Abstract
A close association between salt-sensitive hypertension and insulin resistance has been recognized for more than two decades, although the mechanism(s) underlying this relationship have not been elucidated. Recent data in mice with genetic disruption of the γ-melanocyte stimulating hormone (γ-MSH) system suggest that this system plays a role in the pathophysiological relationship between hypertension and altered glucose metabolism during ingestion of a high sodium diet (8% NaCl, HSD). We tested the hypothesis that these two consequences of interrupted γ-MSH signaling were the result of sympathetic activation by studying rats treated with the dopaminergic agonist bromocriptine (5 mg kg−1 ip qd × one week) (bromo) to cause relative γ-MSH deficiency. Bromo-treated rats fed the HSD developed hypertension, and also exhibited fasting hyperglycemia (p<0.005) and hyperinsulinemia (p<0.025). Furthermore, bromo rats on the HSD had impaired glucose tolerance and blunted insulin-mediated glucose disposal. Intravenous infusion of γ2-MSH, or of the alpha-adrenergic receptor antagonist phentolamine, to bromo-HSD rats lowered both MAP and blood glucose to normal after 15 min, (p<0.001 vs control), but had no effect in rats receiving vehicle and fed the HSD; γ2-MSH infusion also reduced elevated plasma noradrenaline (NA) to control in parallel with the reductions in MAP and blood glucose concentration. Infusion of hydralazine to bromo-HSD rats lowered MAP but had only a trivial effect on blood glucose. We conclude that rats with relative γ-MSH deficiency develop abnormal glucose metabolism, with features of insulin resistance, in association with hypertension when ingesting the HSD. Elevated plasma NA concentration in bromo-HSD rats is normalized with γ2-MSH infusion, suggesting that an adrenergic mechanism may link the salt-sensitive hypertension and the impaired glucose metabolism of relative γ-MSH deficiency.
Keywords: Insulin resistance, Melanocortin system, Sympathetic nervous activity
A close association between hypertension and insulin resistance has been recognized for more than twenty years (Ferrannini et al., 1987; Reaven et al., 1996). Much attention has focused on the basis for this relationship because each condition is an independent determinant of cardiovascular risk. However, there has been no theory yet advanced which adequately explains the occurrence of insulin resistance in many, but not all, hypertensive individuals. Among hypertensives, there is general agreement that about half are salt-sensitive (Campese, 1994; Reaven et al., 1996; Weinberger, 1996), although the mechanism(s) underlying this salt sensitivity of blood pressure are not clearly defined (Campese, 1994; Weinberger, 1996; Meneton et al., 2005; Khalil, 2006). Moreover, salt-sensitivity of blood pressure is associated with insulin resistance (Ogihara et al., 2003).
We have been studying the effect of a high sodium diet (HSD) on the proopiomelanocortin (POMC)-derived natriuretic peptide γ-melanocyte stimulating hormone (γ-MSH). We reported that a HSD increases pituitary content of γ-MSH and doubles its plasma concentration (Mayan et al., 1996). Furthermore, mice with genetic disruption of normal signaling of γ-MSH, whether from impaired secretion from the pituitary or from absence of its receptor Mc3r, develop marked salt-sensitive hypertension (Ni et al., 2003); these mice also exhibit abnormal glucose metabolism (Ni & Humphreys, 2008). Rats treated with the dopaminergic agonist bromocriptine also develop salt-sensitive hypertension due to relative deficiency of γ-MSH (Mayan et al., 2003). The hypertension in both mice and rats is rapidly corrected by infusion of exogenous γ-MSH (Mayan et al., 2003; Ni et al., 2003), and prevented by continuous administration of a stable γ-MSH analog (Ni et al., 2003; Ni & Humphreys, 2007). In γ-MSH-deficient mice, but not resistant, Mc3r−/− mice, infused γ-MSH also rapidly corrects the hyperglycemia (Ni & Humphreys, 2008). Consequently, we hypothesized that the bromocriptine model of pharmacologically induced γ-MSH deficiency and salt-sensitive hypertension in the rat would also be accompanied by altered glucose metabolism with characteristics of insulin resistance. Since γ-MSH microinjected into the nucleus of the tractus solitarius decreases sympathetic outflow (De Wildt et al., 1994; Li et al., 1996), and since states of insulin resistance such as obesity-related hypertension are accompanied by heightened sympathetic nervous activity (Bogaert & Linas, 2009), we also hypothesized that both the hypertension and the altered glucose metabolism in rats with relative γ-MSH deficiency fed a HSD resulted from a common underlying mechanism, namely, activation of the sympathetic nervous system.
Methods
Experimental Animals
All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of UCSF. We studied male Sprague-Dawley rats weighing 250-350g and purchased from Harlan, Hollister, CA. They were kept in the temperature-controlled vivarium with a 12-hr light-dark cycle and ingested either a normal sodium diet (NSD, 0.4% NaCl, Purina Mills Purified Diet 5001, Richmond, IN) or a HSD (8% NaCl, Purina Mills Catalogue #32892) for ≥ seven days. Half the rats received daily injections of bromocriptine, 5 mg kg−1 intraperitoneally for seven days (Bromo); the other half received daily injections of normal saline vehicle (100 μl) (Veh). At the end of the seven day period of diet and drug administration, the animals were anesthetized with thiobutabarbital (Inactin®, Sigma, St. Louis, MO USA) (100 mg kg−1 ip) and underwent acute experimentation as described by us (Mayan et al., 2003) with surgical placement of a tracheostomy tube and catheters in a femoral artery and a femoral vein. The rats received an intravenous infusion of normal saline at 1.8 ml h−1 (30 μL min−1) throughout this surgical preparation and for the duration of the experiment. Supplemental doses of Inactin® were given if response to tail pinch indicated a lightened plane of surgical anesthesia. Animals were sacrificed at the conclusion of the experiments by a lethal injection of thiobutabarbital.
