Abstract
Rat pups, in isolation, produce ultrasonic vocalizations (USVs). These USVs have been used as a diagnostic tool for developmental toxicity. We have shown that neonatal ethanol (ETOH) exposure produces deficits in this behavior. The current study was designed to examine whether agmatine (AG), which binds to imidazoline receptors and modulates n-methyl-d-aspartate receptors (NMDAR), could reduce these deficits. In addition, this study examined critical periods for ETOH’s effects on USVs by administering ETOH during either the 1st or 2nd postnatal week. Neonatal rats received intragastric intubations of either ETOH (6g/kg/day), ETOH and AG (6g/kg/day and 20 mg/kg/day), AG (20mg/kg/day), or maltose on postnatal days (PND) 1–7 or 8–14. A non-intubated control was also included. Subjects were tested on PND 15. Neonatal ETOH exposure significantly increased the latency to vocalize for females and reduced the rate of USVs in both males and females exposed to ETOH on PND 1–7. Agmatine reduced these deficits, in female but not male pups. Subjects exposed to ETOH on PND 8–14 showed no evidence of abnormal USVs. These findings suggest that there may be gender differences in response to AG following neonatal ETOH exposure and also provide further support that the first neonatal week is a particularly sensitive time for the developmentally toxic effects of ETOH in rodents.
Keywords: Fetal Alcohol, Agmatine, Maternal-infant communication, NMDA, Polyamines
Introduction
Prenatal ethanol exposure is associated with a variety of serious and often permanent detrimental effects for the exposed offspring. These children, who can be classified under the umbrella term of Fetal Alcohol Spectrum Disorder (FASD), suffer from a variety of cognitive and behavioral deficits [39,51]. It is estimated that FASD, one of the leading preventable causes of mental retardation affects approximately 9.1 per 1000 live births in the U.S. and Canada [54,71]. Annually, FASD costs approximately 3.4 billion dollars [38], making the consequences of FASD a serious socioeconomic concern as well as a significant health issue for the individual and their family.
Hyperactivity, attention deficits, and poor psychosocial functioning are often reported in clinical populations with histories of chronic prenatal alcohol exposure [31,48,53,59]. Ethanol-induced psychosocial deficits have been observed as early as infancy including nursing and feeding difficulties, as well as increased irritability [11] with the potential to interrupt normal maternal care-giving behavior and bonding [31].
Rodent models of fetal ethanol (ETOH) exposure demonstrate many similar types of deficits including suckling deficits and increased arousal [5,6,37, 44]. These rodent models typically use one of two ETOH exposure paradigms. The prenatal model, which involves administration of ETOH to the pregnant dam, is often used as a model for 1st and 2nd trimester effects in reference to human pregnancy. The 2nd exposure paradigm involves ETOH administration directly to the neonatal rat. The underlying rationale for this exposure model stems from differences in when birth occurs across species relative to CNS development. The “brain growth spurt,” a period characterized by rapid CNS growth and proliferation, occurs during the third trimester of human pregnancy and this same period extends into the first two weeks of neonatal life in the rat [14]. Thus, this provides the rationale for using neonatal ETOH exposure in rodents as a model for the human 3rd trimester exposure, at least in terms of CNS development.
Using this neonatal exposure model, we have reported ETOH related deficits in ultrasonic vocalizations (USVs) in neonatal rats [5,8]. Infant rats in stressful situations, such as isolation, produce USVs [2,9,10,45]. These USVs elicit maternal attention, promote pup grooming and retrieval, and suppress biting and cannibalism by the dam [9,45]. Neonatal ETOH exposure disrupts the normal response to isolation as measured by an increased latency to vocalize and a reduction in the number of vocalizations during isolation [5,8]. The current study was designed to further examine the effect of neonatal ETOH exposure on USVs by focusing on ETOH exposure during either the first or second neonatal week. In addition, this study was designed to examine whether the addition of agmatine (AG) as a potential neuroprotective agent could reduce ETOH induced deficits in USVs.
AG is an endogenous amine that has received considerable interest in recent years due to its neuroprotective effects following a variety of models for CNS and spinal cord injuries [24,25,77]. AG acts via a variety of mechanisms including binding to imidazoline and noradrenergic receptors [49] and suppressing nitric oxide production [17]. Of particular interest for the current study are AG’s potential neuroprotective effects. These properties have been attributed, in part, to its ability to reduce glutamate release [16], and/or block the polyamine modulatory site on the n-methyl-d-aspartate receptor (NMDAR), which could reduce some of the excitotoxic effects of ETOH withdrawal [23].
Polyamines, including spermine and spermidine play an important role in CNS development [57,58] as well as acting as NMDAR modulators [52]. Increased polyamine levels potentiate NMDAR activity. Polyamine levels are increased during CNS development, as well as during CNS damage [3,52,57], and ETOH withdrawal [22]. Polyamine overactivity has been previously implicated in damage associated with ETOH exposure in both adult [34,55,56] and developing organisms [35,55] and AG has been shown to reduce cell death during ETOH withdrawal in organotypic hippocampal cultures [22]. Recently, we have reported that a single oral administration of AG on postnatal day (PND) 8 reduced balance deficits in adolescent rats that had been exposed to ETOH on PND 1–8 [34]. The current study examined whether chronic AG treatment could also reduce deficits in isolation induced ultrasonic vocalizations following neonatal ETOH exposure in vivo and whether there were differences in sensitivity to ETOH and/or AG if exposure was during the first or second postnatal week. An additional question addressed in this study was whether there were sex differences in response to AG treatment. Previous studies have suggested females may be more sensitive to polyamine manipulations [7] with some brain regions showing a higher expression of NMDARs containing NR2B subunits, a receptor subtype known to be particularly sensitive to polyamines during ETOH WD [13].
