Abstract
Tissue engineering utilizing fibrin gel as a scaffold has the advantage of creating a completely biological replacement. Cells seeded in a fibrin gel can induce fibril alignment by traction forces when subjected to appropriate mechanical constraints. While gel compaction is key to successful tissue fabrication, excessive compaction can result due to low gel stiffness. This study investigated using ruthenium-catalyzed photo-cross-linking as a method to increase gel stiffness in order to minimize over-compaction. Cross-links between the abundant tyrosine molecules that comprise fibrin were created upon exposure to blue light. Cross-linking was effective in increasing the stiffness of the fibrin gel by 93% with no adverse effects on cell viability. Long-term culture of cross-linked tubular constructs revealed no detrimental effects on cell proliferation or collagen deposition due to cross-linking. After 4 weeks of cyclic distension, the cross-linked samples were more than twice as long as non-cross-linked controls, with similar cell and collagen contents. However, the cross-linked samples required a longer incubation period to achieve a UTS and modulus comparable to controls. This study shows that photo-cross-linking is an attractive option to stiffen the initial fibrin gel and thereby reduce cell-induced compaction, which can allow for longer incubation periods and thus more tissue growth without compaction below a useful size.
1. Introduction
Fibrin gel is used as a tissue engineering scaffold because it is a natural cell substrate, permissive to ECM deposition [1–3], and conducive to cell-induced compaction that yields alignment of the fibrin fibrils [4, 5]. It is also moldable into physiologically-relevant shapes, being used for fabricating tissue-engineered vessels [6, 7], valves [5, 8, 9], myocardial patches [10, 11], and cartilage [12]. For example, in the development of a tissue-engineered heart valve, circumferential alignment of the fibrin and entrapped fibroblasts was achieved in the valve leaflets by constraining the fibrin gel compaction via design of the casting mold; the collagen fibrils deposited by the cells were found to be aligned with the fibrin fibrils [5, 13]. Over time, as cells deposit stiffer extracellular matrix (ECM) fibrils like collagen, cell-induced gel compaction of the tissue construct should decrease. However, due to relatively slow fibrin remodeling and associated stiffening of the ECM, effects of over-compaction are problematic during long-term incubation, which is required to achieve the tissue mechanical properties needed to withstand physiological forces [14]. In the tissue-engineered heart valve example, over-compaction can reduce the radial dimension of the leaflets beyond coaptation, rendering the valve unusable [5, 8].
A recently reported method to cross-link fibrinogen in solution phase involves covalently linking tyrosine residues that are abundant in two of the three protein chains in fibrinogen (β-chain = 4.9%, γchain =5.6%) [15]. The photochemistry is believed to involve the oxidation of a tyrosine group by a ruthenium metal complex (Ru), activated by blue light in the presence of sodium persulfate (SPS). The reactive tyrosine radical can then react with another tyrosine phenyl group to create a covalent di-tyrosine bond [16]. The relative abundance of tyrosine residues in fibrinogen, comprising about 5% of all residues, is believed to cotribute to the effectiveness of this method.
Previous work by Elvin et al. [15] has shown the photo-cross-linking of solution phase fibrinogen to be highly effective in 150 mg/ml fibrinogen in solution. In tissue engineering application, fibrinogen is treated with thrombin in the presence of Ca2+ to form a fibrin gel by the self-assembly of fibrinopeptides [4, 17–20]. Hence, initial studies were conducted to determine not only the toxic effect of the cross-linking reagents used but also whether any significant change in the stiffness of fibrin gel at 4 mg/ml would result. Additional experiments were performed to evaluate long-term compaction and remodeling of the cross-linked fibrin gel. The objective was to determine if sufficient stiffening of fibrin could be achieved so as to substantially reduce compaction induced by the entrapped cells without a reduction in the tensile mechanical properties of the resulting tissue. If achieved, a longer incubation time could be used to improve the mechanical properties without over-compaction of the tissue formed during fibrin remodeling.
2. Material and Methods
2.1. Cell culture
Neonatal human dermal fibroblasts (nhDFs, Clonetics) were maintained in DMEM/F12 culture medium (Gibco) supplemented with 10% FBS,100 U/ml penicillin, 100 µg/ml streptomycin, and 2.5 µg/ml amphotericin B. Cells were passaged at 100% confluency and harvested for use from passage 7–9.
2.2. Tubular construct (TC) preparation and culture
The details of sample preparation and culture have been previously described (13). Briefly, a cell-seeded fibrin gel was cast in a tubular mold by mixing cells suspended in DMEM into a solution of bovine fibrinogen (Sigma) in 20 mM HEPES-buffered saline. A mixture of bovine thrombin (Sigma) and calcium chloride in DMEM was then added to the fibrinogen/cell mixture. The final concentrations of the suspension were 4.0 mg/ml fibrinogen, 0.4 U/ml thrombin, 3.6 mM Ca2+, and 500,000 cells/ml. Suspensions were well mixed by pipette action and injected into tubular molds of 8 mm ID and 25 mm length having a concentric Teflon mandrel. Constructs were cultured in DMEM media supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.25 µg/mL amphotericin B, 2 µg/ml insulin, and 50 µg/ml ascorbic acid. Constructs were cross-linked (see above) after 1 day or 7 days and cultured for one week, after which they were transferred to a bioreactor for cyclic stretching as previously described [14]). This one-week culture period allowed for cell-mediated fibrin compaction and initial ECM deposition so that the constructs could withstand transfer to the cyclic distension (CD) bioreactor. Samples were cultured in the CD bioreactor for an additional 2 to 4 weeks, as specified. Two sets of experiments were performed, with both sets having paired non-cross-linked controls for all time points. After harvest, each construct was divided into four equal-length sections and then slit longitudinally to form four tissue strips, one for uniaxial tensile testing followed by biochemical analysis, and the others for western blotting, DNA quantification, and histology.