Group I Experiments
After recovery from surgery, a fasting blood sample was obtained for glucose and insulin determinations and the rats underwent an insulin tolerance test (ITT) by receiving an intravenous injection of bovine insulin, 1 USP unit kg−1 body weight. MAP and heart rate were monitored continuously for the next 150 min, and 10 μl arterial blood samples were taken 10, 20, 30, 60, 90, 120, and 150 min following the insulin injection for measurement of glucose concentration. After an additional hour to return to baseline, the rats then underwent a glucose tolerance test (GTT). Glucose, 2 mg g−1 body weight was administered intravenously in a volume of 10 μl g−1 body weight and 30 μl blood samples obtained 3, 5, 15, and 30 min after injection for measurement of blood glucose and plasma insulin concentrations. The experiment was then ended by drawing a large blood sample and the animal sacrificed. Groups studied with this protocol were IA: NSD plus Veh; IB: HSD plus Veh; IC: NSD plus Bromo; and ID: HSD plus Bromo injections; each group contained five animals. Separate groups of five animals each were prepared in the same way but did not undergo the GTT or ITT; their blood was harvested for measurement of plasma prolactin and norepinephrine concentrations.
Group II Experiments
All Group II animals ingested the HSD and were fasted at the time of acute study. After control measurements, rats underwent an intravenous infusion of one of three solutions. Group IIA were Veh rats which received γ2-MSH, 0.8 pmol min−1 (n = 6) in normal saline; Group IIB were Bromo rats receiving the γ2-MSH infusion (n = 6). Group IIC were Veh rats which received phentolamine, 3 μg kg−1 loading dose followed by an infusion of 3 μg kg−1min−1 (n = 6), and Group IID were Bromo rats receiving phentolamine (n = 6). Group IIE were Veh rats which received hydralazine, 0.1 μg kg−1min−1 (n = 5), while Group IIF were Bromo rats infused with hydralazine (n = 5). In all groups, the rate of solution infusion was 30 μl min−1. Infusions were continued for 60 min, with hemodynamic measurements and blood sampling (30 μl) repeated at 15 and 60 min. A large blood sample was then collected and the animals sacrificed. An additional group of five Bromo-treated rats fed the HSD was studied similarly except that they received an infusion of γ2-MSH at a rate of 0.4 pmol min−1 and had blood sampling during control and at 60 min for measurement of plasma noradrenalin.
Blood glucose was measured by glucometer (Accensia Elite XL®, Bayer, Inc., Mishowaka, IN). Plasma insulin was determined in 5 μl of plasma using an ultrasensitive rat insulin ELISA kit (catalog #90060, Crystal Chem Inc, Downers Grove, IL), according to the manufacturer's instructions; its intra- and interassay coefficients of variation (CV) are ≤10%. Plasma noradrenaline concentration was measured using an EIA (catalog #17NORHU-EO1-RES, Alpco Diagnostics, Salem, NH); intraassay CF is 15.6%. γ-MSH concentration was assayed in plasma obtained from blood collected in chilled vials containing 500 KIU aprotinin and 5 mg EDTA (Vacutainer® tubes, Becton Dickinson, Franklin Lakes, NJ).with a commercial radioimmunoassay kit from Peninsula Laboratories using 125I-labeled γ2-MSH as tracer. The characteristics of this assay have been reported by us (Ni et al., 1998); intra- and interassay CVs are 5 and 15%, respectively. Plasma prolactin concentration was measured using a commercially available EIA kit (catalog #12-MKVRP1, Alpco Diagnostics). Bromocriptine, bovine insulin, phentolamine, and hydralazine were purchased from Sigma. γ2-MSH was purchased from Peninsula Laboratories.
Data are presented as means ± SEM. Differences within groups were determined by repeated measures ANOVA, while differences between group means were assessed by one-way ANOVA; the Bonferroni post-hoc test was used to correct for multiple comparisons using GraphPad Instat version 3.05, GraphPad Software Inc, San Diego, CA. When groups being compared exhibited unequal variances, the data were log transformed. Plasma γ-MSH values in groups IIE and IIF were compared using the unpaired t test. Plasma noradrenalin levels before and after γ2-MSH infusion were compared using the paired t test. A p value <0.05 was used to indicate the presence of a significant difference.
Results
Group I Experiments (Table 1; Figures 1,2)
Table 1.
Mean arterial pressure, fasting blood glucose and insulin concentration, and plasma prolactin and norepinephrine concentrations in Group I experiments.
| Group | MAP mm Hg |
Fasting blood glucose, mg mL−1 |
Fasting Plasma Insulin, ng mL−1 |
Plasma prolactin, ng mL−1 |
Plasma norepinephrine, pg mL−1 |
|---|---|---|---|---|---|
| IA, Veh/NSD | 91±1 | 90±1 | 1.18±0.26 | 25.9±2.5 | 102±13 |
| IB, Veh/HSD | 90±3 | 86±4 | 0.69±0.17 | 25.0±2.0 | 103±18 |
| IC, Bromo/NSD | 96±2 | 90±4 | 0.68±0.04 | 2.7±0.6† | 97±26 |
| ID, Bromo/HSD | 126±2* | 113±6* | 1.82±0.4£ | 2.3±0.4† | 212±17* |
Values are means ± SEM of five animals in each group. Veh, vehicle treatment; Bromo, bromocriptine treatment 5 mg/kg ip × 1 week; NSD, normal sodium diet; HSD, high sodium diet.
significantly greater than the other groups, p <0.01
significantly lower than Veh groups, p <0.01
significantly greater than Groups 1B and 1C, p <0.025 by one way ANOVA.
