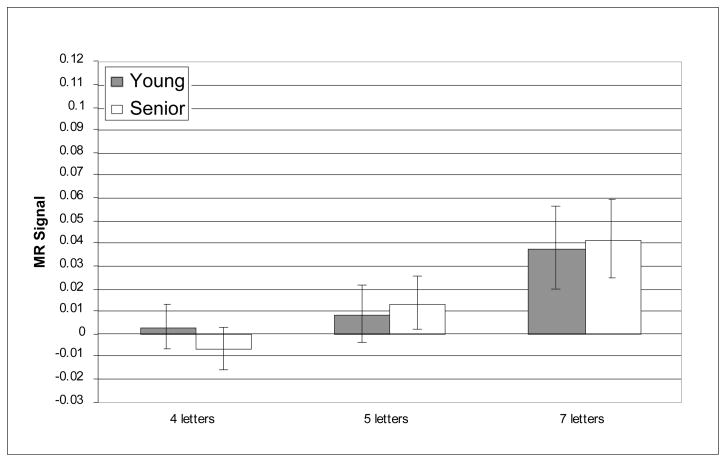
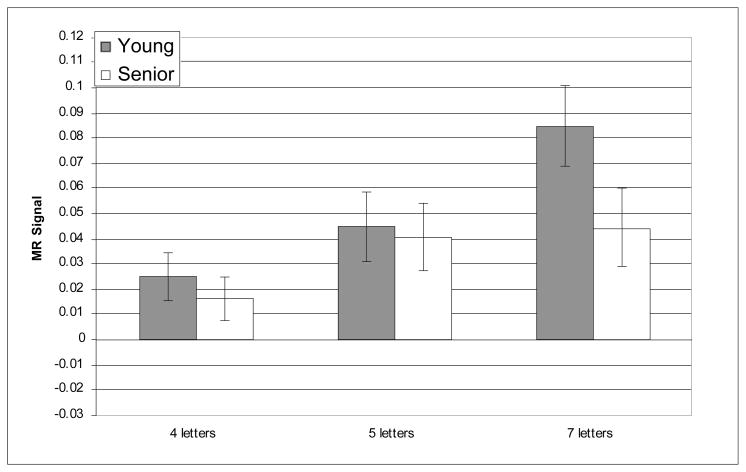
The prefrontal regions that have shown age-related differences in activation in prior reports of verbal working memory were critical for testing age-related differences in the recruitment of executive processes as predicted by CRUNCH. Nevertheless, we also conducted whole-brain analyses and, as noted above, supplementary analyses within other WM-related ROIs in order to examine the regional specificity of the pattern of results observed within the critical regions. The whole-brain analyses did not reveal any reliable age-related differences in activity during verbal WM the target, delay or probe epochs. Furthermore, no reliable age-related differences (main effects or interactions) were observed in any of the supplementary ROIs; this null result persisted even without correction for multiple comparisons, minimizing the possibility that it resulted from an overly conservative statistical threshold. Critically, however, reliable load × age-group interactions were identified only in three of the six prefrontal ROIs and only during the delay epoch; these interactions were all found in the right hemisphere: BA 46, BA 9, and BA 45. Figures 3–8 depict the average MR signal separately for the younger and older age groups in BAs 46, 9, and 45 during the delay period. The following sections present detailed results from each of these right hemisphere prefrontal ROIs and their left hemisphere counterparts.
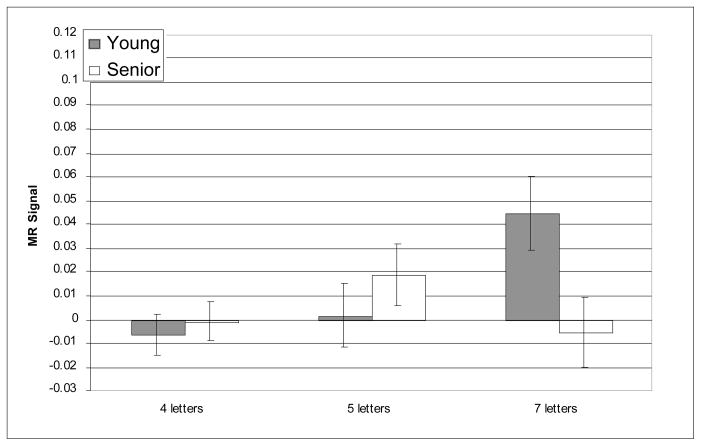
Figure 3.