2.3. Hemisphere constructs (HC) preparation and culture
HC were formed in six-well plates with the bottom of each well being scored to yield a 1 cm diameter etch as previously described [21, 22]. Using the same formulation as above, 200 µl of fibrin-forming cell suspension were placed within each etch, allowed to gel for 30 min, and then incubated with the same medium as for TC. Samples were cross-linked (see below) after 24 h of culture and incubated for an additional 3 weeks in the same medium or immediately tested for stiffness change due to cross-linking.
2.4. Compressive stiffness measurement of HC
For a direct assessment, the compressive stiffness of the HC was measured using a quasi-static force indenter test. The HC was gently detached from the tissue-culture plastic and placed on a flat steel-plate. It was then pressed with a 10 mm diameter Teflon disk attached to a load cell. The vertical movement of the Teflon disk was controlled by a calibrated gauge. The HC was slightly pressed with a force of 0.005 N and compressed in increments of 0.005 N, while measuring the corresponding displacement. The acquired compressive force versus displacement data were used for calculation of compressive stiffness defined as the slope of compressive force vs. displacement plot in the ultimate linear region.
2.5. Ruthenium photo-cross-linking of nhDF-seeded fibrin gel
TC and HC were incubated in culture medium for 24 hrs or more (as specified) before cross-linking. Ruthenium trisbipyridyl chloride [Ru(II)(bpy)3]2+ solution (henceforth identified as Ru for simplicity) was made at 10× (20 mM) concentration by dissolving it in distilled H2O. Sodium persulfate (SPS) solution was prepared at 20× (200 mM) concentration by dissolving it in distilled H2O. Solutions were sterile filtered with a 2 µm filter (Millipore, USA). Solutions were protected from light until used and made fresh for each cross-linking experiment. Prior to cross-linking, the solutions were diluted in sterile PBS, and the TC/HC were incubated in a mixed solution of Ru and SPS at 37°C for 10 min. At the end of the incubation, blue light delivered from a custom mesh of 48 blue LEDs in the shape of cylinder with diameter of 6 cm and height of 10 cm was applied at 28 mW/cm2 (luminous intensity of 144 candela) for 20 sec. Blue LED with the same energy output in a dome-shape format of 8 LEDs was used to crosslink HC. The distance between the light source and fibrin gel was kept the same for TC and HC. After cross-linking, the TC/HC were rinsed 2× in PBS and 1× in DMEM, and then cultured in DMEM medium with the supplements described earlier. Controls were kept in normal culture medium and not exposed to blue light but otherwise subjected to the same culture conditions (including cyclic distension following static incubation).
2.6. SDS-PAGE analysis of photo-cross-linked fibrinogen
To evaluate the extent of cross-linking, a 4 mg/mL fibrinogen solution was cross-linked at several dilutions of Ru, ranging from 0.02 mM to 2 mM, while keeping SPS at 10mM. To assess the kinetics of cross-linking, the fibrinogen solution with Ru at 2 mM concentration was exposed to blue light for 1,5,10, and 20 sec. The cross-linked samples were added to a reducing buffer (4× loading dye: Tris-HCL 0.125 M, glycerol 20%, SDS 4%, bromophenol blue 0.01%, DTT 12.5 mM), heated to 95°C for 5 min, and analyzed by electrophoresis on 10% SDS-polyacrylamide gel. 15 µg of protein was loaded for each treatment group. The gel was stained with blue Bandit protein stain (Amresco, OH, USA).
2.7. Uniaxial Tensile Testing
One tissue strip from each TC was tested for tensile properties in the circumferential direction. The thickness of each strip was measured using a 50 g-force probe attached to a displacement transducer. Tissue strips were placed in compressive grips, attached to the actuator arm and load cell of a Microbionix material testing system (MTS systems) and straightened with a load of 0.005 N. This position was used as the reference length of the strip. Following 6 cycles of 0–10% strain at 2 mm/min, strips were stretched to failure at the same rate. True strain was calculated based on the change in length of the tissue over time. The stress was calculated as force divided by the initial cross-sectional area. Young’s modulus (E) was determined by linear regression of the linear region of the stress-strain curve just prior to failure.
2.8. Histology
Tissue strips were fixed in 4% paraformaldehyde, infiltrated with a solution of 30% sucrose and 5% DMSO, frozen in OCT (Tissue-Tek), and sectioned into 9 µm cross-sections. Sections were then stained with Lillie’s trichrome, Verhoeff’s stain and picrosirius red stain. Images were taken using a color CCD camera. For picrosirius red staining, images were taken with the samples placed between crossed plane polarizers.
2.9. Collagen and Cell quantification
Collagen content was quantified with a hydroxyproline assay assuming 7.46 mg of collagen per 1 mg of hydroxyproline [23]. Tissue strip volume was calculated using the measured length, width, and thickness of the strips (as described above in uniaxial testing). Collagen concentrations were calculated as the amount per unit volume in each strip.