Figure 1.
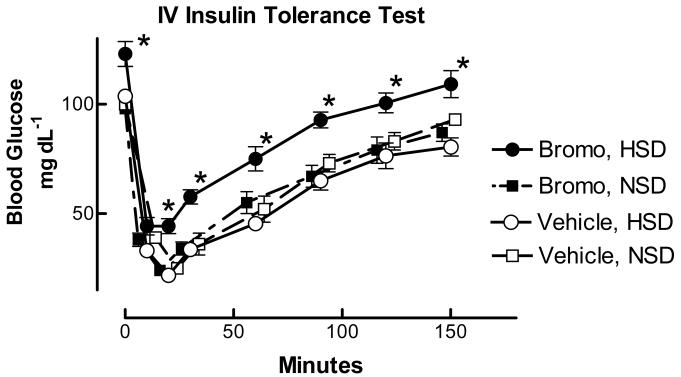
Blood glucose concentration during an intravenous ITT in vehicle or bromocriptine (Bromo)-treated rats ingesting the NSD or HSD. Bromo rats on the HSD were hyperglycemic at the outset, and blood glucose remained elevated throughout the 150 min study period compared to the other three groups. N = 5 rats/group; *, value in Bromo-HSD group significantly elevated over values in other groups, p <0.01 by oneway ANOVA.
Figure 2.
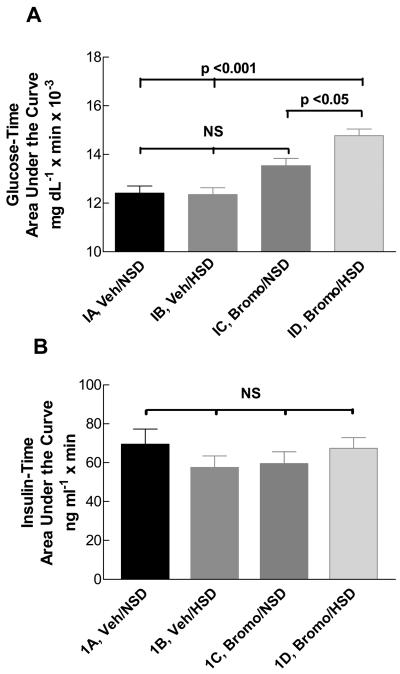
Histogram of the blood glucose-time area under the curve during a high-dose intravenous GTT in vehicle- or bromocriptine (Bromo)-treated rats ingesting either the NSD or the HSD (A), and insulin-time AUC in the same experiments (B). Area under the glucose-time curve was greater in Group ID Bromo-HSD rats compared to vehicle-treated rats on either the NSD (Group IA) or the HSD (Group IB), (p <0.001) or BromoNSD rats (Group IC) (p <0.05) by one-way ANOVA. No significant differences in insulin-time AUC were observed among the groups. N = 5 rats/group.
Anesthetized, fasted Bromo-treated rats fed the HSD for the previous week were hypertensive and exhibited hyperglycemia and hyperinsulinemia (Table 1). In Group I Veh rats, plasma insulin was somewhat less when ingesting the HSD (Group IB) compared to the NSD (Group IA) (Figure 1), but this difference was not significant (p = 0.16). In Bromo-treated rats, this relationship was reversed: Group ID rats, fed the HSD, had a higher fasting insulin concentration than Group IC animals receiving the NSD, and this difference was significant (p < 0.025). Since these Group ID animals were also hyperglycemic, the results suggested the possibility that they had developed insulin resistance or, alternatively, that Bromo treatment was insulin sensitizing in the Group IC animals ingesting the NSD. The values in Groups IA and ID did not differ significantly.
Results of the ITT are shown in Figure 1. Group ID (HSD with Bromo treatment) had higher fasting glucose concentration than Groups IA, IB, or IC. Insulin administered intravenously caused the expected steep reductions in blood glucose in all groups, reaching a nadir 20 min after injection. At each time point blood glucose was significantly higher in Group ID rats than in Groups IA, IB, and IC for the entire 150 min study period, and the percent of the control value at the 20 min time point was lower in Groups IA, IB, and IC than in ID (24.6±1.3% in IA, 24.7±1.3% in IB, 21.1±1.4% in IC, and 36.2±3.1% in ID, p <0.01). These data suggest that Group ID animals with salt-sensitive hypertension may have impaired insulin-mediated glucose uptake compared with the other groups of normotensive rats.
These studies were extended by carrying out an intravenous GTT after the rats had recovered from the ITT. We used a large glucose load to provide a strong stimulus for insulin secretion in addition to measuring the changes in blood glucose concentration. Area under the blood glucose-time curve (AUC) did not differ among Groups IA, B, and C. However, AUC was modestly but significantly greater in Group ID animals vs the other groups (p < 0.05 vs Group IC; p < 0.001 vs Groups IA, IB) (Figure 2A), indicating that these rats had delayed uptake of the iv glucose. The plasma insulin concentration-time AUC are shown in Figure 2B. No differences occurred among the four groups, and peak insulin concentrations were similar in all groups (data not shown). These results suggest that Group ID rats had no major impairment in their ability to secrete insulin in response to a hyperglycemic stimulus.
