Abstract
Background & Aims
Mist1 is a basic helix-loop-helix (bHLH) transcription factor that is important to the proper development of the exocrine pancreas. The aim of this study was to investigate the role of Mist1 in modulating acinar cell proliferation.
Methods
Ductal and acinar pancreatic cell lines were engineered to express an inducible Mist1 cDNA or to express an shRNA that targeted endogenous Mist1. Alterations in RNA and protein levels were detected by real-time RT-PCR and immunoblots. Chromatin immunoprecipitation and reporter gene assays were performed to map Mist1-responsive elements on target genes; the overall proliferation index of acinar cells from Mist1 null pancreata was evaluated by immunohistochemistry.
Results
Expression of Mist1 resulted in a significant decrease in the proliferative potential of cells that was associated with induced expression of p21CIP1/WAF1. shRNA-directed knock-down of p21CIP1/WAF1 generated cells that were refractory to Mist1 expression wheras knockdown of Mist1 transcripts or deletion of Mist1 from the mouse genome led to increased cell proliferation and a concomitant decrease in p21CIP1/WAF1 protein levels. Surprisingly, Mist1-dependent activation of the p21CIP1/WAF1 promoter was independent of classic bHLH protein binding sites. Instead, Sp1 binding sites were essential for Mist1-dependent transcription, suggesting that Mist1 activates p21CIP1/WAF1 expression through a unique Sp1 pathway. Indeed, coimmunoprecipitation studies demonstrated that Mist1 and Sp1 were found within the same transcription complex.
Conclusions
Our results show that Mist1 has a dual role in the development of the exocrine pancreas - controlling cell proliferation and promoting terminal differentiation.
INTRODUCTION
Mouse models of pancreas development have provided important molecular insights into how individual cell lineages are specified and the role that key regulatory factors have in the genesis of pancreatic diseases including diabetes mellitus, pancreatitis, and pancreatic cancer. The pancreas consists of three major cell types: endocrine cells, acinar cells, and duct cells. The endocrine cells are organized into the Islets of Langerhans, which are responsible for producing hormones that regulate blood glucose levels. Acinar cells synthesize and secrete large amounts of digestive enzymes that are transported via an elaborate ductal system to the small intestine to aid in digestion 1, 2. Development of the three main pancreatic cell types is tightly controlled by a regulatory network of transcription factors that modulate gene expression 3. Included in this network are homeobox transcription factors (PDX1, Pbx1, HB9), paired-box homeoprotein transcription factors (Pax4, Pax6), Forkhead box transcription factors (Foxa1, Foxa2), and basic helix-loop-helix (bHLH) transcription factors (ptf1a/p48, Mist1, neurogenin3, NeuroD)2, 4–6. Loss of these individual regulators often results in complete or partial pancreas agenesis that leads to embryonic or early postnatal lethality 4, 7–9. Although the importance of each factor in establishing pancreatic cell lineages and regulating terminal differentiation events is well documented, little is understood about how these regulatory pathways maintain proper cell numbers and cellular identity in the adult.
The bHLH transcription factors are particularly critical to development and differentiation events due to the combinatorial nature of these proteins. bHLH factors are classified into two main groups - Class A proteins that include the widely expressed E12/E47/HEB and Class B proteins that display a tissue-restricted expression pattern. In most cases, the preferred bHLH complex is a heterodimer consisting of a Class A member and a Class B member. These heterodimers bind to E-box sites found in the promoter and enhancer regions of target genes to regulate their transcription 10. Four Class B bHLH proteins have been shown to exhibit a pancreas restricted expression pattern (Neurogenin3, NeuroD, ptf1a/p48, Mist1) 4. The Neurogenin3 gene is a downstream target of Notch signaling and is required for the development of all pancreas endocrine cell lineages 9, 11. NeuroD, a downstream target gene of Neurogenin3, serves as a key regulator of insulin gene transcription in β cells 12. In contrast to these endocrine-restricted bHLH factors, ptf1a/p48 is expressed in early pancreatic progenitor cells but becomes restricted to acinar cells in late embryogenesis and in the adult 8, 13. Together with the Class A bHLH factor HEB and the vertebrate Suppressor of Hairless factor RBPJL, ptf1a/p48 forms a unique trimeric protein complex that initiates transcription of acinar-specific genes 14–16.
Another bHLH transcription factor that is expressed to high levels in pancreatic acinar cells is Mist1 (also called bhlhb8) 17. Deletion of the Mist1 gene (Mist1KO) leads to disorganization of the exocrine pancreas, including loss of cellular polarity and defective exocytosis 18. Mist1KO mice also exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis 19. Unlike most Class B bHLH proteins, Mist1 preferentially forms homodimer complexes that activate gene transcription 20, 21. The altered acinar cell phenotype in Mist1KO pancreata suggested that Mist1 is critical to the maintenance of the acinar cell lineage. This function could influence a variety of intracellular processes, such as regulated exocytosis, but also might be essential for controlling cell growth decisions that are instrumental in maintaining proper cell numbers in the mature organ. To determine if Mist1 has a role in controlling acinar cell growth, we examined the effects of ectopic Mist1 expression in pancreatic cells. Our results show that expression of Mist1 inhibits cellular proliferation through induction of p21CIP1/WAF1. Target gene analysis confirmed that Mist1 regulates p21CIP1/WAF1 gene expression and the activation is dependent on specific Sp1 binding sites located within the p21CIP1/WAF1 proximal promoter. Analysis of intact pancreata also revealed that Mist1KO acinar cells exhibit a higher proliferative index when compared to control littermates. Importantly, this phenotype could be rescued by ectopic Mist1 expression. These findings uncover a novel role for Mist1 - serving as a key regulator of terminal differentiation and controlling acinar cell proliferation events in the exocrine pancreas.
MATERIALS AND METHODS
Mouse strains
Control wild type, Mist1KO and elastasepr-Mist1myc (Elpr-Mist1myc) transgenic mice were maintained on a C57Bl/6 background. All studies were conducted in compliance with NIH and the Purdue University IACUC guidelines.
Cell culture, transfections and viral infections
ARIP and AR42J cells were cultured in 40% F-12 Kaighn's nutrient mixture, 25% F-12 nutrient mixture, 25% high glucose Dulbecco's modified Eagle's medium, 10% fetal bovine serum. To generate the ARIPtet-Mist1 cell line, ARIP cells were electroporated with 18 µg pcDNA6-TR (Invitrogen) and 3 µg pcDNA4-TO-Mist1-Myc/His (Invitrogen). Transfected cells were selected in complete medium containing 5 µg/ml Blasticidin, 200 µg/ml Zeocin. The ARIPtet-Mist1-p21RNAi cell lines were generated by transfecting ARIPtet-Mist1 cells with 20 µg pSuper-p21RNAi constructs and selected in complete medium containing 260 µg/ml G418. The AR42J-Mist1RNAi and control cell lines were generated by electroporating AR42J cells with 20 µg pSuper-Mist1RNAi or pSuper-ControlRNAi and selected in complete medium containing 260 µg/ml G418. To restore Mist1 expression, AR42J-Mist1RNAi cells were infected with pBrit or pBrit-Mist1ER viruses in the presence of 8 µg/ml polybrene and selected in complete medium containing 0.8 µg/ml puromycin.