DNA content was quantified with a modified Hoechst assay [24]. Cell numbers were obtained from DNA contents assuming 7.6 pg of DNA per cell. Cell concentrations were calculated as the number of cells per unit volume using the dimensions of the strips.
2.10. Cell Viability
Immediately after cross-linking and PBS rinse, HC and TC were evaluated for cell viability by incubating construct in DMEM medium with 2 µM Calcein AM and 4 µM Ethidium homodimer-1 (Invitrogen) for 30 min. The constructs were rinsed in PBS for 5 min and then imaged at 10× objective in florescent microscope. Live cells were imaged in the FITC channel and dead cells were imaged in the rhodamine channel.
2.11 Statistics
For experiments with a single time-point of 3 weeks, statistical significance of differences between groups was determined using one-way ANOVA with the Tukey post hoc test in GraphPad Prism® software for Windows. For experiments with 2 time-points of 3 and 5 weeks, two-way ANOVA with Bonferonni post-hoc analysis was performed to evaluate statistical significance. Any reference to a difference in the Results and Discussion sections implies statistical significance at the level p < 0.05. In all cases where the error bars (plus or minus standard deviation) are non-overlapping, the differences are significant; hence, for clarity, no symbols are used. In cases where error bars are overlapping and the difference is significant, paired symbols are used to indicate the difference.
3. Results
3.1. Cross-linking effects on cell viability and gel stiffness
HC were incubated overnight in culture medium before cross-linking. As shown in Figure 1a, 30 min after cross-linking, no cytotoxic effects were observed. To assess whether cross-linking influenced the stiffness of the fibrin gel, the HC were detached from the plates and allowed to float in PBS for 5 min. In a well known phenomenon due to cell traction forces [25], immediate gel compaction occurred in the control HC, which was inhibited in the cross-linked HC, implying an increased stiffness from this functional assessment (Figure 1b). Using direct measurement of gel compression, measured stiffness of the fibrin gel increased with cross-linking by 93% (Figure 1c).
Figure 1.
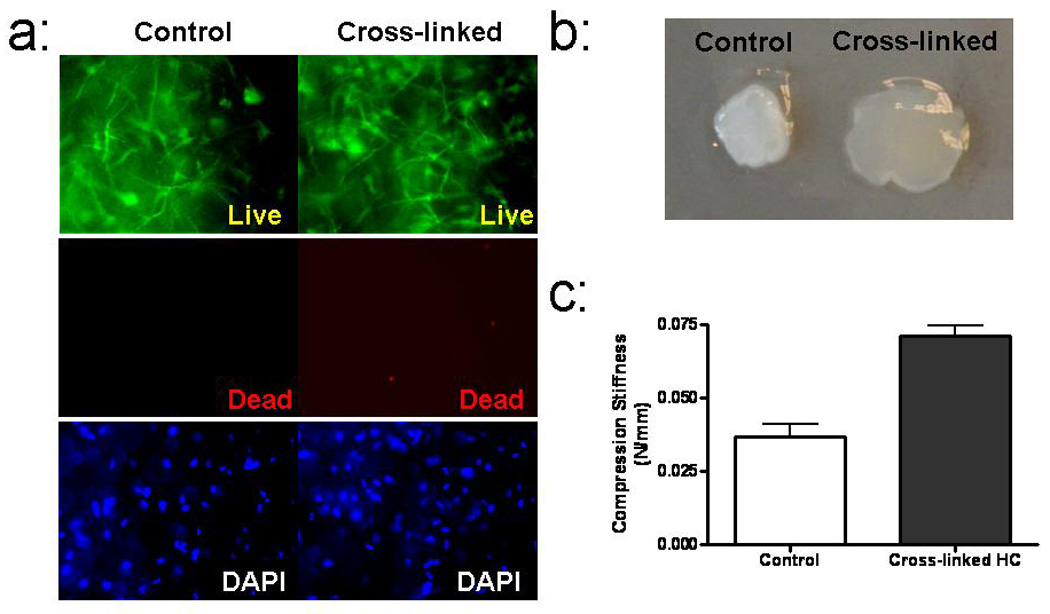
Functional consequences of cross-linking of HC. (a) Live-dead stain comparison of an HC that was photo-cross-linked and a control HC. (b) Visual comparison of HC compaction after detachment from surface. (c) Comparison of compressive stiffness. (* significantly different at p<0.05)
3.2 Cross-linking effects on fibrin gel compaction
TC were cross-linked after day 1 and day 7 of static culture on the Teflon mandrel. At day 7, TC were mounted in the CD bioreactor and cultured for an additional 2 weeks under constant strain amplitude cyclic distension, with length recorded weekly. Figure 2 shows the measured length of TC over 3 weeks. Control samples compacted to 3% of the initial length, while samples cross-linked on day 1 and day 7 compacted to 12% and 17% of the initial length, respectively. Compared to the un-treated control TC, cross-linked TC were thus 4 to 6-fold longer at week 3.
Figure 2.