Group II Experiments (Tables 2,3; Figure 3)
Table 2.
Control Measurements in Group II Experiments before Drug Infusion
| Group | Drug infused |
Body weight, g |
MAP, mm Hg |
Fasting Blood Glucose, mg dL−1 |
|---|---|---|---|---|
| IIA: HSD, Veh (n = 6) |
γ2-MSH | 300 ± 4 | 103 ± 3 | 102 ± 2 |
| IIB: HSD, Bro (n = 6) |
γ2-MSH | 290 ± 5 | 133 ± 3† | 116 ± 4** |
| IIC: HSD, Veh (n = 6) |
Phentol | 354 ± 23£ | 98 ± 3 | 98 ± 3 |
| IID: HSD, Bro (n = 6) |
Phentol | 266 ± 7 | 134 ± 3† | 119 ± 3* |
| IIE: HSD, Veh (n = 5) |
Hydral | 289 ± 15 | 99 ± 3 | 102 ± 1 |
| IIF: HSD, Bro (n = 5) |
Hydral | 309 ± 11 | 131 ± 3† | 117 ± 1† |
Values are means ± SEM of the indicated number of experiments per group. HSD, high sodium diet; Veh, vehicle; Bro, bromocriptine; Phentol, phentolamine; Hydral, hydralazine.
value significantly greater than value in corresponding Veh group, p <0.05
p <0.01
p <0.001
significantly greater than value in Groups IIB, IID, and IIE, p <0.05.
Table 3.
Heart Rate (beats min−1) in Group IIA-F Rats During Control and After Infusions
| Group | Drug Infused | Control | 15 min | 60 min |
|---|---|---|---|---|
| IIA: HSD, Veh | γ2-MSH | 380 ± 10 | 390±13 | 415±14* |
| IIB: HSD, Bromo | γ2-MSH | 365 ± 5 | 360±15 | 370±20 |
| IIC: HSD, Veh | Phentolamine | 365 ± 9 | 390±15* | 390±11* |
| IID: HSD, Bromo | Phentolamine | 360 ± 17 | 420±15** | 415±14** |
| IIE: HSD, Veh | Hydralazine | 372 ± 15 | 378±12 | 366±15 |
| IIF: HSD, Bromo | Hydralazine | 372 ± 15 | 414±25** | 402±15* |
Values are means ± SEM of the same groups shown in Table 2.
significantly greater than corresponding control value, p < .01
p <.001 by repeated measures ANOVA.
Figure 3.
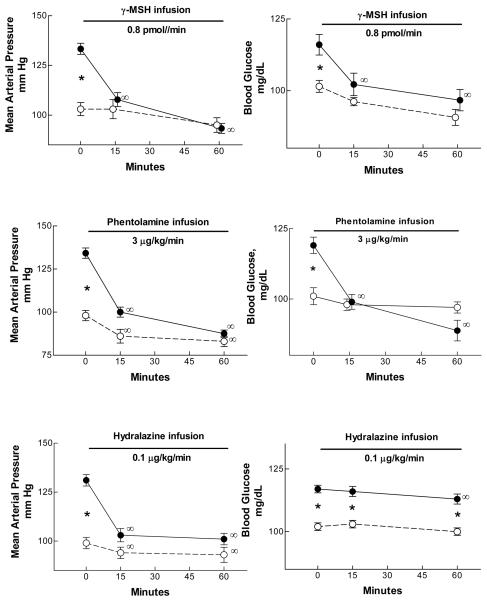
Mean arterial pressure (MAP) and blood glucose concentration in three groups of bromocriptine- (●) or vehicle (○)-treated rats fed the high sodium diet. Bromo-HSD rats were hypertensive and hyperglycemic vs vehicle-treated rats. Intravenous infusion of γ2-MSH (top panels, n = 6/group) or phentolamine (middle panels, n = 6/group) rapidly reduced MAP and blood glucose concentration to values no different than those in vehicle-treated animals. Infusion of hydralazine (lower panels, n = 5/group) promptly lowered MAP but had only a trivial effect on blood glucose concentration. *, BromoHSD value significantly greater than vehicle-HSD value, p <0.01 or greater; ∞, value significantly different from corresponding control value, p <0.01 or greater, repeated measures ANOVA.
We then asked if correction of the salt-sensitive hypertension of Bromo-treated rats on the HSD would have any effect on the elevated blood glucose concentration they exhibited. Control values for the animals studied in these Group II experiments are shown in Table 2. MAP in all groups fed the HSD and treated with bromocriptine was significantly elevated to the range of 131-134 mm Hg compared with vehicle-treated rats fed the HSD, as we have reported before (Mayan et al., 2003); no major differences in body weight occurred among the groups, although rats in Group IIC were significantly heavier than those in Groups IIB, IID or IIE (p <0.05, one way ANOVA). Fasting blood glucose concentration, measured after anesthesia and surgical preparation but before any drug infusion, was also elevated in Bromo rats compared to Veh controls; this elevation was modest but highly significant, and similar to results in the Group I studies.