2. Methods
2.1 Mating procedure
Parent animals were Sprague-Dawley rats, obtained from Harlan Labs (Indianapolis, IN) that were bred in the University of Kentucky Psychology Department Animal Facility. Pregnant dams were individually housed in plastic cages in a temperature and humidity controlled nursery maintained on a 12-h light/dark cycle. As parturition approached, females were checked twice daily with the date of birth designated postnatal day (PND) 0. The day of birth was marked as postnatal day (PND) 0. Twenty-four hours following birth, litters were culled to 10 pups, maintaining equal numbers of males and females in each litter when possible. Litters of less than 6 pups were excluded from the study. Fourteen litters were treated on PND 1–7; and 15 litters were treated on PND 8–14.
2.2 Neonatal Drug Administration
On PND 1, one male and one female pup from each litter were randomly assigned to each treatment condition to preclude possible litter effects [1]. Using a split litter design, the treatment groups included: ETOH (6g/kg/day), isocaloric maltose, agmatine (AG) (20mg/kg/day), or ETOH and AG (ETOH/AG) (6g/kg/day and 20 mg/kg/day). A non-intubated control (NTC) group was also included. A litter received treatments either on PND 1–7 or PND 8–14. This split litter design is routinely used in a number of laboratories including ours [21, 64] and there are no apparent alterations in maternal behaviors directed toward drug exposed pups (Goodlett personal communication and personal observations).
Drugs were added to a milk-based diet prepared in the laboratory and designed to nutritionally mimic rat milk [68]. Litters were intubated twice daily at 1000 and 1400 h. The liquid diets not containing ETOH were made isocaloric with the ETOH diet by the addition of maltose. Each morning, the dams were separated from the pups for approximately 15 min and held in a separate holding cage until intubations were completed. While away from the dam, pups were kept in the home cage on a heating pad to help maintain their body temperature and were weighed and marked with a non-toxic marker on their back for identification. Pups received intubations using a syringe connected to a piece of PE-10 polyethylene tubing (Clay Adams). The feeding tube was dipped in corn oil to ease esophageal passage. The intubation volume was 0.0278 ml/g body weight. NTCs were also weighed daily, but not intubated.
2.3 USV Testing
The USVs were recorded with the aid of an ultrasonic bat detector (Ultra Sound Advice Model #S-25), set at 40.5 kHz with a condenser microphone (SM-1) set 21.5 cm above the test cage floor. The U-30 Ultrasound Advice Bat Detector has a tuning accuracy of ± 0.1kHz, and frequency range of ± 3.0 kHz. The output was recorded on a SONY #WMD8C Cassette Recorder with low noise cassette tapes. Testing was conducted on PND 15. Previous pilot data suggested no evidence of withdrawal symptoms (i.e., tremor, wet dog shakes) at the time of behavioral testing for pups from either exposure period.
On PND15, the dam was removed, placed in a holding cage and returned to the cage rack. Pups were individually tested in a clean plastic cage similar to the home cage (21cm × 11cm) with wood bedding. Each pup was placed in the lower right hand quadrant of the test chamber for a six min test session. Body weights were recorded at the conclusion of testing. Pups from the test litter, when not undergoing individual testing, were kept on a heating pad in their home cage on a table that was located in the opposite side of the test room (10’ × 16’). Small muffin fans were used to generate white noise (measured at 70dB), which masked extraneous environment or litter generated noise. Once all of the subjects in a litter were tested, the dam was returned to the litter. Audiotape recordings were subsequently manually scored by two independent experimenters, blind to treatment condition with an accepted reliability between scorers of 90%. The average of their scores was taken as the dependent variable. Dependent variables included: latency until the first vocalization and the number of vocalizations/min across the 6 min testing period. The number of subjects per treatment condition and sex are presented in the graphs and range from 7 – 12.
2.4 Statistical Analyses
All analyses were performed using a General Linear Model ANOVA (univariate or for repeated measures with Greenhouse-Geisser corrections on Mauchley’s sphericity as warranted). The General Linear Model uses least squares to estimate the mean and standard errors reported throughout this manuscript and a probability level of .05 or less was considered statistically significant. The data were initially examined using a 5 × 2 × 2 × 6 design with neonatal treatment, exposure period and sex as grouping factors and minute (block) as the repeated measure where warranted. If exposure period interacted with neonatal treatment and/or sex, additional ANOVAs were conducted for each exposure period independently. Furthermore, since a key question addressed in this study was whether AG would reduce ETOH-related deficits, the data were also examined using a 2 × 2 × 2 factorial design with ETOH, AG and sex as grouping variables for each exposure period in order to directly assess the ETOH × AG interaction. For these 2 × 2 analyses, the maltose and NTC control groups were pooled since an initial t-test and Levene’s test of homogeneity of variance showed no differences between them following either exposure period.
3. Results
3.1 Latency to the first USV
The overall univariate 5 × 2 × 2 design yielded a significant neonatal drug treatment by exposure period by sex interaction, F(4,176)= 2.56, p=.04. To better understand this interaction, analyses were conducted separately for each of the exposure periods. The ANOVA on the latency to the first USV for pups that received drug treatment on PND 1–7 revealed a significant neonatal treatment by sex interaction, F(4,87)= 4.16, p=.004, while neonatal drug treatment had no effect on animals exposed to drug on PND 8–14.