Plasmid constructs
The 2.4 kb p21CIP1/WAF1 promoter construct was a gift from Dimitris Kardassis 22. Truncated p21 promoter constructs were cloned into the pGL2 vector (Promega) by standard PCR procedures. Point mutations within the Sp1 sites of the p21 −124/+3 construct were generated using the QuickChange Site-Directed Mutagenesis Kit from Stratagene. Mist1 and p21CIP1/WAF1 RNAi sequences that specifically targeted the rat Mist1 and p21CIP1/WAF1 transcripts were designed using the Oligoengine program and cloned into the pSuper vector (Oligoengine).
Immunohistochemistry
Paraffin-embedded pancreas sections were processed as previously described 20 and incubated with primary antibodies at 4°C overnight followed by biotinylated secondary antibodies. Primary antibodies included rabbit anti-Mist1 (1:2000), rabbit anti-Myc (Santa Cruz A-14, 1:100), mouse anti-β-gal (Developmental Studies Hybridoma Bank, 1:100), and rabbit anti-phospho-H3 (Upstate 06–570, 1:200).
RNA isolation and real-time RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen). Reverse transcription assays were performed using the Iscript cDNA synthesis kit (Bio-Rad). PCR reactions (20 µl) included SYBR green PCR master mix (Applied Biosystems, 10 µl), 0.5 µM of each PCR primer, 1 µl of cDNA template, and 8 µl of distilled dH2O. Thermocycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of amplification at 94°C for 15s, 60°C or 63°C for 30s, and 72°C for 30s. Primer sequences for each gene target are available upon request.
Chromatin immunoprecipitation assays
The generation and characterization of the AR42Jtet-Mist1~IRES~BirA cell line will be described elsewhere (Jia and Konieczny, in preparation). Cells were transfected with the WT p21-124/+3 or mut 1–4 p21-124/+3 genes and Mist1 expression was induced with 1 µg/ml tetracycline. 48 hours following transfection, cells were subjected to cross-linking using 1% formaldehyde and incubated at room temperature for 10 min with shaking. Cross-link reactions were stopped by addition of 0.125 M glycine. Nuclear extractions were performed by adding 1 ml of cell lysis buffer (50 mM HEPES, pH 7.9, 1 mM EDTA, 140 mM NaCl, 10% glycerol, 0.5% NP-40, 0.25% Triton X-100) on ice for 30 min. Nuclei were collected and resuspended in 750 µl nuclear lysis buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS). Nuclei were then sonicated three times and soluble nuclear fractions were used for chromatin immunoprecipitation. Briefly, lysates were precleared by incubating 100 µl Protein G Sepharose in binding buffer (0.01% SDS, 1.1% Triton-X 100, 167 mM NaCl, 16.7 mM Tris-HCl, pH 8.1) for 1 hour at 4°C. Precleared lysates were incubated with 20 µl anti-Myc at 4°C overnight. 100 µl of Protein G Sepharose was then added and the incubation continued for 1 hour. Bound protein/DNA complexes were washed and resuspended in TE buffer supplemented with 0.195 M NaCl. Samples were reverse-crosslinked at 65°C overnight and digested with protease K at 56°C for 4 hours. DNA was purified using a Qiaquick PCR purification kit (Qiagen) and used for gene specific PCR.
Pull-down assays
AR42Jtet-BT/Mist1~IRES~BirA and AR42Jtet-Mist1~IRES~BirA cells were induced with 1 µg/ml tetracycline for 48 hours and nuclear extracts were harvested as previously described 20. 100 µg nuclear protein was incubated with 10 µl streptavidin-conjugated Dynal beads (Invitrogen) in TBS-N buffer (10 mM Tris-HCl, 150 mM NaCl, 0.3% NP-40) at 4°C for 2 hours. Samples were washed six times with TBS-N buffer and analyzed by SDS-PAGE, followed by immunoblotting using Mist1 and Sp1 (Santa Cruz, 1:1000) antibodies.
RESULTS
Ectopic expression of Mist1 leads to growth inhibition
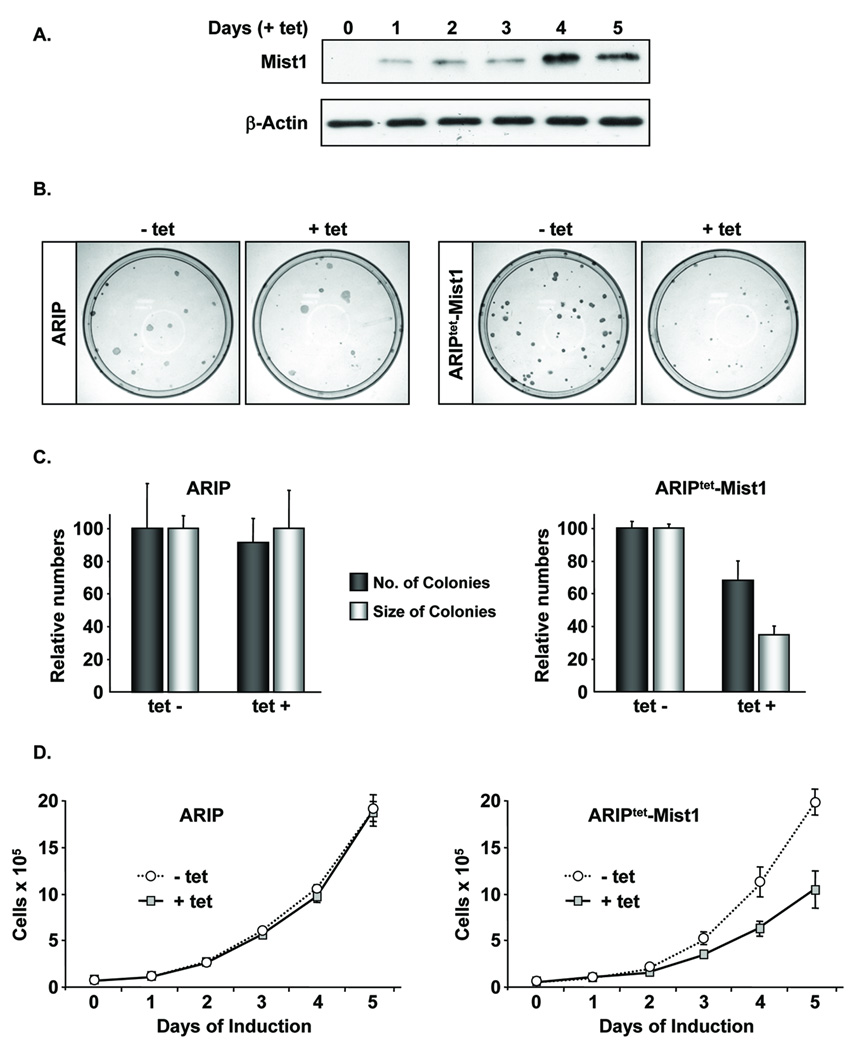
Previous studies have shown that Mist1 accumulates in pancreatic acinar cells where it controls several acinar-specific functions 18, 23–26. To examine the effects of mis-expressing Mist1 in non-acinar cells, a pancreatic ductal cell line (ARIP) was used to generate stable clones (ARIPtet-Mist1) that expressed Mist1 upon tetracycline (tet) induction. ARIPtet-Mist1 cells did not express endogenous Mist1 (data not shown) or the Mist1myc transgene when maintained in the absence of tet. However, following tet treatment, Mist1myc was detected by day 1 (Figure 1A). Induction of Mist1 led to a 30% reduction in colony forming efficiency and a 65% decrease in overall colony size when compared to uninduced ARIPtet-Mist1 or control ARIP cells (Figure 1B,C). Mist1 induction also produced a significant decrease in the growth rate of ARIPtet-Mist1 cells (Figure 1D).