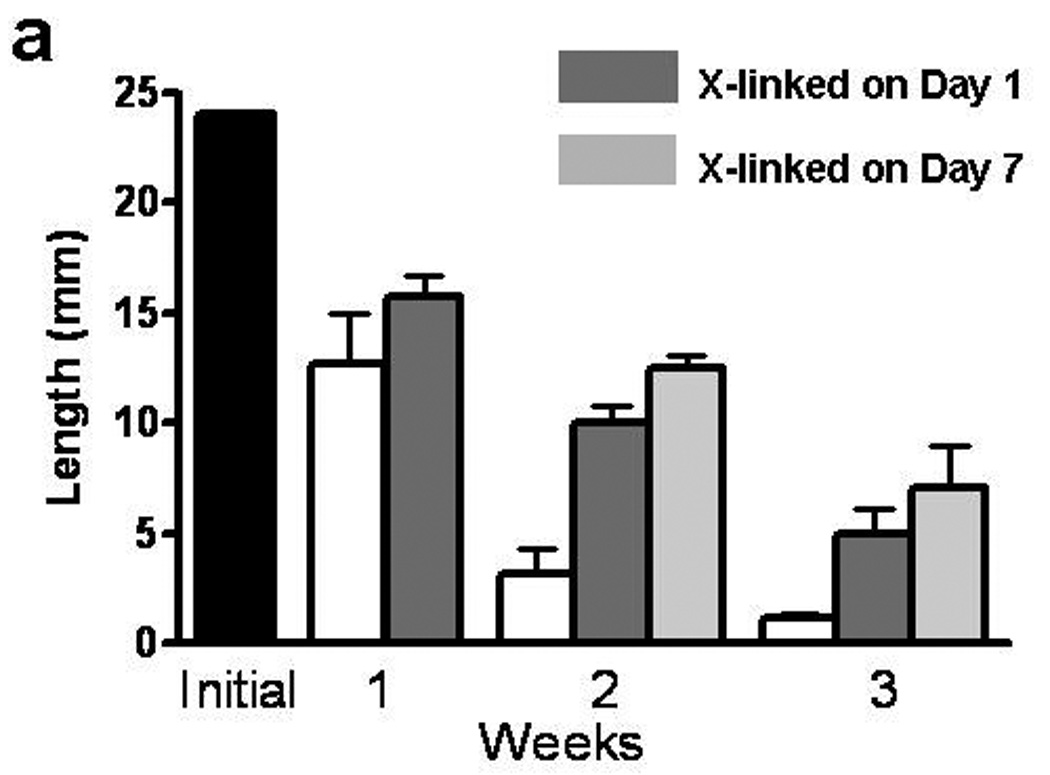
Cross-linking effects on TC compaction. The black bar represents the initial length of TC. White (non-cross-linked control) and shaded grey bars (cross-linked) represent the measured length of TC at weekly time points for the 3 weeks of culture.
3.3. Cross-linking effects on cell proliferation and collagen production
Figure 3 shows the cell count, total deposited collagen, and collagen/cell values. After 3 weeks of culture (including 2 weeks in the CD bioreactor), the cellularity increased by 50% and 125% for control and cross-linked TC, respectively, compared to the initial value (2 million cells per TC). The cellularity was 45% higher in both day 1 (4.4±0.7 million) and day 7 (4.4±0.9 million) cross-linked TC compared to control TC (3.0±0.3 million). There was no difference in collagen deposited by the cells in the control (375±48 µg) and day 1 (409±10 µg) cross-linked TC; however the day 7 cross-linked TC had 45% less collagen (208±77 µg) compared to control TC. Similarly, collagen per cell was not different between control and 1 day cross-linked TC but was 52% less for the day 7 cross-linked TC.
Figure 3.
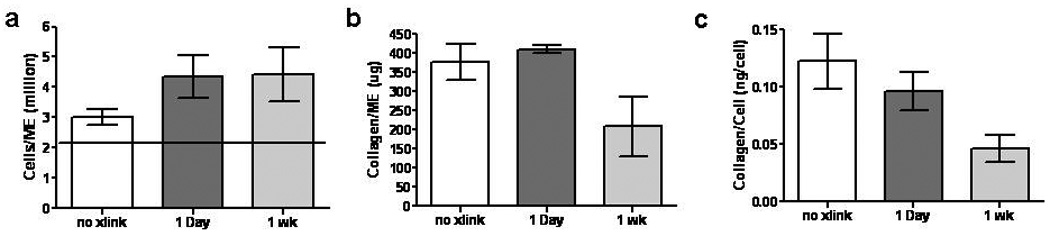
Cross-linking effects on TC cell and collagen content. (a) Cell number, (b) total collagen, and (c) collagen per cell for TC after 2 weeks of culture in the CD bioreactor (3 weeks of culture). The horizontal line in (a) represents the initial cell number.
3.4. Cross-linking effects on tensile mechanical properties
In the experiments described in 3.3, the tensile properties correlated with collagen density, which resulted in cross-linked TC having a lower modulus after 3 weeks of culture compared to control TC (Figure 4). Hence, a second experiment was performed with TC cross-linked at Day 1 and then incubated for additional 3 or 5 weeks (1 week static culture followed by 2 or 4 weeks in the CD bioreactor). For both 3 and 5 week time-points, paired untreated controls were also analyzed. The cross-linked TC further compacted during the additional 2 weeks of CD down to 8.5% of the initial length by week 5 (Figure 5a); however they were still 120% longer than non-cross-linked controls. The UTS and modulus improved by 111% and 293% from week 3 to 5, respectively for the cross-linked TC from week 3 to 5, by 111% and 293% from week 3 to 5, respectively, with 5-week cross-linked TC properties being comparable to the 3-week controls (Figure 5c and d). However, the UTS and modulus of the 5-week controls were 44% and 42% greater, respectively, than the 5-week cross-linked TC values.
Figure 4.