PND 1–7 latency to the first USV - Effects of AG vs ETOH
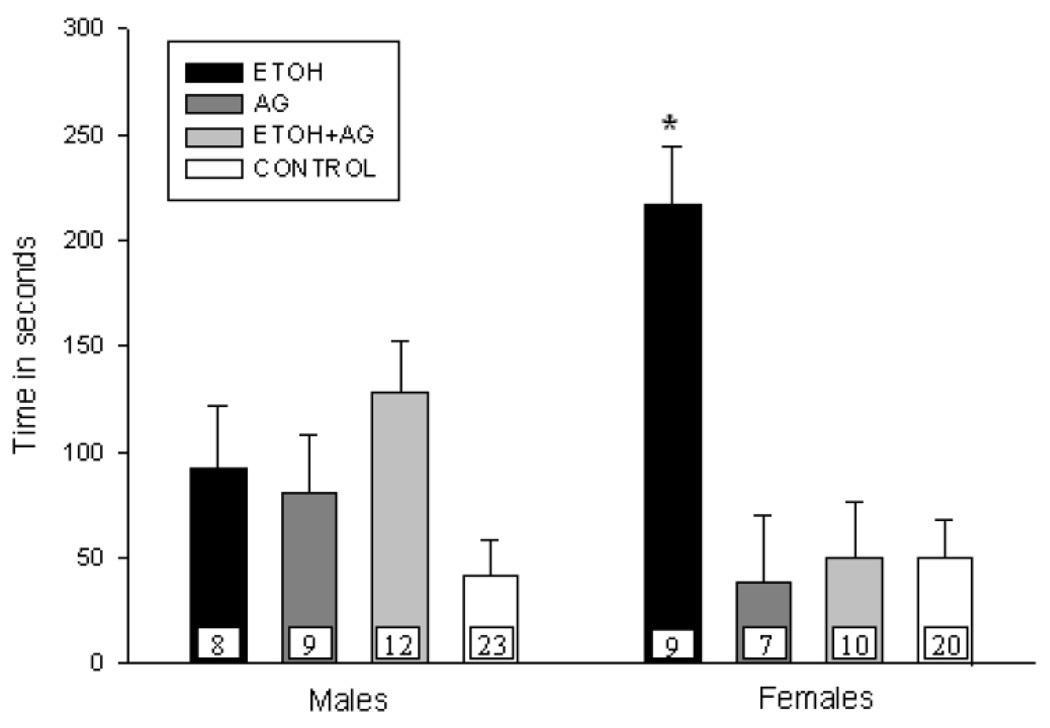
Analysis of the data for pups exposed during PND 1–7 revealed an ETOH × AG × sex interaction, F(1,89)= 4.43, p=.04. To further understand this interaction, univariate analyses were conducted for each sex. As shown in Figure 1a, female ETOH exposed pups took longer to vocalize relative to controls and this deficit was ameliorated by the addition of AG during ETOH exposure [ETOH × AG interaction, F(1,41)= 12.61, p=.001]. AG administration alone had no impact on latency to USV. In contrast, males exposed on PND 1–7 were unaffected by ETOH although there was a trend for the ETOH exposed males to display longer latencies than controls [main effect of ETOH, F(1,48)= 3.09, p=.08] (See Figure 1a). AG alone or in combination with ETOH had no effect on the latency to USV for males.
Figure 1.
Figure 1a. Mean latency (+ S.E.M.) of first vocalization for male and female pups treated on PND 1–7 and tested on PND 15. N’s per treatment cell are presented on each bar (with the two control groups pooled). ETOH exposed females displayed longer latencies to first vocalization and this was eliminated by the addition of AG (* represents groups that differ from controls, p < .05).
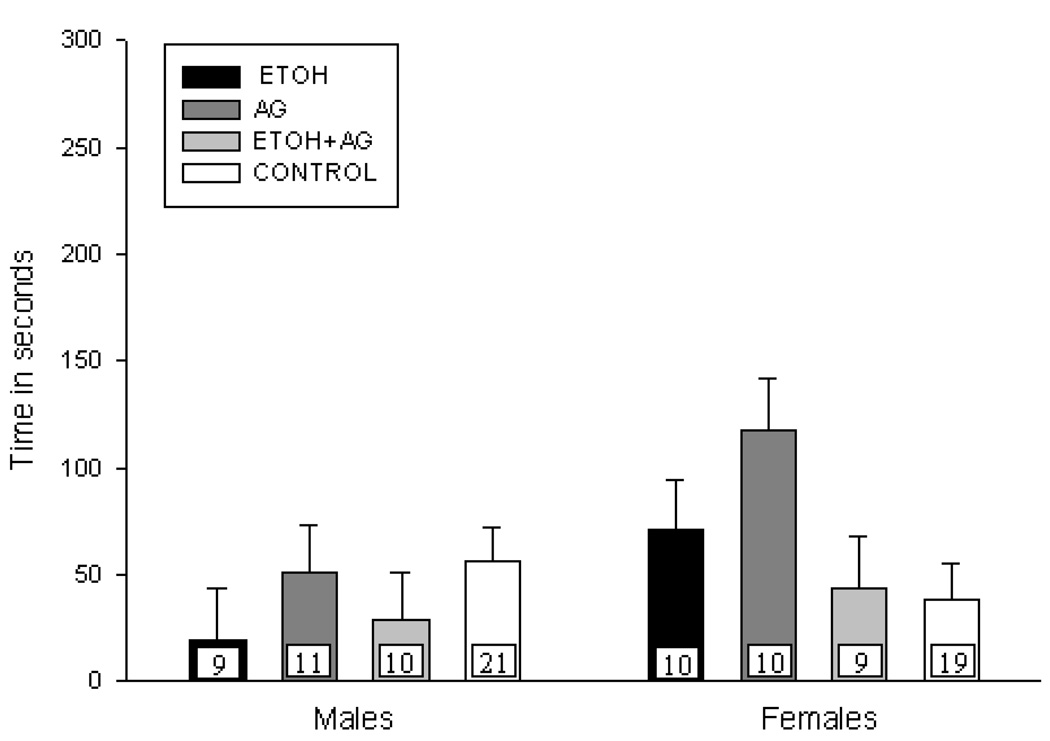
Figure 1b. Mean latency (+ S.E.M.) of first vocalization for male and female pups treated on PND 8 –14 and tested on PND 15. N’s per treatment cell are presented on each bar (with the two control groups pooled). No significant differences in latency to USV were observed.
PND 8–14 latency to the first USV
As stated above, there were no differences in the latency to first vocalization due to neonatal treatment condition for subjects treated on PND 8–14 (See Figure 1b).
