Abstract
Asthma is a complex disorder that displays heterogeneity and variability in its clinical expression both acutely and chronically. This heterogeneity is influenced by multiple factors including age, sex, socioeconomic status, race and/or ethnicity, and gene by environment interactions. Presently, no precise physiologic, immunologic, or histologic characteristics can be used to definitively make a diagnosis of asthma, and therefore the diagnosis is often made on a clinical basis related to symptom patterns (airways obstruction and hyperresponsiveness) and responses to therapy (partial or complete reversibility) over time. Although current treatment modalities are capable of producing control of symptoms and improvements in pulmonary function in the majority of patients, acute and often severe exacerbations still occur and contribute significantly to both the morbidity and mortality of asthma in all age groups. This review will highlight some of the important clinical features of asthma and emphasize recent advances in both pathophysiology and treatment.
Key words: Asthma, respiratory syncytial virus, rhinovirus, allergen, prevention, exacerbation, inception, treatment
Abbreviations used: API, Asthma predictive index; EBC, Exhaled breath condensate; EIB, Exercise-induced bronchospasm; GERD, Gastroesophageal reflux disease; ICS, Inhaled corticosteroid; LABA, Long-acting β-agonist; NSAID, Nonsteroidal anti-inflammatory drug; RBM, Reticular basement membrane; RSV, Respiratory syncytial virus
Asthma is a heterogeneous disorder that is characterized by variable airflow obstruction, airway inflammation and hyperresponsiveness, and reversibility either spontaneously or as a result of treatment. Multiple etiologies no doubt exist for both its inception and symptom exacerbation once the disease is established. Factors underlying inception can range from viral respiratory tract infections in infancy1, 2 to occupational exposures in adults.3 Factors underlying asthma exacerbations include allergen exposure in sensitized individuals, viral infections, exercise, irritants, and ingestion of nonsteroidal anti-inflammatory agents, among others. Exacerbating factors can include one or all of these exposures and vary both among and within patients. Asthma treatment is determined to a large extent after an initial assessment of severity and subsequent establishment of control, both of which can be variable over time and assessed in 2 domains: impairment (current) and risk (long-term consequences).4 Unfortunately, despite the availability of effective therapies, suboptimal asthma control exists in many patients on a worldwide basis.5 The future development of novel therapies and treatment paradigms should address these disparities.
Natural history (inception and progression)
For many asthmatic subjects, the disease has its roots during infancy and early childhood. Viral respiratory tract infections produce wheezing episodes during the first 3 years of life in about 50% of children.6 Some of these children will stop wheezing (transient wheezers), whereas others will go on to have persistent symptoms that will either dissipate before adolescence (primarily nonatopic subjects) or continue into adolescence (atopic wheezers).7 Once in remission, the disease process might remain quiescent, or the subject could relapse in later life.8, 9 The phenotype of severe asthma has also been recently well described.10
The pattern and rate of loss of lung function in asthmatic subjects has been of interest and concern for many investigators. A number of groups have reported that the greatest absolute loss of lung function appears to occur very early in childhood.8, 11, 12 Some have reported that the peak in lung function that is achieved at about 20 years of age in asthmatic subjects can be decreased13 and that the rate of further loss during adulthood can be increased in asthmatic subjects.14 About one fourth of children with asthma might experience greater rates of loss of lung function, and these children have certain phenotypic characteristics: younger age, male sex, higher postbronchodilator FEV1 percent predicted, and greater airway eosinophilic inflammation.15
Molecular and cellular mechanisms in asthma
Children
The performance of invasive procedures in children to evaluate molecular and cellular mechanisms in asthma is obviously not as feasible from a variety of standpoints compared with adults. However, a few carefully and safely conducted studies in young children have provided insights into possible pathophysiologic features as they relate to developmental milestones and disease expression. When bronchoalveolar lavage has been performed in young wheezing children, a 3-fold increase in total cells, most significantly lymphocytes, polymorphonuclear cells, and macrophages/monocytes, compared with counts seen in healthy children has been noted. In addition, levels of leukotriene B4 and C4, prostaglandin E2, and the potentially epithelium-derived 15-hydroxyeicosattetranoic acid were all increased.16
Several bronchial biopsy studies have been performed in children. In 53 infants with reversible airflow obstruction evaluated for severe wheezing or cough, bronchial biopsy specimens demonstrated no reticular basement membrane (RBM) thickening or the eosinophilic inflammation characteristic of asthma in older children and adults, even in the presence of atopic characteristics.17 Conversely, children younger than 6 years with asthma had increased epithelial loss, basement membrane thickening, and eosinophilia compared with control subjects of the same age. However, similar pathologic changes were seen in atopic children without asthma.18 Taken together, it appears that the inflammatory and structural changes associated with asthma occur sometime after infancy during the early preschool years, when children experience more persistent symptoms of airway dysfunction.
