Abstract
Objective
Psychological stress and trauma are risk factors for several medical and psychiatric illnesses. Recent studies have implicated advanced cellular aging as a potential mechanism of this association. Telomeres, DNA repeats that cap the ends of chromosomes and promote stability, shorten progressively with each cell division; their length is a marker of biological aging. Based on previous evidence linking psychosocial stress to shorter telomere length, this study was designed to evaluate the effect of childhood adversity on telomere length.
Method
Thirty-one adults with no current or past major Axis I psychiatric disorder participated. Subjects reported on their history of childhood maltreatment and telomere length was measured from DNA extracted from frozen whole blood using quantitative PCR.
Results
Participants reporting a history of childhood maltreatment had significantly shorter telomeres than those who did not report a history of maltreatment. This finding was not due to effects of age, sex, smoking, BMI, or other demographic factors. Analysis of subscales showed that both physical neglect and emotional neglect were significantly linked to telomere length.
Conclusions
These results extend previous reports linking shortened leukocyte telomere length and caregiver stress to more remote stressful experiences in childhood, and suggest that childhood maltreatment could influence cellular aging.
Introduction
Stressful life experiences have long been identified as risk factors for psychiatric disorders and, more recently, for cardiovascular and immune diseases (1-3) Psychiatric disorders such as major depression and post-traumatic stress disorder are commonly comorbid with disorders of the cardiovascular, metabolic, and immune systems (3, 4). Recent research has focused on identifying how stress might influence common biological mechanisms for these disorders.
The biological response to stress involves the coordinated activity of several systems, including the hypothalamic-pituitary-adrenal (HPA) axis, the sympathetic nervous system, and the immune system. Acute activation of these systems serves to mobilize energy resources and prepare the individual for coping with stressors. With chronic stress exposure, however, attempts at adaptation can result in pathological perturbation of endocrine, immune, and metabolic systems (3, 5). Chronic or excessive activation of these stress-responsive systems may also result in telomere shortening, a cellular marker of biological aging. Telomeres are regions at the ends of linear chromosomes comprised of DNA repeats that serve to “cap” the chromosomal termini, promote stability, and prevent end-to-end fusion of chromosomes. In replicating somatic cells, telomeres shorten progressively with each cell division, ultimately leading to replicative senescence, and exposure to stressors can hasten telomere shortening and lead to premature cell senescence (for a review, see (6)). Shortened leukocyte telomere length has been linked to morbidity and mortality of age-related illnesses (7, 8).
Several genes and proteins that regulate telomere length have been identified (6), and telomere shortening is also induced by physiologic stressors such as exposure to radiation and toxins (9), oxidative stress (10) and cigarette smoke (11, 12). Of particular relevance to psychiatric disorders are recent findings in humans of an association between psychological stress and shortened leukocyte telomere length.
Epel and colleagues (13) found that both perceived stress and chronicity of care giving stress were linked to telomere shortening in peripheral blood leukocytes of healthy adult women. These indices of stress were also linked to higher levels of oxidative stress and lower levels of telomerase, a reverse transcriptase that limits telomere shortening. Damjanovic and colleagues (14) recently found shortened telomeres in caregivers of Alzheimer's disease patients who exhibited higher stress levels and higher average levels of depressive symptoms than a comparison group. Leukocyte telomere shortening has also been documented in relation to pessimism in a sample of healthy post-menopausal women (15), and in patients with unipolar and bipolar mood disorders (16).
Prior studies of the relationship of psychosocial stress to telomere biology have not examined effects of adversity occurring early in development. Neurobiological effects of stress may be greatest in childhood, when the brain and other systems are undergoing profound maturational changes. Rates of telomere shortening are fastest during infancy and early childhood (17, 18), and regulation of telomere length may be programmed in early development (19). In humans, childhood maltreatment is a common form of early adversity, and is a major risk factor for a range of adverse outcomes, including major depression, anxiety disorders, and substance abuse (e.g., (20-22)). The present study was designed to test the hypothesis that reports of childhood maltreatment would be associated with leukocyte telomere shortening in a sample of healthy adults.