3.2 Number of USVs
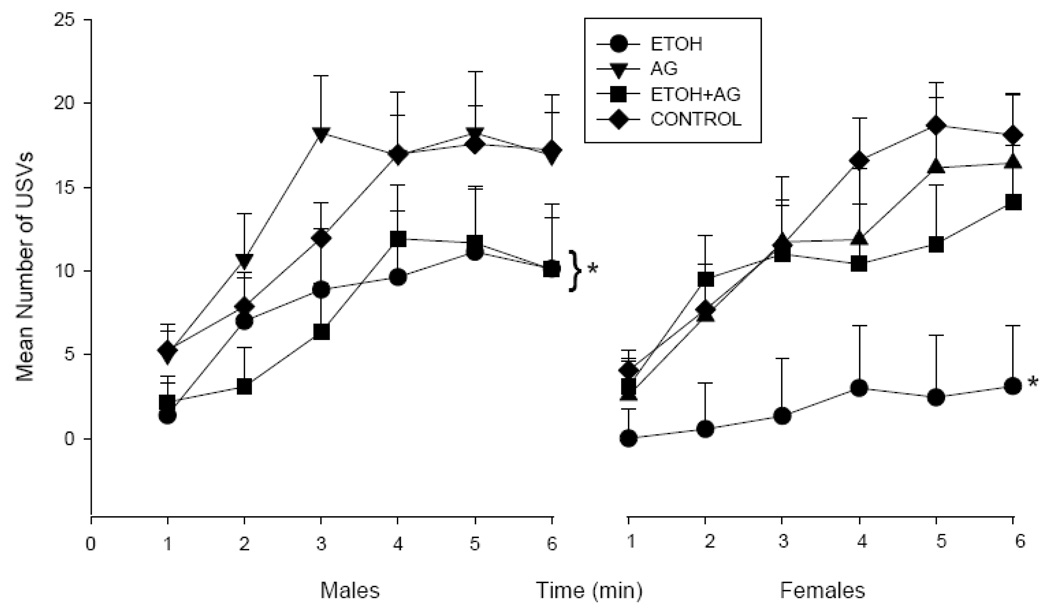
Analysis of the number of USVs in 1 min blocks was initially analyzed by a 5 × 2 × 2 × 6 factorial design (with neonatal drug treatment, exposure period, sex and 1 min block as factors). This ANOVA yielded a neonatal drug treatment by exposure period interaction, F(4,176)= 2.89, p=03. There was also a main effect of sex, F(1,176)= 5.38, p=.02, with males vocalizing more than females. Examination of each exposure period separately revealed a main effect of neonatal treatment for the number of USVS for offspring exposed to drugs on PND 1–7, F(4,87)= 3.80, p=.007, but not on PND 8–14 (See Figure 2b).
Figure 2.
Figure 2a. Mean vocalization rate (+ S.E.M.) for the 6-min test session for male and female pups treated on PND 1–7 and tested on PND 15. N’s ranged from 7–13 per treatment condition. AG in combination with ETOH did not improve performance for males but eliminated the deficit in ETOH exposed females (* represents groups that differ from controls, p < .05).
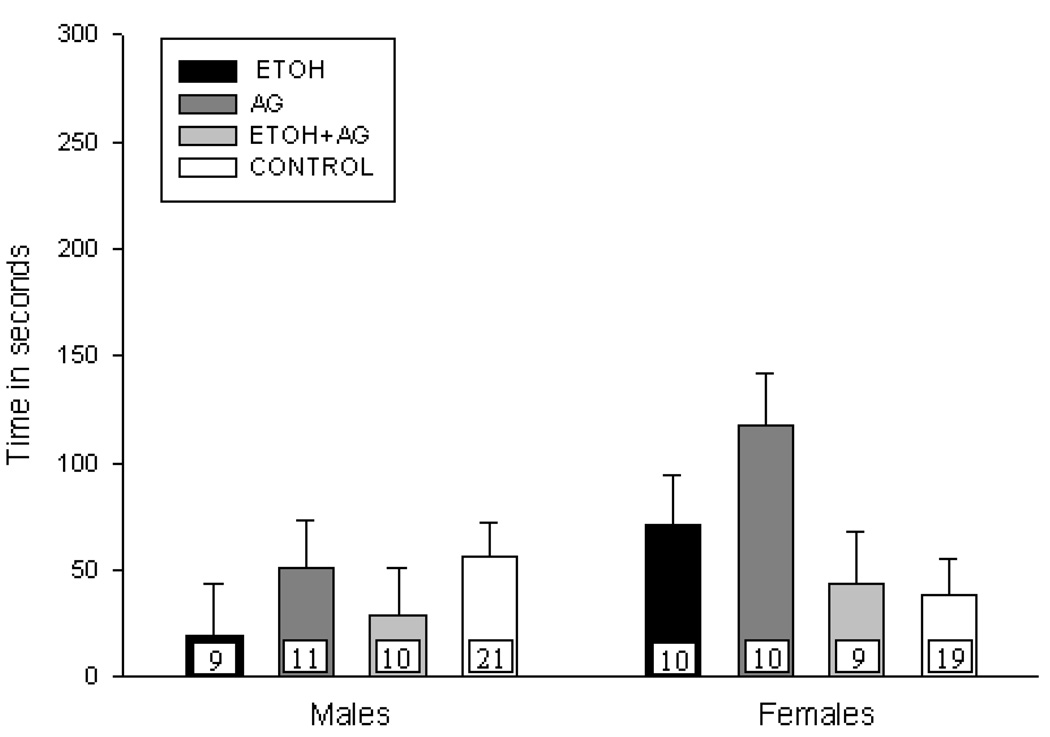
Figure 2b. Mean vocalization rate (+ S.E.M.) for the 6-min test session for male and female pups treated on PND 8 –14 and tested on PND 15. N’s ranged from 9–12 per treatment condition. No significant difference in rate of USVs was observed.
PND 1–7 Number of USVs – Effects of AG vs ETOH
Neonatal ETOH exposure on PND 1–7 reduced the number of USVs, as shown by a main effect of ETOH, F(1,89)= 10.69, p=.002, although there was no ETOH × AG interaction. Additional ANOVAs were conducted for each sex, since sex differences were observed for the latency to first USV and due to the a priori hypothesis that females would be more sensitive to AG. Neonatal ETOH exposure reduced the number of USVs for females and AG eliminated this deficit as shown by an ETOH × AG interaction, F(1,41)= 4.60, p=.05. Males treated neonatally with ETOH also vocalized less than control males but co-administration of AG did not improve outcome. The ANOVA for males showed a main effect of ETOH exposure, F(1,48)= 4.76, p=.03 but no ETOH × AG interaction. AG administration alone had no effect on the number of vocalizations for either sex (See Figure 2a).