In older children 6 to 16 years of age with difficult asthma receiving high-dose inhaled corticosteroids (ICSs), RBM thickening to a similar extent to that seen in adult asthmatic subjects has been demonstrated.19 Additionally, there was no association with RBM thickening and age, symptom duration, lung function, or concurrent eosinophilic airway inflammation. However, unlike adults with asthma, no relationship was observed between RBM thickness and bronchial wall thickening on high-resolution computed tomographic scanning in children with difficult asthma.20 Finally, persistent airflow obstruction has been associated with a greater density of CD4+ T lymphocytes in endobronchial biopsy specimens in 27 school-aged children with difficult asthma after treatment with systemic corticosteroids compared with that seen in control subjects.21
A number of biomarkers have been evaluated to avoid the invasive procedures of bronchial lavage, biopsy, or both in children. Exhaled nitric oxide might be useful as a diagnostic tool and in ongoing management of children with asthma. Exhaled nitric oxide levels have been demonstrated to differentiate young children with asthma from those without,22 to identify children who are likely to respond to ICSs,23 and to predict those children who will experience an asthma relapse after reduction of ICSs.24 However, recent data indicate that when fraction of exhaled nitric oxide monitoring is used in conjunction with a National Asthma Education and Prevention Program guidelines–based asthma management program, it might result in excessive ICS dosing without any significant gains in achieving or maintaining asthma control.25
Exhaled breath condensate (EBC) is obtained by cooling exhaled air and is believed to reflect the contents of the airway lining fluid.26 Hydrogen peroxide, isoprostanes, aldehydes, and nitrotyrosine are considered markers of oxidative stress, and their levels are increased in the EBC of children with asthma, suggesting an imbalance between oxidants and antioxidants. Conversely, levels of glutathione, a protective lung antioxidant, are decreased in children with acute asthma, suggesting a reduced antioxidant capacity.27 Levels of the inflammatory mediators cysteinyl leukotrienes are increased in the EBC of children with atopic asthma, even while receiving corticosteroid treatment.28 Finally, airway pH balance might have a role in asthma because a reduced EBC pH has been reported in children with acute or stable asthma.26
Levels of several other mediators of inflammatory cells have been found to be significantly higher in very young children with asthma, including the number of blood eosinophils, serum eosinophil cationic protein, eosinophil-derived neurotoxin, and urinary eosinophil-derived neurotoxin.29 In addition, both increased eosinophil cationic protein and cysteinyl leukotriene levels30 have been obtained from nasal washings in wheezing children less than 2 years of age.
Adults
Asthma for most, but not all, patients begins in early life. As noted above, the cellular and molecular patterns associated with airway inflammation in asthma are complex, interactive, redundant, and variable.31 In adults, particularly those with established longstanding disease, the factors that contribute to the pathophysiology of airway abnormalities are dependent on the phases of asthma, such as acute, persistent, severe versus nonsevere, or during treatment.
An understanding of the immunopathology of airways in asthma has been markedly advanced with the use of bronchoscopy and biopsy. These airway samples can then be analyzed by using histologic and immunologic methods, and the identified features can be evaluated in relationship to clinical features of asthma to more fully understand the contribution of cellular and molecular events to the resulting physiology and response to treatment.32 In addition, it is now appreciated that the regulation of airway inflammation is distinct in different phases of asthma (ie, early-onset disease largely related to allergic inflammation and in the persistent or chronic phase of the disease).33 It is helpful to arbitrarily consider asthma in terms of the traditional TH2 inflammatory processes and the more chronic inflammatory phase, in which resident airway cells assume the more dominant component contributing to airway dysfunction (Fig 1 ),33 to appreciate the immunopathogenetic mechanisms associated with different phases of asthma.
Fig 1.
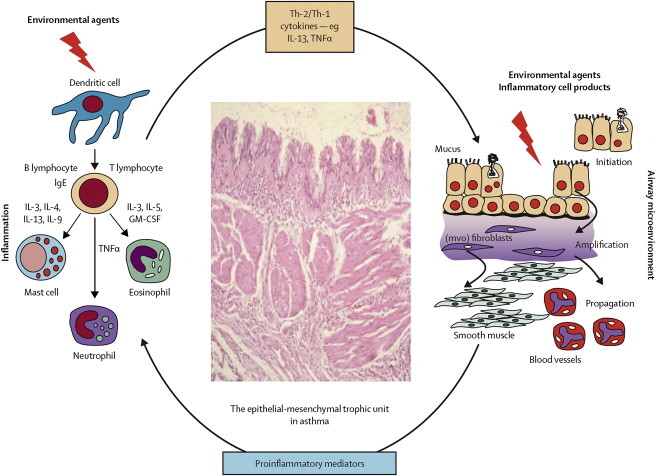
Inflammatory and remodeling responses in asthma with activation of the epithelial mesenchymal trophic unit. The epithelial mesenchymal trophic unit has been defined as bidirectional interaction between the epithelium and underlying mesenchyme involving the release of selective growth factors and cytokines. Epithelial damage alters the set point for communication between bronchial epithelium and underlying mesenchymal cells, leading to myofibroblast activation, an increase in mesenchymal volume, and induction of structural changes throughout airway wall.
Used with permission from the Lancet.33
In the acute inflammatory aspects of asthma, allergen-IgE–directed processes are predominant features of airway pathology, with mast cells, TH2 lymphocytes, and eosinophils the predominant histologic features.32 The cytokine network associated with these processes often includes IL-3, IL-4, IL-5, IL-9, and IL-13.34 Mast cells are important contributors both to the initiation of asthma with release of acute-phase mediators, including cysteinyl leukotrienes, and also inflammatory cytokines, which serve to perpetuate inflammatory events in the airway.35 Subpopulations of lymphocytes polarized toward a TH2 profile further the inflammatory process by release of cytokines, including IL-4, IL-5, and IL-13. It is these factors that serve to drive inflammation (eg, recruitment of eosinophils) and also regulate IgE production.32
Eosinophils are a characteristic feature of allergic inflammation.32 The biology of eosinophils is well designed to cause airway inflammation, enhancement of airway hyperresponsiveness, and airflow obstruction. Eosinophils are recruited to the airway in asthmatic subjects by families of cytokines, and chemokines (eg, IL-5, RANTES, and eotaxin) undergo cell activation through processes not fully identified and release highly inflammatory granule-associated substances, the actions of which injure the airway and cause persistent inflammation. Eosinophils are also a rich source for leukotrienes, products of oxidative metabolism, and inflammatory cytokines and growth factors.36 Although the eosinophil is a prominent feature of airway pathology in asthmatic subjects, its precise contribution to airway pathophysiology is undergoing re-evaluation.