Figure 1. Cell growth inhibition of pancreatic cells by ectopic expression of Mist1.
(A) ARIPtet-Mist1 cells were induced on day 0 by tetracycline (tet) addition and whole cell extracts were harvested daily. Immunoblots were performed using anti-myc to detect Mist1myc. (B,C) Parental ARIP and ARIPtet-Mist1 cells were cultured +/− tet for 2 weeks and colonies were stained and quantified. Values represent the average of 3 independent experiments ± s.d. (D) ARIP or ARIPtet-Mist1 cells were treated +/− tet as indicated and cell numbers were determined over a 5-day period. Each time point corresponds to the average of 3 independent experiments (± s.d.).
To explore the mechanism by which Mist1 exerts its anti-proliferative effects, we next analyzed the expression of key cell cycle regulators in cells treated with or without tet. Although p27, cyclin D, and cyclin A levels remained constant, p21CIP1/WAF1 protein levels increased approximately 3-fold in the tet treated cells (Figure 2A,B). Real-time RT-PCR confirmed that p21CIP1/WAF1 transcript levels were elevated in Mist1-expressing cells, whereas no change was observed in cyclin D, cyclin E, p16, p18, p27, or p57 transcript levels (Figure 2C). To establish if elevated p21CIP1/WAF1 levels were required for Mist1-induced growth inhibition, we generated ARIPtet-Mist1-p21RNAi cell lines in which the endogenous p21CIP1/WAF1 levels were "knocked-down" by p21CIP1/WAF1 shRNA (Figure 2D). Although tet treatment led to the predicted induction of Mist1 expression, p21CIP1/WAF1 levels remained undetectable in the p21CIP1/WAF1 shRNA lines (Figure 2D). Interestingly, induced Mist1 expression did not affect the growth properties of the ARIPtet-Mist1-p21RNAi cells (Figure 2E), confirming that p21CIP1/WAF1 expression was required for Mist1 to induce growth inhibition.
Figure 2. Mist1 inhibits cell proliferation through induction of p21CIP1/WAF1.
(A,B) Immunoblots of cell cycle regulators from ARIPtet-Mist1 cells +/− tet. (C) Real-time RT-PCR analysis of ARIPtet-Mist1 cells treated +/− tet. (D) Immunoblots of ARIPtet-Mist1-p21RNAi cell lines showing that p21CIP1/WAF1 protein levels are undetectable in the presence or absence of tet (arrow, Mist1 protein; asterisk, nonspecific band). (E) Control and ARIPtet-Mist1-p21RNAi cell lines were treated +/− tet and cell numbers were determined over a 5-day period. Each time point corresponds to the average of 3 independent experiments (± s.d.).
Knock-down of endogenous Mist1 leads to increased cell proliferation and decreased p21CIP1/WAF1 levels
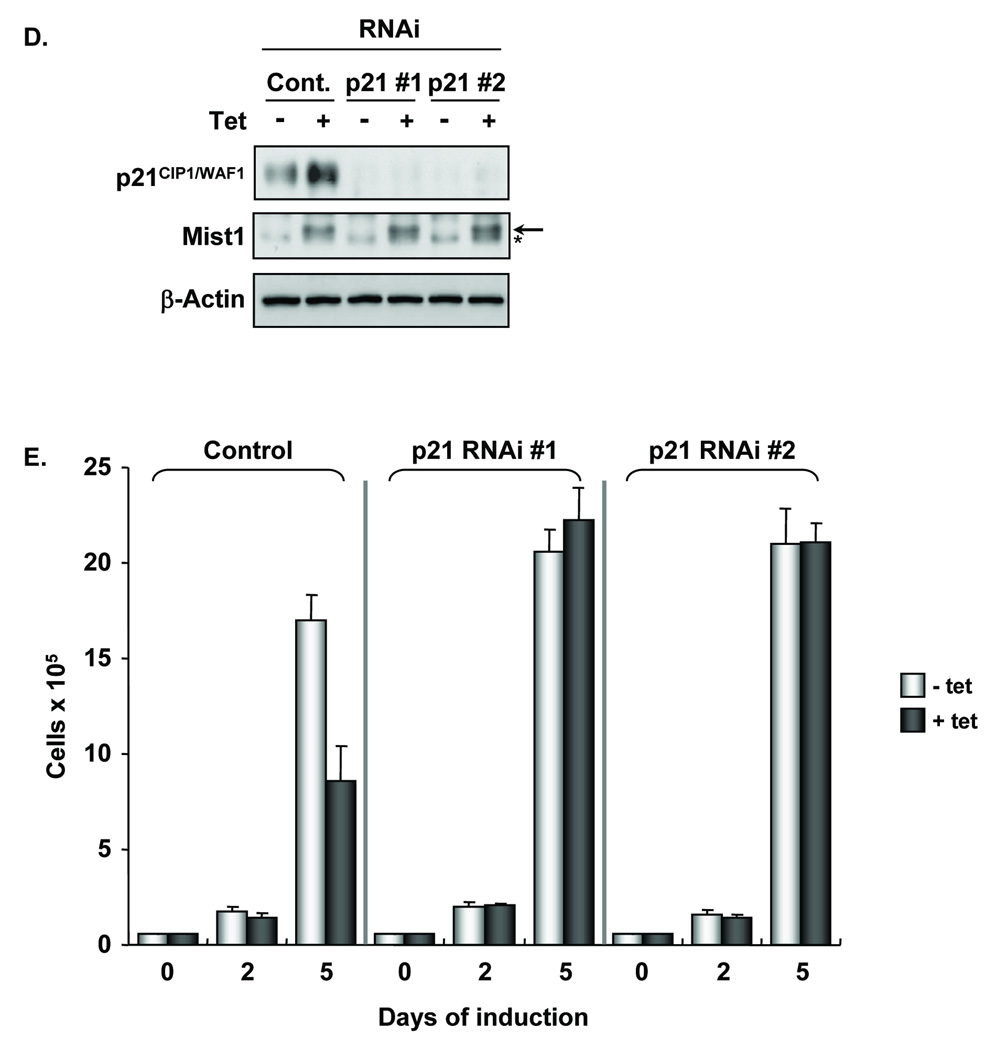
The coordinated induction of p21CIP1/WAF1 with Mist1 expression suggested that Mist1 negatively influenced cell proliferation by transcriptionally activating the p21CIP1/WAF1 gene. To test this hypothesis, we employed a second pancreatic cell line, AR42J, which possesses acinar cell characteristics and accumulates endogenous Mist1 protein (Figure 3A). An AR42J-Mist1RNAi stable cell line was generated in which the endogenous Mist1 transcript levels were knocked-down by an