Our earlier study (Mayan et al., 2003) showed that intravenous infusion of γ2-MSH rapidly corrected the salt-sensitive hypertension resulting from Bromo treatment. We asked if this was also true of the hyperglycemia which accompanied the hypertension. The results in these Group IIA and IIB rats are shown in Figure 3. Intravenous γ2-MSH infusion (0.8 pmol min−1) again rapidly lowered MAP in hypertensive bromocriptine-treated rats fed the HSD; 15 min after the start of the infusion, MAP was no different than that seen in vehicle-treated rats, and remained virtually indistinguishable from the Veh animals for the remainder of the experiment 45 min later. The peptide infusion had a modest and insignificant effect to lower MAP in the vehicle-treated Group IIA. Of interest, the hormone infusion lowered blood glucose concentration in parallel with its reduction in MAP in Bromo-treated Group IIB rats: by 15 min glucose was reduced to 102 ± 4, and by 60 min to 97 ± 4 mg dL−1, both significantly less than the control value, and neither value different from the corresponding value in Group IIA rats, in which a modest and insignificant decline in glucose concentration over time was observed (Figure 3). Thus, infused γ2-MSH to Bromo-treated rats with γ-MSH deficiency and salt-sensitive hypertension rapidly corrected the hyperglycemia as well as the hypertension exhibited by these rats.
To explore further the relationship between elevated blood glucose and hypertension in Bromo-treated rats fed the HSD, we looked at the effect of the alpha-adrenergic receptor antagonist phentolamine on these two variables; the results in these Group IIC and IID rats are also shown in Figure 3. Phentolamine caused a prompt fall in MAP by 15 min of the infusion similar to that seen in the γ2-MSH-infused Group IIB, and remained stable for the duration of the experiment; both values were significantly reduced from Control (p < 0.001). The drug caused a more modest fall in MAP in the Vehicle-treated group, to 86 ± 4 mm Hg at 15 min and 83 ± 3 mm Hg at 60 min (p < 0.001 for both vs the control value); the value at 60 min did not differ from the corresponding value in infused Bromo-treated rats, although the value at 15 min was significantly less (p < 0.04). As with γ2-MSH infusion, phentolamine caused a prompt decrement in blood glucose concentration in Bromo-treated rats that paralleled the fall in MAP (Figure 3); a small and statistically insignificant decrease occurred in Group IIC vehicle-treated animals.
Groups IIE and IIF tested the effects of the peripheral vasodilator hydralazine on the relationship between blood pressure and blood glucose concentration. The results are also presented in Figure 3. In Group IIE Veh-treated animals, infusion of hydralazine caused a modest decrease in MAP and no significant change in blood glucose concentration. In Group IIF Bromo-treated animals, the infusion caused a decrease in MAP which matched closely that observed in Group IIB rats during γ2-MSH infusion and Group IID rats receiving phentolamine. However, in contrast to these Groups, Group IIF rats showed only a modest decrease in blood glucose concentration: at 15 min, blood glucose was 116 ± 1.6 mg dL−1 (p = NS vs Control), and at 60 min, it had fallen only to 113 ± 1.8 mg dL−1 (p < 0.01 vs Control, repeated measures ANOVA). This value was significantly greater than that in Group IIB or IID at 60 min (p < 0.01 vs both). Blood glucose did not change in Group IIE rats. Thus, despite normalization of MAP by hydralazine infusion in Group IIF rats, blood glucose concentration remained elevated. Plasma γ-MSH concentration was measured in samples from the Group IIE and IIF rats at the end of the experiment. In Group IIE it was 72.5 ± 9.8 fmol ml−1, while in Group IIF it was only 34.9 ± 5.7 fmol ml−1 (p = 0.011, unpaired t test), indicating that the Bromo treatment was successful in causing relative γ-MSH deficiency during ingestion of the HSD as we had shown before (Mayan et al., 2003).
Heart rate responses in these experiments are shown in Table 3. Control values did not differ among the six groups. In Bromo-HSD groups infused with phentolamine (IID) or hydralazine (IIF), lowering of MAP was accompanied by reflex tachycardia which was evident at 15 min and persisted for the 60 min of observation. In contrast, Group IIB rats infused with γ2-MSH did not change heart rate during the experiment despite a similar lowering of blood pressure. Groups IIA and IIC vehicle-treated rats also increased heart rate as a consequence of the infusions despite minimal changes in MAP.
Five additional rats treated with Bromo and fed the HSD received a γ2-MSH infusion of 0.4 pmol min−1, a rate we had shown previously achieved a plasma γ-MSH concentration appropriate for rats on the HSD (Mayan et al., 2003). In these rats, MAP fell from 131±3 mm Hg during control to 124±2 mm Hg at 15 min and 104±3 mm Hg at 60 min (p <0.001, repeated measures ANOVA), results similar to those seen with the higher infusion rate (Figure 3); heart rate was again unchanged as in Group IIB (Control, 366±17, 60 min 360±13 beats/min, p = NS). In these studies, plasma noradrenaline concentration during control was 184±37 pg ml−1 and had fallen to 98±17 pg ml−1 after the 60 min infusion of γ-MSH (p <0.03, paired t test).
Discussion
These experimental results demonstrate that the salt-sensitive hypertension which results from suppression of normal γ-MSH secretion is accompanied by a mild abnormality in glucose metabolism. The HSD leads to an increase in pituitary POMC mRNA and immunoreactive γ-MSH content, resulting in a doubling of plasma γ-MSH concentration over the value during ingestion of the NSD (Mayan et al., 1996; Mayan et al., 2003). Bromo treatment prevents the stimulation of pituitary γ-MSH content caused by the HSD and plasma concentration of the peptide is not increased (Mayan et al., 2003). The plasma γ-MSH concentrations we measured in Groups IIE and IIF are consistent with these earlier results: Veh-treated IIE rats fed the HSD had a stimulated value (72.5 fmol ml−1) whereas in Bromo-treated IIF rats the value was only half (34.5 fmol ml−1), and as expected, each of the Bromo-HSD rat groups we studied were hypertensive. As we had shown previously (Mayan et al., 2003), the hypertension was rapidly corrected by administration of exogenous γ2-MSH, and we have also demonstrated that the hypertension develops in conscious, chronically instrumented rats and that a continuous infusion of a γ-MSH analog prevents its occurrence entirely (Ni & Humphreys, 2007). These results indicate that the γ-MSH deficiency, and not some other effect of bromocriptine treatment, is causally related to the hypertension.