Average MR signal change (arbitrary units) in left BA 46 during delay epoch.
Figure 8.
Average MR signal change (arbitrary units) in right BA 45 during delay epoch.
Brodmann’s Area 46
In left BA 46, the main effect of memory load was reliable [F(2, 42) = 14.62, p < 0.001]; however, the effect of age-group was not significant [F(2, 42) = .387, n.s.] and age did not interact with memory load [F(2, 42) = .81, n.s.]. Furthermore, planned t-tests indicated that there were no age differences in left BA 46 at any level of memory load.
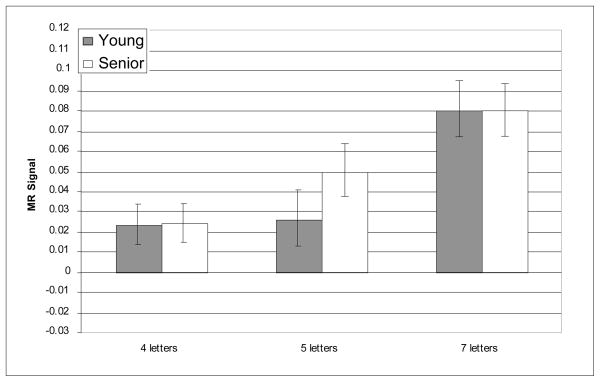
In right BA 46, MR signal increased reliably with increasing memory load [F(2, 42) = 7.08, p = .002], and the effect of memory load was different for the two age groups as indicated by the reliable age-group × memory load interaction [F(2, 42) = 12.38, p < 0.001]. The main effect of age-group was not significant [F(1, 42) = .364, n.s.]. In right BA 46, consistent with our predictions, age-related over-activation was statistically significant at the medium memory load [t(42) = −2.77, p = .024]; however, there was no age difference at the low memory load [t(42) = −1.12, n.s.]. Furthermore, also as predicted, age-related under-activation of right BA 46 was obtained at the high memory load [t(42) = 2.54, p = .045]. Critically, this pattern of age differences cannot be explained by seniors’ slower performance or less accurate performance at the high memory load. When accuracy and RT were included as covariates in the ANOVA, the interaction of age group and memory load remained highly reliable [F(2, 42) = 7.07, p = .002]. Thus, right BA 46 showed a pattern of age-related differences consistent with our prediction of age-related over-activation followed by under-activation with increasing memory load5.
Brodmann’s Area 9
In left BA 9, MR signal increased with increasing memory load [F(2, 42) = 8.56, p < .001]; however, the effect of age group was not significant [F(1, 42) > .001, n.s.] nor did age group interact with memory load [F(2, 42) = .32, n.s.]. Furthermore, planned t-tests indicated that there were no age differences in left BA 9 at any level of memory load.
MR signal in right BA 9 also increased with increasing memory load [F(2, 42) = 5.37, p = .006], and in this region, as in right BA46, the effect of memory load again differed reliably for the two age groups, as indicated by significant interaction of age group and memory load [F(2, 42) = 7.47, p = .001]. The main effect of age was not statistically significant [F(1, 42) = .039, n.s.]. Although none of the planned t-tests of age differences was reliable [4 letters: t(42) = −.256, n.s.; 5 letters: t(42) = −1.62, n.s.; 7 letters: t(42) = 1.91, p =0.190], the reliable interaction of memory load and age group in right BA 9 was consistent with our prediction of age-related over-activation at a relatively low memory load and under-activation at a relatively high memory load. The pattern of effects, and their significance were all preserved when accuracy and RT were included as covariates (age group × memory load [F(2, 42) = 4.70, p = .01])
Brodmann’s Area 45
MR signal in left BA 45, increased reliably with increasing memory load [F(2, 42) = 10.96, p < 0.001]; however, neither the effect of age group [F(1, 42) = 1.43, p = .238] nor the interaction of age group with memory load [F(2, 42) = 2.19, p = .122] were statistically significant. Furthermore, planned t-tests indicated that there were no age differences in left BA 45 at any level of memory load.