shRNA transcript that specifically targeted the rat Mist1 gene. As shown in Figure 3A, expression of Mist1 RNAi produced an 80% reduction in Mist1 protein levels, whereas nonspecific control RNAi had no effect. Importantly, Mist1 RNAi also generated a reduction in p21CIP1/WAF1 protein levels (Figure 3A). As expected, reduced Mist1 and p21CIP1/WAF1 levels permitted a higher proliferation rate and much shorter cell cycle times when compared to the control RNAi cells (Figure 3B; supplemental Table I). Knock-down of endogenous Mist1 led to a significant reduction in the length of G1, indicating that Mist1 expression primarily impacts the activity of G1 cyclin/CDK complexes. As a final test, a non-targeted form of Mist1 (mouse Mist1ER) was introduced into AR42J-Mist1RNAi cells by viral infection (Figure 3C). As predicted, ectopic Mist1 expression restored growth restriction to AR42J-Mist1RNAi cells (Figure 3D), confirming that hyper-proliferation of AR42J-Mist1RNAi cells was due to the reduction in Mist1 levels.
Figure 3. shRNA-directed knock-down of endogenous Mist1 leads to decreased p21CIP1/WAF1 levels and increased proliferation.
(A) Immunoblots of AR42J-ControlRNAi and AR42J-Mist1RNAi cell lines showing that "knock-down" of endogenous Mist1 transcripts results in decreased p21CIP1/WAF1 levels. (B) Growth analysis of AR42J-ControlRNAi and AR42J-Mist1RNAi cells. (C) Immunofluorescence of AR42J-Mist1RNAi cells infected with pBrit or pBrit-mMist1ER virus. 90% of pBrit-Mist1ER cells were strongly or weakly (closed arrows) positive for Mist1ER while a small number of cells remained Mist1ER negative (open arrows). (D) Growth analysis of AR42J-Mist1RNAi cells infected with pBrit or pBrit-mMist1ER virus. Each time point corresponds to the average of 3 independent experiments (± s.d.).
Sp1 sites are essential for Mist1-induced p21CIP1/WAF1 transcription
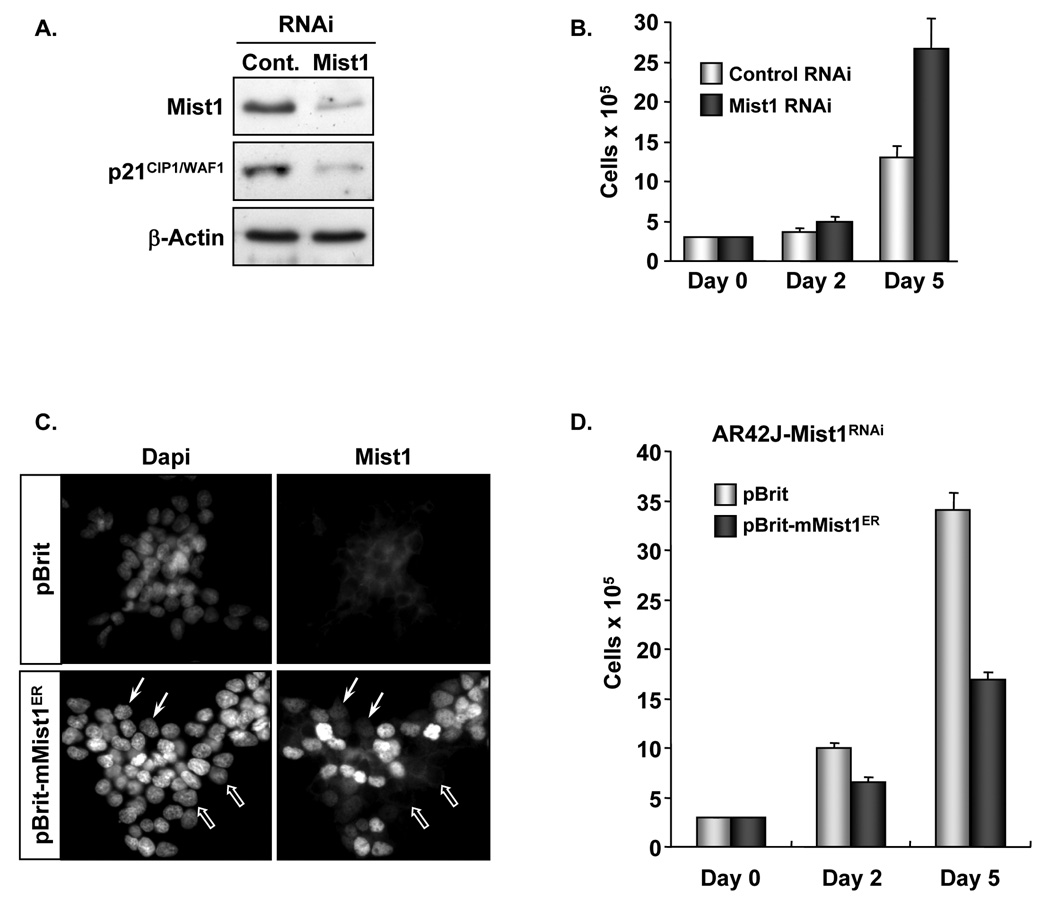
The observation that Mist1 influenced pancreatic cell growth and p21CIP1/WAF1 gene expression suggested that Mist1 binds to the regulatory region(s) of target genes to control their transcriptional expression. To test this hypothesis, we examined if Mist1 could activate a p21-luc reporter gene containing −2.4 kb of the p21CIP1/WAF1 promoter (Figure 4A). As shown in Figure 4B, co-transfection of Mist1 and p21-luc produced a large increase in p21CIP1/WAF1 promoter activity. Sequence analysis revealed a total of 16 E-box regulatory elements within the −2.4 kb promoter which could serve as potential Mist1 binding targets (Figure 4A). To eliminate most sites, we next generated the −194/+3 construct containing 1 E-box (E3) and the −124/+3 and −44/+3 constructs containing no E-boxes (Figure 4A). Expression of the −194/+3 and −124/+3 genes was fully induced by Mist1, whereas the −44/+3 promoter region remained transcriptionally inactive (Figure 4B). Thus, the necessary regulatory elements required for Mist1-induced expression lie within the −124 and −44 proximal region. Surprisingly, this 80 bp sequence lacks a putative Mist1 binding site (E-box).