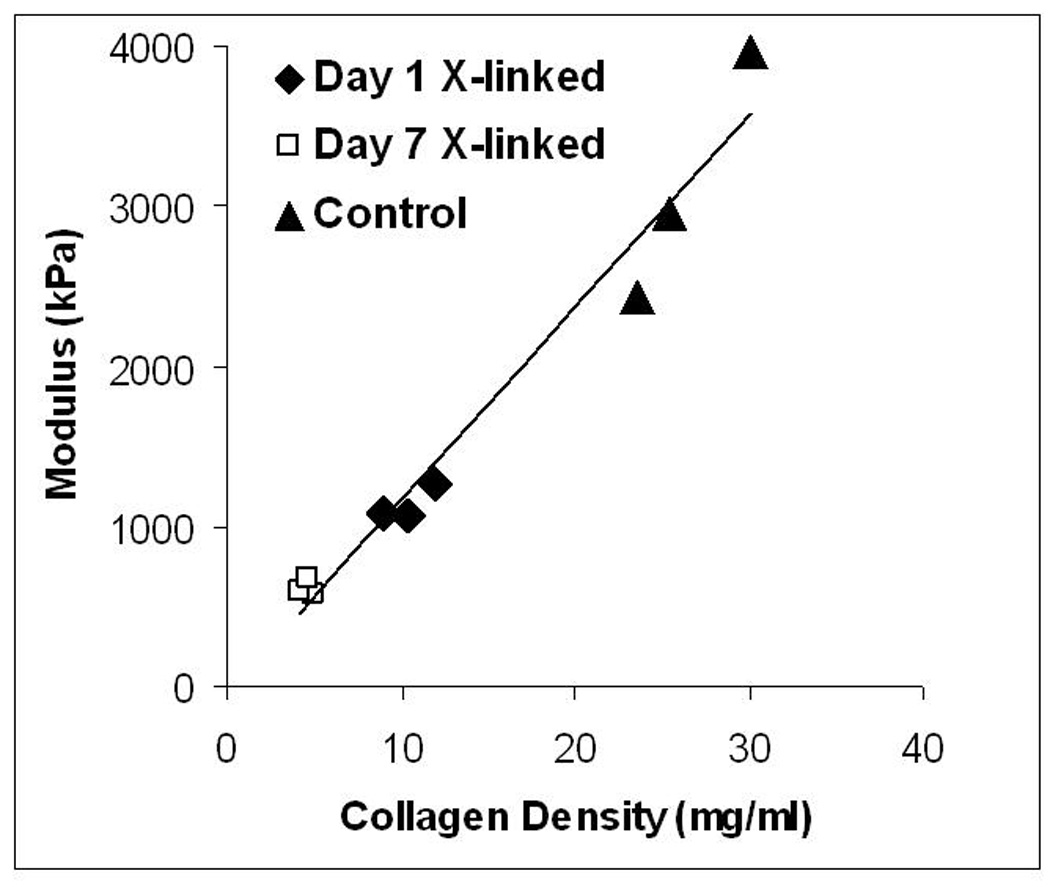
Modulus of TC versus collagen density after 2 weeks of culture in the CD bioreactor. R2 = 0.97 (linear regression line shown).
Figure 5.
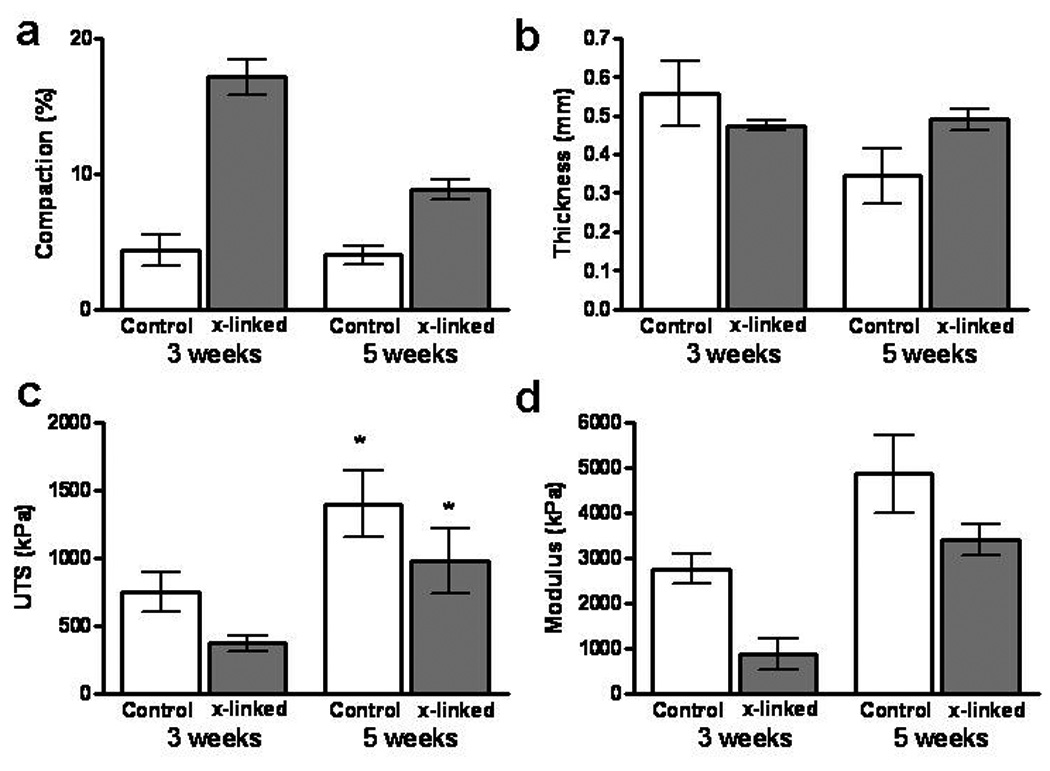
Comparison of compaction and tensile mechanical properties of TC photo-cross-linked on day 1 with non-cross-linked TC control after 3 and 5 weeks of incubation. Samples were statically cultured on mandrel for 1 week followed by 2 or 4 weeks in the CD bioreactor. (a) Compaction as % of initial length. (b) Thickness. (c) UTS. (d) Modulus.