Our results show in addition that the Bromo-treated rats on the HSD were also hyperglycemic and hyperinsulinemic when fasted. These were consequences of both these treatments combined, since neither Bromo rats on the NSD nor Veh rats on the HSD exhibited either hypertension or hyperglycemia. The characteristics of abnormal glucose metabolism in Bromo-HSD rats include fasting hyperglycemia, impaired glucose tolerance, elevated fasting insulin concentration, reduced insulin-mediated glucose uptake, and no gross abnormality of insulin secretion. These characteristics are identical to those we have observed in the genetic mouse models of γ-MSH deficiency or resistance (Ni & Humphreys, 2008); although we did not carry out euglycemic hyperinsulinemic clamp studies, these characteristics are suggestive of the development of insulin resistance. In both the mouse and the rat models, the combination of γ-MSH deficiency (or interrupted signaling) plus the HSD is necessary to elicit both the hypertension and the altered glucose metabolism. The observation of the close association of these two commonly acknowledged cardiovascular risks in rodents with disrupted γ-MSH signaling thus offers a new means to dissect the basis for their relationship.
In this regard, our data provide some clues into a possible mechanism linking salt-sensitive hypertension to abnormal glucose metabolism. Our earlier study (Mayan et al., 2003) demonstrated that the HSD caused similar suppression of plasma renin activity in Bromo- and Veh-treated rats, arguing that the renin-angiotensin system was not involved in the hypertension of Bromo-HSD rats. Similarly, our results are not likely to be confounded by the effects of general anesthesia on the cardiovascular system, since we observed a similar elevation in MAP in conscious, chronically instrumented and unrestrained rats fed the HSD and treated with Bromo (Ni & Humphreys, 2007). In the present study, correction of hypertension by infusion of either γ-MSH or phentolamine also rapidly corrected the hyperglycemia in Bromo-HSD treated rats, whereas blood glucose was only minimally affected when MAP was equivalently lowered by the smooth muscle vasodilator hydralazine. This suggests that some consequence of the hypertension, such as decreased blood flow to skeletal muscle with resulting reduction in glucose uptake, is not a major contributor to the hyperglycemia. Rather, the data with phentolamine, an α-adrenergic receptor antagonist, makes an effect mediated by the sympathetic nervous system seem likely. Insulin has been shown to stimulate sympathetic outflow by a central mechanism, an effect which is amplified in animals ingesting the HSD (Muntzel et al., 2007). Heightened sympathetic nervous activity has also been linked to impaired glucose metabolism in obesity (Landsberg, 2001; Bogaert & Linas, 2009). We have suggested that γ-MSH acts centrally as a tonic brake on sympathetic outflow, so that in states of γ-MSH deficiency sympathetic nervous activity would be increased (Humphreys, 2004, 2007). If true, such a mechanism could therefore account both for the hypertension and the hyperglycemia observed in γ-MSH-deficient Bromo-treated rats ingesting the HSD as well as the rapid correction of each with infused γ-MSH and phentolamine but not with hydralazine. In support of this possibility is our observation that plasma noradrenaline concentration in hypertensive Bromo-HSD rats was double the value in the other experimental groups, and infusion of a physiologically relevant dose of γ-MSH lowered both MAP and plasma noradrenaline to control. The failure of heart rate to increase when MAP was abruptly lowered by γ2-MSH infusion in Group IIB studies (Table 3) suggests that the peptide may have interrupted the baroreflex control of heart rate and is also consistent with inhibition of sympathetic outflow. A high salt diet is known to activate neurons and centers involved in cardiovascular regulation (Bealer & Metcalf, 2005; Isogai et al., 2005; Adams et al., 2007) and γ-MSH microinjected into one of these regions (the nucleus of the tractus solitarius) lowers blood pressure by reducing sympathetic outflow (De Wildt et al., 1994; Li et al., 1996). The peptide could interact with neurons in this or other regions to modulate sympathetic outflow.
The development of hypertension in Bromo-treated rats is surprising given the known cardiovascular effects of this agent. Studies in normal rats indicate that it has an acute renal vasodilatory and hypotensive action (Stier et al., 1982) and also lowers blood pressure in the spontaneously hypertensive rat (SHR) (Nagahama et al., 1984; Racz et al., 1986; Kanayama et al., 1987; van den Buuse & Lambrechts, 1989; Oguro et al., 1992), DOCA-salt hypertensive rats (Nagahama et al., 1985) and normal and hypertensive humans (Sowers, 1981; Mannelli et al., 1984; Franchi et al., 2001; Kok et al., 2006). The possible basis for this hypotensive action is a reduction in sympathetic activity, as plasma and urine noradrenaline levels are reduced in most studies in rats (Nagahama et al., 1984, 1985; Racz et al., 1986; Kanayama et al., 1987; Oguro et al., 1992) and humans (Sowers, 1981; Carey et al., 1983; Mannelli et al., 1984). Our observation of elevated plasma noradrenaline concentration in Bromo-HSD rats indicates that the combination of Bromo treatment with ingestion of the HSD leads to sympathoexcitation in contrast to the above effects of Bromo by itself; restoration of γ-MSH levels by exogenous infusion lowers this sympathoexcitation as reflected in the fall to normal levels of plasma noradrenaline concentration. It is also noteworthy that the Bromo-treated rats fed the HSD in the present study also developed hyperglycemia and hyperinsulinemia, since Bromo has been shown to ameliorate glucose metabolism and increase insulin sensitivity in obese humans (Pijl et al., 2000; Kok et al., 2006) and the obese Syrian hamster (Luo et al., 1999). Our data indicate an interaction of the effect of Bromo treatment with the HSD that is not present with either treatment alone and indeed overcomes the hypotensive and insulin-sensitizing actions of Bromo expected during ingestion of more normal of amounts dietary sodium.