Figure 4. Sp1 binding sites are essential for Mist1-induced p21CIP1/WAF1 expression.
(A) Schematic representation of the full-length and truncated p21CIP1/WAF1 promoter luciferase gene constructs. Boxes (E1–E16) represent E-box sites. (B) Control pcDNA or pcDNA-Mist1 expression plasmids were co-transfected with the indicated p21-luc promoter genes into AR42J cells and p21-luc activity was quantified. (C) Schematic representation of WT and mutated −124/+3 p21-luc constructs. Open boxes: WT Sp1 binding sites (1–6). Solid boxes: mutated Sp1 binding sites. (D) Sp1 binding sites 1-4 are essential for Mist1 activity. Control pcDNA or pcDNA-Mist1 expression plasmids were co-transfected with the indicated −124/+3 p21-luc promoter constructs into AR42J cells and p21-luc activity was quantified. (E) Cells were transfected with the corresponding plasmids and luciferase assays were performed. Immunoblots reveal Mist1 and Sp1 expression levels. Mist1 and Sp1 synergistically activated the −124/+3 p21-luc reporter gene. Values in B, D, E are the average of 3 or more independent experiments (± s.d.).
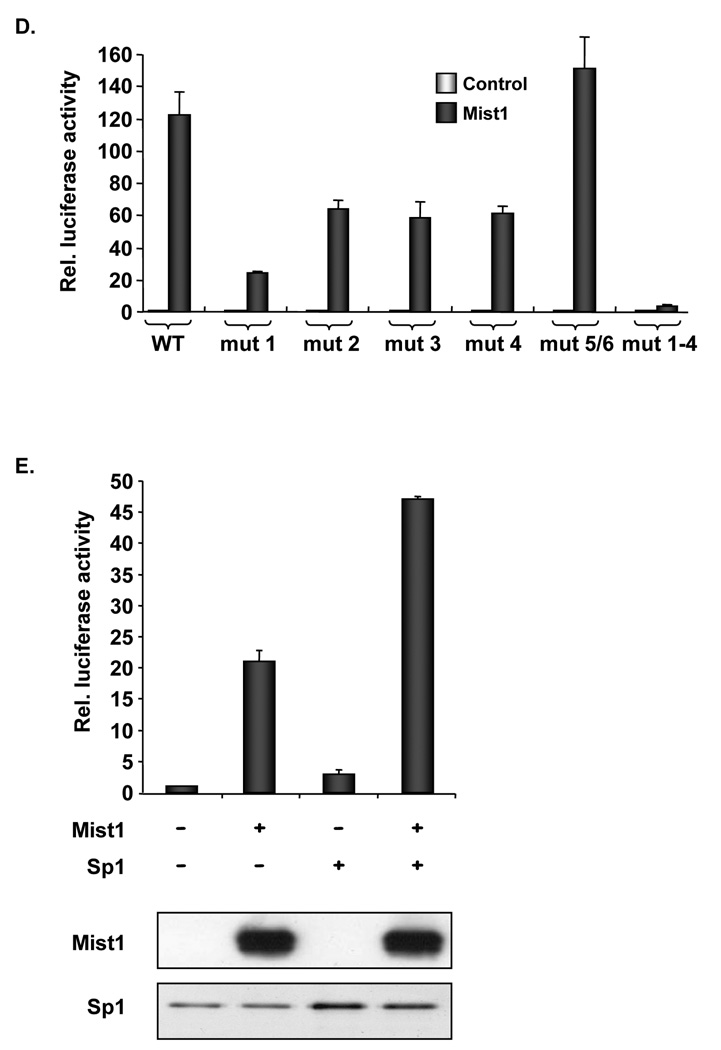
Further analysis of the −124/+3 p21CIP1/WAF1 promoter region revealed 6 Sp1 binding sites that have been implicated in p21CIP1/WAF1 transcriptional expression 22. To determine their importance for Mist1-induced transcription, point mutations were introduced into each Sp1 binding site (Figure 4C). Luciferase assays revealed that mutations within Sp1 sites 1–4 significantly diminished Mist1-induced expression (Figure 4D). When sites 1–4 were simultaneously mutated (mut 1–4), Mist1-induced activity was completely abolished. As a further test, Sp1 and Mist1 were tested independently and together with the −124/+3 p21-luc reporter gene. As expected, Mist1 and Sp1 individually induced expression of the truncated p21CIP1/WAF1 promoter (Figure 4E), although the response to Sp1 was minimal due to the low Sp1 levels obtained from this expression plasmid. Nonetheless, cotransfection of Mist1 and Sp1 resulted in synergistic activation (Figure 4E), demonstrating that together both transcription factors significantly elevate p21CIP1/WAF1 expression.
Mist1:Sp1 complexes bind to the p21CIP1/WAF1 proximal promoter
Our studies support a model where Mist1 interacts with Sp1 to elicit transcriptional activation of p21CIP1/WAF1. To examine this directly, we performed modified co-immunoprecipitation assays using AR42J cells that contained a tet-inducible Mist1myc or a tet-inducible biotinylated Mist1myc (BT-Mist1myc) protein. Following tet-induction, nuclear extracts were harvested and subjected to "pull-down" assays using streptavidin-conjugated beads (Figure 5A). As expected, endogenous Mist1 protein co-purified with BT-Mist1myc, confirming that Mist1 forms homodimers in vivo 20, 21. Interestingly, endogenous Sp1 also co-purified with BT-Mist1myc, revealing for the first time the existence of a Mist1:Sp1 complex in pancreatic acinar cells.