The pattern of airway injury in patients with chronic asthma tends to be more variable, with a shift in the histologic picture toward resident cells of the airway as the more likely cause of persistent disease. In some patients there will be a progressive decrease in lung function and the development of chronic irreversible changes in lung function with their asthma. Although these changes likely have their origins at the onset of asthma, many questions remain as to who is at risk for airway remodeling, when this process begins, and what factors regulate the transition from acute to chronic inflammation. The recognition of progressive loss of lung function in asthmatic subjects has led to a renewed interest in the role of resident airway cells in persistent inflammation.
The airway epithelium is both a target and contributor of persistent inflammatory airway changes in asthmatic subjects.37 Histologic evaluation of airways in asthmatic subjects, particularly those with more severe disease, reveals injury to epithelium and often a loss of these cells. Epithelial cells are also a rich source of inflammatory mediators and growth factors. In addition, airway smooth muscle often shows hypertrophic and hyperplastic changes in subjects with persistent severe asthma. Moreover, the airway smooth muscle can be a source of both inflammatory cytokines and growth factors.38
There are other airway cells involved in asthma histopathology, including mucous glands and blood vessels. In subjects with asthma, mucous glands hypertrophy occurs. Activation of these cells leads to the release of mucus to occlude airways and, in severe exacerbations, to become the principal cause for resistance to treatment. Many factors generated in asthma (ie, vascular endothelial growth factor) can act on airway vessels to cause proliferation and, as a process, narrow the airways.
Understanding that heterogeneity exists in the pattern of airway inflammation and the likely molecular factors regulating these processes explains why current therapy is not effective in all subjects with asthma. As the phenotypic features of asthma unfold and with them a recognition of the associated cellular and molecular events, a more specific approach to treatment will follow accompanied by improved control of disease.
Risk factors
Risk factors in relationship to asthma have been evaluated in the context of disease inception (eg, viral infections1, 2, 39), environmental exposures (eg, aeroallergens,40 pollution,41, 42, 43 and tobacco smoke)44, 45, 46, 47 and lifestyle (eg, living on a farm,48 diet,49 and antibiotic use50), comorbid conditions (eg, atopic dermatitis51 and obesity52), and occupational exposures,3 among others, as well as disease severity (as defined by the risk domain, which is discussed subsequently; hospitalizations,53, 54 frequency and severity of exacerbations,55 and loss of lung function8, 56). Genetic factors also contribute significantly to disease expression and severity. Asthma is genetically classified as a complex disorder; as such, it does not follow simple Mendelian inheritance characteristics. Hundreds of genetic association studies on asthma-related phenotypes have been conducted in different populations; these have been recently reviewed.57 Although the importance of gene-environment interactions in the expression of disease has recently been highlighted,58 the complexities involved in analyzing these relationships from a functional perspective have proved challenging.59 Recent pharmacogenetic evaluations in relationship to chronic β-agonist use60 and corticosteroid efficacy have provided new insights into the variability of response in asthmatic patients.
Exacerbating factors
Allergens
Allergen exposure is important in host allergic sensitization and as a common precipitant of asthmatic symptoms in both children and adults. The formation of antigen-specific IgE antibody to aeroallergens (eg, mites, trees, grasses, and animal dander)—the development of allergic sensitization but not necessarily of allergic disease—does not usually occur until 2 to 3 years of life. Thus aeroallergen-induced asthma is uncommon during the first year of life, begins to increase in prevalence during later childhood and adolescence, and peaks in the second decade of life. Once established in genetically predisposed individuals, IgE-mediated reactions are a major contributor both to acute asthmatic symptoms and chronic airway inflammation. Chronic low-level exposure to indoor allergens, dust mite and cockroach in particular, might play a major role in both asthma inception and subsequent provocation of symptoms.61 Although a wide variety of inhaled allergens can provoke asthma symptoms, sensitization to house dust mite,62 cockroach,63 Alternaria species,64 and possibly cat40 are important in the pathogenesis of asthma. Dog, but not cat, ownership during infancy has been shown to reduce the subsequent development of allergic sensitization and atopic dermatitis65; numbers of pets and not the type of furred pet might also reduce future risk.66 These diverse findings indicate that these relationships are indeed complex and might involve gene-environment interactions. Pollen immunotherapy in school-aged children with only allergic rhinitis at the start of treatment has been demonstrated to reduce significantly the subsequent risk of the development of airway hyperresponsiveness and asthma.67
Infections
Respiratory tract infections caused by viruses,1, 68, 69 Chlamydia species,70 and Mycoplasma species70 have been implicated in the pathogenesis of asthma. Of these respiratory pathogens, viruses have been demonstrated to be epidemiologically associated with asthma in at least 3 ways.
First, during infancy, certain viruses have been implicated as potentially being responsible for the inception of the asthmatic phenotype. The viruses most convincingly demonstrated in this regard have been rhinovirus and respiratory syncytial virus (RSV).1, 2 The propensity to respond to these infections differently in persons destined to have asthma might be due to aberrations in innate immune responses, epithelial cell barrier alterations that enhance viral replication, and potentially increased virulence of pathogenic viral strains. However, because nearly every child has been infected at least once with this virus by 2 years of age, additional genetic, environmental, or developmental factors must contribute to the propensity of RSV to be epidemiologically linked with childhood asthma.
Second, in patients with established asthma, particularly children, viral upper respiratory tract infections play a significant role in producing acute exacerbations of airway obstruction that might result in frequent outpatient visits or hospitalizations.1, 71 Rhinovirus, the common cold virus, is the most frequent cause of exacerbations, but other viruses, including parainfluenza, RSV, influenza, and coronavirus, also have been implicated, albeit to a lesser extent. The increased tendency for viral infections to produce lower airway symptoms in asthmatic subjects might be related, at least in part, to interactions among allergic sensitization, allergen exposure, and viral infections acting as cofactors in the induction of acute episodes of airflow obstruction.72, 73 Abnormalities in the innate immune response that would prevent viral replication in airway epithelial cells from asthmatic subjects have recently been demonstrated.68
Third, and paradoxically, infections have been considered to have the potential of actually preventing the development of allergic respiratory tract diseases, including asthma. Interest in this area increased after the advancement of the hygiene hypothesis,74 which proposed that increasing family size coincident with an increased number of infections might protect against these developments. Based on a progressively broader interpretation of this initial hypothesis,75 a number of other epidemiologic (eg, living on a farm76 and early pathologic bacterial colonization of the airway77) and biologic (eg, probiotics78) factors have been evaluated regarding their ability to influence the development of allergic sensitization, asthma, or both.