PND 8–14 Rate of USVs
Neonatal drug treatment on PND 8–14 had no effect on the number of USVs. Further analyses to investigate the a priori hypothesis also failed to reveal any significant effect of neonatal drug treatment on the rate of USVs for either sex (See Figure 2b).
3.3 Body Weights
Body weights were measured on PND 15 after completion of testing. Due to experimenter error, the body weight was not recorded for 26 subjects (16 from PND 1–7 subjects and 10 from PND 8–14). The missing data were distributed across neonatal treatment conditions. The ANOVA on the remaining subjects showed that rats intubated on PND 8–14 weighed more than those intubated on PND 1–7. The 5 × 2 × 2 ANOVA yielded a main effect of exposure period, F(1,150)= 5.23, p=.02. There was no main effect of neonatal treatment, nor any significant interactions. To remain consistent with previous analyses, the data were also analyzed separately as a function of exposure period. While this weight analysis demonstrated no significant effect of neonatal drug treatment on body weight in the animals in the later exposure group, this was not the case for animals exposed to drugs on PND 1–7, F(4,71)= 3.52, p=.01. Tukey post hoc comparisons revealed that the only groups that were statistically different from each other were the ETOH groups and the non-intubated controls. No other groups differed from each other and there was no main effect or interaction with sex.
Correlational Analyses Between Body Weight and USV Measures
Since the ETOH exposed offspring weighed less than the non intubated controls when exposure occurred on PND 1–7, correlational analyses were conducted between PND 15 body weight and the USV measures (latency and total number of USVs). Analyses were conducted two ways; collapsing across treatment condition, and separately for each treatment group. No significant correlations between body weight and USV outcomes were observed in either analysis. Correlations were not conducted on data from animals exposed on PND 8–14, since these animals demonstrated no USV deficits.
4. Discussion
This study provides further support that there are clear temporal windows of vulnerability following ETOH exposure during the “brain growth spurt”. Both male and female rat pups exposed to ETOH during the first postnatal week displayed fewer vocalizations than controls while pups exposed on PND 8–14 were not impaired. Thus, the first neonatal week is clearly a more sensitive period for ETOH’s effects on the USV response to isolation than the second. These findings are similar to other results from our laboratory examining activity levels [26] and motor coordination on a balance task [34], suggesting this sensitivity to ETOH during the first neonatal week generalizes to a variety of behaviors. An additional intriguing finding from the current study was that AG reduced these deficits although this effect was only apparent for female offspring.
As stated in the Introduction, an underlying hypothesis for the current work was that blocking or reducing polyamine effects, particularly during ETOH withdrawal, could reduce ETOH’s effects on the developing brain. Increased polyamine levels result in increased NMDAR activity and NMDAR activity has been widely implicated as a contributor to the neurotoxic and behavioral deficits associated with prenatal/neonatal ETOH exposure [46,47,61–63]. The NR2B subunit of the NMDAR is particularly sensitive to alcohol’s effects [75] and to polyamine potentiation [70]. This receptor subtype is prominent during early development and as the CNS matures, they are replaced by other subunits [66, 67]. Therefore, periods of development where NR2B subunits are more highly expressed, such as during the early neonatal period, could result in the increased vulnerability to ETOH and to polyamines [20,22,43,52,62,70] and consequently result in greater functional impairments. This could also help explain the neuroprotective effects of AG observed in this study since AG can block polyamine activity [23]. However, it does not explain why AG’s neuroprotective effects were observed only in female ETOH exposed offspring.
There are a number of hypotheses that might explain these sex differences. First, as stated earlier in this paper, there is evidence that there are sex differences in hippocampal NR2B subunit expression in response to and particularly in recovery from chronic ETOH exposure and withdrawal with females showing a more persistent up-regulation than males [13]. Thus, AG administration following ETOH exposure could result in sex differences in outcome as a consequence although this likely also depends on factors not yet well understood (e.g. timing of treatment, CNS region, etc). In addition, recent in vitro work suggests that organotypic hippocampal cultures taken from female neonatal rats are more sensitive to polyamine challenge than those taken from males [7,50]. If female brains are more sensitive to polyamines, drugs that reduce polyamine activity may have a greater effect for females relative to males. Sex differences in pharmacokinetics and/or sensitivity to polyamines have also been reported although this literature is also somewhat ambiguous [19,27,42,60]. Clearly, further work is needed to determine if there are sex differences in the interaction between polyamines, response to AG and ethanol and/or ethanol withdrawal.
Neither the sex differences nor agmatine effects can be explained by differences in blood alcohol concentrations (BAC). Previous results from our laboratory have shown no differences in BACs as a function of sex or agmatine treatment. There was also no evidence of differences in BACs as a consequence of whether ETOH exposure was on PND 1–7 or PND 8–14 [34].
Animals exposed to ETOH on PND 1–7 weighed less than non-intubated controls although they did not weigh less than intubated controls or agmatine (alone) treated pups. Despite these weight differences, it is still unlikely that the deficits in USVs were due to weight differences alone since body weights of ETOH+AG females did not differ from the ETOH females yet their USV response resembled controls. Furthermore, there were no significant correlations between body weight and USV behaviors providing further support that the behavioral outcomes observed were the result of ETOH exposure and not body weight differences per se.
AG has been shown to be protective in other models of CNS injury/damage including glucocorticoid induced cell death [78], ischemia [25], spinal cord injury [15], glutamate induced cell death [65], ETOH withdrawal in an organotypic hippocampal cell culture model [23], and now in vivo neonatal ETOH exposure. It is also important to note that AG administered alone had no effects on behavior which suggests that this agent might be more useful than typical NMDAR antagonists that have a variety of adverse side effects.
AG does have other non-NMDAR mediated mechanisms of action. It is certainly possible that AG’s ability to reduce ETOH’s effects in the current study may be mediated by some of these other mechanisms as well, although other studies using more specific polyamine antagonists and/or NR2B antagonists such as eliprodil [62] and ifenprodil [40] provide additional support for the role of polyamines or at the very least, the involvement of the NR2B receptor subtype in ethanol’s behavioral teratogenic effects.