Figure 5. Mist1:Sp1 complexes are associated with the p21CIP1/WAF1 promoter.
(A) AR42J cells that expressed tet-inducible Mist1myc (M) or biotinylated Mist1myc (BT) were induced with tetracycline and nuclear extracts were subjected to pull-down assays using streptavidin conjugated beads. Sp1 co-purifies with BT-Mist1myc. (B) AR42Jtet-Mist1 cells were treated +/− tet and real-time PCR was performed on Mist1 ChIP products using primers specific to the endogenous p21CIP1/WAF1, β-actin and tubulin promoters. Values are the average of 3 or more independent experiments (± s.d.). (C) AR42Jtet-Mist1 cells were used to introduce the WT −124/+3 p21-luc or mut 1–4 −124/+3 p21-luc gene construct into the cells. Real-time PCR was performed on Mist1 ChIP products using primers specific to the flanking region of the −124/+3 p21CIP1/WAF1 promoter. Mist1 specifically bound the WT p21CIP1/WAF1 promoter but not the mutated p21CIP1/WAF1 promoter. Values are the average of 3 independent experiments (± s.d.).
The ability of Mist1 to interact with Sp1 and activate p21CIP1/WAF1 gene expression suggested that the Mist1:Sp1 protein complex must associate with the endogenous p21CIP1/WAF1 gene promoter. To test this hypothesis, we next performed chromatin immunoprecipitation (ChIP) assays using our tet-inducible Mist1 cell lines and gene-specific primers. As shown in Figure 5B, binding of Mist1 to the proximal AR42J p21CIP1/WAF1 promoter was enhanced ~3-fold in the +tet group compared to the −tet samples. In contrast, control genes (actin, tubulin) were not significantly enriched. Importantly, the association of Mist1 with the p21CIP1/WAF1 promoter was completely dependent on intact Sp1 binding sites. Whereas p21CIP1/WAF1 promoter-Mist1 complexes were readily detected by ChIP, no complexes were immunoprecipitated when the Sp1 sites were destroyed (Figure 5C). We conclude that p21CIP1/WAF1 is a direct target of Mist1 and that Sp1 sites are required for interaction with the proximal promoter.
Loss of Mist1 leads to increased acinar cell proliferation in the exocrine pancreas
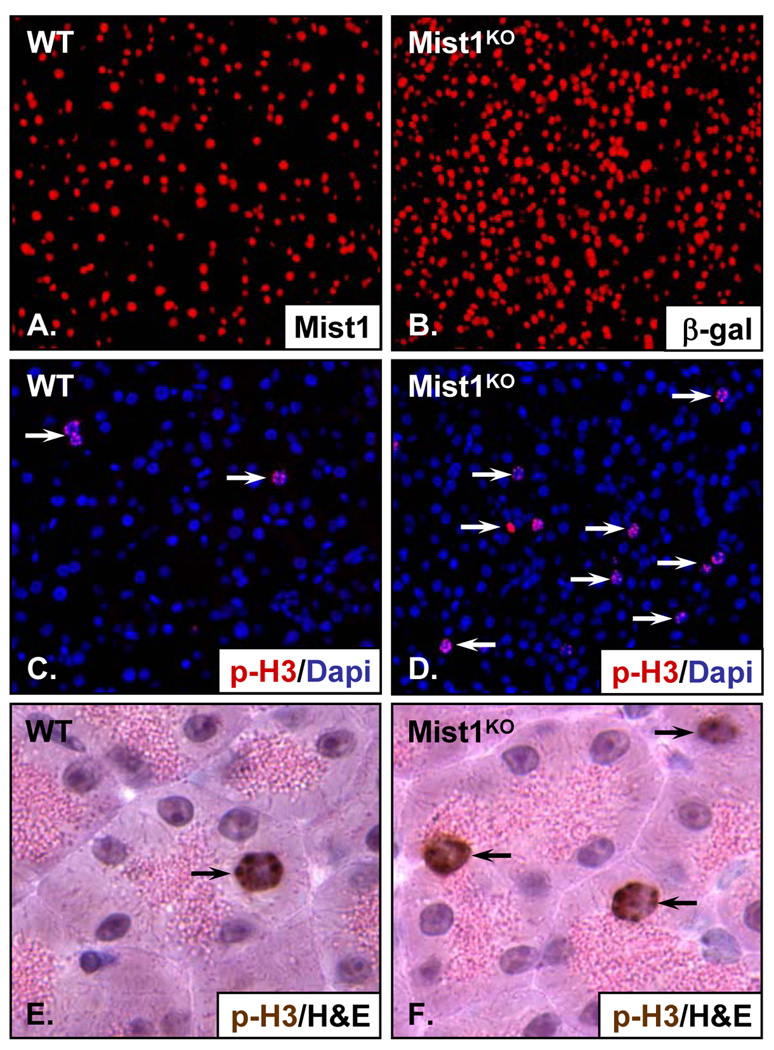
Our studies have shown that Mist1 regulates cell growth of cultured pancreatic cell lines through induction of p21CIP1/WAF1. Thus, one would predict that p21CIP1/WAF1 levels and cell numbers would be similarly influenced in intact pancreata by the presence or absence of Mist1. To examine this, we first analyzed protein levels of p21CIP1/WAF1 in wild type (WT) and Mist1KO pancreata 18. As expected, deletion of Mist1 resulted in decreased levels of p21CIP1/WAF1 (Supplemental Figure 1). Quantification of pancreatic acinar cells in WT and Mist1KO mice revealed that adult Mist1KO pancreata always contained a 2-fold higher acinar cell content when compared to control WT littermates (Figure 6A,B). Detailed analysis revealed a slight increase in the number of Mist1KO acinar cells at 3 weeks of age, with a KO/WT cell ratio of 1.2 (Supplemental Table II). However, as animals aged, the KO/WT ratio increased so that by 3 months Mist1KO pancreata had twice the number of acinar cells as control samples. The acinar cell increase in Mist1KO pancreata was due to an increased proliferation rate and not due to loss of cells in WT samples (data not shown). This was confirmed by anti-phospho-Histone 3 (p-H3) immunohistochemistry in which Mist1KO pancreata exhibited an elevated acinar cell proliferation index (Figure 6C–F; Supplemental Table II). Interestingly, p21CIP1/WAF1 null pancreata exhibited a similar increase in acinar cell numbers (Supplemental Figure 2), supporting the hypothesis that Mist1 and p21CIP1/WAF1 are critical regulators of acinar cell growth.
Figure 6. The Mist1KO pancreas has an increased acinar cell proliferation index.