For infections with other microbial agents, recent attention has focused on Chlamydia and Mycoplasma species as potential contributors to both exacerbations and the severity of chronic asthma in terms of loss of lung function or medication requirements.70 Finally, infections involving the upper airways (ie, sinusitis) have been considered to contribute to asthma control instability, evoking the concept of a unified airway in relationship to inflammatory responses and alterations in airway physiology.
Exercise
Exercise is one of the more common precipitants of airway obstruction in asthmatic subjects.79 The symptoms of exercise-induced bronchospasm (EIB) can include any or all of the following: wheezing, coughing, and shortness of breath and, in children, chest pain or discomfort. The symptoms are most intense for 5 to 10 minutes and usually resolve within 15 to 30 minutes after exercise cessation. Under most circumstances, the degree of bronchoconstriction is rarely severe enough to be life-threatening, and such a situation almost invariably reflects advanced untreated disease, confounding triggering factors (ie, concomitant allergen or irritant exposure), or both. Objective documentation of airflow obstruction after an exercise challenge test (≥15% decrease in FEV1; ≥10% if symptoms accompany the decrease in FEV1)79 or a convincing history with an appropriate response to prophylactic or rescue medication is required to make the diagnosis of EIB. Exercise challenge testing, particularly in elite athletes,80 must be of sufficient intensity and duration to be able to accurately diagnose the condition, keeping in mind that such confounding problems as vocal cord dysfunction might need to be considered in the differential diagnosis.81 The pathophysiology of EIB can involve exaggerated responses to heat and water loss and the release of inflammatory mediators as a consequence of these thermodynamic alterations.82
Nonsteroidal anti-inflammatory drugs
Approximately 5% to 10% of adult asthmatic patients will have an acute worsening of symptoms to nonsteroidal anti-inflammatory drugs (NSAIDs).83 The aspirin triad, asthma, nasal polyps, and aspirin sensitivity, is usually found in adult asthmatic patients. The response to aspirin or other NSAIDs begins within an hour of aspirin ingestion and is associated with profound rhinorrhea, eye lacrimation, and, potentially, severe bronchospasm. Patients sensitive to aspirin usually are reactive to all other NSAIDs, and variations in the frequency and severity of adverse responses appear to depend on the potency of each drug within this class of compounds to inhibit the activity of the COX-1 enzyme.83
The sensitivity to NSAIDs is not IgE mediated but involves the modulation of eicosanoid production. It has been suggested that NSAIDs act by reducing the formation of prostaglandins, which help maintain normal airway function, while increasing the formation of asthma-provoking eicosanoids, including hydroxyeicosatetraenoic acids and large quantities of cysteinyl leukotrienes.83 In addition, there is evidence that mast cell activation occurs, and its mediators can be detected in nasal secretions during an episode of aspirin-induced asthma.84 This syndrome should be of concern in any asthmatic subject with nasal polyposis, chronic sinusitis, and eosinophilia, although the polyposis and sinusitis might precede the onset of recognized NSAID sensitivity by years.
Aspirin desensitization is available for the aspirin-sensitive patient who might need anti-inflammatory treatment or for use in patients with ischemic heart disease. In patients with aspirin-induced asthma, desensitization with aspirin has proved beneficial in improved asthma control, as well as improved sense of smell, reduced purulent sinus infections, and need for further polyp surgery.85, 86
Gastroesophageal reflux
The true incidence of gastroesophageal reflux disease (GERD) in asthmatic subjects and as a causative factor in disease severity has yet to be established. However, it has been estimated that as many as 45% to 65% of adults and children with asthma have GERD. The mechanisms by which GERD affects asthma are also not established but might include microaspiration or irritation of the esophagus with reflux bronchospasm. Although often asymptomatic in its presentation, many patients have nighttime exacerbations or difficult-to-control symptoms. Confirmation of the importance of GERD to asthma often requires endoscopy and 24-hour monitoring of intraesophageal pH levels with concomitant measures of peak expiratory flow rates.
A number of clinical trials have begun to evaluate the effect of suppressing acid reflux on asthma symptoms. A systematic review of 12 small trials of proton-pump inhibitors used in asthma showed an improvement in asthma-related outcomes, but many studies had design flaws and variability in their outcomes.87 In one study with patients experiencing nocturnal symptoms and symptomatic gastroesophageal reflux, comparisons were made between placebo and 40 mg of esomeprazole twice daily.88 Improvements in peak flow were noted but not in FEV1, rescue inhaler use, or nocturnal awakenings. Finally, the American Lung Association–Asthma Clinical Research Center evaluated 40 mg of esomeprazole twice daily versus placebo in subjects who were asymptomatic for acid reflux disease but had documented acid reflux in 40% of the subjects. Proton-pump inhibitors had no significant effect on a variety of asthma outcomes.89 These studies suggest that treatment of acid reflux is beneficial, but improvement in symptoms from this condition had no effect on asthma outcomes.