AG was given chronically with daily ETOH exposure in the current study. The rationale for this administration schedule was to reduce the cell damage/death following the daily withdrawal associated with chronic intermittent ETOH administration. It is possible that this chronic AG regime itself resulted in neuroadaptations that may have reduced the effectiveness of AG (at least for males) and that acute administration of AG may be more beneficial if given during a single (the last) ethanol withdrawal. This does appear to be the case at least in a cerebellar-dependent balance paradigm [34]. Research is currently underway to further examine this hypothesis.
Isolation-induced USVs have been used to study stress responses, anxiolytics, as well as to assess the effects of a variety of teratogens including drugs of abuse [5,30,31,74]. During the first neonatal week, environmental temperature plays a key role in the emission of isolation induced USVs [10]. During the second neonatal week, significant developmental changes occur. At this age, social factors and social cues play an increasingly important role in USV emission relative to environmental temperature [28]. Prenatal alcohol exposure can impair thermoregulatory response to an extended thermal challenge (1–4 hrs) in younger pups (PND 5 or PND 10) but not older preweanlings (PND 15 or PND 20) [79]. It is not known whether 3rd trimester ETOH has an effect on thermoregulatory response. However, based on the postnatal age and developmental maturity of the pups tested (PND 15) and the role that social cues play at this age, it is unlikely that this ETOH related deficit is simply related to a thermoregulatory deficit.
The underlying pathways and neurotransmitters that play a role in USVs are complex and not well understood [12,29,72,73]. The periaqueductal gray (PAG) has received some attention for its role in the production and control of isolation-induced USVs in the developing rat pup [69]. Both in vivo and in vitro studies have shown that ETOH withdrawal results in PAG hyperexcitability [36,76] although it is not known whether this also occurs following neonatal ETOH exposure or whether it plays a role in the USV related deficits observed. However, it is known that the PAG receives inputs from a number of limbic structures, which are known to be damaged by neonatal ETOH exposure [32] and thus one or more aspects of this circuitry could be playing a role in the observed behavioral deficits.
ETOH induced deficits in USVs can have potential long-term effects on learning and social behaviors as a consequence of altered maternal/infant interactions [4,33,41]. The current findings provide some intriguing insight into a possible underlying mechanism for the damaging effects of ethanol on the developing brain and demonstrate the potential efficacy of AG to attenuate such damage, at least in females.
Table 1.
Mean body weight on PND 15 (in grams) ± S.E.M.
| Exposure Period | |||||
|---|---|---|---|---|---|
| PND 1–7 | PND 8–14 | ||||
| Group | Sex | M ± SEM | N’s | M ± SEM | N’s |
| Ethanol | |||||
| Male | 29.1 ± 1.8 | 7 | 38.7 ± 2.3 | 8 | |
| Female | 32.4 ± 1.5 | 9 | 36.7 ± 2.2 | 9 | |
| Agmatine | |||||
| Male | 34.3 ± 1.7 | 6 | 40.7 ± 2.1 | 10 | |
| Female | 33.0 ± 1.6 | 6 | 37.2 ± 2.2 | 9 | |
| Ethanol+Agmatine | |||||
| Male | 33.3 ± 1.4 | 9 | 34.5 ± 2.2 | 9 | |
| Female | 32.6 ± 1.4 | 8 | 34.9 ± 2.3 | 8 | |
| Maltose | |||||
| Male | 36.7 ± 1.7 | 6 | 38.6 ± 2.8 | 8 | |
| Female | 33.2 ± 1.4 | 9 | 41.2 ± 2.2 | 9 | |
| Non-intubated Controls | |||||
| Male | 35.8 ± 1.2 | 11 | 36.3 ± 2.1 | 11 | |
| Female | 35.2 ± 1.3 | 10 | 39.7 ± 2.3 | 8 | |
The ethanol exposed pups treated on PND 1 – 7 weighed less than non-intubated controls on PND 15 (p < .05).
Acknowledgements
The authors would like to thank William Stewart III, Pooja Krishnappa, and Greg Hardin for their assistance in data collection, and Clay Adams for the supply of polyethylene tubing. This research was supported, in part, by NIAAA Grant # AA-014032 to SB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Abbey H, Howard E. Statistical procedure in developmental studies on species with multiple offspring. Dev. Psychobiol. 1973;6:329–335. doi: 10.1002/dev.420060406. [DOI] [PubMed] [Google Scholar]
- 2.Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus) Anim. Behav. 1972;20:175–185. doi: 10.1016/s0003-3472(72)80189-1. [DOI] [PubMed] [Google Scholar]
- 3.Babu GN, Sailor KA, Beck J, Sun D, Dempsey RJ. Ornithine decarboxylase activity in in vivo and in vitro models of cerebral ischemia. Neurochem Res. 2003;28:1851–1857. doi: 10.1023/a:1026123809033. [DOI] [PubMed] [Google Scholar]
- 4.Barbazanges A, Vallee M, Mayo W, Day J, Simon H, LeMoal M, Maccari SA. Early and later adoptions have different long-term effects on male rat offspring. J. Neurosci. 1996;16:7783–7790. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron S, Gilbertson R. Neonatal ethanol exposure but not neonatal cocaine selectively reduces specific isolation-induced vocalization waveforms in rats. Behav. Genet. 2005;35:93–102. doi: 10.1007/s10519-004-0859-2. [DOI] [PubMed] [Google Scholar]
- 6.Barron S, Kelly SJ, Riley EP. Neonatal alcohol exposure alters suckling behavior in neonatal rat pups. Pharmacol. Biochem. Behav. 1991;39:423–427. doi: 10.1016/0091-3057(91)90202-d. [DOI] [PubMed] [Google Scholar]
- 7.Barron S, Mulholland PJ, Littleton JM, Prendergast MA. Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal culture. Alcohol. Clin. Exp. Res. 2008;32:929–936. doi: 10.1111/j.1530-0277.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effects of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharmacol. Biochem. Behav. 2000;67:1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 9.Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups' ultrasonic calls in a particular maternal behavior in Wistar rat: pups' anogenital licking. Behav. Brain Res. 1992;50:147–154. doi: 10.1016/s0166-4328(05)80296-7. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus Norvegicus) elicit and direct maternal search behavior. J. Comp. Psychol. 1994;108:298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Coles CD, Platzman KA. Behavioral development in children prenatally exposed to drugs and alcohol. Int. J. Addict. 1993;28:1393–1433. doi: 10.3109/10826089309062192. [DOI] [PubMed] [Google Scholar]