Bromo treatment has actions in addition to suppression of pituitary POMC processing. Its primary use in clinical medicine has been to suppress release of prolactin, and studies in both rats and humans indicate that it does cause reductions in plasma prolactin concentration (Stier et al., 1982; Mannelli et al., 1984; Nagahama et al., 1984, 1985; Kok et al., 2006). We observed that Bromo treatment lowered prolactin concentration to ~10% of the control value whether on the NSD or the HSD (Table 1). Thus, although prolactin infusion leads to natriuresis (Ibarra et al., 2005), so that reduced prolactin levels after Bromo treatment could favor sodium retention and lead to hypertension, the normal MAP of Bromo-NSD rats argues that reduced prolactin levels do not contribute to the salt-sensitive hypertension. Furthermore, the observation that the hypertension and insulin resistance were corrected by γ-MSH infusion provides direct support for the idea that γ-MSH is necessary and sufficient to prevent HSD-induced insulin resistance.
There are several limitations to the data presented in this study. While the abnormal glucose metabolism exhibited by the Bromo-HSD is clearly present, its magnitude is only modest, raising the question of its importance to the human condition. We have shown that genetically altered mice with γ-MSH deficiency or resistance have a qualitatively similar but quantitatively more pronounced defect in glucose metabolism that is also elicited during ingestion of the HSD (Ni & Humphreys, 2008). It must also be acknowledged that the 8% NaCl diet is excessive, and even though this amount of dietary sodium has been the standard means of studying experimental salt-sensitive hypertension in rodents, exploration of the effect of lower amounts on the variables we have measured is clearly desirable. Finally, the rapid correction of both blood pressure and blood glucose concentration by infused γ-MSH and phentolamine raises the possibility that these reflect transient responses which might not be maintained with chronic administration. We do not believe this to be the case, at least with respect to blood pressure, since we have shown that chronic infusion of a γ-MSH analog completely prevents the development of hypertension in Bromo-treated rats fed the HSD (Ni & Humphreys, 2007). Nevertheless, this possibility should be addressed directly in studies tracking the response to chronic infusion of these agents once the hypertension and hyperglycemia have been established.
Despite these limitations, our data show that rats with salt-sensitive hypertension caused by γ-MSH deficiency also develop abnormal glucose metabolism consistent with insulin resistance. The close association of this abnormality in glucose regulation with the hypertension suggests that the two may be linked by a common mechanism involving increased sympathetic nervous outflow, although the mechanism remains unknown. These observations thus offer a new means of exploring the relationship between hypertension and insulin resistance, which are so commonly associated.
Acknowledgements
Portions of this work have appeared in abstract form in J Am Soc Nephrol 2006; 17:213A and 17:213A. This work was supported by grant HL68871 from the National Institutes of Health. Dr. Van Dijk's current address is 1750 Pierce Street, Lakewood, CO 80214, USA.
References
- Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- Bealer SL, Metcalf CS. Increased dietary sodium enhances activation of neurons in the medullary cardiovascular pathway during acute sodium loading in the rat. Auton Neurosci. 2005;117:33–40. doi: 10.1016/j.autneu.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bogaert YE, Linas S. The role of obesity in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2009;5:101–111. doi: 10.1038/ncpneph1022. [DOI] [PubMed] [Google Scholar]
- Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- Carey RM, Van Loon GR, Baines AD, Kaiser DL. Suppression of basal and stimulated noradrenergic activities by the dopamine agonist bromocriptine in man. J Clin Endocrinol Metab. 1983;56:595–602. doi: 10.1210/jcem-56-3-595. [DOI] [PubMed] [Google Scholar]
- De Wildt DJ, Van der Ven JC, Van Bergen P, De Lang H, Versteeg DH. A hypotensive and bradycardic action of gamma 2-melanocyte-stimulating hormone (gamma 2-MSH) microinjected into the nucleus tractus solitarii of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:50–56. doi: 10.1007/BF00178205. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- Franchi F, Lazzeri C, Barletta G, Ianni L, Mannelli M. Centrally mediated effects of bromocriptine on cardiac sympathovagal balance. Hypertension. 2001;38:123–129. doi: 10.1161/01.hyp.38.1.123. [DOI] [PubMed] [Google Scholar]
- Humphreys MH. Gamma-MSH, sodium metabolism, and salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286:R417–430. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- Humphreys MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- Ibarra F, Crambert S, Eklof AC, Lundquist A, Hansell P, Holtback U. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005;68:1700–1707. doi: 10.1111/j.1523-1755.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- Isogai O, Tsukamoto K, Masubuchi Y, Tomioka S, Suzuki T, Kawato H, Yajima Y, Kasamaki Y, Ito S, Kanmatsuse K. High salt diet enhances cardiovascular responses from the nucleus tractus solitarius and ventrolateral medulla of Sprague-Dawley rats. Clin Exp Hypertens. 2005;27:33–44. doi: 10.1081/ceh-200044252. [DOI] [PubMed] [Google Scholar]