The results for right BA 45, were similar to the other right prefrontal regions: activation increased reliably within increasing memory load [F(2, 42) = 3.57, p = .039], the effect of memory load differed reliably for the two age groups, [F(2, 42) = 8.48, p = .001] and the main effect of age was not statistically significant [F(1, 42) = .41, n.s.]. Planned t-tests indicated that the age-differences in right BA 45 were not reliable at the two lower levels of memory load [4 letters: t(42) = .67, n.s.; 5 letters: t(42) = .22, n.s.]. However, the effect of age was marginally reliable at the high memory load [7 letters: t(42) = 1.84, p =0.07]. Therefore, while the activation of right BA 45 followed a general pattern consistent with our predictions, the pattern received only partial statistical support. Once again, the pattern of effects and their significance were all preserved when accuracy and RT were included as covariates (age group × memory load [F(2, 42) = 5.50, p = .008])6.
Activation-Performance Correlations
There were no significant correlations of MR signal in left or right BA 9, BA 46, or BA 45 with accuracy or RT within the young or senior groups for any of the memory loads. The lack of significant correlations between MR signal and accuracy may be in part due to the limited range of accuracies obtained in the task (41/44 subjects had mean accuracies greater than 75%).
Discussion
We observed, within the same participants, both age-related over-activation and age-related under-activation of right prefrontal regions. Consistent with the predictions of CRUNCH, age-related over-activation was observed when participants maintained a relatively low WM load, and under-activation was observed during the maintenance of a relatively high memory load. This pattern was robust in right BA 46, but also evident in right BA 9 and BA 45. Also consistent with CRUNCH, over-activation occurred in senior adults despite equivalent accuracy across age groups, and under-activation occurred with increased memory load and reduced performance. These results are consistent with the proposal that over-activation reflects a limited-resource functional compensation for age-related declines in WM.
A question that is relevant to CRUNCH that we raised in the introduction is whether young adults and seniors recruit similar brain regions in response to increases in task demand. Here, we have obtained evidence that young adults and seniors recruited the same cortical regions in the face of increased verbal WM load. Compared to maintaining four letters, a 5-item memory load, produced no change in right DLPFC activity (Figures 4 and 6). However, when the memory load was increased to seven letters, young adults recruited the same additional right DLPFC regions recruited by the seniors at a lower memory load (Figures 4 and 6). The similar pattern of increased right DLPFC recruitment in young and older adults in response to an increase in task demand is consistent with the proposal that activation of this region in seniors reflects compensation rather than non-specific or dysfunctional activation.
Figure 4.
Average MR signal change (arbitrary units) in right BA 46 during delay epoch.
Figure 6.
Average MR signal change (arbitrary units) in right BA 9 during delay epoch.
Additionally, while pronounced age-related differences were observed in right DLPFC, these differences were absent in left DLPFC despite robust, load-dependent activations in left BA 46 and left BA 9 in both young and older adults. The effectiveness of our parametric load manipulations for both age groups is critical to ruling out potential contributions of baseline activation (e.g., default network; Persson, et al., 2007) differences between the age groups that could otherwise lead to spurious age-related differences in DLPFC.
There are several features of our results that should assuage potential concerns about how age differences in hemodynamic coupling may have affected our results (e.g., D’Esposito et al., 1999; Huettel et al., 2001). First, age differences were evident in only a subset of the regions that we examined. For key task-relevant regions in left prefrontal cortex, the effects of parametric variation of memory load did not differ due to age. Second, in the regions where we did find activation differences due to age, these differences consisted of both over-activation and under-activation, whereas weaker hemodynamic coupling would produce primarily age-related under-activation in older adults. Finally, observed age-related differences in PFC activation were confined to the delay period and were not observed during target or probe epochs. The absence of age differences in activation during the probe phase indicates that age differences in response speed, and the possibility that seniors were more likely to make their response following the offset of the probe, did not confound our estimates of retrieval-related activation across age groups.