Methods
Subjects
Participants were 31 adults (22 women, nine men), ages 18-64 (mean, 26.9 ± SD 10.1). Subjects were recruited via advertisements in the community for a larger study of stress reactivity and psychiatric symptoms. Included in this study were individuals with no current or past major Axis I psychiatric disorder who reported either no history of childhood maltreatment (n=21) or a history of moderate or severe childhood maltreatment (n=10). Participants completed a medical history, physical and neurological exams, blood chemistries, EKG, and urine analysis/toxicology to rule out acute or unstable medical illness, endocrine disease, or ongoing treatment with drugs which might influence HPA axis function, including psychotropics, beta blockers, angiotensin-converting enzyme inhibitors, ketoconazole, metyrapone, and corticosteroids. Oral contraceptives were permitted. After complete description of the study to the subjects, written informed consent was obtained. This study was approved by the Butler Hospital Institutional Review Board.
Measures
Demographic Characteristics
Height and weight were measured in the laboratory by a research nurse and body mass index (BMI) was calculated. Participants reported the highest education level they had attained. Socioeconomic adversity was assessed with the following true/false statement: “My family was generally financially stable when I was growing up, and all of my basic needs (food, shelter, and clothing) were met during my childhood.”
The Structured Clinical Interview for DSM-IV (SCID I and II, (23, 24)
Current and lifetime history of Axis I psychiatric diagnoses were assessed using the SCID for DSM-IV, and participants with no lifetime major Axis I disorder were included in this study. Current Axis II diagnoses were assessed but did not form the basis for inclusion or exclusion. One participant was missing SCID II data, one participant met the criteria for obsessive-compulsive personality disorder, and one met the criteria for personality disorder not otherwise specified. Both of the latter participants reported a history of childhood maltreatment as defined below.
The Childhood Trauma Questionnaire (CTQ)
The 28-item version of the CTQ (25) was used to assess childhood maltreatment. This measure has excellent psychometric properties (26) including high test-retest reliability (27, 28), as well as convergent validity (25, 29, 30). Participants were selected if they reported either no childhood maltreatment (no maltreatment group) or moderate to severe levels of childhood maltreatment (maltreatment group) on any of five subscales (physical abuse, sexual abuse, emotional abuse, physical neglect, emotional neglect).
The Perceived Stress Scale
(PSS, (31)), a 14-item questionnaire, was used to measure the degree to which life situations were perceived as stressful over the past month.
Telomere Measurement
DNA was extracted from frozen whole blood using standard methods. The real-time quantitative polymerase chain reaction (qPCR) was used to obtain a measure of telomere length using the method of Cawthon (32). The number of telomere repeats was determined in conjunction with the number of copies of the beta-hemoglobin gene, which served as a reference single-copy gene to account for genome copy number in each sample. Thus, telomere length was expressed as a ratio of telomere copy number over single gene copy number (T/S), which was normalized to the T/S from a reference DNA each time the assay was performed. PCRs were performed in 96-well reaction plates; the quantification of telomere length and the single-copy gene were performed separately in triplicate. Each 96-well plate contained 6 serial dilutions of the same DNA standard spanning the estimated DNA concentrations of the samples to be assayed. All measurements on the same plate were normalized to the DNA standards, and a conversion factor applied to control for batch-to-batch variation. Following the method of Shen and colleagues ((33)), we standardized our values to the average of all the white blood cell T/S ratios. Data are reported as the average of the three triplicate measurements. Intra-assay variability for the standard ranged from a standard deviation of 2.5 to 15% of the control (a set of dilutions of the standard DNA). Inter-assay variability ranged from 1.7% to 7.3% (standard deviation) for actual samples quantitated in triplicate.
Statistical Analysis
T-tests and chi square tests were used to test for differences between the maltreatment and comparison groups on demographic factors and the measure of telomere length. The primary analysis assessed whether the maltreatment group and the no maltreatment group differed with respect to telomere length. In addition, exploratory analyses examined the association of the individual maltreatment types with telomere length.
Results
Subject Characteristics and Analysis of Potential Confounds
Most participants reporting a history of moderate or severe maltreatment endorsed more than one form of maltreatment (minimal, moderate, or severe, n=7, 70%). Eight subjects (89%) reported emotional abuse, eight (80%) emotional neglect, seven (70%) physical neglect, four (40%) physical abuse, and two (20%) sexual abuse. The individual maltreatment subscales of the CTQ were highly inter-correlated (r's > .5), with the exception of the sexual abuse subscale (only two participants reported sexual abuse). Mean scores on the subscales are shown for each group in Table 1.
Table 1.