The collagen density of cross-linked TC (9±2 mg/ml) was 150% lower than controls (23±2 mg/ml) at 3 weeks, but improved to 31±2 mg/ml by 5 weeks, which was not different from the controls at 5 weeks (28±6 mg/ml) (Figure 6a). There was a 100% and 65% increase in cell concentration and collagen per cell, respectively for cross-linked TC from week 3 to week 5 (Figure 6b and c). At both 3 and 5 weeks, the collagen deposited per cell was comparable between cross-linked and control TC (Figure 6c).
Figure 6.
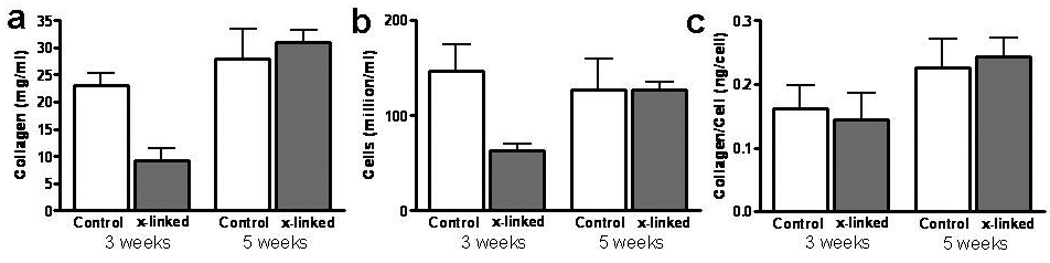
Comparison of cell and collagen contents of TC photo-cross-linked on day 1with non-cross-linked TC control after 3 and 5 weeks of incubation. (a) Collagen concentration. (b) Cell concentration. (c) Collagen per cell.
Remodeling was also evaluated by staining cross-sections of TC at 3 and 5 weeks (Figure 7). Trichrome stain showed the presence of more fibrin (indicated by abundance of red color) in cross-linked TC compared to the controls. Comparing the cross-linked TCs, more green stain (indicating collagen) is visible at 5 weeks compared to 3 weeks. Picrosirius red stained brighter for cross-linked TC at 5 weeks compared to 3 weeks, which suggests collagen maturation between the two time-points.
Figure 7.
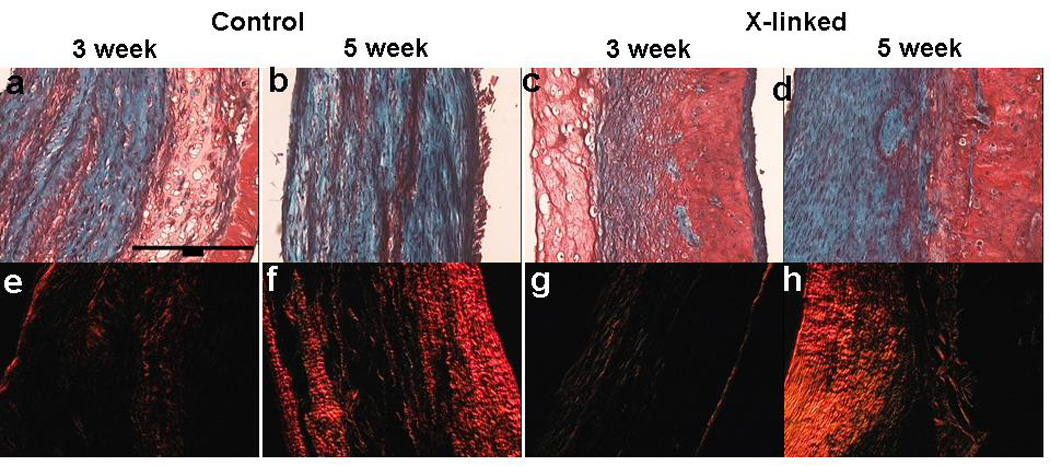
Trichrome and Picrosirius red stain comparison of control TC after 3 (a&e) and 5 week (b&f) with cross-linked TC after 3 (c&g) and 5 (d&h) weeks of culture. In trichrome stained sections, collagen stains green and fibrin stains red. In cross-linked samples, more fibrin is visible at the 3 and 5 week time-point compared to control samples. Picrosirius red stain shows brighter red stain, which is an indicator of more mature collagen, after 5 weeks compared to 3 weeks. A 200 µm scale-bar is shown in the top left image.
4. Discussion
In this study, we investigated whether a ruthenium-catalyzed photo-chemical process, shown to induce dityrosine crosslinking in solution phase fibrinogen to form a tissue sealant [15], could also be used to cross-link fibrin gel with entrapped tissue cells as a means to circumvent over-compaction of fibrin-based tissue constructs during culture. Specifically, we evaluated the effects of photo-cross-linking on the stiffness of nhDF-seeded fibrin gel and long-term compaction and remodeling of seeded fibrin gel during cyclic stretching.