It is important to note, however, that while we parametrically varied delay period and ITI in order to minimize collinearity between target and probe regressors, some delay-period activity is necessarily collinear with target presentation. Therefore, executive operations, such as chunking, that are associated with encoding into WM, especially at higher loads, may also contribute to the recruitment of PFC regions. However, we also point out that the reliable interactions involving age group were associated only with the modeled delay-interval activity-- there were no concomitant effects for the modeled target epoch, a pattern of results which, while not conclusive, is consistent with a locus of age-related processing differences in WM maintenance. Finally, CRUNCH does not make strong claims about which particular task epoch or executive processes should be affected by load.
The Function of Age-Related Over-activation
If over-activation is compensatory, what processes are performing the compensation and what are they compensating for? Our results do not provide a specific answer for these questions but they are consistent with our earlier proposal that seniors recruit DLPFC-mediated executive processes for a rote maintenance task that young adults need not recruit at relatively low WM loads (Reuter-Lorenz, et al., 2001; Reuter-Lorenz and Mikels, 2006; Rypma, et al., 2001). While we have evidence that young adults and seniors recruit the same cortical regions in the face of increased WM demand, the question of whether this represents the groups’ recruitment of the same cognitive operation(s) remains unanswered. If the functions of brain regions remain constant with adult aging, then the result obtained in the current experiment suggests that the young adults and seniors recruit the same right DLPFC-mediated cognitive process(es) in the face of increased verbal WM load. However, if aging alters the functions of brain regions, the cognitive interpretation of the result obtained in DLPFC becomes more complicated.
On the assumption that the functions of the right DLPFC remain constant with age, what are some of the possible roles that this region might play with respect to coping with increases in WM demand? The DLPFC is a large and heterogeneous region and its precise functions are still largely unknown. However, we discuss two proposals regarding the role of the DLPFC in WM that are relevant to the present results. First, it has been suggested that DLPFC, especially BA 46, provides a top-down control signal that is critically involved in protecting WM representations from distraction during maintenance (Jha et al., 2004; Sakai, et al., 2002; Yoon, et al., 2006). It may be that as WM load increases, the representation of individual items becomes more fragile and hence more reliant on the DLPFC-mediated mechanisms that protect them from distraction. Distraction could take the form of task-irrelevant environmental stimuli, internally generated task-irrelevant thoughts or emotions, or task-specific interference (e.g., proactive interference from previous trials). Second, other investigators have suggested that the DLPFC is involved in the manipulation or transformation of information in WM (D’Esposito, et al., 1999; Postle, et al., 1999; Veltman, et al., 2003). While rote maintenance of information in WM may suffice at lower levels of memory load, it may be that the maintenance of a large amount of information in WM requires the use of mnemonic strategies that involve manipulation or transformation of the information. For instance, many of the participants in the current experiment reported organizing (“chunking”) the memoranda into smaller, meaningful units by creating words or sentences from the individual letters.
With regard to the precise locus within DLPFC in which age-related over-activation was observed, the results of this study are consistent both with those that have found over-activation in BA 46 (Cabeza, et al., 2004) and those that have found them in BA 9 (Mattay, et al., 2006). At present, the respective roles of BA 9 and BA 46 in the maintenance of WM representations are largely unknown. In the future, ascertaining the precise functions of the diverse subregions of prefrontal cortex will greatly assist in providing a neurocognitive interpretation of the role of age-related DLPFC over-activation (Rajah and D’Esposito, 2005).
Age-Related Under-activation
In addition to observing age-related over-activation of right DLPFC at a relatively low WM load, we also observed under-activation in seniors relative to the younger group at the high memory load. This under-activation was associated with reliably lower accuracy in the older group (Figure 1). These results are consistent with the CRUNCH prediction of under-activation, associated with performance drop, when task demand exceeds available resources. At this “crunch” point, activation could either remain at an asymptotic level or drop off relative to lower task demand, as we observed in the present results. Either possibility is consistent with CRUNCH because once the effective capacity limit has been reached, the neurocognitive system may no longer fully engage task-appropriate processes, leading to a drop in activation relative to lower levels of demand.