Subject Characteristics
|
No Maltreatment (n = 21) |
Maltreatment (n = 10) |
|
|---|---|---|
| Age (mean ± SD years) | 27.6 ± 10.8 | 25.5 ± 8.5 |
| Sex, Women, n (%) | 14 (67%) | 8 (80%) |
| BMI, (mean ± SD) | 25.4 ± 4.6 | 26.2 ± 6.3 |
| Smoker, n (%) | 1 (4.8%) | 2 (20%) |
| Oral Contraceptive, n, (% of females) | 6 (43%) | 2 (25%) |
| Race, n (%) | ||
| Caucasian | 16 (76.2%) | 5 (50%) |
| Black | 1 (4.8%) | 2 (20%) |
| Hispanic | 0 | 1 (10%) |
| Asian | 2 (9.5%) | 1 (10%) |
| Other | 2 (9.5%) | 1 (10%) |
| Highest Education Level, n (%) | ||
| High school | 1 (4.8%) | 1 (10%) |
| Partial college | 7 (33.3%) | 5 (50%) |
| College | 8 (38.1%) | 3 (30%) |
| Professional degree | 5 (23.8%) | 1 (10%) |
| Childhood Socioeconomic Adversity, n (%) | ||
| Experienced | 2 (9.5%) | 4 (40%) |
| Did not experience | 19 (90.5%) | 6 (60%) |
| Perceived Stress Scale, (Mean ± SD) | 16.5 ± 10.9 | 20.9 ± 6.3 |
| CTQ Subscale Score, (mean ± SD) | ||
| Physical Abuse | 5.2 ± 0.5 | 7.8 ± 2.6 |
| Sexual Abuse | 5.0 ± 0.0 | 5.6 ± 1.4 |
| Emotional Abuse | 5.4 ± 0.9 | 12.6 ± 3.3 |
| Emotional Neglect | 6.1 ± 1.3 | 14.7 ± 4.1 |
| Physical Neglect | 5.0 ± 0.0 | 9.4 ± 3.0 |
BMI, body mass index; CTQ, Childhood Trauma Questionnaire (Subscale scores range from 5-25). T-tests and chi-squares were used to test for differences between the maltreatment and no maltreatment groups on the demographic variables, hormonal contraception use among women, and the Perceived Stress Scale. There were no significant differences between the groups on these variables. Due to the small numbers of participants from different racial groups, race was dichotomized as Caucasian versus non-Caucasian. Education was dichotomized as completed college versus did not.
Table 1 shows the demographic and abuse characteristics of the maltreatment and no maltreatment groups. The maltreatment group did not differ significantly from the comparison group with respect to age, smoking status, BMI, hormonal contraception use in females, race, education, socioeconomic adversity, or perceived stress on the PSS.
Telomere Length in Relation to Childhood Maltreatment
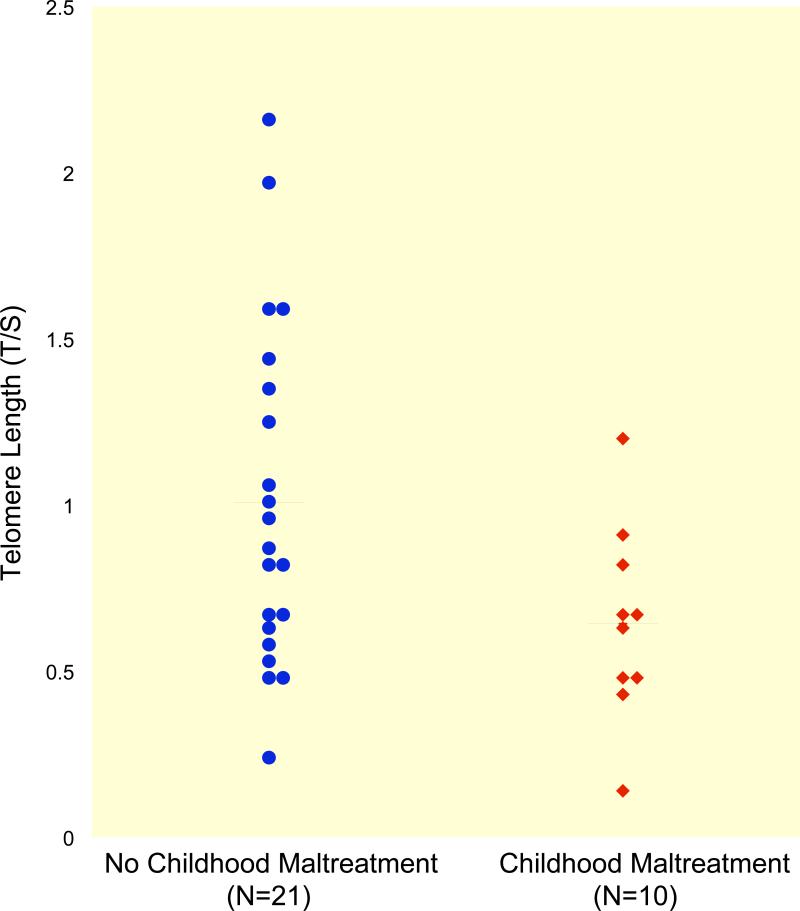
A t test showed that the maltreatment group had significantly shorter telomeres than the group reporting no childhood maltreatment (0.70 ± 0.24 versus 1.02 ± 0.52; t(29) = 2.4, p = .03; Fig. 1). Analysis of the CTQ subscales (excluding sexual abuse which was endorsed by only two subjects) showed that shorter telomeres were associated with both physical neglect (0.59 ± 0.15 versus 0.99 ± 0.49; t(26.9) = 3.5, p = .002) and emotional neglect (0.69 ± 0.24 versus 0.99 ± 0.51; t(25.6) = 2.2, p = .04). This association did not reach statistical significance for physical abuse or emotional abuse (p = .057 and p = .054, respectively).