Cross-linking of fibrinogen in solution by Ru was first evaluated by running cross-linked samples on an SDS gel, which showed complete cross-linking of fibrinogen at 4 mg/ml under conditions described in section 2.5 for tubular constructs (Supplementary Figure 1). These results are consistent with results previously reported [15]. The initial HC study demonstrated the photo-cross-linking to be non-toxic to nhDFs entrapped in the fibrin gel while increasing the fibrin gel stiffness, indicative of successful cross-linking.
Given the success in fibrin gel stiffening without loss of cell viability, further long-term effects were evaluated in the tubular samples, which were subsequently mounted in the CD bioreactor to assess remodeling under cyclic stretching [14]. Further, to evaluate whether cross-linking could be beneficial in partially remodeled fibrin, a subset of TC were cross-linked on day 7 rather than day 1. Since fibrin compaction leads to alignment of its fibrils [5], which is important to achieve the desired anisotropic structure, a delayed cross-linking could be beneficial to accommodate the compaction and alignment prior to cross-linking. The three groups (day 1, day 7 and control) were mounted in the CD bioreactor and subjected to cyclic stretching at 10% for 2 weeks after 1 week of static incubation on the rigid Teflon mandrel. The data showed that cross-linking was effective in slowing, but not abolishing, the compaction of the fibrin gel (Figure 2). As noted above, some gel compaction is desirable, as under appropriate mechanical constraints it leads to the circumferential alignment of the fibrin fibrils as well as the deposited ECM fibers [4].
Biochemical data showed that cross-linked TC developed higher cell content (Figure 3a). One plausible explanation would be that cell proliferation is a function of cell density and nutrient availability. It was observed that control TC rapidly compacted by week 2 (Figure 2), and at 3 weeks, the cell density was greater in control TC, compared to cross-linked TC. Due to higher cell density induced by rapid compaction in the control TC, cell proliferation could have slowed between weeks 2 and 3 compared to cross-linked TC. The total deposited collagen was comparable between the control and day 1 cross-linked TC, with day 7 cross-linked TC having 50% less collagen (Figure 3b). Even though no toxic effects of cross-linking were observed immediately after crosslinking (Figure 1), it is possible that the exposure to ruthenium, sodium persulfate, or blue light led induced a lag phase in the cells so they required longer before they could further remodel the surrounding ECM. This is supported in the trend of collagen/cell (Figure 3c), which is similar for controls and 1 day but lower in 7 day samples.
The total collagen was comparable between the control group and 1-day cross-linked TC, but since they possessed dramatically different volumes (Figure 2), the collagen density was 2-fold higher in the controls. As shown in Figure 4, the tensile modulus correlated with the collagen density. To facilitate improvement in the tensile properties of the cross-linked TC, additional experiments with longer culture time in the CD bioreactor were performed. An additional 2 weeks of culture improved the UTS and modulus by 111% and 293% respectively, of cross-linked TC, which are values comparable to the 3-week control samples. The trichrome and picrosirius red stains confirmed further remodeling with more visible (trichrome) and mature (picrosirius red stain) collagen in 5 week TC compared to the 3-week cross-linked TC (Figure 7). However, during the additional 2 weeks of culture, the length further decreased down to 8.5% (percent of original length) compared to 16% at 3 weeks. Nonetheless, the 5-week cross-linked TC were still 120% longer than control TC.
Approaches to reduce/control the compaction of cell-seeded fibrin gel generally include reducing the cell’s ability to exert cell traction forces and thereby compact the fibril network, inhibiting enzymes that reduce fibrin degradation, or stiffening the initial fibrin gel. Aprotinin and ε-aminocaproic acid (ACA) have been used to prevent plasmin-mediated degradation of fibrin; however, at low doses these fibrinolytic inhibitors are not effective and at high doses they inhibit cell proliferation and collagen production [7]. Cell traction force can be inhibited by using myosin [26] or Rho kinase blockers [27, 28]. Blebbistatin has been shown to be effective in reducing cell induced compaction of collagen and fibrin gels [29]. Similarly, the Rho kinase inhibitor Y-67423 has also been shown to be effective in reducing fibrin gel compaction [27]. However, these drugs are relatively expensive and our studies have revealed an inhibition of collagen deposition at concentrations required to inhibit compaction (unpublished data).
Since enzyme inhibitors like ACA and cellular inhibitors like blebbistatin can potentially affect the cell’s ability to produce ECM proteins, stiffening the initial fibrin gel has been considered an attractive option for reducing gel compaction. Though fibrin gel stiffness can be increased by increasing the density of fibrin fibrils, previous research has shown this to also reduce the cell’s ability to remodel the fibrin and achieve desirable tensile properties [7, 30]. Thus, a more attractive approach is to stiffen the fibrin network by cross-linking the fibrin fibrils during or soon after fibrin formation. Previous research in our lab has used Factor XIII and other transglutaminases to cross-link fibrin (unpublished data). They were successful in increasing the initial stiffness of the fibrin gel (the increase was modest, likely due to contaminating Factor XIII in the fibrinogen). Due to the expensive cost of these enzymes and only modest increase in stiffness, this approach was not pursued.
The Ru-based photo-cross-linking method used in this study could be further optimized. While Ru is effective at low concentrations since it is recycled during the photochemical reaction, SPS is consumed, and thus varying the initial SPS concentration can be used to vary the cross-link density more so than Ru (Misook Kim, CSIRO, personal communication).