In this respect, it is worth comparing the present results to the earlier work of Mattay et al. (2006). They used an n-back task that included three level of task demand (1-back to 3-back). Older adults performed more poorly than younger adults except in the 1-back condition, where the memory load was lightest. Only in the 1-back condition did older adults also show over-activation of the DLPFC. For 2- and 3-back, where older adults performed substantially worse than their younger counterparts, DLPFC activation decreased relative to the level associated with 1-back, and relative to the activation demonstrated by younger adults. Critically, younger adults also appeared to reach a resource ceiling in that by 3-back they showed a drop in performance and a drop in DLPFC activation, paralleling the effects observed in seniors at lower levels of task demand. We speculate that for younger and older adults alike the inflexion point on the function relating task demand to activation corresponds with the inability to effectively recruit the processing operations that were instrumental for mediating successful performance at the lower loads. That is, people may fail to effectively engage the necessary neurocognitive mechanisms when the mismatch between available resources and task demands is too great.
Two other explanations for age-related under-activation have previously been suggested and should also be considered here: (1) an irreversible loss of task-specific neural resources and (2) a decreased ability to spontaneously engage the neural resources that are in fact available. The former mechanism is consistent with the well-documented neurophysiological losses that occur with age (for reviews, see Hedden and Gabrieli, 2004; Raz and Rodrigue, 2006). Evidence for the operation of the latter mechanism, however, was recently obtained by Logan, Sanders, Snyder, Morris, and Buckner (2002), who showed that seniors can engage the very networks that they failed to recruit spontaneously, when provided with a strategy for task performance. In the case of the present results, the fact that older adults did spontaneously recruit right DLPFC at low and intermediate levels of memory load suggests that the under-activation observed at the highest memory load was not due to a lack of spontaneous, strategic engagement of neural resources. Rather, it suggests that the senior group reached a limitation in processing capacity.
One additional explanation for senior’s under-activation at load 7 is that they may have been unable to perceive the entire memory set during the encoding period, and were therefore maintaining a lower memory load compared to younger adults. However, the pattern of activation in left DLPFC is inconsistent with this possibility. Left BA 46 and left BA 9 are more active for both seniors and younger adults at load 7 than at load 5 (no reliable interaction between these loads and age group), suggesting that both groups are attempting to maintain more items in the high than medium load condition. Thus, the left DLPFC regions are recruited in a load-dependent manner that is inconsistent with the possibility that older adults, but not younger adults, failed to encode additional items at load 7.
Resources and CRUNCH
Although CRUNCH is an hypothesis about how age-specific patterns of brain activation relate to task demands and performance, it has some commonalities with earlier ideas about age-related changes in the availability and distribution of resources that emerged from behavioral and neuropsychological studies of cognitive aging (see e.g, Craig & Byrd, 1983; Kinsbourne, 1980; Baltes, 1993, Reuter-Lorenz et al., 1999). Despite previous criticisms about its utility and falsifiability (e.g., Navon, 1984; Salthouse, 1988), the concept of resources has been somewhat revitalized by brain imaging methods which provide new means for operationalizing and quantifying neural resource (e.g. level of activation) recruited for a particular task. Moreover these methods allow improved specification and distinctions between types of resources by virtue of their localization and interconnectivity with other the brain regions (see e.g., Cabeza, Park & Nyberg, 2005). In the current report we demonstrate that specific regions of the left and right prefrontal cortex become active as the demands of a verbal task increase. Importantly, only a subset of those regions, all of which are right lateralized, show age-differential responses to verbal load, and this age-specific response profile shows an intriguing relationship with overall performance levels for the older adults. Clearly, many questions remain about the specific resources provided by these prefrontal regions, but there is good reason to infer they are executive in nature.
Conclusions
The present results are consistent both with studies that have found age-related over- activation of DLPFC and those that have observed age-related under-activation of DLPFC and provide a means of reconciling the seemingly inconsistent findings. At relatively low memory loads, seniors recruit more neural resources than do young adults in order to maintain good performance. At high memory loads, seniors have reached the limits of their neural resources, whereas young adults have resources to spare. One prediction that awaits future testing, is that young adults should also show a decline in task-relevant regions when the memory demands of the task exceed the neural resources available to reach those demands.