FIGURE 1.
Telomere Length in No Childhood Maltreatment and Childhood Maltreatment Groups
Discussion
These results extend previous reports linking shortened leukocyte telomere length and caregiver stress to more remote stressful experiences in childhood. In this study, reported maltreatment was moderate-severe in degree, and a variety of types of abuse and neglect were represented. Analysis of subtypes of maltreatment suggested that neglect may have the most robust effect, but given the modest sample size we cannot rule out effects of other forms of abuse. Both emotional neglect and physical neglect were linked to shorter telomeres, thus it is possible that in addition to the psychological effects of stress, physical stressors such as inadequate nutrition or illness contributed to the findings.
In contrast with previous work, this study did not find an effect of recent perceived stress on leukocyte telomere length; it is of note that childhood maltreatment was not correlated with recent perceived stress in this sample. This study included only individuals with no current or past Axis I psychiatric disorder because some psychiatric disorders have been linked to telomere shortening. Shortened telomeres may represent a risk factor for such disorders or other adverse health outcomes in this sample. On the other hand, in addition to such a risk factor, these participants may have some factors that confer resilience, given the absence of psychiatric disorder even in the maltreatment group.
This study is limited by the modest sample size and the cross-sectional nature of the study. In addition, the CTQ is a brief, retrospective self-report questionnaire that may be subject to recall and other biases. We did not find an association of telomere length with age in this sample which is not surprising given that all but three subjects were younger than age 40. The rate of telomere sequence loss in human leukocytes varies with age, and little change in telomere length occurs in young adults (34) Given the range of influences on telomere length, it is possible that other variables, including genetic factors (35), could have influenced telomere length. However, the findings cannot be explained by effects of age, sex, smoking status, BMI, level of education, medical or psychiatric illness, or a measure of childhood socioeconomic adversity.
The pathway from psychological stress to telomere shortening remains to be elucidated, but glucocorticoid-associated oxidative stress damage is a likely mediator of this association (36, 37). Glucocorticoids are released from the adrenal gland in response to stress and have been shown to increase neuronal oxidative stress damage (36-39). Increased levels of oxidative stress have been seen in subjects reporting psychological stress (13, 40) and depressive symptoms (41), and oxidative stress reduces telomere length in vitro (42).
The present findings suggest that links of childhood maltreatment with psychopathology and associated medical problems could be due to effects of stress on cellular aging. Further work is needed to replicate these preliminary findings and determine whether potential mediators of this effect, including glucocorticoid activity, oxidative stress, reduced telomerase levels, and immune activation, may be responsible for telomere shortening in response to psychological stress. Moreover, longitudinal studies on the effects of psychosocial stress and trauma on changes in telomere length over time are needed to more fully characterize this relationship.
Disclosures and Acknowledgements
In the last year, Drs. Tyrka, Price, and Carpenter have received grant/research support from the National Institutes of Health, the US Department of the Interior, the US Department of Defense, Sepracor, Pfizer, Cyberonics UCB Pharma, and Medtronic. Dr. Tyrka has received honoraria for continuing medical education from Lundbeck and Takeda Pharmaceuticals. Dr. Price has received speakers bureau honoraria from Jazz Pharmaceuticals and has served as a consultant for Gerson Lehrman, Wiley, and Springer. Dr. Carpenter has served as a consultant or on the advisory board for Cyberonics, Neuronetics, Novartis and Wyeth, and has received honoraria for continuing medical education from AstraZeneca and spears; bureau honoraria for Cyberonics. Drs. Kao and Porton and Ms. Marsella report no biomedical financial interests or potential conflicts of interest.