(A,B) Pancreas sections from WT and Mist1KO littermates were subjected to IHC using Mist1 or β-gal antibodies to detect acinar cells. (C,D) Anti-p-H3 IHC showing that Mist1KO acinar cells exhibit a higher cell proliferation index when compared to WT samples. (E,F) Pancreas sections from WT and Mist1KO littermates were subjected to IHC using the p-H3 antibody and hematoxylin and eosin staining to identify acinar cells containing zymogen granules.
Expression of Mist1myc in the exocrine pancreas leads to lower acinar cell proliferation indices
Our in vitro experiments demonstrated that Mist1 controlled the growth potential of pancreatic cells. To test if a similar phenomenon could be observed in vivo, we took advantage of Elpr-Mist1myc mice in which acinar cells expressed a Mist1myc transgene from the elastase promoter (Figure 7A,B). Crossing the Elpr-Mist1myc transgene into the Mist1KO line restored the normal acinar-specific growth inhibition that is a hallmark of WT pancreata (Figure 7C). Similarly, WT mice expressing Mist1myc exhibited even lower proliferation indices than control littermates lacking the transgene (Figure 7C). These results, combined with our in vitro studies, support the hypothesis that Mist1 negatively regulates acinar cell proliferation potency through induction of p21CIP1/WAF1.
Figure 7. Expression of a Mist1myc transgene results in decreased acinar cell proliferation.
(A,B) Pancreas sections from WT and Elpr-Mist1myc transgenic littermates were subjected to IHC to detect Mist1myc protein (arrows). (C) Pancreas sections from Mist1KO, Mist1KO/Elpr-Mist1myc, WT and WT/Elpr-Mist1myc littermates were subjected to IHC using anti-p-H3. Each value corresponds to the average of 10 or more sections.
DISCUSSION
Specification of pancreas cell lineages is orchestrated by key transcription factors that play important roles in promoting differentiation and in controlling cell growth. Cell numbers of the endocrine and exocrine compartments must be carefully regulated to insure the proper function of the organ, since misregulation of cell mass often results in pancreatic diseases. Defects in maintenance or expansion of β-cell mass can lead to impaired glucose metabolism and diabetes 27, while improper growth control of the exocrine pancreas is often associated with pancreatitis and pancreatic cancer 28, 29. Although recent studies suggest that the overall size of the pancreas is controlled by the initial number of embryonic progenitor cells 30, little is known about how cellular proliferation events are regulated in the adult exocrine pancreas.
In this study, we addressed whether Mist1 contributes to maintaining a proper acinar cell population. We found that Mist1KO pancreata exhibited elevated acinar cell numbers as well as a higher acinar cell proliferation index. Expression of an acinar specific Mist1myc transgene led to decreased proliferation potency. As predicted, ectopic expression of Mist1 in pancreatic cell lines also led to a lower proliferation rate that was coupled to induced p21CIP1/WAF1 gene expression. The growth inhibition effect of Mist1 was solely dependent on the induction of p21CIP1/WAF1, suggesting that Mist1 controls p21CIP1/WAF1 transcription. Indeed, chromatin immunoprecipitation and reporter gene assays confirmed that Mist1 transcriptionally activated p21CIP1/WAF1 gene expression. Since p21CIP1/WAF1 is a cyclin dependent kinase inhibitor and serves to arrest the cell cycle 31, we propose that Mist1 controls acinar cell proliferation through the induction of p21CIP1/WAF1.
The classic mechanism by which bHLH transcription factors activate gene transcription is through binding to E-box sequences located in the proximal regions of target promoters. Our results reveal that transcriptional activation of the p21CIP1/WAF1 promoter by Mist1 is independent of E-box sequences. Indeed, mutations within the proximal Ebox sites E1–E3 resulted in no significant alteration in Mist1-dependent promoter activity (data not shown). Instead, our analysis defined four proximal Sp1 binding sites that were essential for p21CIP1/WAF1 expression. Previous studies have shown that Sp1 serves as a key regulator of p21CIP1/WAF1 transcription, not only by activating p21CIP1/WAF1 gene transcription, but also by functioning as a docking site for additional transcriptional activators and/or repressors including Smad proteins 32, c-Jun 33, and c-Myc 34. In this study, we showed that a similar mechanism operates in acinar cells in concert with Mist1. Indeed, co-immunoprecipitation and ChIP experiments revealed a common Mist1:Sp1 complex bound to the p21CIP1/WAF1 promoter, supporting the hypothesis that Mist1 activates p21CIP1/WAF1 transcription through Sp1. These results offer a unique glimpse into how a tissue-restricted factor (Mist1) influences the activation of the ubiquitous cell cycle machinery in pancreatic acinar cells.
The ability of Mist1 to regulate p21CIP1/WAF1 expression and cell proliferation is reminiscent of activities reported for other bHLH transcription factors, such as ptf1a/p48 and MyoD 35, 36. Aside from their essential role in pancreas and muscle development, respectively, these proteins also function to inhibit cell proliferation through activation of the p21CIP1/WAF1 gene. Additional studies have shown that other bHLH transcription factors, including E12 in osteoblasts 37, NeuroD in neuronal cells 38, and myogenin in muscle cells 39, participate in regulating cell growth through activating transcription of cyclin-dependent kinase inhibitor genes. Thus, members of the bHLH superfamily often control terminal differentiation events by inhibiting cell proliferation. However, Mist1 regulates p21CIP1/WAF1 gene expression by a mechanism that is distinct from other bHLH proteins. Instead of direct binding to E-box sequences, Mist1 controls p21CIP1/WAF1 transcription through binding to the ubiquitously expressed transcription factor Sp1.
The antiproliferative activity of Mist1 correlates with its function to serve as a gate keeper to the development of exocrine pancreas diseases such as pancreatitis and pancreatic cancer. For instance, Mist1KO mice are hypersensitive to caerulein-induced pancreatitis 19 and recent studies have shown that Mist1 also may be critical to controlling the development of pancreatic ductal adenocarcinoma (PDA). Although PDAs are thought to originate from duct cells upon activation of the Kras oncogene 40, several studies have shown that acinar cells can also contribute to the precursor lesions of PDA, termed pancreatic intraepithelial neoplasia (PanIN) 41–43. This process involves silencing acinar-specific gene expression (amylase, Mist1), turning on duct-specific genes (keratin 19), and activating the cell cycle machinery. In heterogeneous acinar-ductal metaplastic units, acinar cells that remain Mist1 positive are growth arrested, while cells exhibiting a ductal phenotype are Mist1 negative and highly proliferative 42. Activating the Kras pathway within Mist1 expressing cells leads to the rapid formation of PanINs, but this process is dramatically accelerated when tested in a Mist1KO background (L. Zhu, G. Shi, and S. Konieczny, unpublished data). These findings suggest that Mist1 also may function as a key anti-cancer factor. Future studies will be designed to elucidate the regulatory pathways that impinge on Mist1 activity in normal pancreas function as well as in disease states.