These results provide direct empirical support for predictions of Compensation-Related Utilization of Neural Circuits Hypothesis: seniors over-activated right DLPFC at a nominally low level of task demand while maintaining age-equivalent performance, but under-activated this same region at a high level of task demand that resulted in age-related decrements in performance. We also show that this age-related over-activation occurs in a region (right DLPFC) that was also recruited by young adults at higher memory loads suggesting that increased recruitment of this regions is not an aberrant sign of aging, but may instead be a typical compensatory neural response to increased cognitive demand (see also Schneider-Garces et al., 2009). Together these findings suggest that aging results in a shift in the function relating task demand to neural activity in right DLPFC, such that the processing resources provided by DLPFC activation are exhausted at a lower level of task demand. The proposal that such over-activation in older adults serves a compensatory function by bringing more neural resources to bear on task performance gains some credibility from the similarity with younger adults. However, further evidence relating individual patterns of activation to performance will be needed to fully evaluate the compensatory role of age-related over-activation. At present, we believe that CRUNCH provides a useful framework for interpreting age-related under- and over-activations and for relating these patterns to overall brain health and capacity for functional reorganization and redistribution of neural resources with age.
Figure 5.
Average MR signal change (arbitrary units) in left BA 9 during delay epoch.
Figure 7.
Average MR signal change (arbitrary units) in left BA 45 during delay epoch.
Acknowledgments
We thank Megan Walsh and Laura Zahodne for their assistance with data collection and analysis. This work was supported by NIH AG18286 (PARL).
Footnotes
The simple maintenance tasks that are the focus of this report use a single recognition probe following the retention interval, and do not therefore require memory for serial order (cf. Emery, et al., 2008; Sun, et al., 2005).
The phrase “neural efficiency” and the related phrase “cognitive efficiency” have been used by others as constructs to account for individual differences in intelligence and processing speed (Haier, Siegel, Tang, Abel & Buchsbaum, 1992; Neubauer and Fink, 2009; Rypma, Berger, Prabhakaran, et al., 2006). We use the phrase generically here to refer to general age-related neurophysiological declines that compromise the rate and/or quality of neural computation.
All participants had previously completed four blocks of the verbal working memory task outside of the scanner on testing day 1. Additionally, all participants completed 4 blocks of a visuospatial delayed response task during both the behavioral and the fMRI sessions. One half of the participants received the verbal and visuospatial task blocks in ABBABAAB order; the remaining half received them in BAABABBA order. The visuospatial data are not presented in the current report.
This pattern of results was unchanged when error trials were removed from the analysis of response times.
To test for differences within the older sample, young-old (61–66) and old-old (67–82) subgroups were identified based on a median split of the sample according to age. ANOVAs with subgroup and load as factors were computed for each ROI. No effects or interactions with subgroup were reliable, indicating that the patterns of activation did not vary reliably across the age range that constituted the older group.
A reviewer raised the interesting possibility that under-activation with high memory load by older adults might be due to their inability to sustain maintenance-related activity especially across the longer retention intervals (e.g., Paxton, Barch, Racine & Braver, 2008; Kim & Braver, 2009). To test this idea, for each ROI we calculated a three-way ANOVA with age group, retention interval and load as factors. Contrary to the hypothesis that older adults were especially disadvantaged at longer delays, the three-way interaction was not significant for any of the ROIs (all p’s greater than .20). Furthermore, analysis of BOLD time courses associated with the right hemisphere regions of under-activation indicated that older adults showed reduced amplitude responses in early as well as late time points within the retention interval. These results suggest that age-related under-recruitment was not restricted to long retention intervals nor to time points late in the retention interval. Note, however, that there were only a maximum of 8 trials for each delay by retention interval combination, so these results should be interpreted with caution.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychology and Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J. Using the Talairach atlas with the MNI template. NeuroImage. 2001;13:S85. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in old adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Hillsdale; Erlbaum: 1992. pp. 51–109. [Google Scholar]
- Craik FIM, Rabinowitz J. Age differences in the acquisition and use of verbal information: A tutorial review. In: Bouma H, Bouwhuis DE, editors. Attention and Performance. Vol. 10. Hillsdale; Erlbaum: 1984. pp. 471–499. [Google Scholar]