- 12.Dastur FN, McGregor IS, Brown RE. Dopaminergic modulation of rat pup ultrasonic vocalizations. Eur. J. Pharmacol. 1999;382:53–67. doi: 10.1016/s0014-2999(99)00590-7. [DOI] [PubMed] [Google Scholar]
- 13.Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on gamma-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol. Clin. Exp. Res. 2004;28:957–965. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- 14.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 15.Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc. Natl. Acad. Sci. U S A. 2000;97:10584–10589. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, LeBlanc MH, Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: A potential mechanism for the anticonvulsive effects. Neurosci. Lett. 2005;390:129–133. doi: 10.1016/j.neulet.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2002;52:606–611. doi: 10.1203/00006450-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez K, Caul WF, Haenlein M, Vorhees CV. Effects of prenatal alcohol on homing behavior, maternal responding and open-field activity in rats. Neurobehav. Toxicol. Teratol. 1983;5:351–356. [PubMed] [Google Scholar]
- 19.Ferioli ME, Pinotti O, Pirona L. Gender-related differences in polyamine oxidase activity in rat tissues. Amino Acids. 1999;17:139–148. doi: 10.1007/BF01361877. [DOI] [PubMed] [Google Scholar]
- 20.Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Mol. Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- 21.Gass JT, Jenkins WJ, Marino MD, Lugo JN, Jr., Kelly SJ. Alcohol exposure during development: analysis of effects on female sexual behavior. Alcohol. Clin. Exp. Res. 2007;31:2065–2072. doi: 10.1111/j.1530-0277.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA, 2nd, Holley RC, Pedigo NW, Littleton JM. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol. Clin. Exp. Res. 2003;27:1099–1106. doi: 10.1097/01.ALC.0000075824.10502.DD. [DOI] [PubMed] [Google Scholar]
- 23.Gibson DA, Harris BR, Rogers DT, Littleton JM. Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil. Brain Res. 2002;952:71–77. doi: 10.1016/s0006-8993(02)03198-0. [DOI] [PubMed] [Google Scholar]
- 24.Gilad GM, Gilad VH. Accelerated functional recovery and neuroprotection by agmatine after spinal cord ischemia in rats. Neurosci. Lett. 2000;296:97–100. doi: 10.1016/s0304-3940(00)01625-6. [DOI] [PubMed] [Google Scholar]
- 25.Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:41–46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 26.Gilbertson RJ, Barron S. Neonatal ethanol and nicotine causes locomotor activity changes in preweanling animals. Pharmacol. Biochem. Behav. 2005;81:54–64. doi: 10.1016/j.pbb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Henningsson S, Rosengren E. Biosynthesis of histamine and putrescine in mice during post-natal development and its hormone dependence. J. Physiol. 1975;245:467–479. doi: 10.1113/jphysiol.1975.sp010856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer MA, Shair H. Sensory processes in the control of isolation-induced ultrasonic vocalization by 2-week-old rats. J. Comp. Physiol. Psychol. 1980;94:271–279. doi: 10.1037/h0077665. [DOI] [PubMed] [Google Scholar]
- 29.Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: Further pharmacological characterization. Pharmacol. Biochem. Behav. 2005;82:652–657. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Insel TR, Harbaugh CR. Central administration of corticotropin releasing factor alters rat pup isolation calls. Pharmacol. Biochem. Behav. 1989;32:197–201. doi: 10.1016/0091-3057(89)90233-5. [DOI] [PubMed] [Google Scholar]
- 31.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol. Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol. Teratol. 1994;16:337–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 33.Levy F, Melo AI, Galef BG, Jr., Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev. Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- 34.Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol. Biochem. Behav. 2007;88:114–121. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S. Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol. Clin. Exp. Res. 2001;25 Suppl ISBRA:132S–136S. doi: 10.1097/00000374-200105051-00023. [DOI] [PubMed] [Google Scholar]
- 36.Long C, Yang L, Faingold CL, Steven Evans M. Excitatory amino acid receptor-mediated responses in periaqueductal gray neurons are increased during ethanol withdrawal. Neuropharmacology. 2007;52:802–811. doi: 10.1016/j.neuropharm.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Lugo JN, Jr., Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol. Behav. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 38.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am. J. Med. Genet. C. Semin. Med. Genet. 2004;127:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 39.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol. Clin. Exp. Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 40.Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, Littleton J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol. Clin. Exp. Res. 2002;26:1468–1478. doi: 10.1097/01.ALC.0000033261.14548.D2. [DOI] [PubMed] [Google Scholar]
- 41.Moore CL, Wong L, Daum MC, Leclair OU. Mother-infant interactions in two strains of rats: Implications for dissociating mechanism and function of a maternal pattern. Dev. Psychobiol. 1997;30:301–312. [PubMed] [Google Scholar]
- 42.Murakami Y, Marumo M, Hayashi S. Ornithine decarboxylase antizyme in kidneys of male and female mice. Biochem. J. 1988;254:367–372. doi: 10.1042/bj2540367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J. Neurochem. 2002;80:850–860. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- 44.Ness JW, Franchina JJ. Effects of prenatal alcohol exposure on rat pups' ability to elicit retrieval behavior from dams. Dev. Psychobiol. 1990;23:85–99. doi: 10.1002/dev.420230109. [DOI] [PubMed] [Google Scholar]