Psychosocial factors
The role of psychosocial factors, or “stress,” has undergone an important re-evaluation both in terms of a disease risk factor and a concomitant component of severity. Evidence has shown that parental stress is a risk factor for asthma expression in some children.90 The mechanisms by which this occurs have not been defined but might include the promotion of allergic inflammation. For example, Liu et al91 found stress from final examinations to enhance eosinophil recruitment to the airway after an antigen inhalation challenge. Chen et al92 evaluated the influence of socioeconomic status, which they related to stress, on cytokine generation. With peripheral blood cells from asthmatic subjects but not healthy control subjects, lower socioeconomic status was associated with greater generation of the proinflammatory cytokines IL-5 and IL-13. These data are a further indication that stress can, in asthmatic subjects, promote an inflammatory profile. Recent work has also demonstrated dose-response–type relationships between panic and asthma and bidirectional longitudinal associations between the 2 conditions.93
Disease progression, prevention, and treatment
Although a number of research groups are investigating strategies aimed at asthma prevention,94, 95 this goal has not yet been achieved. Therefore therapy at present is directed primarily at achieving optimal control while attempting to minimize both short- and long-term side effects from any therapeutic intervention. Asthma control is defined by an understanding of the patient's asthma severity, which can be viewed in 2 domains: impairment and risk. Impairment is an evaluation of the concurrent degree of control in achieving the following: minimal (or none) chronic symptoms, including nocturnal awakenings caused by asthma; minimal (or none) need for acute rescue therapy, such as inhaled β2-agonists; establishment of a normal lifestyle with no limitations on activities, including exercise; and normalization of pulmonary function. The risk domain includes criteria that deal with future events that the treatment program should either prevent or reduce to the greatest extent possible: reduction (or elimination of) in the frequency and severity of asthma exacerbations; minimal or no loss of lung function over time (considered to be a potential consequence of airway remodeling); and minimal or no adverse effects from medications.
The initial selection of pharmacologic treatment is determined based on the age of the patient and the severity of his or her asthma at the time of evaluation. Because asthma is a variable but chronic disease (or syndrome), specific treatment will need to be adjusted both acutely, or during exacerbations, and chronically in the context of eliminating or reducing both impairment and risk because they dynamically fluctuate over time to achieve acceptable control. a stepwise approach has been adapted for treatment to accomplish these goals (http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm).4 The basis of the stepwise approach is to increase the number, frequency, and dose of medications with increasing asthma severity until the patient's disease has been put under “control” (ie, achieving optimal control for that patient). Once control has been established, step-down therapy can be attempted to minimize medication burden, when possible. Recently, the concept of response to therapy has also received increasing attention. Responsiveness is the ease with which asthma control is achieved by therapy. Responsiveness to an asthma treatment is highly variable, and it is likely that both genetic and phenotypic characteristics contribute to this intrapatient and interpatient variability in response over time.96, 97
In the last few years, a number of published clinical trials with new therapeutic agents or novel treatment strategies are noteworthy based on their potential effect in initiating or adjusting medication based on this stepwise severity scheme. The first set of trials pertains to the treatment of preschool wheezing children. One trial98 evaluated continuous ICS treatment (2 years receiving therapy with an ICS and the third year receiving as-needed medication, which served as the observation year) in children who had a positive asthma predictive index (API). Children with positive APIs in the first 3 years of life have about a 65% chance of having clinically diagnosed asthma by age 6 years. During the 2 years of treatment with a low-dose ICS (fluticasone, 88 μg twice daily) compared with matching placebo, treated children had significantly greater numbers of episode-free days and reduced exacerbations requiring oral steroid treatment. However, after discontinuation of the ICS treatment at the beginning of the observation period, episode-free days were no different than in the placebo group within about 3 months. Reduced airway resistance in the ICS group at the end of the treatment period was no longer evident at the end of the observation period. Thus early recognition and treatment of high-risk children can reduce symptom burden while receiving therapy but does not appear to alter the natural history of asthma.98 Similar negative results were seen when intermittent ICS therapy was prescribed.99 Intermittent therapy with either an ICS or montelukast at the onset of respiratory tract symptoms was able to reduce symptom burden during these illnesses; however, these beneficial effects were only seen in children with positive APIs.100 In a third study in preschool-aged children with moderate-to-severe, presumed virus-induced wheezing, pre-emptive treatment with high-dose fluticasone (750 μg twice daily at the start of upper respiratory tract symptoms) compared with placebo reduced the use of rescue oral corticosteroids. However, treatment with fluticasone was associated with a smaller gain in height and weight.101 Finally, in preschool children presenting to a hospital with mild-to-moderate wheezing associated with a presumed viral infection, oral prednisolone was not superior to placebo in reducing the duration of the hospital stay.102 These disparate results in this age group might relate to host (eg, presence or absence of atopy103), viral (cause/pathogenicity of viral infection, such as rhinovirus vs RSV104), or both factors that confer differential responses to these types of interventions. More studies are obviously needed before precise recommendations can be made in this age group.
The second set of trials pertains to the use of long-acting β-agonists (LABAs) in combination with ICSs for the treatment of persistent asthma. Although a number of clinical trials have demonstrated both safety and efficacy in terms of asthma control in both the impairment and risk domains,105 concern has been raised about the potential for adverse outcomes in a small number of patients with the use of LABAs.106 Recent continued review of the available data has re-emphasized these potential safety issues in both children and adults.107 Unfortunately, the possible mechanisms underlying these rare events are unknown. Moreover, the numbers of patients needed to be prospectively evaluated to ascertain the precise risks involved might be too large to realistically enroll.108 Overall, however, the benefits of combination therapy appear to outweigh the risks in the majority of patients. Monotherapy with LABAs in asthmatic subjects should not be prescribed.
Summary
Asthma is a complex genetic disorder that is characterized by airway inflammation and reversible airflow obstruction. It is further distinguished by multiple phenotypes that might differ based on age of onset, triggering factors, and patterns of severity both during acute exacerbations and on a more chronic basis, as reflected by variably reversible loss of lung function. As a result of this clinical heterogeneity, treatment approaches need to be individualized and modified to obtain and maintain adequate symptom and disease control over time. Although current therapy is targeted at the development of secondary and tertiary prevention strategies, ongoing research is evaluating the prospects of primary prevention as well.
Footnotes
Supported by National Institutes of Health grants 1P01HL70831-01, HL56396, and AI50500.