5. Conclusions
This study shows that use of Ru-catalyzed photo-cross-linking after fibrin formation can be achieved at a relatively low initial fibrin concentration of 4 mg/ml, which is typical for use in creating tissue constructs, based on gel stiffening. The photochemistry had no toxic effects on the entrapped fibroblasts. In long-term culture, the cell proliferation and collagen deposition were comparable between cross-linked and control samples, but tubular constructs that were cross-linked at day 1 were twice as long after 5 weeks. Further, photo-cross-linking can be applied to partially compacted and remodeled fibrin after 1 week of incubation to inhibit further compaction. The study demonstrates ruthenium cross-linking as a successful method to control compaction of fibrin-based tissue constructs.
Supplementary Material
Figure S1: Kinetics of cross-linking of fibrinogen solution. (a) Evaluation of cross-linking at Ru concentrations of 0.02, 0.2, 0.5, 1 and 2 mM for a fixed blue light exposure (20 sec at 10 mW/cm2). (b) Evaluation of cross-linking at blue light exposure times of 1, 5, 10 and 20 seconds at 10 mW/cm2 for a fixed Ru concentration (2 mM). The first lane on the left side of each gel has a protein ladder (L) followed by untreated fibrinogen solution loaded at 15 µg (F).
Acknowledgments
The authors acknowledge Naomi Ferguson and Cary Valley for technical assistance. Funding was provided by NIH BRP HL71538 to R.T.T
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108(Pt 3):1251–1261. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 2.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type I collagen for fabrication of a media-equivalent. J Biomed Mater Res. 2002;60(4):607–612. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 3.Long JJ, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22(4):339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 4.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66(3):550–561. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 5.Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 6.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and Flow Conditioning of Fibrin-Based Media-Equivalents. Ann Biomed Eng. 2006;34(6):971–985. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 7.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11(7–8):991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan TC, Cornelissen C, Koch S, Tschoeke B, Sachweh JS, Schmitz-Rode T, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28(23):3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Syedain ZH, Tranquillo RT. Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials. 2009;30(25):4078–4084. doi: 10.1016/j.biomaterials.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black LD, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0502. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birla R, Dhawan V, Huang YC, Lytle I, Tiranathanagul K, Brown D. Force characteristics of in vivo tissue-engineered myocardial constructs using varying cell seeding densities. Artificial organs. 2008;32(9):684–691. doi: 10.1111/j.1525-1594.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 12.Dare EV, Griffith M, Poitras P, Wang T, Dervin GF, Giulivi A, et al. Fibrin Sealants from Fresh or Fresh/Frozen Plasma as Scaffolds for In Vitro Articular Cartilage Regeneration. Tissue Eng Part A. 2009;15(8):2285–2297. doi: 10.1089/ten.tea.2008.0228. [DOI] [PubMed] [Google Scholar]
- 13.Neidert MR, Tranquillo RT. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12(4):891–903. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 14.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105(18):6537–6542. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elvin CM, Brownlee AG, Huson MG, Tebb TA, Kim M, Lyons RE, et al. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials. 2009;30(11):2059–2065. doi: 10.1016/j.biomaterials.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 16.Fancy DA, Kodadek T. Chemistry for the analysis of protein-protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc Natl Acad Sci U S A. 1999;96(11):6020–6024. doi: 10.1073/pnas.96.11.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and flow conditioning of fibrin-based media-equivalents. Annals of Biomedical Engineering. 2006;34(6):971–985. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 18.Balestrini JL, Billiar KL. Equibiaxial cyclic stretch stimulates fibroblasts to rapidly remodel fibrin. J Biomech. 2006;39(16):2983–2990. doi: 10.1016/j.jbiomech.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Ahlfors JE, Billiar KL. Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials. 2007;28(13):2183–2191. doi: 10.1016/j.biomaterials.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25(17):3699–3706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Tuan TL, Song A, Chang S, Younai S, Nimni ME. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res. 1996;223(1):127–134. doi: 10.1006/excr.1996.0065. [DOI] [PubMed] [Google Scholar]
- 22.Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGF-b1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23(17):3717–3731. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 23.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using HOECHST-33258. Analytical Biochemistry. 1988;174(1):168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 25.Tuan TL, Grinnell F. Fibronectin and fibrinolysis are not required for fibrin gel contraction by human skin fibroblasts. J Cell Physiol. 1989;140(3):577–583. doi: 10.1002/jcp.1041400324. [DOI] [PubMed] [Google Scholar]
- 26.Abe M, Ho CH, Kamm KE, Grinnell F. Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction. J Biol Chem. 2003;278(48):47707–47712. doi: 10.1074/jbc.M306228200. [DOI] [PubMed] [Google Scholar]
- 27.Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456(2):224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57(5):976–983. [PubMed] [Google Scholar]
- 29.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12(4):378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 30.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12(6):1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kinetics of cross-linking of fibrinogen solution. (a) Evaluation of cross-linking at Ru concentrations of 0.02, 0.2, 0.5, 1 and 2 mM for a fixed blue light exposure (20 sec at 10 mW/cm2). (b) Evaluation of cross-linking at blue light exposure times of 1, 5, 10 and 20 seconds at 10 mW/cm2 for a fixed Ru concentration (2 mM). The first lane on the left side of each gel has a protein ladder (L) followed by untreated fibrinogen solution loaded at 15 µg (F).