- Curtin F, Schulz P. Multiple correlations and Bonferroni’s correction. Biological Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Fleck MS, Daselaar SM, Cabeza R. Que PASA?: The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Prospective memory and self-reports of memory abilities in older adults. Canadian Journal of Psychology. 1987;41:209–222. doi: 10.1037/h0084152. [DOI] [PubMed] [Google Scholar]
- Emery L, Heaven TJ, Paxton JL, Braver TS. Age-related changes in neural activity during performance matched working memory manipulation. NeuroImage. 2008;42:1577–1586. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. In: Miller M, Kingstone A, editors. The Year in Cognitive Neuroscience. Oxford: Blackwell; 2008. pp. 127–144. [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. NeuroImage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: New evidence from neuroimaging of the aging brain. Current Opinion in Neurology. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel B, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–426. [Google Scholar]
- Jha AP, Fabian SA, Aguirre GK. The role of prefrontal cortex in resolving distractor interference. Cognitive Affective Behavioral Neuroscience. 2004;4:517–27. doi: 10.3758/cabn.4.4.517. [DOI] [PubMed] [Google Scholar]
- Jimura K, Braver TS. Age-related shifts in brain activity dynamics during task switching. Cerebral Cortex. 2009 October 5; doi: 10.1093/cercor/bhp206. eprint, advanced access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Linderberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neuroscience Letters. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychological Science. 1993;4:326–330. [Google Scholar]
- Meegan DV, Purc-Stephenson R, Honsberger MJ, Topan M. Task analysis complements neuroimaging: An example from working memory research. NeuroImage. 2004;21:1026–1036. doi: 10.1016/j.neuroimage.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processsing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Hillsdale; Erlbaum: 1992. pp. 315–372. [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JD. The role of the prefrontal cortex in the maintenance of verbal working memory: An event-related FMRI analysis. Neuropsychology. 2005;19:223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Navon D. Resources- A theoretical soupstone? Psychological Review. 1984;91:216–234. [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural ef ciency: Measures of brain activation versus measures of functional connectivity in the brain. Intelligence. 2009;37:223–229. [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI - II. Analysis. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz PA. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2009;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2. Lawrence Erlbaum Associates; 2000. pp. 1–90. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Marshuetz C, Jonides J, Smith EE, Hartley A, Koeppe R. Neurocognitive ageing of storage and executive processes. European Journal of Cognitive Psychology. 2001;13:257–278. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe R. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Mikels JA. The aging brain: The implications of enduring plasticity for behavioral and cultural change. In: Baltes P, Reuter-Lorenz PA, Roesler F, editors. Lifespan Development and the Brain: The Perspective of Biocultural Co-Constructivism. Cambridge University Press; 2006. pp. 255–287. [Google Scholar]
- Reuter-Lorenz PA, Sylvester CY. The cognitive neuroscience of aging and working memory. In: Cabeza R, Nyberg L, Park D, editors. The Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; 2005. pp. 186–217. [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Age -related change in brain-behaviour relationships: Evidence from event-related functional MRI studies. European Journal of Cognitive Psychology. 2001;13:257–278. [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation performance relations in delayed response task: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The role of processing resources in cognitive aging. In: Howe ML, Brainerd CJ, editors. Cognitive Development in Adulthood. New York: Springer-Verlag; 1988. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime Users Guide. Pittsburgh, PA: Psychology Software Tools; [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH and beyond: working memory capacity and the aging brain. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21230. (epublication ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;12:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Sternberg S. Discovery of processing stages - extensions of Donders’ method. Acta Psychologica. 1969;30:276–315. [Google Scholar]
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, Chen J, He S, Hu X. Age- dependent brain activation during forward and backward digit recall revealed by fMRI. NeuroImage. 2005;26:36–47. doi: 10.1016/j.neuroimage.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: An Approach to Medical Cerebral Imaging. Stuttgart: Thieme Medical Publishers; 1988. [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: An fMRI study. NeuroImage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, and Behavioural Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- West R. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:72–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. general methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D’Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage. 2006;29:1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]