Disclosure of potential conflict of interest: R. F. Lemanske is a Speaker for Merck, Doembecher Children's Hospital, Washington University, Medicus Group, Park Nicolet Institute, ACAAI, LA Allergy Society, Michigan Allergy/Asthma Society, Medical College of Wisconsin, Fund for Medical Research and Education (Detroit), Children's Hospital of Minnesota, Toronto Allergy Society, AAAAI, Beaumont Hospital, University of Illinois, Canadian Society of Allergy and Clinical Immunology, and New York Presbyterian; is a consultant and speaker for AstraZeneca; is a consultant for Map Pharmaceuticals, Gray Consulting, Smith Research, Inc., Merck Childhood Asthma Network, Novartis, Quintiles/Innovax, RC Horowitz & Co, Inc., International Meetings and Science, and Scienomics; is an author for Up-to-Date; and is an Editor for Elsevier, Inc. W. W. Busse is on the Advisory Board for Altair, Amgen, Abbott Laboratories, Asthmatx, Bristol-Meyer Squibb, Centocor, Genentech, GlaxoSmithKline, Merck, Pfizer, Schering-Plough, and Wyeth; is a speaker for Merck, and a consultant for Alexion, AstraZeneca, Boehringer Ingelheim, Dainippon Sumitomo, Funxional Therapeutics Ltd, Novartis, and TEVA; and has received research support from Novartis, Centocor, GlaxoSmithKline, MedImmune, Ception, and the National Institutes of Health–National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute.
References
- 1.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E., et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusel M.M., de Klerk N.H., Kebadze T., Vohma V., Holt P.G., Johnston S.L., et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush R.K., Peden D.B. Advances in environmental and occupational disorders in 2008. J Allergy Clin Immunol. 2009;123:575–578. doi: 10.1016/j.jaci.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 4.Busse W.W., Lemanske R.F., Jr. Expert panel report 3: moving forward to improve asthma care. J Allergy Clin Immunol. 2007;120:1012–1014. doi: 10.1016/j.jaci.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Rabe K.F., Adachi M., Lai C.K., Soriano J.B., Vermeire P.A., Weiss K.B., et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Stern D.A., Morgan W.J., Halonen M., Wright A.L., Martinez F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sly P.D., Boner A.L., Bjorksten B., Bush A., Custovic A., Eigenmann P.A., et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spahn J.D., Covar R. Clinical assessment of asthma progression in children and adults. J Allergy Clin Immunol. 2008;121:548–557. doi: 10.1016/j.jaci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Sears M.R., Greene J.M., Willan A.R., Wiecek E.M., Taylor D.R., Flannery E.M., et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel S.E., Busse W.W. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:14–21. doi: 10.1016/j.jaci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Taussig L.M., Wright A.L., Holberg C.J., Halonen M., Morgan W.J., Martinez F.D. Tucson children's respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 12.Borrego L.M., Stocks J., Leiria-Pinto P., Peralta I., Romeira A.M., Neuparth N., et al. Lung function and clinical risk factors for asthma in infants and young children with recurrent wheeze. Thorax. 2009;64:203–209. doi: 10.1136/thx.2008.099903. [DOI] [PubMed] [Google Scholar]
- 13.Xuan W., Peat J.K., Toelle B.G., Marks G.B., Berry G., Woolcock A.J. Lung function growth and its relation to airway hyperresponsiveness and recent wheeze. Results from a longitudinal population study. Am J Respir Crit Care Med. 2000;161:1820–1824. doi: 10.1164/ajrccm.161.6.9809118. [DOI] [PubMed] [Google Scholar]
- 14.Lange P., Parner J., Vestbo J., Schnohr P., Jensen G.A. 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 15.Covar R.A., Spahn J.D., Murphy J.R., Szefler S.J. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004;170:234–241. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 16.Krawiec M.E., Westcott J.Y., Chu H.W., Balzar S., Trudeau J.B., Schwartz L.B., et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 17.Saglani S., Malmstrom K., Pelkonen A.S., Malmberg L.P., Lindahl H., Kajosaari M., et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 18.Barbato A., Turato G., Baraldo S., Bazzan E., Calabrese F., Panizzolo C., et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 19.Payne D.N., Rogers A.V., Adelroth E., Bandi V., Guntupalli K.K., Bush A., et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 20.Saglani S., Papaioannou G., Khoo L., Ujita M., Jeffery P.K., Owens C., et al. Can HRCT be used as a marker of airway remodelling in children with difficult asthma? Respir Res. 2006;7:46. doi: 10.1186/1465-9921-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne D.N., Qiu Y., Zhu J., Peachey L., Scallan M., Bush A., et al. Airway inflammation in children with difficult asthma: relationships with airflow limitation and persistent symptoms. Thorax. 2004;59:862–869. doi: 10.1136/thx.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmberg L.P., Pelkonen A.S., Haahtela T., Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58:494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeiger R.S., Szefler S.J., Phillips B.R., Schatz M., Martinez F.D., Chinchilli V.M., et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Zacharasiewicz A., Wilson N., Lex C., Erin E.M., Li A.M., Hansel T., et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 25.Szefler S.J., Mitchell H., Sorkness C.A., Gergen P.J., O'Connor G.T., Morgan W.J., et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110:28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 27.Corradi M., Folesani G., Andreoli R., Manini P., Bodini A., Piacentini G., et al. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 28.Zanconato S., Carraro S., Corradi M., Alinovi R., Pasquale M.F., Piacentini G., et al. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol. 2004;113:257–263. doi: 10.1016/j.jaci.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Moss M.H., Gern J.E., Lemanske R.F., Jr. In: Middleton's allergy: principles and practice. 5th ed. Adkinson N.F., Yunginger J.W., Busse W.W., Bochner B.S., Holgate S.T., editors. Mosby; Philadelphia: 2003. Asthma in infancy and childhood; pp. 1225–1255. [Google Scholar]