Supplementary Material
Supplemental Figure 1: The Mist1KO pancreas has decreased p21CIP1/WAF1 protein levels. Protein extracts were harvested from 4 week WT and Mist1KO pancreata. Immunoblots were performed using anti-p21CIP1/WAF1 and anti-β-actin.
Supplemental Figure 2: p21KO mouse pancreata exhibit an increase in acinar cell numbers when compared to control littermates. p21KO and control mouse pancreata were evaluated for the number of acinar cells/area as described in the text.
Supplemental Table I: Knock-down of Mist1 results in shortened cell doubling and G1 times. Cell doubling time was calculated based on cell growth curves. The length of each cell cycle phase was determined by FACS analysis of AR42J-ControlRNAi and AR42J-Mist1RNAi cells.
Supplemental Table II: Mist1KO acinar cells exhibit an increase in acinar cell numbers and in cell proliferation indices. The number of acinar cells/area was quantified in pancreas sections from WT and Mist1KO littermates at the indicated ages. The percentage of p-H3 positive acinar cells was determined by dividing the number of p-H3 cells by the total number of acinar cells per area. Each number corresponds to the average of 10 independent slides.
ACKNOWLEDGEMENTS
We thank Dimitris Kardassis for supplying the p21CIP1/WAF1 promoter construct, Michael Collingswood for generating pcDNA4-TO-Mist1-Myc/His, and Judy Hallett and the Purdue Transgenic Mouse Core Facility for generating the mouse lines used in this study.
Grant Support: This work was supported by grants to S.F.K. from the National Institutes of Health (DK55489, CA124586), the Department of Defense (BC043093), and the Lustgarten Foundation for Pancreatic Research. D.J. was supported by a Purdue Research Foundation Graduate Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist
REFERENCES
- 1.Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 2.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 3.Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- 4.Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 5.Murtaugh LC, Leach SD. A case of mistaken identity? Nonductal origins of pancreatic "ductal" cancers. Cancer Cell. 2007;11:211–213. doi: 10.1016/j.ccr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 7.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 9.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 12.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. Embo J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer L, Hagenbuchle O, Wellauer PK, Strubin M. Nuclear targeting of the transcription factor PTF1 is mediated by a protein subunit that does not bind to the PTF1 cognate sequence. Cell. 1991;67:987–994. doi: 10.1016/0092-8674(91)90371-5. [DOI] [PubMed] [Google Scholar]
- 15.Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masui T, Long Q, Beres TM, Magnuson MA, MacDonald RJ. Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev. 2007;21:2629–2643. doi: 10.1101/gad.1575207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–167. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio E, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004;24:2673–2681. doi: 10.1128/MCB.24.7.2673-2681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran T, Jia D, Sun Y, Konieczny SF. The bHLH domain of Mist1 is sufficient to activate gene transcription. Gene Expression. 2007 doi: 10.3727/000000006780666975. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21(WAF1/Cip1) gene by the p53 tumor suppressor protein. J Biol Chem. 2001;276:29116–29125. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- 23.Rukstalis JM, Kowalik A, Zhu L, Lidington D, Pin CL, Konieczny SF. Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1. J Cell Sci. 2003;116:3315–3325. doi: 10.1242/jcs.00631. [DOI] [PubMed] [Google Scholar]
- 24.Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004 doi: 10.1016/j.mod.2004.01.003. In Press. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, Shin DM, Wang X, Konieczny SF, Muallem S. Aberrant localization of intracellular organelles, Ca2+ signaling, and exocytosis in Mist1 null mice. J Biol Chem. 2005;280:12668–12675. doi: 10.1074/jbc.M411973200. [DOI] [PubMed] [Google Scholar]
- 26.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1123–G1132. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 28.Meggiato T, Calabrese F, Valente M, Favaretto E, Baliello E, Del Favero G. Spontaneous apoptosis and proliferation in human pancreatic cancer. Pancreas. 2000;20:117–122. doi: 10.1097/00006676-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bateman AC, Turner SM, Thomas KS, McCrudden PR, Fine DR, Johnson PA, Johnson CD, Iredale JP. Apoptosis and proliferation of acinar and islet cells in chronic pancreatitis: evidence for differential cell loss mediating preservation of islet function. Gut. 2002;50:542–548. doi: 10.1136/gut.50.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 32.Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci U S A. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kardassis D, Papakosta P, Pardali K, Moustakas A. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J Biol Chem. 1999;274:29572–29581. doi: 10.1074/jbc.274.41.29572. [DOI] [PubMed] [Google Scholar]
- 34.Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, Tyner AL. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci U S A. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodolosse A, Chalaux E, Adell T, Hagege H, Skoudy A, Real FX. PTF1alpha/p48 transcription factor couples proliferation and differentiation in the exocrine pancreas [corrected] Gastroenterology. 2004;127:937–949. doi: 10.1053/j.gastro.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 36.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 37.Funato N, Ohtani K, Ohyama K, Kuroda T, Nakamura M. Common regulation of growth arrest and differentiation of osteoblasts by helix-loop-helix factors. Mol Cell Biol. 2001;21:7416–7428. doi: 10.1128/MCB.21.21.7416-7428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Encinas M, Comella JX, Aldea M, Gallego C. Basic helix-loop-helix proteins bind to TrkB and p21(Cip1) promoters linking differentiation and cell cycle arrest in neuroblastoma cells. Mol Cell Biol. 2004;24:2662–2672. doi: 10.1128/MCB.24.7.2662-2672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC, Ziemiecki A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- 40.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–859. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- 42.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: The Mist1KO pancreas has decreased p21CIP1/WAF1 protein levels. Protein extracts were harvested from 4 week WT and Mist1KO pancreata. Immunoblots were performed using anti-p21CIP1/WAF1 and anti-β-actin.
Supplemental Figure 2: p21KO mouse pancreata exhibit an increase in acinar cell numbers when compared to control littermates. p21KO and control mouse pancreata were evaluated for the number of acinar cells/area as described in the text.
Supplemental Table I: Knock-down of Mist1 results in shortened cell doubling and G1 times. Cell doubling time was calculated based on cell growth curves. The length of each cell cycle phase was determined by FACS analysis of AR42J-ControlRNAi and AR42J-Mist1RNAi cells.
Supplemental Table II: Mist1KO acinar cells exhibit an increase in acinar cell numbers and in cell proliferation indices. The number of acinar cells/area was quantified in pancreas sections from WT and Mist1KO littermates at the indicated ages. The percentage of p-H3 positive acinar cells was determined by dividing the number of p-H3 cells by the total number of acinar cells per area. Each number corresponds to the average of 10 independent slides.