- 45.Noirot E. Ultrasounds and maternal behavior in small rodents. Dev. Psychobiol. 1972;5:371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- 46.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 47.Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57Bl/6 mouse brain. Dev. Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- 48.Olson HC, Streissguth AP, Sampson PD, Barr HM, Bookstein FL, Thiede K. Association of prenatal alcohol exposure with behavioral and learning problems in early adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1187–1194. doi: 10.1097/00004583-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Piletz JE, Chikkala DN, Ernsberger P. Comparison of the properties of agmatine and endogenous clonidine-displacing substance at imidazoline and Alpha-2 adrenergic receptors. J. Pharmacol. Exp. Ther. 1995;272:581–587. [PubMed] [Google Scholar]
- 50.Prendergast MA, Harris BR, Blanchard JA, 2nd, Mayer S, Gibson DA, Littleton JM. In vitro effects of ethanol withdrawal and spermidine on viability of hippocampus from male and female rat. Alcohol. Clin. Exp. Res. 2000;24:1855–1861. [PubMed] [Google Scholar]
- 51.Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp. Biol. Med. (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 52.Rock DM, MacDonald RL. Polyamine regulation of N-methyl-d-aspartate receptor channels. Annu. Rev. Pharmacol. Toxicol. 1995;35:463–482. doi: 10.1146/annurev.pa.35.040195.002335. [DOI] [PubMed] [Google Scholar]
- 53.Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol. Clin. Exp. Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- 54.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 55.Sessa A, Desiderio MA, Perin A. Ethanol and polyamine metabolism in adult and fetal tissues: Possible implication in fetus damage. Adv. Alcohol Subst. Abuse. 1987;6:73–85. doi: 10.1300/J251v06n04_06. [DOI] [PubMed] [Google Scholar]
- 56.Shibley IA, Jr., Gavigan MD, Pennington SN. Ethanol's effect on tissue polyamines and ornithine decarboxylase activity: A concise review. Alcohol. Clin. Exp. Res. 1995;19:209–215. doi: 10.1111/j.1530-0277.1995.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 57.Slotkin TA, Bartolome J. Role of ornithine decarboxylase and the polyamines in nervous system development: A review. Brain Res. Bull. 1986;17:307–320. doi: 10.1016/0361-9230(86)90236-4. [DOI] [PubMed] [Google Scholar]
- 58.Slotkin TA, Ferguson SA, Cada AM, McCook EC, Seidler FJ. Neonatal polyamine depletion by alpha-difluoromethylornithine: Effects on adenylyl cyclase cell signaling are separable from effects on brain region growth. Brain. Res. 2000;887:16–22. doi: 10.1016/s0006-8993(00)02961-9. [DOI] [PubMed] [Google Scholar]
- 59.Spohr HL, Steinhausen HC. Clinical, psychopathological and developmental aspects in children with the fetal alcohol syndrome: A four-year follow-up study. Ciba Found. Symp. 1984;105:197–217. doi: 10.1002/9780470720868.ch12. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka T, Kurai K, Kunitoh S, Kondo K, Goto Y, Kawai S, Warashina M, Yamashita T, Toda T, Monna T, et al. Gender-related differences in the inhibitory effect on liver regeneration in alcohol-treated rats: study of polyamine metabolism. Alcohol Alcoho,l Suppl. 1993;1A:15–20. doi: 10.1093/alcalc/28.supplement_1a.15. [DOI] [PubMed] [Google Scholar]
- 61.Thomas JD, Fleming SL, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol. Clin. Exp. Res. 2001;25:764–773. [PubMed] [Google Scholar]
- 62.Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175:189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- 63.Thomas JD, Riley EP. Fetal alcohol syndrome: Does alcohol withdrawal play a role? Alcohol Health. Res. World. 1998;22:47–53. [PMC free article] [PubMed] [Google Scholar]
- 64.Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Dev. Psychobiol. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- 65.Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006;1084:210–216. doi: 10.1016/j.brainres.2006.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: Differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- 68.West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 69.Wiedenmayer CP, Goodwin GA, Barr GA. The effect of periaqueductal gray lesions on responses to age-specific threats in infant rats. Dev. Brain Res. 2000;120:191–198. doi: 10.1016/s0165-3806(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 70.Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the n-methyl-d-aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- 71.Williams RJ, Odaibo FS, McGee JM. Incidence of fetal alcohol syndrome in northeastern Manitoba. Can. J. Public Health. 1999;90:192–194. doi: 10.1007/BF03404505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winslow JT, Insel TR. Endogenous opioids: Do they modulate the rat pup's response to social isolation? Behav. Neurosci. 1991;105:253–263. doi: 10.1037//0735-7044.105.2.253. [DOI] [PubMed] [Google Scholar]
- 73.Winslow JT, Insel TR. The infant rat separation paradigm: A novel test for novel anxiolytics. Trends Pharmacol. Sci. 1991;12:402–404. doi: 10.1016/0165-6147(91)90616-z. [DOI] [PubMed] [Google Scholar]
- 74.Winslow JT, Insel TR, Trullas R, Skolnick P. Rat pup isolation calls are reduced by functional antagonists of the NMDA receptor complex. Eur. J. Pharmacol. 1990;190:11–21. doi: 10.1016/0014-2999(90)94107-9. [DOI] [PubMed] [Google Scholar]
- 75.Wirkner K, Poelchen W, Koles L, Muhlberg K, Scheibler P, Allgaier C, Illes P. Ethanol-induced inhibition of NMDA receptor channels. Neurochem. Int. 1999;35:153–162. doi: 10.1016/s0197-0186(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, Long C, Evans MS, Faingold CL. Ethanol withdrawal results in aberrant membrane properties and synaptic responses in periaqueductal gray neurons associated with seizure susceptibility. Brain Research. 2002;957:99–108. doi: 10.1016/s0006-8993(02)03609-0. [DOI] [PubMed] [Google Scholar]
- 77.Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport. 2000;11:3203–3207. doi: 10.1097/00001756-200009280-00031. [DOI] [PubMed] [Google Scholar]
- 78.Zhu MY, Wang WP, Bissette G. Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience. 2006;141:2019–2027. doi: 10.1016/j.neuroscience.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmerberg B, Ballard GA, Riley EP. The development of thermoregulation after prenatal exposure to alcohol in rats. Psychopharmacology. 1987;91:479–484. doi: 10.1007/BF00216014. [DOI] [PubMed] [Google Scholar]