- 30.van Schaik S.M., Tristram D.A., Nagpal I.S., Hintz K.M., Welliver C.I.I., Welliver R.C. Increased production of IFN-γ and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630–636. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 31.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bousquet J., Jeffery P.K., Busse W.W., Johnson M., Vignola A.M. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 33.Holgate S.T., Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006;368:780–793. doi: 10.1016/S0140-6736(06)69288-X. [DOI] [PubMed] [Google Scholar]
- 34.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 35.Bradding P., Walls A.F., Holgate S.T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen E.A., Taranova A.G., Lee N.A., Lee J.J. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Holgate S.T., Davies D.E., Lackie P.M., Wilson S.J., Puddicombe S.M., Lordan J.L. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 38.Amrani Y., Tliba O., Panettieri R.A., Jr. In: Middleton's allergy principles & practice. 7th ed. Adkinson N.F. Jr., Bochner B.S., Busse W.W., Holgate S.T., Lemanske R.F. Jr., Simons F.E.R., editors. Elsevier; Philadelphia: 2009. Biology of airway smooth muscle cells; pp. 399–411. [Google Scholar]
- 39.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A., et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Arbes S.J., Jr., Gergen P.J., Vaughn B., Zeldin D.C. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–1145. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brauer M., Hoek G., Smit H.A., de Jongste J.C., Gerritsen J., Postma D.S., et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 42.Holgate S.T. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 43.Peden D.B. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol. 2005;115:213–219. doi: 10.1016/j.jaci.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Wang C., Salam M.T., Islam T., Wenten M., Gauderman W.J., Gilliland F.D. Effects of in utero and childhood tobacco smoke exposure and beta2-adrenergic receptor genotype on childhood asthma and wheezing. Pediatrics. 2008;122:e107–e114. doi: 10.1542/peds.2007-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergeron C., Boulet L.P., Page N., Laviolette M., Zimmermann N., Rothenberg M.E., et al. Influence of cigarette smoke on the arginine pathway in asthmatic airways: increased expression of arginase I. J Allergy Clin Immunol. 2007;119:391–397. doi: 10.1016/j.jaci.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Lazarus S.C., Chinchilli V.M., Rollings N.J., Boushey H.A., Cherniack R., Craig T.J., et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dijkstra A., Vonk J.M., Jongepier H., Koppelman G.H., Schouten J.P., ten Hacken N.H., et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006;61:105–110. doi: 10.1136/thx.2004.039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douwes J., Cheng S., Travier N., Cohet C., Niesink A., McKenzie J., et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32:603–611. doi: 10.1183/09031936.00033707. [DOI] [PubMed] [Google Scholar]
- 49.Greer F.R., Sicherer S.H., Burks A.W. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 50.Kusel M.M., de Klerk N., Holt P.G., Sly P.D. Antibiotic use in the first year of life and risk of atopic disease in early childhood. Clin Exp Allergy. 2008;38:1921–1928. doi: 10.1111/j.1365-2222.2008.03138.x. [DOI] [PubMed] [Google Scholar]
- 51.Lowe A.J., Carlin J.B., Bennett C.M., Hosking C.S., Abramson M.J., Hill D.J., et al. Do boys do the atopic march while girls dawdle? J Allergy Clin Immunol. 2008;121:1190–1195. doi: 10.1016/j.jaci.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Shore S.A. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F., et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan V., Diette G.B., Rand C.S., Bilderback A.L., Merriman B., Hansel N.N., et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174:633–638. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Byrne P.M., Pedersen S., Lamm C.J., Tan W.C., Busse W.W. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 56.Bai T.R., Vonk J.M., Postma D.S., Boezen H.M. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 57.Ober C., Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 58.Yang I.A., Holloway J.W. Asthma: advancing gene-environment studies. Clin Exp Allergy. 2007;37:1264–1266. doi: 10.1111/j.1365-2222.2007.02798.x. [DOI] [PubMed] [Google Scholar]
- 59.von Mutius E. Genes and the environment: two readings of their interaction. J Allergy Clin Immunol. 2008;122:99–100. doi: 10.1016/j.jaci.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Wechsler M.E., Israel E. How pharmacogenomics will play a role in the management of asthma. Am J Respir Crit Care Med. 2005;172:12–18. doi: 10.1164/rccm.200412-1635OE. [DOI] [PubMed] [Google Scholar]
- 61.Platts-Mills T.A. Allergen avoidance in the treatment of asthma: problems with the meta-analyses. J Allergy Clin Immunol. 2008;122:694–696. doi: 10.1016/j.jaci.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 62.Celedon J.C., Milton D.K., Ramsey C.D., Litonjua A.A., Ryan L., Platts-Mills T.A., et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120:144–149. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruchalla R.S., Pongracic J., Plaut M., Evans R., III, Visness C.M., Walter M., et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Bush R.K., Prochnau J.J. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 65.Bufford J.D., Reardon C.L., Li Z., Roberg K.A., Dasilva D., Eggleston P.A., et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–1643. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 66.Ownby D.R., Johnson C.C., Peterson E.L. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 67.Moller C., Dreborg S., Ferdousi H.A., Halken S., Host A., Jacobsen L., et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 68.Kelly J.T., Busse W.W. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. doi: 10.1016/j.jaci.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu P., Dupont W.D., Griffin M.R., Carroll K.N., Mitchel E.F., Gebretsadik T., et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newcomb D.C., Peebles R.S., Jr. Bugs and asthma: a different disease? Proc Am Thorac Soc. 2009;6:266–271. doi: 10.1513/pats.200806-056RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston N.W., Johnston S.L., Norman G.R., Dai J., Sears M.R. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Green R.M., Custovic A., Sanderson G., Hunter J., Johnston S.L., Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G., et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 74.Liu A.H., Leung D.Y. Renaissance of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:1063–1066. doi: 10.1016/j.jaci.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 75.von Mutius E. Of attraction and rejection—asthma and the microbial world. N Engl J Med. 2007;357:1545–1547. doi: 10.1056/NEJMe078119. [DOI] [PubMed] [Google Scholar]