- Kanayama Y, Kohno M, Takaori K, Itoh S, Yasunari K, Takeda T. Involvement of sympathetic nervous system inhibition in the hypotensive effect of bromocriptine in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1987;14:141–144. doi: 10.1111/j.1440-1681.1987.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Khalil R. Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol. 2006;290:R509–R513. doi: 10.1152/ajpregu.00600.2005. [DOI] [PubMed] [Google Scholar]
- Kok P, Roelfsema F, Frolich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006;291:E1038–1043. doi: 10.1152/ajpendo.00567.2005. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Liang Y, Cincotta AH. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology. 1999;69:160–166. doi: 10.1159/000054415. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Delitala G, De Feo ML, Maggi M, Cuomo S, Piazzini M, Guazzelli R, Serio M. Effects of different dopaminergic antagonists on bromocriptine-induced inhibition of norepinephrine release. J Clin Endocrinol Metab. 1984;59:74–78. doi: 10.1210/jcem-59-1-74. [DOI] [PubMed] [Google Scholar]
- Mayan H, Ling KT, Lee EY, Wiedemann E, Kalinyak JE, Humphreys MH. Dietary sodium intake modulates pituitary proopiomelanocortin mRNA abundance. Hypertension. 1996;28:244–249. doi: 10.1161/01.hyp.28.2.244. [DOI] [PubMed] [Google Scholar]
- Mayan H, Ni XP, Almog S, Humphreys MH. Suppression of gamma-melanocyte-stimulating hormone secretion is accompanied by salt-sensitive hypertension in the rat. Hypertension. 2003;42:962–967. doi: 10.1161/01.HYP.0000097601.83235.F8. [DOI] [PubMed] [Google Scholar]
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- Muntzel MS, Crespo R, Joseph T, Onwumere O. Dietary salt loading exacerbates the increase in sympathetic nerve activity caused by intravenous insulin infusion in rats. Metabolism. 2007;56:373–379. doi: 10.1016/j.metabol.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Nagahama S, Chen YF, Oparil S. Mechanism of the depressor effect of bromocriptine in the spontaneously hypertensive rat. J Pharmacol Exp Ther. 1984;228:370–375. [PubMed] [Google Scholar]
- Nagahama S, Chen YF, Oparil S. Enhanced depressor effect of bromocriptine in the DOCA/NaCl hypertensive rat. Am J Physiol. 1985;249:H64–70. doi: 10.1152/ajpheart.1985.249.1.H64. [DOI] [PubMed] [Google Scholar]
- Ni XP, Humphreys MH. Prevention of salt-induced hypertension by an analog of gamma-melanocyte-stimulating hormone in the rat. Am J Hypertens. 2007;20:862–865. doi: 10.1016/j.amjhyper.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Ni XP, Humphreys MH. Abnormal glucose metabolism in hypertensive mice with genetically interrupted gamma-melanocyte stimulating hormone signaling fed a high-sodium diet. Am J Hypertens. 2008;21:1284–1287. doi: 10.1038/ajh.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni XP, Kesterson RA, Sharma SD, Hruby VJ, Cone RD, Wiedemann E, Humphreys MH. Prevention of reflex natriuresis after acute unilateral nephrectomy by melanocortin receptor antagonists. Am J Physiol. 1998;274:R931–938. doi: 10.1152/ajpregu.1998.274.4.R931. [DOI] [PubMed] [Google Scholar]
- Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara T, Asano T, Fujita T. Contribution of salt intake to insulin resistance associated with hypertension. Life Sci. 2003;73:509–523. doi: 10.1016/s0024-3205(03)00315-1. [DOI] [PubMed] [Google Scholar]
- Oguro M, Takeda K, Itoh H, Takesako T, Tanaka M, Takenaka K, Hirata M, Nakata T, Tanabe S, Hayashi J, et al. Role of sympathetic nerve inhibition in the vasodepressor effect of bromocriptine in normotensive and hypertensive rats. Jpn Circ J. 1992;56:943–949. doi: 10.1253/jcj.56.943. [DOI] [PubMed] [Google Scholar]
- Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, Pipek R, Iozzo P, Lancaster JL, Cincotta AH, DeFronzo RA. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23:1154–1161. doi: 10.2337/diacare.23.8.1154. [DOI] [PubMed] [Google Scholar]
- Racz K, Kuchel O, Buu NT. Bromocriptine decreases blood pressure of spontaneously hypertensive rats without affecting the adrenomedullary synthesis of catecholamines. J Cardiovasc Pharmacol. 1986;8:676–680. [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Dopaminergic control of circadian norepinephrine levels in patients with essential hypertension. J Clin Endocrinol Metab. 1981;53:1133–1137. doi: 10.1210/jcem-53-6-1133. [DOI] [PubMed] [Google Scholar]
- Stier CT, Jr., Cowden EA, Allison ME. Effects of bromocriptine on single nephron and whole-kidney function in rats. J Pharmacol Exp Ther. 1982;220:366–370. [PubMed] [Google Scholar]
- van den Buuse M, Lambrechts AC. Bromocriptine-induced decrease in blood pressure in conscious spontaneously hypertensive rats: evidence for a peripheral site of action. J Pharm Pharmacol. 1989;41:644–646. doi: 10.1111/j.2042-7158.1989.tb06549.x. [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]