This study was supported by National Institute of Mental Health grants 1 K23 MH067947 (ART), R01 MH070898 (BP), and R01 MH068767-01 (LLC), and National Institute of Neurological Disorders and Stroke grant R01 NS047209 (HTK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browne A, Finkelhor D. Impact of child sexual abuse: a review of the research. Psychol Bull. 1986;99(1):66–77. [PubMed] [Google Scholar]
- 2.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160(8):1453–60. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 3.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry. 2009;22(1):32–6. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 6.Gilley D, Herbert BS, Huda N, Tanaka H, Reed T. Factors impacting human telomere homeostasis and age-related disease. Mech Ageing Dev. 2008;129(1-2):27–34. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–80. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 9.Derradji H, Bekaert S, De Meyer T, Jacquet P, Abou-El-Ardat K, Ghardi M, Arlette M, Baatout S. Ionizing radiation-induced gene modulations, cytokine content changes and telomere shortening in mouse fetuses exhibiting forelimb defects. Dev Biol. 2008;322(2):302–13. doi: 10.1016/j.ydbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Bull C, Fenech M. Genome-health nutrigenomics and nutrigenetics: nutritional requirements or ‘nutriomes’ for chromosomal stability and telomere maintenance at the individual level. Proc Nutr Soc. 2008;67(2):146–56. doi: 10.1017/S0029665108006988. [DOI] [PubMed] [Google Scholar]
- 11.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 12.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 13.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179(6):4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190(2):157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, Goodenow M, Biggar R, Dimitrov D. Rapid telomere shortening in children. Blood. 1999;93(9):2824–30. [PubMed] [Google Scholar]
- 19.Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;(Suppl 35):159–84. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 20.Burns BJ, Phillips SD, Wagner HR, Barth RP, Kolko DJ, Campbell Y, Landsverk J. Mental health need and access to mental health services by youths involved with child welfare: a national survey. J Am Acad Child Adolesc Psychiatry. 2004;43(8):960–70. doi: 10.1097/01.chi.0000127590.95585.65. [DOI] [PubMed] [Google Scholar]
- 21.Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 2007;166(8):966–74. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Negl. 2007;31(5):517–30. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) A.P. Association; Washington, DC: 1997. [Google Scholar]
- 24.Maffei C, Fossati A, Agostoni I, Barraco A, Bagnato M, Deborah D, Namia C, Novella L, Petrachi M. Interrater reliability and internal consistency of the structured clinical interview for DSM-IV axis II personality disorders (SCID-II), version 2.0. J Pers Disord. 1997;11(3):279–84. doi: 10.1521/pedi.1997.11.3.279. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein DP, Fink LA. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. The Psychological Corporation; San Antonio, Texas: 1998. [Google Scholar]
- 26.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 27.Paivio SC. Stability of retrospective self-reports of child abuse and neglect before and after therapy for child abuse issues. Child Abuse Negl. 2001;25(8):1053–68. doi: 10.1016/s0145-2134(01)00256-3. [DOI] [PubMed] [Google Scholar]
- 28.Paivio SC, Cramer KM. Factor structure and reliability of the Childhood Trauma Questionnaire in a Canadian undergraduate student sample. Child Abuse Negl. 2004;28(8):889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152(9):1329–35. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 32.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, Neugut AI, Santella RM. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009;124(7):1637–43. doi: 10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lung FW, Fan PL, Chen NC, Shu BC. Telomeric length varies with age and polymorphisms of the MAOA gene promoter in peripheral blood cells obtained from a community in Taiwan. Psychiatr Genet. 2005;15(1):31–5. doi: 10.1097/00041444-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Ceccatelli S, Tamm C, Zhang Q, Chen M. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav. 2007;92(1-2):87–92. doi: 10.1016/j.physbeh.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh LJ, Sapolsky RM. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996;17(3-4):873–82. [PubMed] [Google Scholar]
- 38.McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998;791(1-2):209–14. doi: 10.1016/s0006-8993(98)00115-2. [DOI] [PubMed] [Google Scholar]
- 39.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141(2):201–6. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 40.Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health. 2001;74(2):153–7. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- 41.Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311(4):1014–8. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- 42.Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40(12):1277–83. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]