- 76.Braun-Fahrlander C., Riedler J., Herz U., Eder W., Waser M., Grize L., et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 77.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bonnelykke K., et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 78.Prescott S.L., Bjorksten B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol. 2007;120:255–262. doi: 10.1016/j.jaci.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 79.Weiler J.M., Bonini S., Coifman R., Craig T., Delgado L., Capao-Filipe M., et al. American Academy of Allergy, Asthma & Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349–1358. doi: 10.1016/j.jaci.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 80.Fitch K.D., Sue-Chu M., Anderson S.D., Boulet L.P., Hancox R.J., McKenzie D.C., et al. Asthma and the elite athlete: summary of the International Olympic Committee's consensus conference, Lausanne, Switzerland, January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254–260. doi: 10.1016/j.jaci.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Watson M.A., King C.S., Holley A.B., Greenburg D.L., Mikita J.A. Clinical and lung-function variables associated with vocal cord dysfunction. Respir Care. 2009;54:467–473. [PubMed] [Google Scholar]
- 82.Hallstrand T.S., Moody M.W., Aitken M.L., Henderson W.R., Jr. Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;116:586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szczeklik A., Stevenson D.D. Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–921. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- 84.Fischer A.R., Rosenberg M.A., Lilly C.M., Callery J.C., Rubin P., Cohn J., et al. Direct evidence for role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–1056. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 85.Lee J.Y., Simon R.A., Stevenson D.D. Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2007;119:157–164. doi: 10.1016/j.jaci.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 86.Stevenson D.D. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol. 2003;24:159–168. doi: 10.1385/CRIAI:24:2:159. [DOI] [PubMed] [Google Scholar]
- 87.Coughlan J.L., Gibson P.G., Henry R.L. Medical treatment for reflux oesophagitis does not consistently improve asthma control: a systematic review. Thorax. 2001;56:198–204. doi: 10.1136/thorax.56.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiljander T.O., Harding S.M., Field S.K., Stein M.R., Nelson H.S., Ekelund J., et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2006;173:1091–1097. doi: 10.1164/rccm.200507-1167OC. [DOI] [PubMed] [Google Scholar]
- 89.Mastronarde J.G., Anthonisen N.R., Castro M., Holbrook J.T., Leone F.T., Teague W.G., et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–1499. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright R.J., Cohen S., Carey V., Weiss S.T., Gold D.R. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 91.Liu L.Y., Coe C.L., Swenson C.A., Kelly E.A., Kita H., Busse W.W. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 92.Chen E., Hanson M.D., Paterson L.Q., Griffin M.J., Walker H.A., Miller G.E. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 93.Hasler G., Gergen P.J., Kleinbaum D.G., Ajdacic V., Gamma A., Eich D., et al. Asthma and panic in young adults: a 20-year prospective community study. Am J Respir Crit Care Med. 2005;171:1224–1230. doi: 10.1164/rccm.200412-1669OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holloway J.W., Yang I.A., Holgate S.T. Interpatient variability in rates of asthma progression: can genetics provide an answer? J Allergy Clin Immunol. 2008;121:573–579. doi: 10.1016/j.jaci.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Martinez F.D. Asthma treatment and asthma prevention: a tale of 2 parallel pathways. J Allergy Clin Immunol. 2007;119:30–33. doi: 10.1016/j.jaci.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 96.Knuffman J.E., Sorkness C.A., Lemanske R.F., Jr., Mauger D.T., Boehmer S.J., Martinez F.D., et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009;123:411–416. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szefler S.J., Phillips B.R., Martinez F.D., Chinchilli V.M., Lemanske R.F., Jr., Strunk R.C., et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Guilbert T.W., Morgan W.J., Zeiger R.S., Mauger D.T., Boehmer S.J., Szefler S.J., et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 99.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 100.Bacharier L.B., Phillips B.R., Zeiger R.S., Szefler S.J., Martinez F.D., Lemanske R.F., Jr., et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122:1127–1135. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ducharme F.M., Lemire C., Noya F.J.D., Davis G.M., Alos N., Leblond H., et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–353. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 102.Panickar J., Lakhanpaul M., Lambert P.C., Kenia P., Stephenson T., Smyth A., et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 103.Bush A. Practice imperfect—treatment for wheezing in preschoolers. N Engl J Med. 2009;360:409–410. doi: 10.1056/NEJMe0808951. [DOI] [PubMed] [Google Scholar]
- 104.Jartti T., Lehtinen P., Vanto T., Vuorinen T., Hiekkanen H., Hartiala J., et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42:1125–1133. doi: 10.1002/ppul.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaeschke R., O'Byrne P.M., Mejza F., Nair P., Lesniak W., Brozek J., et al. The safety of long-acting beta-agonists among patients with asthma using inhaled corticosteroids: systematic review and metaanalysis. Am J Respir Crit Care Med. 2008;178:1009–1016. doi: 10.1164/rccm.200804-494OC. [DOI] [PubMed] [Google Scholar]
- 106.Martinez F.D. Safety of long-acting beta-agonists—an urgent need to clear the air. N Engl J Med. 2005;353:2637–2639. doi: 10.1056/NEJMp058299. [DOI] [PubMed] [Google Scholar]
- 107.Kramer J.M. Balancing the benefits and risks of inhaled long-acting beta-agonists—the influence of values. N Engl J Med. 2009;360:1592–1595. doi: 10.1056/NEJMp0810561. [DOI] [PubMed] [Google Scholar]
- 108.Drazen J.M., O'Byrne P.M. Risks of long-acting beta-agonists in achieving asthma control. N Engl J Med. 2009;360:1671–1672. doi: 10.1056/NEJMe0902057. [DOI] [PubMed] [Google Scholar]


